Abstract
Objectives. We examined the effect of routine screening on breast cancer staging by race/ethnicity.
Methods. We used a 1990 to 1998 mammography database (N = 5182) of metropolitan Denver, Colo, women to examine each racial/ethnic cohort's incident cancer cases (n = 1902) and tumor stage distribution given similar patterns of routine screening use.
Results. Regardless of race/ethnicity, women participating in routine screenings had earlier-stage disease by 5 to 13 percentage points. After control for possible confounding factors, White women were more likely to have early-stage disease compared with Black and Hispanic women.
Conclusions. Lack of screening coverage in certain racial/ethnic populations has often been cited as a reason for tumor stage differences at detection. In this study, correcting for screening did not completely reduce stage differentials among Black and Hispanic women. (Am J Public Health. 2002;92:1144–1150)
Breast cancer incidence rates have risen in the United States for the past 2 decades. Although the lifelong chance of developing breast cancer is higher for White women than for Black and Hispanic women, Black women and subgroups of Hispanic women have a lower breast cancer survival rate.1–6 In the United States, Black and Hispanic women disproportionately have poor breast cancer outcomes. Black women diagnosed with breast cancer are twice as likely to die from the disease within 5 years after diagnosis and Hispanic women are 1.5 times as likely to die as White women.1,3 Black and Hispanic women undergo fewer baseline and routine mammography screenings and have more advanced stage of disease at diagnosis, which in part explains the observed decreases in survival rates. Several investigators contend that race/ethnicity is in part a determinant of resource access and that it is a contributing factor in the racial/ethnic disparity.7–13
Racial/ethnic differentials in breast cancer incidence rates, staging, and survival seen nationwide are similar to those observed in the Colorado and the 6-county Denver metropolitan area study data. Non-Hispanic White (hereafter labeled White), Hispanic, and non-Hispanic Black (Black) women in the Denver metropolitan area demonstrated an 11- to 21-percentage-point increase in early-stage disease between the period 1985 to 1987 and the period 1996 to 1997. Although increases in early detection were seen in all groups, presumably as a result of screening, tumor stage differentials were still apparent among Black, Hispanic, and White women. In 1996 to 1997, more Black (48%) and Hispanic (46%) women than White women (40%) received a diagnosis of advanced-stage breast cancer.1
Several randomized controlled trials of screening found significant decreases in mortality rates. Notably, the Stockholm and Malmo trials reported that 85% to 100% of breast cancer deaths occurred among women diagnosed with stage II, III, or IV breast cancer disease.14–16 Screening mammography for early breast cancer detection has been investigated quite thoroughly, but studies examining the association of screening with race/ethnicity have been limited. The current investigation was undertaken in light of the observational evidence regarding the association of race/ethnicity with breast cancer staging and survival and the public health importance attributed to showing that access to routine screening can reduce racial/ethnic differentials in tumor staging.
Conducting a randomized controlled trial of sufficient size to answer these questions was not feasible in our environment. Thus, we elected to explore data from a 9-year observational study designed to examine mammography performance in a community setting. From these data, we assessed the effect of routine screening on the identification of primary breast cancer and examined whether routine screening would have eliminated the excess of late-stage breast cancer found in Black and Hispanic women.
METHODS
Sources of Data
The Colorado Mammography Project longitudinal database (from 1990 to 1998) was used to examine the comparative experience of Black, Hispanic, and White women with respect to tumor stage and histological grade, controlling for education (as a surrogate for socioeconomic status), age, and screening practices. The Colorado Department of Public Health began the Colorado Mammography Project in 1989. As part of the Breast Cancer Screening Consortium, the National Cancer Institute currently funds the Colorado Mammography Project, which collects data from mammography facilities serving women in the 6-county Denver metropolitan area.
Facilities were recruited to the study by the use of outreach project coordinators who explained the project and offered evaluation data for the facility and the radiologists involved. In Denver, single radiologist groups serve multiple mammography facilities. This provides patient population diversity with some consistency in the radiological review. Colorado Mammography Project participation is voluntary for each facility and woman, but participating-facility compliance is nearly 100%. The Colorado Mammography Project population consists of women who attend these clinics throughout the 6-county metropolitan area, complete personal history forms, and receive a mammogram and radiological report. Because these facilities serve all metropolitan areas and facility participation is driven more by the participating radiologist groups than by the individual patient, no specific biases in patient selection were obvious.
