Abstract
Objectives. The purpose of this study was to determine the impact of iron status on cadmium dose among pregnant women.
Methods. Iron status and cadmium concentration in blood, urine, and placenta were determined among women followed for 2 years from early pregnancy.
Results. Blood cadmium and urinary cadmium were correlated with iron status throughout the study period. Urinary cadmium increased longitudinally among women with exhausted iron stores during their pregnancy. The increase in urinary cadmium with age was more pronounced in multiparous than in nulliparous women.
Conclusions. Iron deficiency during pregnancy leads to increased cadmium absorption and body burden. Multiparous women exhibit additional increases with increasing age.
There is growing evidence that current dietary cadmium levels may induce renal tubular damage an end-stage renal disease in the general population.1–4 Associations between cadmium and osteoporosis further emphasize the public health concern.5,6 Cadmium absorption increases when iron stores are depleted,7–10 but the consequences for long-term body burden are not known. Because iron absorption is greatly elevated in late pregnancy, particularly among iron-deficient women,11 there is reason to believe that cadmium uptake is also affected. Placental accumulation of cadmium hinders transfer to the fetus,12 but little else is known about maternal cadmium uptake and body burden related to pregnancy.13–15
In the present prospective study, we investigated concentrations of cadmium in the blood (recent exposure), urine (kidney burden), and placenta in relation to iron status among women followed for 2 years beginning in early pregnancy.
METHODS
We recruited women residing in Stockholm, Sweden, who were aged 20 to 45 years and who were early in their pregnancies; 216 (85%) of the women reported no current smoking.16–18 Study recruitment took place from 1994 to 1996, and data were collected through 1997. Smoking information was validated via analysis of urinary levels of cotinine (NicoMeter Serex, Inc, Maywood, NJ). Blood and urine were collected at gestational weeks 11 and 36 and at 3 days (puerperium), 3 months (lactation), and 15 months (postlactation) postpartum. Also, placentas (n = 106) and cord blood (n = 32) were collected at delivery.
Reasons for study attrition have been described elsewhere.16,18 The women who left the study at various points in time did not differ from women who continued with respect to age, parity, iron status markers, or blood and urine cadmium levels (Mann–Whitney test). For example, blood cadmium levels at gestational week 11 were 0.15 μg/L in those remaining until lactation and 0.16 μg/L in those who left the study (P = .20). The corresponding urinary cadmium levels were 0.30 μg/L and 0.31 μg/L (P = .28).
Iron status measurements included serum ferritin and soluble transferrin receptor.16 Maternal soluble transferrin receptor values above 8.3 mg/L indicate tissue iron deficiency, and a soluble transferrin receptor–serum ferritin ratio of 500 indicates exhausted iron stores.19
The standard error for graphite furnace atomic absorption spectrophotometry measurement7 of blood cadmium was 0.032 μg/L. Six percent of the samples were below the limit of detection (0.05 μg/L). Inductively coupled plasma mass spectrophotometry was used in determining cadmium levels in urine, adjusted to a density of 1.018 g/mL, and cadmium levels in whole homogenized placentas.3,17 Extensive quality control was applied.7
RESULTS
Median cadmium concentrations in early gestation among nonsmokers were 0.16 μg/L (range: below limit of detection to 0.73 μg/L) in blood, 0.31 μg/L (0.11 to 1.1 μg/L) in urine, and 4.8 μg/kg (1.1 to 18.6 μg/kg) in placentas. Because smokers had 4 to 5 times higher blood cadmium levels, they were excluded from further analysis.
In general, cadmium in both blood and urine was negatively correlated with serum ferritin and positively correlated with soluble transferrin receptor and soluble transferrin receptor–serum ferritin ratio (Table 1 ▶). Results were not confounded by age. In late gestation, more than 50% of the women developed exhausted iron stores, and 15% developed tissue iron deficiency. The latter had 40% to 50% higher blood and urinary cadmium levels during the lactation period than those who did not develop tissue iron deficiency (P < .01; Mann–Whitney test).
TABLE 1.
