Abstract
Objectives. This study measured age-specific seroprevalence of HIV, hepatitis B virus, and hepatitis C virus (HCV) infection among injection drug users (IDUs) admitted to drug treatment programs in 6 US cities.
Methods. Remnant sera collected from persons entering treatment with a history of illicit drug injection were tested for antibodies to HIV, hepatitis C (anti-HCV), and hepatitis B core antigen (anti-HBc).
Results. Prevalence of anti-HBc and anti-HCV increased with age and reached 80% to 100% among older IDUs in all 6 cities. Although overall age-specific HIV prevalence was lower than anti-HCV or anti-HBc, this prevalence was greater in the Northeast than in the Midwest and West.
Conclusions. The need continues for effective primary prevention programs among IDUs specifically targeting young persons who have recently started to inject drugs. (Am J Public Health. 2002;92:385–387)
Injection drug use has played an important role in parenteral transmission of HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV). Several studies have described high prevalence of antibodies to HCV (anti-HCV) and hepatitis B core antigen (anti-HBc) among injection drug users (IDUs) in the United States and other countries1–3 and have measured coinfection rates of HIV, HBV, and HCV.1,2,4–6
Sera were originally collected from 1993 to 1994 for the Centers for Disease Control and Prevention (CDC) national HIV seroprevalence surveys to measure the prevalence of anti-HBc, anti-HCV, and HIV among IDUs entering treatment in 6 US cities: Newark, NJ; Baltimore, Md; Detroit, Mich; Denver, Colo; San Francisco, Calif; and Seattle, Wash. By using standardized methods7,8 for measuring prevalence in these 6 cities, we added age-specific and geographic data to the existing body of information about the national HBV, HCV, and HIV endemic among IDUs.
METHODS
We used remnant sera collected from persons entering treatment for use of illicit drugs other than alcohol during the CDC's 1993 to 1994 national HIV seroprevalence surveys of IDUs. These serum samples were tested for HIV antibodies after all personal identifiers (client's name, medical record number) had been permanently removed.7 In consultation with state and local health departments, sites (primarily methadone maintenance programs) were selected on the basis of geographic representation and population characteristics. Limited information on demographic characteristics and drug use was abstracted from client files at each survey site.
Routine HIV-1 antibody testing was done with an enzyme immunoassay licensed by the US Food and Drug Administration. Sera that were repeatedly reactive were confirmed by Western blot. Western blot band patterns were interpreted according to the recommendations of the Association of Public Health Laboratories and the CDC.9
After HIV testing, stored sera were tested for anti-HBc and anti-HCV at the Hepatitis Reference Laboratory, CDC. Anti-HBc testing was performed with a radioimmunoassay (CORAB; Abbott Laboratories, Inc, Abbott Park, North Chicago, Ill), and anti-HCV testing was done with an enzyme immunoassay (HCV EIA 2.0; Abbott Laboratories, Inc); the first 100 specimens that were repeatedly reactive were tested with a supplemental assay (HCV MATRIX; Abbott Laboratories, Inc). Because 99 of the 100 repeatedly reactive specimens were also positive with the supplemental assay, the remainder of the specimens was considered anti-HCV positive if repeatedly reactive by enzyme immunoassay.
Prevalence was calculated by city and demographic group; age was categorized into 5-year groups.
RESULTS
Demographic characteristics and prevalence of HIV, anti-HBc, and anti-HCV among the 1717 persons tested in the 6 cities are shown in Table 1 ▶. Overall, 35% of the persons tested were female, 37% were younger than 35, and 43% were White. A higher proportion of the sampled IDUs were younger than 35 years in San Francisco (49%), Newark (40%), and Seattle (45%) than in the other cities. HIV seroprevalence was 28% to 29% in cities in the Northeast, compared with 3% to 5% in cities in the Midwest and West. However, the prevalence of anti-HBc (50%–81%) and anti-HCV (66%–93%) was high in all geographic regions.
