Abstract
Objectives. This study determined trends in diabetes prevalence among young American Indians and Alaska Natives.
Methods. American Indian and Alaska Native children (< 15 years), adolescents (15–19 years), and young adults (20–34 years) with diabetes were identified from the Indian Health Service (IHS) outpatient database. The population living within IHS contract health service delivery areas was determined from census data.
Results. From 1990 to 1998, the total number of young American Indians and Alaska Natives with diagnosed diabetes increased by 71% (4534 to 7736); prevalence increased by 46% (6.4 per 1000 to 9.3 per 1000 population). Increases in prevalence were greater among adolescents and among young men.
Conclusions. Diabetes should be considered a major public health problem among young American Indians and Alaska Natives.
Type 2 diabetes has been recognized as a significant public health problem in American Indian communities for almost 40 years.1,2 The Pima Indians in Arizona have the highest recorded prevalence of diabetes in the world.3 Compared with other US populations, American Indians and Alaska Natives have been disproportionately affected with diabetes since the early 1960s,1 and its increasing prevalence in this population has been documented since 1983.2,4
Although type 2 diabetes has traditionally been regarded as a disease of adults,5 its prevalence among children and youth has emerged as a public health concern for American Indian communities.6 As early as 1979, young Pima Indians were noted to have a high prevalence of type 2 diabetes.7 More recently, the emergence of type 2 diabetes among children and adolescents has been described in other US populations, such as African Americans and Hispanics, and among Canada’s First Nations.8 The American Diabetes Association issued a statement in 2000 alerting the health care community to the growing problem of type 2 diabetes among children and adolescents.9
Among American Indian and Alaska Native adults, diabetes is a major cause of morbidity (such as blindness, kidney failure, lower-extremity amputation, and cardiovascular disease), disability, decreased quality of life, and premature mortality2 as well as a major cause of congenital anomalies, malformations, and perinatal death.10 Little is known, however, about the prevalence of diabetes among young American Indians and Alaska Natives in the United States and how this prevalence varies geographically.
The purpose of this study was to determine the magnitude of this public health problem and to document national and regional trends in diabetes prevalence among young American Indians and Alaska Natives. Additionally, we describe initial efforts in affected communities to address prevention of diabetes among young people.
METHODS
Established in 1955, the Indian Health Service (IHS) is the agency of the US Public Health Service responsible for providing health care to American Indians and Alaska Natives.11 Of the estimated 2.4 million American Indians and Alaska Natives in the United States in 1998,12 1.46 million (61%) resided in IHS contract health service delivery areas. Out of this group, approximately 1.40 million (96%) used IHS or tribal facilities for their health care.13
The IHS outpatient computerized database contains clinical and demographic information on outpatient encounters—including laboratory and pharmacy data—in 550 IHS and tribal health facilities. The facilities are grouped into 151 service units.11 After we excluded missing or incompletely reported data, we analyzed IHS outpatient data from 105 service units, representing approximately 84% of the IHS population. We used the IHS database to identify American Indians and Alaska Natives younger than 35 years with at least 1 of the 10 diagnostic codes for diabetes according to the ninth revision of the International Classification of Diseases (Clinical Modification, codes 250.0–250.9) for each fiscal year from October 1, 1990, to September 30, 1998. Compared with chart review, 1 diagnostic code for diabetes from the IHS outpatient computerized database identified diabetes cases a sensitivity of 92% and a specificity of 99%.14 The data for fiscal year 1998 are provisional and subject to change; in past years, however, the change between provisional and final data has been approximately 1%–2% (Steve Kaufman, MS, August 23, 2001, telephone communication).