Race/ethnicity information is obtained by self-description. Only women identifying themselves as exclusively Black, Hispanic, or White were selected as study participants. The study area includes approximately 60% of the state of Colorado's female population. The study population is representative of the Denver area in terms of racial/ethnic subgroups and income and educational levels.
In 1999, the Colorado Mammography Project collects as data more than 50% of all mammograms conducted in the study area (T. Byers; 1999 CMAP Coverage Analysis; Department of Preventive Medicine and Biometrics, University of Colorado Health Science Center, Denver; 1999). Through linkages with the statewide population-based Colorado Central Cancer Registry, a total of 5182 breast cancers were identified during the observation period and determined eligible for the study. The Colorado Central Cancer Registry follows Surveillance, Epidemiology, and End Results (SEER) guidelines for collection and reporting of cancer data. Non–Colorado Mammography Project cases were defined as the breast cancer cases of racial/ethnic eligible women residing in the Denver metropolitan area and in the Colorado Central Cancer Registry that were not linked to a Colorado Mammography Project record during the observation period (n = 5163).
Case Selection
A major goal of this investigation was to ascertain whether screening can eliminate stage differences found between Black and Hispanic women compared with White women. Few populations have sufficient numbers of routinely screened minorities with detected cancers. To address the 2 types of screening behavior—episodic screenings, which are more like prevalent screens, and routine screenings, which yield incident cases—we defined 2 types of cases: prevalent and incident. Our assumption for defining incident cases was that a single negative screen sufficiently conveys incident status.
Prevalent cases were defined as women with breast cancer detected on their first screen or women who had an interval of longer than 25 months between the detection mammographic sequence and their prior screen. Incident cases were defined as women with at least 1 documented negative mammogram result 10 to 25 months before their primary breast cancer diagnosis (mammogram results were defined as negative based on American College of Radiology BI-RADS codes 1, 2, and 3), excluding the detection mammographic sequence, and no positive mammogram results (American College of Radiology BI-RADS codes 0, 4, or 5).
Breast cancer cases were considered unconfirmed incident if a woman had stated on her completed questionnaire or if the radiology report indicated that a previous negative mammogram result existed. No record of a negative result, however, was available in the database for status verification.
Stage of disease was defined with the American Joint Committee on Cancer TNM system. Early-stage disease was defined as TNM stage 0 or I, and late-stage disease was defined as stage II, III, or IV cancer. Histological grade of tumor (grade 1–4) was determined with the International Classification of Diseases for Oncology. A tumor not identified as belonging to the original tissues is considered undifferentiated (grade 4) and is the most aggressive grade. The tumor data were obtained from the Colorado Central Cancer Registry records.
Analysis
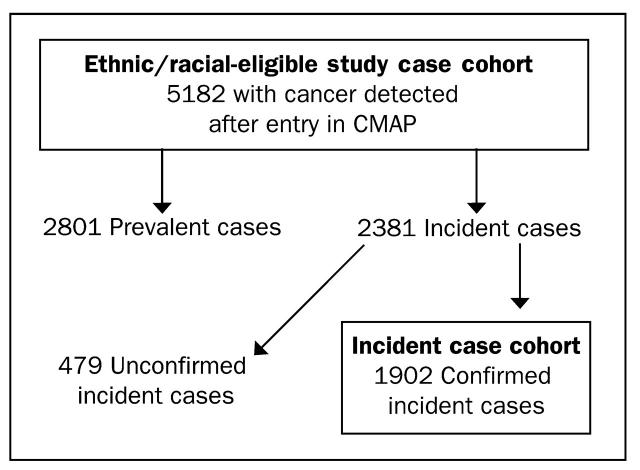
To characterize the racial/ethnic cohorts, frequencies and cross-tabulations for the variables of interest were generated in both the study case cohort of all incident and prevalent cancers combined and the incident case cohort (Figure 1▶). Bivariate relationships between racial/ethnic groups and the independent variables were evaluated with a χ2 test for independence using SAS.17 A logistic regression model was developed via a backward elimination approach to evaluate the effects of race/ethnicity, education, and other related explanatory variables on the dependent variable—stage of disease at diagnosis. In all comparisons, the cohort of White women was used as the reference cohort. Odds ratios were calculated for each dichotomous and continuous independent variable to test the statistical significance. Because variables were found not to be associated with the results, simpler models were used to assess the differences among racial/ethnic groups, adjusting for the remaining covariates.
