Abstract
Objectives. Prenatal iron supplementation has been the standard recommendation for reducing maternal anemia in developing countries for the past 30 years. This article reviews the efficacy of iron supplementation on hemoglobin levels in pregnant women in developing countries.
Methods. Data from randomized controlled trials published between 1966 and 1998 were pooled. Meta-analyses of the relative change in maternal hemoglobin associated with iron supplementation were stratified by initial hemoglobin levels, duration of supplementation, and daily gestational supplement dose and supplementation with other nutrients.
Results. Iron supplementation raises hemoglobin levels. Its effects are dose dependent and are related to initial hematologic status. The extent to which iron supplementation can reduce maternal anemia is unclear.
Conclusions. The extent to which maternal hemoglobin levels can be increased by recommended prenatal supplementation is limited and has uncertain physiological benefits. Other approaches, including food fortification and prevention and treatment of other causes of anemia, require methodologically rigorous evaluation to find effective answers to this global problem.
Iron-deficiency anemia is the most common nutritional deficiency in the world.1 It is most prevalent in pregnant women, infants, and children. Anemia is caused by inadequate diet (mostly insufficient iron but also dietary deficiencies of folate and vitamin B12); impaired absorption; or blood loss resulting from hemorrhage or helminths or, in women, from menstruation, childbirth, or repeated pregnancies. Nonnutritional anemia also may be caused by thalassemia and other disorders such as malaria and sickle cell disease.
Despite much research, the relationship between anemia and adverse pregnancy outcome is unclear. The evidence that maternal anemia can reduce a pregnant woman's ability to withstand sudden blood loss or that it increases the risk of spontaneous abortion, preterm delivery, low birthweight, and maternal mortality1–4 is inconclusive.5–7 Associations between maternal anemia and adverse pregnancy outcome may be better explained by other factors. For example, women who deliver before the 35th week of gestation (many of whom have low-birthweight babies) will have lower hemoglobin levels because of pregnancy-related hemodilution.2,3 The relationship between maternal anemia and mortality in developing countries may be accounted for by differences in the socioeconomic status of anemic and nonanemic women. Wealthier women eat better, are less anemic, and present themselves more promptly (and in better condition) to health care facilities for delivery or pregnancy termination than poorer women. Women who receive prompt management by trained providers are more likely to survive obstetric complications than those who do not.
Iron supplementation is almost universally recommended during pregnancy to correct or prevent iron deficiency,1,2,8 because dietary consumption of iron is unlikely to meet the daily dietary recommendation of 30 mg.9 Iron absorption generally is poor in otherwise well-nourished women, although it improves in pregnancy. Absorption depends on the form of iron ingested and the composition of the diet (tea and phytates inhibit absorption).3 Heme iron is more efficiently absorbed than nonheme iron, but it is available only from animal foods that generally are relatively expensive and therefore less likely to be consumed by poor women in developing countries. Iron absorption may be particularly poor where parasitic and infectious diseases, including malaria, are prevalent, although absorption may be more efficient in response to iron deficiency.2,9
METHODS
There have been many iron supplementation trials involving pregnant women over the past 30 years. The effects of prenatal oral iron supplementation on proteinuric hypertension, antepartum hemorrhage, maternal infection, short gestation and low birthweight, and pre- and postdelivery hematologic status have been reviewed for a small sample of the available studies, but few of the reviewed studies were from developing countries.5,6 In this paper, we present a comprehensive review of the impact of prenatal iron supplementation on maternal hemoglobin in studies from developed and developing countries published between 1966 and 1998 in refereed journals identified by MEDLINE computer search for the terms “maternal anemia” and “ ‘pregnancy,' ‘hemoglobin or hematocrit,' ‘iron,' or ‘iron supplementation.' ” In this review, we present data on hemoglobin and hematocrit levels, which are the most common measures of anemia but do not distinguish iron deficiency from other causes of anemia.10 Although serum ferritin is thought to be the single measurement that is most indicative of iron stores and thus iron deficiency, hemoglobin and hematocrit levels are the measures used by public health programs to identify maternal anemia.1 The World Health Organization has a uniform definition of anemia in pregnancy—hemoglobin levels below 11.0 g/dL—whereas the US Centers for Disease Control and Prevention defines anemia as hemoglobin levels below 11.0 g/dL, 10.5 g/dL, and 11.0 g/dL in the first, second, and third trimesters, respectively, to reflect normal gestational hematologic variation.2