—Correlations Between Cadmium Concentration and Iron Status Among Nonsmoking Women During and After Pregnancy: Stockholm, Sweden, 1997
| GW 11/12 | GW 36/37 | Lactation | Postlactation | |||||
| B-Cd, SCC | U-Cd, SCC | B-Cd, SCC | U-Cd, SCC | B-Cd, SCC | U-Cd, SCC | B-Cd, SCC | U-Cd, SCC | |
| (No. of Samples) | (No. of Samples) | (No. of Samples) | (No. of Samples) | (No. of Samples) | (No. of Samples) | (No. of Samples) | (No. of Samples) | |
| Ferritin | ||||||||
| GW 11 | –0.23*** (210) | –0.02 (188) | –0.14* (120) | –0.21** (114) | –0.16* (97) | –0.22** (84) | –0.09 (56 | –.36* (20) |
| GW 36 | –0.07 (123) | 0.09 (111) | –0.14 (80) | –0.25** (70) | –0.12 (34) | –0.95*** (10) | ||
| Puerperium | –0.16 (54) | –0.34*** (50) | –0.34** (28) | –0.86*** (7) | ||||
| Lactation | –0.20** (99) | –0.10 (85) | –0.42*** (33) | –0.36 (11) | ||||
| Postlactation | –0.10 (56) | –0.006 (20) | ||||||
| Transferrin receptor | ||||||||
| GW 11 | 0.03 (191) | 0.007 (171) | 0.012 (111) | 0.08 (108) | 0.10 (90) | 0.11 (77) | –0.12 (50 | 0.32 (17) |
| GW 36 | 0.08 (122) | 0.02 (110) | 0.24** (78) | 0.22** (69) | 0.30** (33) | 0.93*** (10) | ||
| Puerperium | 0.08 (54) | 0.15 (50) | 0.10 (28) | 0.86*** (7) | ||||
| Lactation | 0.08 (98) | 0.31*** (84) | –0.03 (33) | 0.66** (11) | ||||
| Postlactation | –0.07 (51) | 0.50** (19) | ||||||
| Transferrin receptor/ferritin | ||||||||
| GW 11 | 0.20*** (191) | 0.015 (171) | 0.08 (111) | 0.16* (108) | 0.15* (90) | 0.24** (77) | 0.001 (50) | 0.35* (17) |
| GW 36 | 0.08 (122) | –0.06 (110) | 0.18* (78) | 0.23** (69) | 0.18 (33) | 0.98*** (10) | ||
| Puerperium | 0.15 (54) | 0.28** (50) | 0.32** (28) | 0.89*** (7) | ||||
| Lactation | 0.16* (98) | 0.16* (84) | 0.26* (33) | 0.46* (11) | ||||
| Postlactation | 0.063 (51) | 0.068 (19) | ||||||
Note. GW = gestational week; B-Cd = blood cadmium; U-Cd = urinary cadmium (adjusted to a density of 1.018 g/mL); SCC = Spearman correlation coefficient.
*P ≤ .1; **P ≤ .05; ***P ≤ .01. All P values are 1 = failed.
Longitudinal evaluation showed that blood cadmium concentration increased about 20% from early gestation to lactation and to the postlactation period, irrespective of iron status (P ≤.0.01; repeated measures analysis of variance, designed to handle missing values). Increased urinary cadmium at lactation relative to early gestation (P = .01) was detected only in the iron-depleted group (soluble transferrin receptor–serum ferritin ratio above 500). There was a further increase to the postlactation period (P<.001).
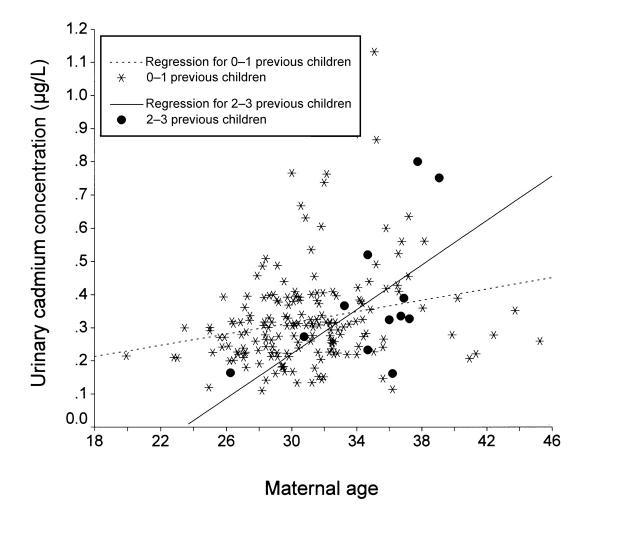
Urinary cadmium levels (Figure 1 ▶) increased with age (r = 0.32, P < .001) and parity (r = 0.22, P = .001). Multiple regression analysis (with normally distributed residuals) showed an interaction between age and parity. While the increase in cadmium level among women with 1 child or no children was 0.009 μg per liter of urine per year (P = .002), women with 2 or 3 children evidenced an additional increase of 0.025 μg per liter of urine per year (P = .046).
FIGURE 1.
—Urinary cadmium concentrations(adjusted to a density of 1.018 g/mL) in nonsmokers at gestational week 12 in relation to maternal age, stratified by parity: Stockholm, Sweden, 1997.