FIGURE 1.
—Study case selection process: selection of incident cases.
The models reported show the effect of various covariates on the resulting dependent variable. The dependent variable, stage of disease, was classified into 2 groups: early and late stage of disease.
RESULTS
The Colorado Mammography Project database contained approximately 290 500 women who had received at least 1 mammogram through a participating screening facility. In the Colorado Mammography Project, 45% of the Black women, 40% of the Hispanic women, and 48% of the White women underwent at least 2 screens in the system.
The distribution of racial/ethnic groups shows a predominantly White female population. Of the women who reported their race/ethnicity, 83.5% were White, 9.6% were Hispanic, and 3.6% were Black. In Denver, approximately 66.4% of the population who report Hispanic ethnicity are of Mexican American descent.
Figure 1▶ shows the number of cases in the total study cohort (N = 5182) and the distribution into incident (n = 2381, or 45.9%) and prevalent cases (n = 2801, or 54.1%). Note that the percentage of incident cases in our cohort—45.9%—was only slightly lower than the percentage of women with more than 1 mammogram in the total Colorado Mammography Project registry (55%). Of the 5182 cancers found, 1902 incident cases were confirmed (36.7%). The racial/ethnic breakdown of confirmed incident cases of the total (confirmed incident, unconfirmed incident, and prevalent cases) was as follows: Hispanic women, 89 of 324 (27.5%); Black women, 49 of 149 (32.9%); and White women, 1764 of 4709 (37.5%). Among the total incident cases (2381), 479 (20.1%) were unconfirmed cases, and 79.9% were confirmed.
Results From All Women (Prevalent and Incident Cases)
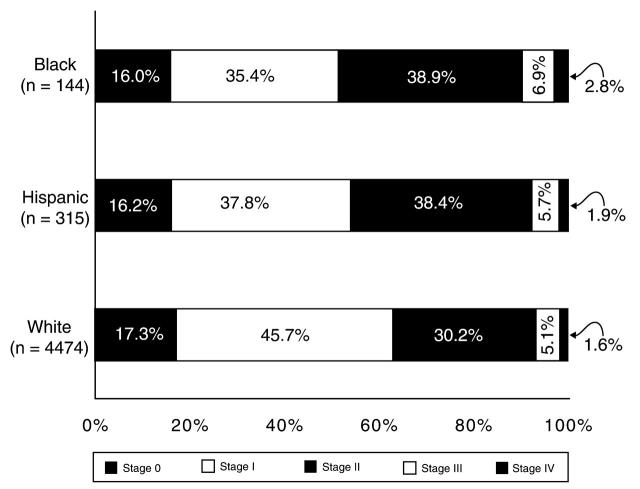
Stage information was available for 4933 (95.2%) of the 5182 women diagnosed with breast cancer. Among the White women, 63.0% of the cancer cases were detected in early stages (TNM stage 0 or I), compared with 51.4% of the Black women and 54.0% of the Hispanic women (Figure 2▶). Among the three racial/ ethnic cohorts, there was very little difference in the percentage of stage of disease that was classified as “unknown.” White women had a more than 10–percentage point excess of early-over late-stage disease. Conversely, Black (P = .01) and Hispanic women (P = .03) had a statistically significant greater percentage of advanced-stage disease compared with White women and had less difference between late- and earlier-stage disease.
FIGURE 2.
—Stage of disease at diagnosis, by race/ethnicity.
The breast cancer risk factor variables—personal cancer history, higher educational attainment, and age 50 years and older—were correlated with lower staging. These variables, when introduced into the model, had some level of explanatory power.