We identified and reviewed approximately 70 studies of iron supplementation in pregnant women; two thirds of these studies were from developing countries. The criteria we used to determine whether to include a study in our meta-analyses were that the study must have been a randomized controlled trial and must have reported the initial sample size, baseline and follow-up or change in hemoglobin concentrations or hematocrit and their associated variance, the daily dose of supplemental elemental iron, duration of therapy, proportion of women with whom follow-up was conducted, general reasons for loss to follow-up, and additional nutritional supplementation or medical therapy provided. Only 23 studies—15 of which were conducted in developing countries—met these criteria. Almost all studies drew their samples from women attending prenatal clinics; 2 drew their samples from rural antenatal programs.11,12
We conducted meta-analyses by pooling data weighted by the inverse variance method for risk differences (for dichotomous outcome) and for weighted mean differences (for continuous outcome) used by the Cochrane Library of systematic reviews.13 When follow-up was less than 100%, we used the harmonic mean number of subjects at initial visit and follow-up visit.14 We measured the effect of iron supplementation as the difference in the average change in hemoglobin between initial and last gestational measurement in women receiving iron supplementation relative to the average change in hemoglobin in women receiving no iron or other supplementation (except where otherwise specified). Although the most meaningful analysis would be the reduction in the proportion of anemic women associated with iron supplementation, these data were very sparse; we report them whenever possible.
For studies reporting hematocrit only, we calculated hemoglobin (Hb) levels as hematocrit levels divided by 3. We report iron supplementation as the equivalent daily dose (mg) of elemental iron. We stratified the meta-analyses by initial hemoglobin level, dose, and duration of supplementation to reduce the variability of study subjects within each stratum and to demonstrate the effect across strata. We combined data from developed and developing countries in the analysis except where otherwise specified. We calculated the effect of iron supplementation on maternal hemoglobin with the following equation and tested the results by t test and by linear regression, using SPSS for Windows version 9.0 and Stata 6 (SPSS Inc, Chicago, Ill).
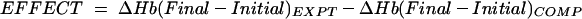 |
We report all effects as mean ± standard error (SE); we report sample size and supplement dose as mean ± standard deviation (SD).
RESULTS
Sample characteristics and data from the trials included in our meta-analyses, as well as data from 6 studies that are not included in the analyses because of the absence of measures of variance, are available from the authors. Some studies have more than 1 experimental group to test the effects of different doses or the additional effect of other nutrients or antimalarial prophylaxis. Most of the studies had small samples and minimal statistical power to detect significant differences. The mean number of participants per study group in the 15 studies conducted in developing countries was 50 ± 43; the mean number of participants in the 8 studies conducted in developed countries was 24 ± 13.
The average baseline Hb level of women in 13 of the 15 randomized controlled trials conducted in developing countries reviewed in this article was less than 11 g/dL—the World Health Organization definition for anemia during pregnancy.15–21
Relative to no supplementation (by any nutrient or medication), iron supplementation alone increased Hb change by 1.00 ± 0.013 g/dL (P < .001, n = 1118, df = 13). The average daily dose of iron in women receiving supplementation was 114 ± 65 mg. In combination with other nutrients or antimalarial prophylaxis, iron supplementation increased Hb change by 1.20 ± 0.023 g/dL (P < .001, n = 1077) relative to no supplementation, and the average daily dose of iron was higher: 132 ± 76 mg (P < .001). In studies reporting serum ferritin change and its variance,16,22–24 iron supplementation alone increased serum ferritin levels by 9.48 ± 0.0174 μ/L (the mean effect on Hb was 0.87 ± 0.017 g/dL with an average daily dose of 132 ± 77 mg iron, n = 578, P < .001) relative to no supplementation. In the few studies reporting iron deficiency,16,17,23 iron supplementation alone reduced the percentage of women with Hb levels of less than 11 g/dL by 38% ± 0.101% (the mean effect on Hb was 1.20± 0.039 g/dL with an average daily dose of 167 ± 82 mg, n = 431, P < .001). All but 1 study,24 with 30 subjects, of the effect of iron supplementation alone used placebos. The average effect of iron supplementation in the subsample of studies using placebos was 0.99 ± 0.013 g/dL (n = 1088) at an average daily dose of iron supplementation of 112 ± 64 mg.