Population data from the US Census Bureau were used to determine the number of American Indians and Alaska Natives who resided in IHS contract health service delivery areas; these individuals may or may not have received care at an IHS or tribal health facility.11 We used these population data and the number of persons younger than 35 years identified in the IHS database with diagnosed diabetes to estimate the age-specific prevalence of diabetes among American Indians and Alaska Natives younger than 15 years and aged 15–19, 20–24, and 25–34 years. Prevalence estimates were age-adjusted by the direct method, with the projected 2000 US population used as the standard.15
Because diabetes prevalence varies by tribe and by geographic area,16 we also examined trends in prevalence in 7 regions: Alaska; the Great Lakes (Michigan, Minnesota, and Wisconsin); the Northern Plains (Iowa, Montana, Nebraska, North Dakota, South Dakota, and Wyoming); the Pacific (California, Idaho, Oregon, and Washington); the Southeast (Mississippi and North Carolina); the Southern Plains (Kansas and Oklahoma); and the Southwest (Arizona, Colorado, Nevada, New Mexico, and Utah). Nineteen (41%) of the 46 service units that had missing or incompletely reported data were located in the eastern part of the United States, and many states in this area were not represented after exclusion of data from these service units.
RESULTS
Between 1990 and 1998, the number of American Indian and Alaska Native children, adolescents, and young adults with diagnosed diabetes who used IHS or tribal health facilities increased by 71% (from 4534 to 7736 persons); the crude prevalence of diagnosed diabetes increased by 46% (6.4 per 1000 to 9.3 per 1000) (Table 1 ▶). Throughout the period, the prevalence increased by 68% among adolescents aged 15–19 years (3.2 per 1000 to 5.4 per 1000), by 47% among adults aged 20–24 years (7.8 per 1000 to 11.5 per 1000), and by 50% among adults aged 25–34 years (18.0 per 1000 to 26.9 per 1000). Prevalence among children younger than 15 years remained unchanged (1.2 per 1000).
TABLE 1.
—American Indians/Alaska Natives Aged < 35 Years With Diagnosed Diabetes and Prevalence of Diagnosed Diabetes, by Age Group and Sex, 1990–1998
| Prevalence per 1000 | |||||||
| Year | n | < 15 | 15–19 | 20–24 | 25–34 | < 35 | < 35a |
| Males and Females | |||||||
| 1990 | 4534 | 1.23 | 3.24 | 7.84 | 17.95 | 6.36 | 7.06 |
| 1991 | 4757 | 1.23 | 3.46 | 7.86 | 18.71 | 6.57 | 7.31 |
| 1992 | 5050 | 1.40 | 3.63 | 7.67 | 19.37 | 6.82 | 7.57 |
| 1993 | 5431 | 1.34 | 4.04 | 8.83 | 20.10 | 7.16 | 7.96 |
| 1994 | 5869 | 1.36 | 4.46 | 9.30 | 21.34 | 7.59 | 8.44 |
| 1995 | 6062 | 1.43 | 4.23 | 9.06 | 21.74 | 7.66 | 8.51 |
| 1996 | 6465 | 1.29 | 4.48 | 10.09 | 22.91 | 8.03 | 8.95 |
| 1997 | 6862 | 1.30 | 4.56 | 10.67 | 23.90 | 8.35 | 9.32 |
| 1998 | 7736 | 1.23 | 5.42 | 11.49 | 26.91 | 9.27 | 10.36 |
| Males | |||||||
| 1990 | 1705 | 1.13 | 2.27 | 5.57 | 13.57 | 4.75 | 5.35 |
| 1991 | 1811 | 1.14 | 2.41 | 5.67 | 14.34 | 4.97 | 5.61 |
| 1992 | 2033 | 1.30 | 2.72 | 5.37 | 16.05 | 5.46 | 6.15 |
| 1993 | 2171 | 1.16 | 2.75 | 6.39 | 16.77 | 5.69 | 6.44 |
| 1994 | 2478 | 1.23 | 3.10 | 7.47 | 18.75 | 6.37 | 7.21 |
| 1995 | 2568 | 1.42 | 2.85 | 7.01 | 19.07 | 6.45 | 7.29 |
| 1996 | 2770 | 1.13 | 3.22 | 7.86 | 20.69 | 6.84 | 7.78 |
| 1997 | 2882 | 1.19 | 3.54 | 8.07 | 20.85 | 6.97 | 7.92 |
| 1998 | 3217 | 1.11 | 4.10 | 8.53 | 23.36 | 7.66 | 8.73 |
| Females | |||||||
| 1990 | 2829 | 1.34 | 4.25 | 10.16 | 22.13 | 7.99 | 8.73 |
| 1991 | 2946 | 1.31 | 4.55 | 10.08 | 22.87 | 8.19 | 8.96 |
| 1992 | 3017 | 1.52 | 4.57 | 10.02 | 22.55 | 8.20 | 8.95 |
| 1993 | 3260 | 1.52 | 5.38 | 11.33 | 23.28 | 8.65 | 9.46 |
| 1994 | 3391 | 1.50 | 5.89 | 11.17 | 23.81 | 8.82 | 9.65 |
| 1995 | 3494 | 1.44 | 5.66 | 11.15 | 24.29 | 8.88 | 9.72 |
| 1996 | 3695 | 1.45 | 5.78 | 12.36 | 25.03 | 9.23 | 10.11 |
| 1997 | 3980 | 1.42 | 5.62 | 13.32 | 26.80 | 9.75 | 10.69 |
| 1998 | 4519 | 1.35 | 6.81 | 14.50 | 30.30 | 10.90 | 11.97 |
aAge–adjusted based on the 2000 US population.