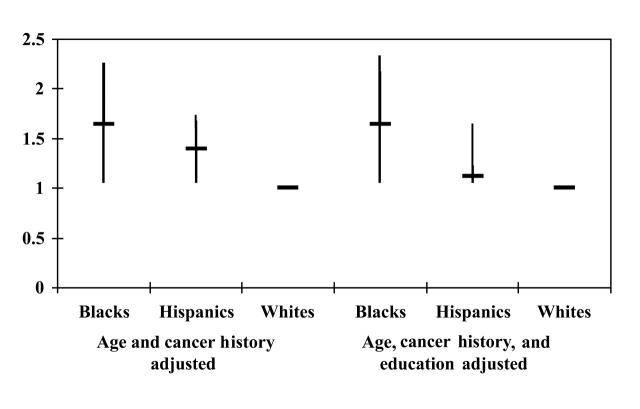
Black women had a 1.6-fold increased odds (P = .01) of late-stage cancer compared with White women (95% confidence interval [CI] = 1.1, 2.2) when the covariates age and cancer history were controlled (Figure 3▶). In contrast, Hispanic women had a 1.4-fold increased odds (P < .01) of late-stage disease compared with White women (95% CI = 1.1, 1.7) when age and cancer history were controlled for in the model (Figure 3▶).
FIGURE 3.
—Association between race/ethnicity and late stage of disease (≥ stage II).
When education was included in the equation, Hispanic women had a 1.2-fold increased odds (with a confidence interval that includes 1) of late-stage disease; the difference was not statistically significant (Figure 3▶). This was not the case for Black women, however; when the covariates age, cancer history, and education were controlled, Black women continued to have a l.6-fold increased odds (P = .02) of late-stage disease at diagnosis (95% CI = 1.1, 2.3).
After age, cancer history, and education were controlled, there was an approximately 60% increase in late stage of disease among Black women compared with White women when all cancers (prevalent or incident cases) were examined.
Results From the Incidence Cohort
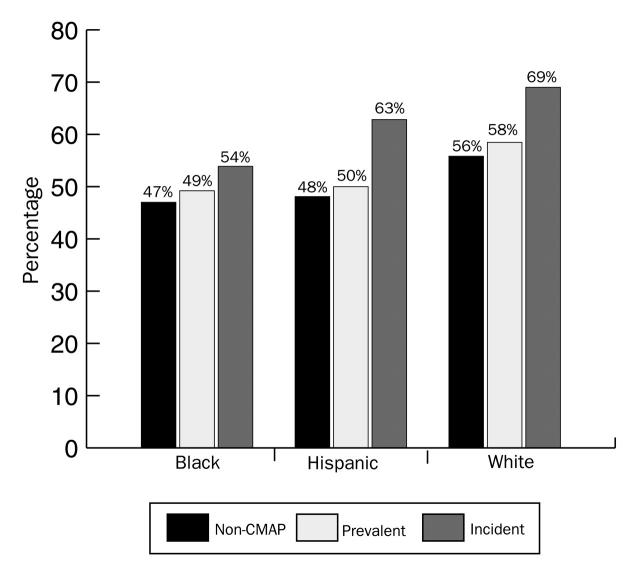
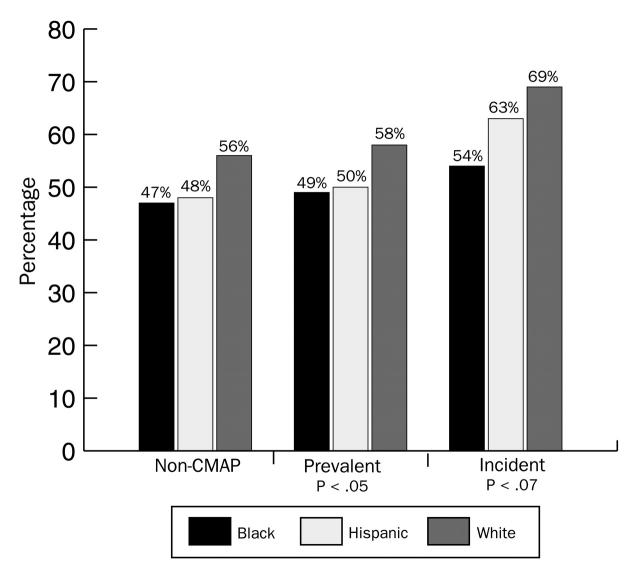
Among the White women, 69% of the confirmed incident cases were early stage (compared with 58% of the prevalent cases); the rates were 63% (vs 50%) for Hispanic women and 54% (vs 49%) for Black women (Table 1▶). The frequency distribution of early-detection breast cancer, by race/ethnicity, among non–Colorado Mammography Project, Colorado Mammography Project–prevalent, and Colorado Mammography Project–incident cases is shown in Figure 4▶.