Most studies reported 100% follow-up. However, women who were not followed through the end of pregnancy actually were excluded from analysis by these studies. This methodology produces comparable groups for those who completed the trial but reduces the generalizability of the studies. Poor follow-up may affect validity because many women included in the reported initial mean data were not included in the reported final mean data. The effect of iron supplementation alone on maternal levels in studies with at least 75% follow-up was 0.98 ± 0.183 g/dL (n = 1063), compared with 1.64 ± 0.077 g/dL (n = 56, P < .001) in studies with follow-up rates of less than 75%; the mean daily doses also were different (114 ± 65 mg vs 124 ± 66 mg, P < .05). Studies with less than 75% follow-up contribute to only 5% of the sample in studies in which women received iron supplementation alone.17
Exclusions within studies could have variable effects on findings. Exclusion of women with premature deliveries—as in most studies—would underestimate the effects of supplementation if iron supplementation reduces the incidence of prematurity, but there is no evidence of this effect.5–7 Excluding women who developed anemia and were treated with iron leaves a group of women with higher Hb levels in the study. Excluding women from the final sample who were included in the initial sample but developed anemia and were treated would be expected to occur more often in the comparison group than in the iron supplementation group, but the effects of this bias cannot be determined beyond recognition that follow-up was similar in these groups.
Adherence to the regimen affects the effectiveness of the supplementation.2,25,36 Side effects of iron supplementation include constipation, diarrhea, vomiting, or epigastric pain. These effects are reported to increase with dose21,26 and may have caused some women to abandon therapy or take less than the recommended dose. Although many of the studies we reviewed report that adherence was a problem, few measured compliance. Adherence to iron supplementation was found to be poor (approximately 42%) in a study that carefully measured this factor in Tanzania27; it was better (61%) with a slow-release gastric delivery system in another study.21
Initial Hemoglobin Status
The effect of iron supplementation was greater in women from developing countries with initial levels of less than 11 g/dL relative to unsupplemented women (P < .001). The effect of iron supplementation in women with initial Hb values of less than 10 g/dL18 and 10 to less than 11 g/dL11,13,17 was 1.13 ± 0.120 g/dL (P < .05) and 1.10 ± 0.045 g/dL, respectively (P < .001, Table 1 ▶). The effect was smaller—0.85 ± 0.018 g/dL (P < .001)—in women from developing countries with initial levels of 11 to less than 12 g/dL.17,23 No data were available on iron supplementation alone for women from developing countries with initial levels of 12 g/dL or higher.
TABLE 1.
—Relative Hemoglobin Change by Initial Hemoglobin (Hb) Level: Pregnant Women, 1966–1998
| Initial Hb (g/dL) | Daily Dose (mg), Mean (SD) | Effect (g/dL), Mean (SE) | Experimental (n) | Comparison (n) | References in Analysis (df) |
| Developing Countriesa | |||||
| <10 | 153 (543) | 1.13(0.120)* | 147 | 108 | 16,18 (1) |
| ≥10–<11 | 134 (76) | 1.10 (0.045)*** | 148 | 128 | 11,15–17 (3) |
| ≥11–<12 | 71 (33) | 0.85 (0.018)** | 140 | 135 | 17,22 (1) |
| ≥12 | NA | NA | NA | NA | NA |
| Developed Countriesa | |||||
| <10 | NA | NA | NA | NA | NA |
| ≥10–<11 | NA | NA | NA | NA | NA |
| ≥11–<12 | 64 (47) | 1.17 (0.022)** | 26 | 69 | 28,29 (2) |
| ≥12 | 162 (49) | 1.16 (0.045)*** | 61 | 57 | 23,24,30 (2) |
Note. NA = Not available.
aComparison group received no iron supplementation.