TABLE 1—
Demographic Characteristics and Prevalence of HIV, Anti-HBc, and Anti-HCV Among Injection Drug Users Admitted to Drug Treatment, by US City, 1993–1994
| Total | Newark | Baltimore | Detroit | Denver | San Francisco | Seattle | |
| (N = 1717), % | (n = 300), % | (n = 267), % | (n = 235), % | (n = 289), % | (n = 355), % | (n = 271), % | |
| Female | 35 | 32 | 35 | 29 | 38 | 33 | 46 |
| White | 43 | 27 | 18 | 9 | 61 | 67 | 64 |
| <35 y | 37 | 40 | 35 | 20 | 24 | 49 | 45 |
| HIV positive | 12 | 29 | 28 | 3 | 3 | 5 | 3 |
| Anti-HBc positive | 64 | 60 | 81 | 60 | 72 | 50 | 62 |
| Anti-HCV positive | 79 | 70 | 93 | 66 | 92 | 69 | 84 |
Note. Anti-HBc = antibody to hepatitis B core antigen; anti-HCV = antibody to hepatitis C virus.
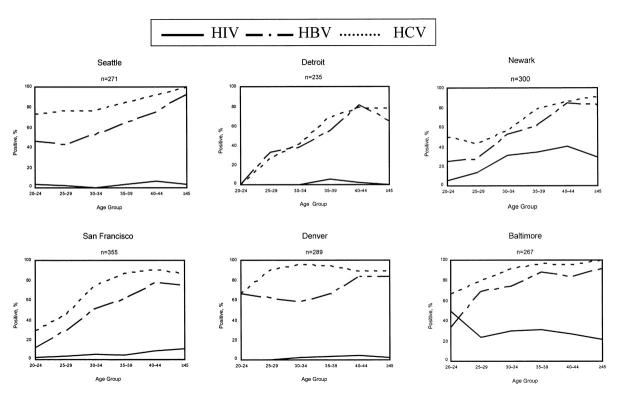
The prevalence of anti-HBc and anti-HCV increased with age and reached 80% to 100% among older IDUs in all 6 cities (Figure 1 ▶). Prevalence of anti-HCV was consistently higher than that of anti-HBc across all age groups in the 6 cities. In contrast, HIV seroprevalence also increased with age but peaked at 30% to 40% in Newark and Baltimore and at approximately 5% in the other 4 cities.
FIGURE 1—
Prevalence of HIV, antibody to hepatitis B core antigen (anti-HBc), and antibody to hepatitis C virus (anti-HCV) among injection drug users admitted to drug treatment, by age group and city, 1993–1994.
Seroprevalence of HIV did not differ significantly by sex or race/ethnicity in the 6 cities (data not shown). Anti-HBc prevalence was significantly higher in non-White than in White persons in Baltimore (85% vs 63%: χ2 = 12.8, P < .01), Seattle (74% vs 55%: χ2 = 9.5, P < .01), and San Francisco (71% vs 40%: χ2 = 31.0, P < .01). Anti-HBc seroprevalence was higher in men than in women in San Francisco (56% vs 39%: χ2 = 9.1, P < .01). Anti-HCV seroprevalence differed significantly between non-White and White persons in San Francisco (87% vs 60%: χ2 = 28.3, P < .01) and between men and women in Baltimore (96% vs 88%: χ2 = 6.1, P = .01).
DISCUSSION
Our finding that HIV, anti-HBc, and anti-HCV prevalence increased with age is consistent with the results of previous studies and suggests that the prevalence of infection is associated with duration of drug use.1,10 Longer duration of injection drug use may lead to more sharing of needles and other equipment such as cotton and cookers, resulting in a greater likelihood of transmission.10,11
Among IDUs entering treatment in 6 cities in the United States during 1993 to 1994, the greater HIV seroprevalence in the Northeast compared with that in the Midwest and West has been observed in other studies.7,8 In contrast, HBV and HCV prevalence rates were high in all 6 cities.
The higher overall prevalence of HBV and HCV than of HIV in IDUs in all 6 cities was likely related to the size of the reservoirs of these viruses in these communities. Geographic differences in HIV prevalence among IDUs in the United States have remained remarkably consistent since at least the late 1980s,8 an observation that has not been explained. One hypothesis is that higher HIV prevalence is observed in regions with higher population densities; larger social networks in these areas could increase risk for contact with a person who is HIV infected.12
Our study population was limited to a selected group of IDUs who were entering drug treatment programs in 6 US cities. Therefore, the observed seroprevalences of HIV, HBV, and HCV are not generalizable to all IDUs in these 6 cities. IDUs in treatment programs tend to be older than those not in treatment programs because programs usually admit only clients with well-established drug habits; therefore, these seroprevalence rates may be different from those among IDUs in general. However, the observed geographic differences were consistent with those observed in other studies.1,6,13
Given the high prevalence of both HBV and HCV among IDUs, effective primary prevention programs are needed because the likelihood that an uninfected novice IDU will come in contact with an infected IDU is great. Such programs should offer hepatitis B vaccination, provide treatment to stop the use of illicit drugs, and promote the use of sterile needles and the nonsharing of equipment used to prepare and inject drugs (cotton, cookers, and water) for those who are unable to discontinue injection drug use.14 To prevent HBV and HCV infection among IDUs, these programs will need to target young persons who have recently started to inject drugs. Effective programs also should use street-based interventions to locate IDUs currently not accessing treatment programs.15 This approach applies especially to younger IDUs without well-established habits. Intervention research should be conducted to monitor the effectiveness of such programs.