TABLE 1.
—Variable Composition (Percentage), by Race/Ethnicity, for Incident Cases (n = 1902)a
| Hispanic | White | Black | |
|---|---|---|---|
| Age group, y | |||
| < 50 | 32.58 | 20.98 | 32.65 |
| ≥ 50 | 67.42 | 79.03 | 67.35 |
| Education level | |||
| ≤ Eighth grade or some high school | 17.97 | 4.04 | 4.76 |
| High school graduate or some college | 49.25 | 54.99 | 59.52 |
| College graduate or postgraduate degree | 32.83 | 40.98 | 35.72 |
| American Joint Committee on Cancer stage | |||
| 0 | 24.14 | 19.63 | 13.04 |
| I | 39.08 | 49.13 | 41.30 |
| II | 31.03 | 26.85 | 36.96 |
| III | 2.30 | 3.37 | 6.52 |
| IV | 3.45 | 1.02 | 2.17 |
| Histological grade | |||
| 1 (well) | 21.35 | 17.52 | 10.20 |
| 2 (moderate) | 28.09 | 29.25 | 26.53 |
| 3 (poor) | 15.73 | 18.42 | 28.57 |
| 4 (undifferentiated) | 4.49 | 5.05 | 10.20 |
| 9 (unknown) | 30.34 | 29.76 | 24.49 |
Note. CMAP = Colorado Mammography Project.
aNot all variables are present for each and every woman.
FIGURE 4.
—Case percentage with early detection (< stage II), by race/ethnicity and by case group.
Within each racial/ethnic group's incident cases, the percentage of early-stage cancers was higher in the incident cancer cases than in the prevalent cases, suggesting that cancer detected under an incident screening protocol, regardless of race/ethnicity, leads to earlier-stage detection. The early-stage cancer differential between incident and prevalent cancers was smallest for Black women. Figure 4▶ shows for incident cases that the 3 racial/ethnic cohorts differed in regard to early detection (P = .07). There was a 15–percentage point difference between White and Black women in early-stage detection (69% vs 54%) and a 6–percentage point difference between White and Hispanic women (69% vs 63%). These results suggest that after correcting for screening behavior by selecting only the confirmed incident cancer cases, racial/ethnic stage differentials were not eliminated. Whites had higher rates of early-stage cancers in both of the other categories (non–Colorado Mammography Project and Colorado Mammography Project–prevalent cases), whereas Black and Hispanic women were more similar, differing by only 1 percentage point in these other groupings.
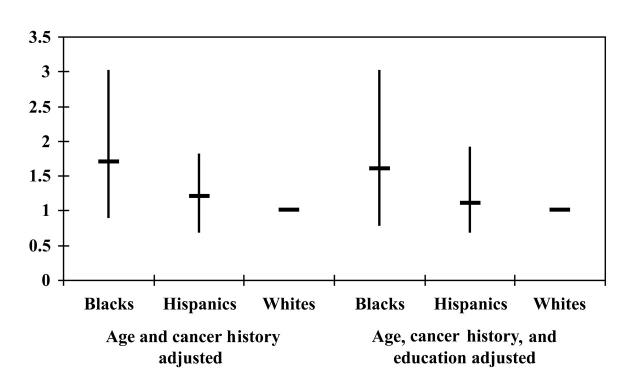
With the expectation that the residual differences in early-stage distribution among Black and Hispanic incidence cohorts would be eliminated when covariates were included, a formal logistic model for the adjusted odds ratio of late-stage disease was constructed. When the covariates age and cancer history were introduced into the model, the 95% confidence interval for both Black and Hispanic women overlapped unity (Figure 5▶). Black women, however, still had a 1.7-fold increased odds of detection of advanced-stage disease compared with White women (P = .08). Therefore, adjusting for age and cancer history did not completely eliminate stage-of-disease differences. These incident cases of Black women yielded an estimated odds ratio almost identical to the unadjusted values and the values based on the entire cohort of women. No statistically significant difference in the adjusted odds ratio was seen for Hispanic women compared with White women (Figure 5▶).