*P < .05; **P < .01; ***P < .001.
There were no studies of women in developed countries with low initial Hb levels. The effect of iron supplementation alone in women in developed countries with initial mean Hb levels of more than 11 g/dL to less than 12 g/dL28,29 and at least 12 g/dL24,25,30 averaged 1.17 ± 0.022 g/dL and 1.16 ± 0.045 g/dL above unsupplemented comparisons (both P < .001), respectively.
Dose–Response
Based on studies from developed and developing countries, a positive dose–response relationship exists between iron dose and change in studies with unsupplemented comparison groups (P < .001, Table 2 ▶). Women receiving no more than 60 mg daily iron supplementation alone, with an average daily dose of 42 ± 23 mg iron,11,28,39 had a 0.41 ± 0.027 g/dL increase in change compared with unsupplemented women (P < .01, Table 2 ▶). Women receiving between 61 and 90 mg daily iron supplementation with an average dose of 76 ± 4 mg iron20,32 showed an average effect of 0.86 ± 0.018 g/dL (P < .01). Women receiving between 91 and 120 mg daily iron supplementation with an average dose of 117 ±6 mg iron15,16,18,29,30 had an average effect of 1.87 ± 0.027 g/dL (P < .001), and those receiving more than 120 mg daily iron supplementation with an average dose of 223±20 mg iron16,17,23,24 had an average increase of 1.78 ± 0.042 g/dL (P < .001), compared with women not receiving supplementation.
TABLE 2.
—Relative Hemoglobin Change by Daily Dose of Iron Supplementation: Pregnant Women, 1966–1998
| Daily Dose (mg) | Daily Dose (mg), Mean (SD) | Effect (g/dL), Mean (SE) | Experimental (n) | Comparison (n) | References in Analysis (df) |
| ≤60 | 42 (23) | 0.41 (0.027)** | 135 | 131 | 11,28,29 (2) |
| 61–90 | 76 (4) | 0.86 (0.018)* | 140 | 135 | 17,22 (1) |
| 91–120 | 117 (6) | 1.87 (0.027)*** | 214 | 164 | 14,18,29,30 (3) |
| >120 | 223 (20) | 1.78 (0.042)*** | 133 | 119 | 16,17,23,24 (4) |
Note. Comparison group received no iron supplementation.
*P < .05; **P < .01; ***P < .001.
Only 2 studies compared hematologic change associated with iron alone in women receiving higher (167 ± 26 mg) versus lower (45 ± 22 mg) average daily supplementation.29,31 These 2 studies showed a 0.33 ± 0.012 g/dL improvement with higher iron supplementation (P < .05).
Duration of Therapy
Studies from developed and developing countries providing up to 10 weeks,18,22 11 to 13 weeks,17 and 14 to 19 weeks15,16,29 of iron supplementation alone found benefits of 0.84 ± 0.017 g/dL (P < .001), 1.37 ± 0.062 g/dL (P < .05), and 1.16 ± 0.022 g/dL (P < .001, Table 3 ▶), respectively, relative to no supplementation. The effect of 20 weeks or more of iron supplementation in women in developed countries23,24,28,30 (1.00 ± 0.039 g/dL, P < .001) was smaller than that in other women receiving more than 10 weeks but less than 20 weeks of supplementation. The effect of duration of supplementation is attributable to differences in iron supplementation dose (data not shown).
TABLE 3.