Total placenta cadmium content (but not concentration) was correlated with soluble transferrin receptor–serum ferritin ratio in late gestation (r = 0.25, P = .019; n = 71). It was also correlated with soluble transferrin receptor in cord serum (r = 0.45, P = .01; n = 26).
DISCUSSION
The present longitudinal study of women followed from early pregnancy to postlactation is the first to show a persistent effect of low iron status on body burden of cadmium, as reflected in urinary cadmium concentrations. The effect on urinary cadmium was manifested several months after the onset of exhausted iron stores or iron deficiency and was maintained throughout the study period. Previous cross-sectional studies have revealed associations between iron status and cadmium in blood but not urine.10 This lack of associations may be due to the long half-life of cadmium in the kidney (10–30 years) relative to that in the blood (1–3 months), resulting in slow accumulation.12
In the present study, the causal relationship was supported by the longitudinal increase in urinary cadmium levels only among the women with exhausted iron stores in late gestation. On the basis of this and previous findings showing increased absorption of radiolabeled cadmium at low serum ferritin levels,9 increased blood cadmium with decreasing serum ferritin,7,10,20 and binding of cadmium to the recently identified duodenal iron transporter,21 which is up-regulated by iron deficiency, it seems clear that low iron status leads to increased body burden of cadmium via increased intestinal absorption of dietary cadmium. This is probably the main reason why the body burden of cadmium is generally higher among women,1,2,5,22 whose prevalence of iron depletion is higher than that of men.
The present study involved multiple losses to follow-up, especially during postlactation. However, in all of the tests performed, the women continuing to take part in the study were found to be representative of the recruited group, indicating no discernible selection bias. In addition, associations between blood cadmium levels and iron status were obvious before any attrition occurred. We controlled for possible confounding as a result of age and unreported current smoking, and comprehensive quality control eliminated analytic errors. Moreover, use of the recently introduced soluble transferrin receptor as a marker for iron status diminished many of the uncertainties associated with other indices of iron status during pregnancy.16,23 The soluble transferrin receptor–serum ferritin ratio24 is inversely proportional to iron status,19 thereby reflecting iron absorption.
Interestingly, the effect of age on urinary cadmium concentrations in the present study was much more pronounced among multiparous women than among women with 1 child or no children. This may be explained by deteriorations in iron status as well as other essential elements with increasing parity in these women.16,17 However, pregnancy per se also seemed to increase cadmium uptake, concomitantly with increased iron absorption.11 Our findings can explain why severe cadmium-induced osteomalacia and osteoporosis almost exclusively affected elderly, malnourished, multiparous women who ate cadmium-polluted rice.25
Only 30% of the variation in placental cadmium could be explained by maternal cadmium concentrations (data not shown). The observed negative correlation between placental cadmium content and maternal iron status was probably caused by increasing placental weight with low iron status. We hypothesize that placental cadmium does not increase until the placenta itself is iron deficient. Because iron supply to the placenta and fetus is highly prioritized,26 placental iron deficiency was unlikely in the present study.
However, placental cadmium content, but not placental weight, increased with increasing cord soluble transferrin receptor. The way in which cord soluble transferrin receptor is regulated is not known.18 If the number of receptors in the placenta mediating maternal–fetal iron transport26 correlates with soluble transferrin receptor in cord serum, the results may imply that placental cadmium uptake is mediated by the transferrin receptor.
Taken together, our results support the notion that the higher cadmium burden in women than in men is caused by low iron status and pregnancy. Thus, women with low iron stores, especially those with multiple pregnancies, constitute a risk group for health effects of cadmium such as osteoporosis. This is a major public health problem. High consumption of cereals and vegetables is recommended for a variety of health reasons, yet these foods are the main contributors of dietary cadmium. Every effort must therefore be made to reduce cadmium concentrations in food.
Acknowledgments
This study was supported by grants from the Swedish Environmental Protection Agency, the Council for Swedish Forestry and Agricultural Research, and the Lund University Medical Faculty. The study was carried out in accordance with the Helsinki declaration and was approved by the ethics committee at Karolinska Institutet.
We thank the participants, the midwives, Tuula Eklöf, Brita Palm, Katarina Osman, Bo Nilsson, Birger Lind, Anders Ekholm, Anna Akantis, Elisabeth Berg, and Ingvar Krakau.
All authors contributed to the design and implementation of the study and edited the manuscript. A. Åkesson performed the statistical analyses and wrote the manuscript. M. Vahter made substantial contributions to the interpretations and conclusions.