FIGURE 5.
—Association between race/ethnicity and late stage of disease (≥ stage II).
To further assess these apparent stage differences, we examined the distribution of another dependent variable separate from tumor stage—the histological grade—for early- and late-stage cancer by racial/ethnic group. A slide review was not performed. Specific multivariate modeling with histological grade as the outcome variable was conducted. Because Hispanic and Black women have a higher percentage of late-stage disease, we included stage as a confounding variable in the model.
When the variables of stage of disease, age, and cancer history were controlled in the model, Black women had a 2.2-fold increased odds (P = .03) of higher histological grade (95% CI = 1.2, 4.3) compared with White women. No statistically significant difference was found in the adjusted odds ratio for Hispanic women compared with White women. The results confirmed that despite the similar screening histories in these incidence cohorts, and similarity in education level and other variables, Black women in the incident cohort had an excess of higher histological grades (P = .03).
Finally, we reported only on the combined cases (i.e., symptomatic and asymptomatic women). As expected, the percentage of women with later-stage disease was higher among the symptomatic or diagnostically screened women. The patterns, however, were similar. For diagnostic mammograms in the series, the rates of late-stage disease were 44.0% (11 of 25) for the Hispanic women, 42.3% (148 of 350) for the White women, and 50.0% (6 of 12) for the Black women. For asymptomatic or screen-detected women, the rates were 36.2% (17 of 47) for the Hispanic women, 28.0% (330 of 1180) for the White women, and 39.3% (11 of 28) for the Black women.
DISCUSSION
This article examines whether routine screening can be expected to eliminate racial/ethnic disparities in breast cancer staging. We specifically studied proper assignment of a cancer case as incident or prevalent. This study confirmed the results of numerous studies that reported a more advanced stage of disease in Black and Hispanic women at the time of diagnosis.18–24 This finding is important because researchers consider advanced stage at diagnosis to be a strong contributor to survival differences seen between Black women and Hispanic women.3,25 It also has been determined in the literature that to a large extent, racial/ethnic differentials seen in staging are the result of lower screening rates.26–29 This study also has shown that although screening lowers stage at detection, even among women who participate in routine screening and who began the study observation period with a documented negative screening result, racial/ethnic stage differentials exist.
Adjusting for measurable risk and socioeconomic factors did not completely eliminate differences in stage of diagnosis by racial/ethnic group. White women were still more likely to have early-stage diagnosis compared with Black and Hispanic women. When routine screening was part of the equation, the odds of advanced stage were reduced, but residual effects still were seen, especially among Black women.
Hispanic women were younger and less educated than White women (2 factors correlated with late-stage disease), yet the difference in advanced-stage disease between Hispanic and White women was smaller than that between Black and White women. Overall, adjustment of individual variables produced modest modifications of the racial/ethnic group–stage association. This was clearly seen when education was added to models. Modifications to the estimates and confidence intervals were minimal to nonexistent. Routine screening may operate differently on stage of disease in Black women compared with Hispanic women. Screening does not appear sufficient to remove the staging differential seen in Black women.
By definition, incident cases reflected a fairly compliant screening population of women. In comparison to White women, no statistically significant difference (P = .07) in the distribution of advanced-stage disease among incident cases of Black and Hispanic women existed. The lack of significance, however, may be due to the decreasing sample size of Black and Hispanic women rather than to a lack of real differences between the groups, because the results parallel those of the total group in magnitude of effect.
Of course, it is difficult to determine whether these findings result from small sample size. The 70% increased odds of late-stage disease in Black women compared with White women, however, seems large enough not to ignore. To further investigate the plausibility of these increased odds of late-stage disease in Black women, we approached the race/ethnicity–screening behavior relationship by examining histological grade. Histological grade is considered a marker for the biological behavior of tumors.28
More-advanced histological grade tumors, which are considered more proliferative, were found among Black women who participated in routine screening, even after controlling for stage of disease. We believe that this intriguing result is consistent with the late-stage excess also shown.