—Relative Hemoglobin Change by Duration of Therapy of Iron Supplementation: Pregnant Women, 1966–1998
| Duration of Supplementation (Weeks) | Daily Dose (mg), Mean (SD) | Effect (g/dL), Mean (SE) | Experimental (n) | Comparison (n) | References in Analysis (df) |
| ≤10 | 90 (24) | 0.84 (0.017)*** | 292 | 249 | 11,18,22 (2) |
| 11–13 | 124 (66) | 1.37 (0.062)* | 33 | 23 | 17 (1) |
| 14–19 | 138 (87) | 1.16 (0.022)*** | 182 | 161 | 15,16,29 (4) |
| ≥20 | 136 (62) | 1.00 (0.039)*** | 74 | 82 | 23,24,28,30 (3) |
Note. Comparison group received no iron supplementation.
*P < .05; ***P < .001.
Iron and Folate
Women receiving combined average daily iron (176 ± 65 mg) and folate (5 ± 0 mg) supplementation had 1.37 ± 0.093 g/dL ( df = 8, P < .001) better change than did women not receiving supplementation,15,16,28 but there was no additional effect (–0.07 ± 0.009 g/dL, df = 19, P < .001) from folate (5 ± 0 mg with 170 ± 64 mg iron) compared with iron supplementation alone (158 ± 74 mg in those without folate).15,16,28,31 The combined effect of folate and iron supplementation (average daily doses: 66 ± 18 mg iron and 4.6 ± 0.5 mg folate) on maternal hematologic change above folate supplementation alone (average daily dose: 4.7 ± 0.4 mg folate) was 1.22 ± 0.047 g/dL (df = 3, P < .001).12,15,21,28
Iron and Vitamin C
Combining iron and vitamin C supplementation (average daily dose 113 ± 64 mg iron and 100 ± 0 IU vitamin C) had no demonstrable benefit, 0.10 ± 0.104 g/dL (not significant [NS]), compared with women in the same 2 small studies17 receiving iron supplementation alone (average daily dose 126 ± 66 mg, NS).
Iron and Antimalarials
All of the studies that examined the effects of iron supplementation in combination with antimalarial prophylaxis (for Plasmodium vivax) were conducted in sub-Saharan Africa,16,39,40 but only 1 very small (n = 10) study reported measures of variance. Antimalarials in combination with 30 mg iron per day alone had no effect on improving Hb (–0.01 ± 0.473 g/dL, NS) in comparison with antimalarials alone.
Iron and Vitamin B12
One study33 found that vitamin B12 supplementation in combination with iron and folate supplementation improved Hb by 1.20 ± 0.134 g/dL, 1.35 ± 0.160 g/dL, 1.63 ± 0.162 g/dL, and 1.76 ± 0.172 g/dL (all P < .001) in groups receiving daily 30, 60, 120, and 240 mg iron supplementation, respectively, and 5 mg folate compared with no supplementation. The additional effect of combined folate and B12 in women receiving 120 mg daily iron was 0.54 ± 0.170 g/dL (P < .001). Vitamin B12 and folate without iron supplementation had a smaller effect in this sample (0.15 ± 0.131 g/dL, NS).
Iron and Thiamine
One very small study in which women received antimalarial prophylaxis20 found that thiamine with or without iron supplementation was associated with a 1.70 to 1.75 g/dL (NS) decrement in Hb change.
Iron and Vitamin A
In one study11 the effect of adding 2.4 mg retinol to 60 mg iron daily increased Hb change by an additional 0.50 ± 0.054 g/dL (P < .001).
Iron and Multivitamins
No data were available to compare the effects of multivitamins with iron with the effects of iron supplementation alone. Two studies18,32,34,39 estimated the effects of iron (average daily dose 72 ± 7 mg) and multivitamin supplementation compared with multivitamin supplementation alone and found increases in Hb change of 1.37 ± 0.059 (df = 1, P < .05). This effect (and average daily dose) is similar to those observed with iron supplementation alone.