Peer Reviewed
References
- 1.Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(suppl 1):1–52. [PubMed] [Google Scholar]
- 2.Buchet JP, Lauwerys R, Roels H, et al. Renal effects of cadmium body burden on the general population. Lancet. 1990;336:699–702. [DOI] [PubMed] [Google Scholar]
- 3.Järup L, Hellström L, Alfvén T, et al. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000;57:668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellström L, Elinder CG, Dahlberg B, et al. Cadmium exposure and end-stage renal disease. Am J Kidney Dis 2001;38:1001–1008. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Roels HA, Emelianov D, et al. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet. 1999;353:1140–1144. [DOI] [PubMed] [Google Scholar]
- 6.Alfvén T, Elinder CG, Carlsson MD, et al. Low level cadmium exposure and osteoporosis. J Bone Miner Res. 2000;15:1579–1586. [DOI] [PubMed] [Google Scholar]
- 7.Berglund M, Åkesson A, Nermell B, Vahter M. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Perspect. 1994;102:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahter M, Berglund M, Nermell B, Åkesson A. Bioavailability of cadmium from shellfish and mixed diet in women. Toxicol Appl Pharmacol. 1996;136:332–341. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan PR, McLellan JS, Haist J, Cherian G, Chamberlain MJ, Valberg LS. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology. 1978;74:841–846. [PubMed] [Google Scholar]
- 10.Staessen JA, Vyncke G, Lauwerys RR, et al. Transfer of cadmium from a sandy acidic soil to man: a population study. Environ Res. 1992;58:25–34. [DOI] [PubMed] [Google Scholar]
- 11.Svanberg B. Absorption of iron in pregnancy. Acta Obstet Gynaecol Scand. 1975;48(suppl):1–108. [PubMed] [Google Scholar]
- 12.Environmental Health Criteria 134: Cadmium. Geneva, Switzerland. IPCS, World Health Organization. 1992:280.
- 13.Bonithon-Kopp C, Huel G, Grasmick C, Sarmini H, Moreau T. Effects of pregnancy on inter-individual variations in blood levels of lead, cadmium and mercury. Biol Res Pregnancy Perinatol. 1986;7:37–42. [PubMed] [Google Scholar]
- 14.Hernandez M, Schuhmacher M, Fernandez JD, Domingo JL, Llobet JM. Urinary cadmium levels during pregnancy and postpartum. A longitudinal study. Biol Trace Elem Res. 1996;53:205–212. [DOI] [PubMed] [Google Scholar]
- 15.Lagerkvist BJ, Söderberg HA, Nordberg GF, Ekesrydh S, Englyst V. Biological monitoring of arsenic, lead and cadmium in occupationally and environmentally exposed pregnant women. Scand J Work Environ Health. 1993;19(suppl 1):50–53. [PubMed] [Google Scholar]
- 16.Åkesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am J Clin Nutr. 1998;68:1241–1246. [DOI] [PubMed] [Google Scholar]
- 17.Osman K, Åkesson A, Berglund M, et al. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33:131–138. [DOI] [PubMed] [Google Scholar]
- 18.Åkesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Soluble transferrin receptor: Longitudinal assessment from pregnancy to postlactation. Obstet Gynecol 2002 (in press). [DOI] [PubMed]
- 19.Baynes RD. Refining the assessment of body iron status. Am J Clin Nutr. 1996;64:793–794. [DOI] [PubMed] [Google Scholar]
- 20.Osman K, Schutz A, Åkesson B, Maciag A, Vahter M. Interactions between essential and toxic elements in lead exposed children in Katowice, Poland. Clin Biochem. 1998;31:657–665. [DOI] [PubMed] [Google Scholar]
- 21.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian protoncoupled metal-ion transporter. Nature. 1997;338:482–488. [DOI] [PubMed] [Google Scholar]
- 22.Whittemore AS, DiCiccio Y, Provenzano G. Urinary cadmium and blood pressure: results from the NHANES II survey. Environ Health Perspect. 1991;91:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letsky EA. The haematological system. In: Chamberlain G, Broughton Pipkin F, eds. Clinical Physiology in Obstetrics. Oxford, England: Blackwell Scientific Publications; 1998:71–110.
- 24.Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptorferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92:2934–2939. [PubMed] [Google Scholar]
- 25.Friberg L, Piscator M, Nordberg GF, Kjellström T. Health effects of cadmium in the general environment in Japan. In: Friberg L, Piscator M, Nordberg GF, Kjellström T, eds. Cadmium in the Environment. Cleveland, Ohio: CRC Press Inc; 1974:137–195.
- 26.Petry CD, Wobken JD, McKay H, et al. Placental transferrin receptor in diabetic pregnancies with increased fetal iron demand. Am J Physiol. 1994;267:E507–E514. [DOI] [PubMed] [Google Scholar]