This study offers no evidence that the observed remaining differences in tumor histology were due to genetic makeup or imply a biological uniqueness in any one group. The differences seen may be caused by other factors including incomplete control for screening behavior, postmenopausal estrogen exposure or obesity, diet, or subtle measures of socioeconomic status. Investigation of any and all of these factors may provide insight into why we may continue to see racial/ethnic staging differentials in cohorts with similar screening histories. The relation between staging and histological grade among Black women needs to be further explored within a larger cohort, because small numbers limit the interpretive power of this finding.
Routine screening alone may not, however, be sufficient to remove the staging differentials seen among racial/ethnic subgroups of women. Even though the literature suggests that the higher proportions of late-stage disease found in Black and Hispanic women relative to White women is related to these subgroups' greater difficulty in accessing mammography screening and early intervention, our data indicate that this explanation is insufficient—or, alternatively, that perhaps a longer observation period is needed to see the positive effects of screening. Nevertheless, the finding that routine screening does not completely eliminate the staging differentials finding is quite important in refocusing expectations by public health programs regarding the markers of success expectations of the amount of morbidity and mortality that it is possible to prevent.
Limitations
This study had several potential limitations. Education and cancer history depended on study participants' responses; therefore, misclassification was possible because of potential differential recall bias among the groups. We are reasonably confident regarding the generalizability of this cohort sample, given that the database captures more than 50% of the women undergoing mammography in the Denver metropolitan area. Also, the racial/ethnic composition and racial/ethnic educational attainment of Colorado Mammography Project study women were similar to those of the Denver metropolitan population. Colorado Mammography Project women reflect the overall Denver metropolitan population except for their tendency to have been screened.
Although the screening interval for all racial/ethnic cohorts was 10 to 25 months, variation in the average number of months before cancer diagnosis among the racial/ethnic cohorts could contribute to the differences seen. These differences, however, did not explain the staging differences. Interestingly, among all the incident cohorts, additional negative screening results (i.e., over 1) were not associated with lower stages of disease (women who had more than 1 negative screening result before the screening in which cancer was detected did not evidence lower stages of disease than women who had only 1 negative screen).
Summary
Despite mandatory mammography insurance coverage; free screening programs for low-income women; screening guidelines; national, state, and local public health outreach campaigns; and a public health agenda to eliminate disparities, Black and Hispanic women undergo fewer periodic screenings and have more-advanced disease at diagnosis. Although the research shows that screening leads to improvement across all racial/ethnic groups, a result evidenced in our data as well, the present study—admittedly based on a single study site and a relatively small population—suggests that screening may not completely reduce staging differentials.
Our results may need to be confirmed in a larger cohort, but they illustrate the complex relationship between stage and race/ethnicity. Great strides have been made in the screening of Black and Hispanic women. However, a better understanding of modifiable risk factors and the racial/ethnic beliefs and practices that affect screening is essential.
Acknowledgments
We would like acknowledge Monika Baier and Keri Holthaus for their assistance with statistical analysis, Jack Finch for his help in linking the data sets used in the analysis, and Dr Holly Hedegaard for her helpful comments in reviewing the final paper. We also wish to thank the Colorado Central Cancer Registry staff and the Colorado Mammography Project staff for their diligent attention to data quality and Louann Dicus for her technical and editing assistance.
J. Jacobellis planned and designed the study, analyzed the data, and wrote the paper. G. Cutter was a major contributor to the study design and data analysis and contributed to the writing of the paper.
Peer Reviewed
References
- 1.Colorado Central Cancer Registry. Cancer in Colorado 1992-1997 Incidence Mortality and Survival. Denver: Colorado Department of Public Health and Environment; 1999.