Acknowledgments
We gratefully acknowledge the contributions of the following collaborative state and local health departments: Seattle–King County Health Department, San Francisco Department of Public Health, Colorado Department of Public Health, City of Detroit Health Department, Michigan Department of Public Health, New Jersey Department of Health and Senior Services, and Maryland Department of Health and Mental Hygiene. We also thank Steve Lambert at the Centers for Disease Control and Prevention for performing serologic hepatitis testing and Janet Royalty, Stephanie Behel, and Amy Metzger for additional data management and analytical support.
C. S. Murrill was the principal author. H. Weeks coordinated specimen and data collection. B. C. Castrucci assisted with data analyses. H. S. Weinstock was technical adviser at the time of study design and implementation and of data analyses. B. P. Bell was Hepatitis Program adviser and provided editorial support. C. Spruill provided logistical support at the time of specimen collection and testing at the Centers for Disease Control and Prevention. M. Gwinn was senior technical adviser and provided editorial support for the final draft.
Peer Reviewed
References
- 1.Garfein R, Vlahov D, Galai N, et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loxley W, Phillips M, Carruthers S, Bevan J. The Australian study of HIV and injecting drug use, part 1: prevalence for HIV, hepatitis B and hepatitis C among injecting drug users in four Australian cities. Drug Alcohol Rev. 1997;16:207–214. [DOI] [PubMed] [Google Scholar]
- 3.Crofts N, Nigro L, Oman K, et al. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction. 1997;92:999–1005. [PubMed] [Google Scholar]
- 4.Levine O, Vlahov D, Brookmeyer R, et al. Differences in the incidence of hepatitis B and human immunodeficiency virus infections among injecting drug users. J Infect Dis. 1996;173:579–583. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa de Carvalho H, Mesquita F, Massad E, et al. HIV and infections of similar transmission patterns in a drug injectors community of Santos, Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:282–289. [DOI] [PubMed] [Google Scholar]
- 6.Zeldis J, Jain S, Kuramoto I, et al. Seroepidemiology of viral infections among intravenous drug users in northern California. West J Med. 1992;156:30–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Prevots D, Allen D, Lehman J, et al. Trends in human immunodeficiency virus seroprevalence among injecting drug users entering drug treatment centers, United States, 1988–93. Am J Epidemiol. 1996;143:733–742. [DOI] [PubMed] [Google Scholar]
- 8.National HIV Prevalence Surveys, 1997 Summary. Atlanta, Ga: Centers for Disease Control and Prevention; 1998:11.
- 9.Centers for Disease Control and Prevention. Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep. 1989;38(S-7):1–7. [Google Scholar]
- 10.Levine O, Vlahov D, Koehler J, et al. Seroepidemiology of hepatitis B virus in a population of injecting drug users: association with drug injecting patterns. Am J Epidemiol. 1995;142:331–341. [DOI] [PubMed] [Google Scholar]
- 11.Stark K, Bienzle U, Vonk R, et al. History of syringe sharing in prison and risk of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection among injecting drug users in Berlin. Int J Epidemiol. 1997;26:1359–1366. [DOI] [PubMed] [Google Scholar]
- 12.Neaigus A, Friedman S, Jose B, et al. High-risk personal networks and syringe sharing as risk factors for HIV infection among new drug injectors. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:499–509. [DOI] [PubMed] [Google Scholar]
- 13.Garfein R, Williams I, Monterosso E, et al. HCV, HBV and HIV infections among young, street recruited injection drug users (IDUs): the Collaborative Injection Drug Users Study (CIDUS II). Antivir Ther. 2000;5(suppl 1):64. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Hepatitis B vaccination program for injecting drug users—Pierce County, Washington, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(19):388–390, 399. [PubMed] [Google Scholar]