During the study period, the prevalence of diagnosed diabetes increased with age among both males and females (Table 1 ▶). The prevalence among females aged 15 years and older was generally about 2 times that among males aged 15 years and older. This sex differential decreased throughout the study period for persons aged 25–34 years, and by 1998, prevalence among females was only 1.3 times that among males (30.3 per 1000 vs 23.4 per 1000). The prevalence among girls aged less than 15 years was generally about 1.2 times that among boys.
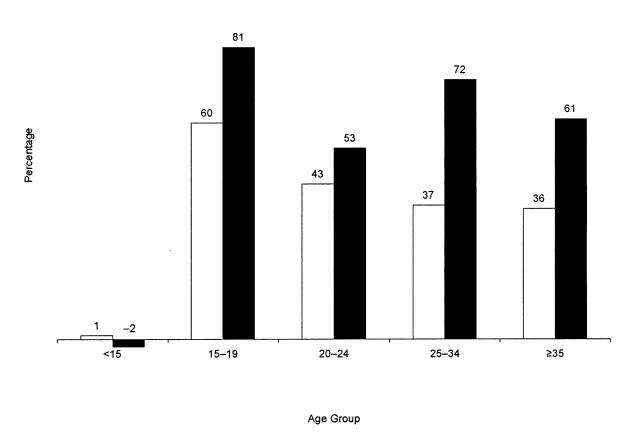
Although the prevalence of diagnosed diabetes was higher among females than among males in each age group, the relative increase in prevalence in each age group was greater among males than among females, except among children younger than 15 years (Figure 1 ▶). Among females aged 15–34 years, the rate of increase in prevalence ranged from 37% among those aged 25–34 years to 60% among those aged 15–19 years. Among males aged 15–34 years, the rate of increase in prevalence ranged from 53% among those aged 20–24 years to 81% among those aged 15–19 years. The overall crude prevalence increased by 61% (4.8 per 1000 to 7.7 per 1000) among males and by 36% (8.0 per 1000 to 10.9 per 1000) among females.
FIGURE 1.
—Rate of increase in diabetes prevalence among American Indians and Alaska Natives aged < 35 years, by age group and sex, 1990 and 1998.
The overall age-adjusted prevalence of diagnosed diabetes increased by 47% (7.1 per 1000 to 10.4 per 1000); the age-adjusted prevalence increased by 63% among males (5.4 per 1000 to 8.7 per 1000) and by 37% among females (8.7 per 1000 to 12.0 per 1000) (Table 1 ▶). The rate of increase in age-adjusted prevalence was similar to the rate of increase in crude prevalence (47% vs 46%).
The age-adjusted prevalence of diagnosed diabetes among American Indians and Alaska Natives younger than 35 years varied by region, ranging from (in 1998) 3.0 per 1000 in the Alaska region to 34.9 per 1000 in the Southeast region (Table 2 ▶). Between 1990 and 1998, the age-adjusted prevalence increased in all regions, with increases ranging from 6% in the Pacific region to 152% in the Alaska region. Increases in the other 5 regions—Great Lakes, Northern Plains, Southeast, Southern Plains, and Southwest—ranged from 40% to 89%.