- 2.Delgado DJ, Lin WY, Coffey M. The role of Hispanic race/ethnicity and poverty in breast cancer survival. P R Health Sci J. 1995;14:103–116. [PubMed] [Google Scholar]
- 3.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer—results of the National Cancer Institute Black/White Survival Study. JAMA. 1994;272:947–954. [DOI] [PubMed] [Google Scholar]
- 4.Fox SA, Stein JA, Gonzalez RE, Farrenkopf M, Dellinger A. A trial to increase mammography utilization among Los Angeles Hispanic women. J Health Care Poor Underserved. 1998;9:309–321. [DOI] [PubMed] [Google Scholar]
- 5.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. [DOI] [PubMed] [Google Scholar]
- 6.Weiss SE, Tartter PI, Ahmed S, et al. Ethnic differences in risk and prognostic factors for breast cancer. Cancer. 1995;76:268–274. [DOI] [PubMed] [Google Scholar]
- 7.Breen N, Kessler LG, Brown ML. Breast cancer control among the underserved—an overview. Breast Cancer Res Treat. 1996;40:105–115. [DOI] [PubMed] [Google Scholar]
- 8.Kerner JF. Breast cancer prevention and control among the medically underserved. Breast Cancer Res Treat. 1996;40:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Hahn RA, Stroup DF. Race and ethnicity in public health surveillance: criteria for scientific use of social categories. Public Health Rep. 1994;109:7–15. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu ET. The uncoupling of race and cancer genetics. Cancer. 1998;83(suppl):1765–1769. [Google Scholar]
- 11.Neale AV. Racial and marital status influences on 10 year survival from breast cancer. J Clin Epidemiol. 1994;47:475–483. [DOI] [PubMed] [Google Scholar]
- 12.Wells BL, Horm JW. Stage at diagnosis in breast cancer: race and socioeconomic factors. Am J Public Health. 1992;82:1383–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst. 1993;85:1644–1656. [DOI] [PubMed] [Google Scholar]
- 15.Frisell J, Eklund G, Hellstrom L, et al. Randomized study of mammography screening—preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat. 1991;18:49–56. [DOI] [PubMed] [Google Scholar]
- 16.Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ. 1988;297:943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAS System for Regression. Cary, NC: SAS Institute Inc; 1991.
- 18.Ansell D, Whitman S, Lipton R, Cooper R. Race, income, and survival from breast cancer at two public hospitals. Cancer. 1993;72:2974–2978. [DOI] [PubMed] [Google Scholar]
- 19.Villar HV, Menck HR. The National Cancer Data Base report on cancer in Hispanics: relationships between ethnicity, poverty, and the diagnosis of some cancers. Cancer. 1994;74:2386–2395. [DOI] [PubMed] [Google Scholar]
- 20.Bentley JR, Delfino RJ, Taylor TH, Howe S, Anton-Culver H. Differences in breast cancer stage at diagnosis between non-Hispanic white and Hispanic populations, San Diego County 1988–1993. Breast Cancer Res Treat. 1998;50:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Trapido EJ, Davis K. Differences in stage at presentation of breast and gynecologic cancers among whites, blacks, and Hispanics. Cancer. 1994;73:2838–2842. [DOI] [PubMed] [Google Scholar]
- 22.Horm JW, Devisa SS, Berhansstipanov L. Cancer incidence, mortality, and survival among racial and ethnic minority groups. In: Schottenfeld D, Fraumeni JF, eds. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 1996:192–235.
- 23.Perkins P, Cooksley CD, Cox JD. Breast cancer: is ethnicity an independent prognostic factor for survival? Cancer. 1996;78:1241–1247. [DOI] [PubMed] [Google Scholar]
- 24.Mandeblatt J, Andrews H, Lerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast cancer and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassett MT, Krieger N. Social class and black–white differences in breast cancer survival. Am J Public Health. 1986;76:1400–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaloznik AJ. Breast cancer stage at diagnosis: Caucasians versus Afro-Americans. Breast Cancer Res Treat. 1995;34:195–198. [DOI] [PubMed] [Google Scholar]
- 27.Zaloznik AJ. Breast cancer stage at diagnosis: Caucasians versus Hispanics. Breast Cancer Res Treat. 1997;42:121–124. [DOI] [PubMed] [Google Scholar]
- 28.Hunter CP, Redmond CK, Chen VW, et al. Breast cancer: factors associated with stage at diagnosis in black and white women. Black/White Cancer Survival Study Group. J Natl Cancer Inst. 1993;85:1129–1137. [DOI] [PubMed] [Google Scholar]
- 29.Richardson JL, Langholz L, Bersnstein C, Burciaga C, Danley K, Ross RK. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer. 1992;65:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]