DISCUSSION
The data we reviewed demonstrate that iron supplementation during pregnancy increases hemoglobin and serum ferritin levels. The decrease in hemoglobin levels that normally occurs in the second trimester of pregnancy3 has been shown to be partially reversed in controlled studies of well-nourished and malnourished pregnant women receiving iron supplementation. The gestation at which supplementation occurs does not affect our findings beyond the effect of dose and duration of supplementation; hematologic samples were obtained at the same gestational age for control and supplemented groups (in essence controlling for gestational differences between the groups). The effect of iron supplementation is directly related to dose. The statistical significance of the results is largely an effect of data pooling or large differences in smaller, unrepresentative samples. Substantial benefits, however, are evident only with supplemental doses of more than 91 mg per day—higher than the daily multivitamins with iron (18 mg iron) or the recommended daily supplement dose of 60 mg but less than the less-usual but recommended 120 mg iron. The effect also is slightly more pronounced in women with lower initial hemoglobin levels. The effect of duration of therapy was mediated by dose. Inferences about the effects of other nutrients in addition to iron are limited because data from multiple studies are available only for folate and vitamin C. These studies show that iron supplementation was not greatly improved by folate or vitamin C supplementation. Inferences about the effect of iron supplementation on maternal anemia also are limited by the small number of studies and subjects from which these data were available.
The prevalence of maternal anemia globally has remained high over the past 30 years.35-37 Oral iron supplementation programs have been hindered by many factors, including supply problems, limited duration of therapy, and poor adherence to regimens.2,25,26
Recent recommendations include providing weekly supplements to improve adherence and extending the duration or increasing the dose of iron supplementation to achieve greater effect.1,2 Prolonging the duration of supplementation has limitations because studies have shown that adherence declines consistently with increased duration of pill-taking regimens.38–41 Weekly supplements appear to be slightly less efficacious than daily supplements.42 Furthermore, severely anemic women who are diagnosed late in pregnancy may require alternative therapeutic strategies such as intramuscular or intravenous iron to correct anemia, which are not viable public health strategies.43
It has been suggested that patient and provider education, community-based and mass media promotion, and beautification of oral iron supplements and their packaging will improve compliance.2 Data from developing countries are limited, but numerous studies in developed countries indicate that these tactics have minimal effect on compliance.38–41 Extensive and expensive communications efforts to promote oral iron supplements to women in rural Indonesia had virtually no effect on reported adherence beyond that attained by an inexpensive system to improve the availability of the supplements at the village level.44 Compliance in the Indonesia study was so low that little improvement in individuals' hematologic status could be expected. Supervision had no impact on the effectiveness of iron supplementation in Thailand, whereas supervision increased the effect of high-dose iron supplementation in Burma.16
It is time to question recommendations for large-scale, public health oral iron supplementation programs as a means of reducing global maternal anemia.7,42 Methodologically rigorous evaluation is needed to determine the physiological benefits of iron supplementation and the effectiveness of other approaches—including prevention of hookworm infection,1 food fortification,45 and prenatal prophylactic treatment for falciparum malaria46—in reducing maternal anemia.
Acknowledgments
This work was supported by the MotherCare Project under USAID contract DPE-5966-Z-00–8083–00, project 936–5966, and the Population Council.
N. L. Sloan, E. Jordan, and B. Winikoff conceptualized the meta-analysis and wrote the report. N. L. Sloan conducted the data analysis and, together with E. Jordan, conducted the literature review.
Peer Reviewed
References
- 1.Stoltzfus RJ, Dreyfuss ML. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington, DC: International Nutritional Anemia Consultative Group, International Life Sciences Institute; 1998.
- 2.Institute of Medicine. Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990.
- 3.Rosso P. Nutrition and Metabolism in Pregnancy: Mother and Fetus. New York, NY: Oxford University Press Inc; 1990.
- 4.Llewellyn-Jones D: Severe anaemia in pregnancy. Aust N Z J Obstet Gynecol. 1965;5:191–197. [DOI] [PubMed] [Google Scholar]
- 5.Mahomed K, Hytten F. Iron and folate supplementation in pregnancy. In: Chalmers I, Enkin M, Keirse MJNC, eds. Effective Care in Pregnancy and Childbirth. New York, NY: Oxford University Press Inc; 1989:301–317.
- 6.World Health Organization. The WHO Reproductive Health Library No. 2. Geneva, Switzerland: World Health Organization; 1999.
- 7.Rush D. Nutrition and maternal mortality in the developing world. Am J Clin Nutr. 2000;1(suppl):212S–240S. [DOI] [PubMed] [Google Scholar]
- 8.Preventing Iron Deficiency in Women and Children: Technical Consensus on Key Issues. New York, NY: United Nations Children's Foundation; 1999.
- 9.National Research Council. Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press; 1989.
- 10.International Nutritional Anemia Consultative Group. Measurements of Iron Status. Washington, DC: Nutrition Foundation; 1985.
- 11.Suharno D, West CE, Muhilal, et al. Supplementation with vitamin A and iron for nutritional Anaemia in Pregnant Women in West Java, Indonesia. Lancet. 1993;342:1325–1328. [DOI] [PubMed] [Google Scholar]
- 12.Menendez C, Todd J, Alonso PL, et al. The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Trans R Soc Trop Med Hyg. 1994;88:590–593. [DOI] [PubMed] [Google Scholar]
- 13.Deeks J. Statistical methods programmed in Meta View version 4. In: Mulrow CD, Oxman AD, eds. Cochrane Collaboration Handbook. Oxford, England: Update Software; 2000:Appendix.
- 14.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977.
- 15.Aung-than-Batu, Thane-Toe, Hla-Pe, Khin-Kyi-Nyunt. A prophylactic trial of iron and folic acid supplements in pregnant Burmese women. Isr J Med Sci. 1976; 12:1410–1417. [PubMed] [Google Scholar]
- 16.Charoenlarp P, Dhanamitta S, Kaewvichit R, et al. A WHO Collaborative Study on Iron Supplementation in Burma and in Thailand. Am J Clin Nutr. 1988;47:280–97. [DOI] [PubMed] [Google Scholar]
- 17.Kuizon MD, Platon TP, Ancheta LP, Angeles JC, Nunez CB, Macapinlac MP. Iron supplementation studies among pregnant women. Southeast Asian J Trop Med Public Health. 1979;10:520–527. [PubMed] [Google Scholar]
- 18.Sood SK, Ramachandran K, Mathur M, et al. WHO sponsored collaborative studies on nutritional anaemia in India. Q J Med. 1975;44:241–258. [PubMed] [Google Scholar]
- 19.Thane-Toe, Thein-Than. The effects of oral iron supplementation on ferritin levels in pregnant Burmese women. Am J Clin Nutr. 1982;35:95–99. [DOI] [PubMed] [Google Scholar]
- 20.Powers HJ, Bates CJ, Lamb WH. Haematological response to supplements of iron and riboflavin to pregnant and lactating women in rural Gambia. Hum Nutr Clin Nutr. 1985; 39C:117–129. [PubMed] [Google Scholar]
- 21.Simmons WK, Cook JD, Bingham KC, et al. Evaluation of a gastric delivery system for iron supplementation in pregnancy. Am J Clin Nutr. 1993;58:622–626. [DOI] [PubMed] [Google Scholar]
- 22.Freire WB. Hemoglobin as a predictor of response to iron therapy and its use in screening and prevalence estimates. Am J Clin Nutr. 1989;50:1442–1449. [DOI] [PubMed] [Google Scholar]
- 23.Romslo I, Haram K, Sagen N, Augensen K. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynecol. 1983;90:101–107. [DOI] [PubMed] [Google Scholar]
- 24.Puolakka J. Serum ferritin as a measure of iron stores during pregnancy. Acta Obstet Gynecol Scand Suppl. 1980;95:1–31. [DOI] [PubMed] [Google Scholar]
- 25.Cook J, Skikne BS, Baynes RD. Iron deficiency: the global perspective. In: Hershko C, ed. Progress in Iron Research. New York, NY: Plenum Press; 1994:219–228. [DOI] [PubMed]
- 26.Galloway R, McGuire J. Determinants of compliance with iron supplementation: supplies, side-effects, or psychology? Soc Sci Med. 1994;39:381–390. [DOI] [PubMed] [Google Scholar]
- 27.Ekstrom EC, Kavishe FP, Habicht JP, et al. Adherence to iron supplementation during pregnancy in Tanzania: determinants and hematologic consequences. Am J Clin Nutr. 1996;64:368–374. [DOI] [PubMed] [Google Scholar]
- 28.Fleming AF, Martin JD, Hahnel R, Westlake AJ. Effects of iron and folic acid antenatal supplements on maternal haematology and fetal well-being. Med J Aust. 1974;4:429–436. [DOI] [PubMed] [Google Scholar]
- 29.Paintin DB, Thomson AM, Hytten FE. Iron and the haemoglobin level in pregnancy. J Obstet Gynecol Br Commonw. 1966;73:181–190. [Google Scholar]
- 30.Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L. Absorption of supplemental iron during pregnancy—a longitudinal study with repeated bonemarrow studies and absorption measurements. Acta Obstet Gynecol Scand Suppl. 1975;48:87–108. [DOI] [PubMed] [Google Scholar]
- 31.Srisupandit S, Pootrakul P, Areekul S, et al. A prophylactic supplementation of iron and folate in pregnancy. Southeast Asian J Trop Med Public Health. 1983;14:317–323. [PubMed] [Google Scholar]
- 32.Dommisse J, Bell DJ, du Toit ED, Midgely V, Cohen M. Iron-storage deficiency and iron supplementation in pregnancy. S Afr Med J. 1983;64(27):1047–1051. [PubMed] [Google Scholar]
- 33.Fleming AF, Ghatoura GBS, Harrison KA, et al. The prevention of anaemia in pregnancy in primigravidae in the Guinea Savanna of Nigeria. Ann Trop Med Parasitol. 1986;80:211–233. [DOI] [PubMed] [Google Scholar]
- 34.Cantlie GSD, de Leeuw NKM, Lowenstein L. Iron and folate nutrition in a group of private obstetrical patients. Am J Clin Nutr. 1971;24:637–641. [DOI] [PubMed] [Google Scholar]
- 35.DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38:302–316. [PubMed] [Google Scholar]
- 36.Royston E. The prevalence of nutritional anaemia in women in developing countries: a critical review of available information. World Health Stat Q. 1982;35:52–91. [PubMed] [Google Scholar]
- 37.The Prevalence of Anaemia in Women: A Tabulation of Available Information. 2nd ed. Geneva, Switzerland: World Health Organization; 1992.
- 38.Cramer JA, Spilker B. Patient Compliance in Medical Practice and Clinical Trials. New York, NY: Raven Press; 1991.
- 39.Potter L, Wright S, Berrio D, et al. Oral contraceptive compliance in rural Colombia: knowledge of users and providers. Int Fam Plann Perspect. 1988;14(1):27–31. [Google Scholar]
- 40.Sbarbaro JA. The patient-physician relationship: compliance revisited. Ann Allergy. 1990;64:325–332. [PubMed] [Google Scholar]
- 41.Sloan NL. Pill Taking Behavior: Implications for Micronutrient Supplementation for Safe Motherhood. New York, NY: Population Council; 1998.
- 42.Beaton GH, McCabe GP. Efficacy of Intermittent Iron Supplementation in the Control of Iron Deficiency Anemia in Developing Countries: An Analysis of Experience. Ottawa, Canada: The Micronutrient Initiative; 1999.
- 43.Becker CE, MacGregor RR, Walker KS, Jandl JH. Fatal anaphylaxis after intramuscular iron-dextran. Ann Intern Med. 1966;65:745–748. [DOI] [PubMed] [Google Scholar]
- 44.Utomo B, Riono P, Budiono T, et al. The Alleviation of Maternal Anemia in Indramayu Regency, Indonesia. Arlington, VA: John Snow Inc; 1993. MotherCare Working Paper 23.
- 45.Sommer A, West KP Jr. Fortification of dietary items with vitamin A. In: Vitamin A Deficiency: Health, Survival and Vision. New York, NY: Oxford University Press Inc; 1996:410–431.
- 46.Schulman CE, Dorman EK, Cutts F, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353:632–636. [DOI] [PubMed] [Google Scholar]