TABLE 2.
—Age-Adjusted Prevalence, by Region, of Diagnosed Diabetes Among American Indians/Alaska Natives Aged < 35 Years, 1990–1998.
| Prevalence per 1000 by Region | |||||||
| Year | Alaskaa | Great Lakesb | Northern Plainsc | Pacificd | Southeaste | Southern Plainsf | Southwestg |
| 1990 | 1.19 | 7.94 | 8.86 | 4.13 | 24.97 | 7.14 | 8.22 |
| 1991 | 1.05 | 8.71 | 8.83 | 4.06 | 26.10 | 7.55 | 8.47 |
| 1992 | 1.38 | 8.85 | 9.93 | 4.27 | 27.04 | 7.34 | 8.77 |
| 1993 | 1.24 | 8.46 | 10.17 | 4.73 | 28.40 | 8.47 | 9.01 |
| 1994 | 1.76 | 8.85 | 10.44 | 4.58 | 31.70 | 9.23 | 9.54 |
| 1995 | 1.77 | 10.66 | 10.19 | 4.82 | 33.55 | 8.89 | 9.62 |
| 1996 | 1.86 | 11.70 | 11.85 | 5.05 | 36.40 | 8.50 | 10.15 |
| 1997 | 2.29 | 11.39 | 12.26 | 4.60 | 34.68 | 9.45 | 10.62 |
| 1998 | 3.00 | 14.98 | 13.72 | 4.38 | 34.93 | 10.30 | 11.84 |
Note. Prevalences are age-adjusted based on the 2000 US population.
aAlaska.
bMichigan, Minnesota, and Wisconsin.
cIowa, Montana, Nebraska, North Dakota, South Dakota, and Wyoming.
dCalifornia, Idaho, Oregon, and Washington.
eMississippi and North Carolina.
f Kansas and Oklahoma.
gArizona, Colorado, Nevada, New Mexico, and Utah.
Regional trends by age and sex were similar to trends in the total American Indian and Alaska Native population younger than 35 years. In general, regional estimates of diagnosed diabetes prevalence were higher among females than among males, increased with age, and rose throughout the study period, with greater relative increases among males. Although the national prevalence of diagnosed diabetes among American Indian and Alaska Native children younger than 15 years did not increase, the prevalence in this age group increased in 3 regions—Great Lakes (27%, from 1.8 per 1000 to 2.3 per 1000), Southeast (38%, from 1.2 per 1000 to 1.7 per 1000), and Alaska (114%, from 0.4 per 1000 to 0.9 per 1000).
CONCLUSIONS
Diabetes should be considered a major public health problem among young American Indians and Alaska Natives; in less than a decade, diabetes prevalence among American Indians and Alaska Natives younger than 35 years increased by 46%. In contrast, diabetes prevalence among the US general population younger than 45 years increased by 14% between 1990 and 1996.17 The large increase in diabetes prevalence among young American Indians and Alaska Natives further contributes to the already large and disproportionate burden of diabetes on these populations.4,18 Furthermore, this increase poses a major public health challenge for affected communities, because young persons with diabetes will have more years of disease burden and a higher probability of developing costly and disabling diabetes-related complications early in life.16
Throughout the study period, young American Indian and Alaska Native males had a lower prevalence of diabetes than did females but had a higher relative increase in prevalence (61% vs 36%). The reason for the larger relative increase among males is unknown; however, a national examination survey indicated that men have a higher prevalence of impaired fasting glucose than do women and thus may have a greater chance of developing and being diagnosed with diabetes.19 Although the increase in prevalence was smaller among females than among males, it was nonetheless substantial.
The increase in diabetes prevalence among young women of childbearing age constitutes an additional public health concern. Diabetes is not only a major cause of congenital anomalies, malformations, and perinatal death10 but also may start a vicious cycle—diabetes during pregnancy, diabetes onset at a young age in offspring, diabetes during pregnancy—that would be detrimental for future generations of American Indians and Alaska Natives.20 For example, the prevalence of diabetes among Pima Indians aged 15–19 years who were exposed to diabetes in utero was 9 times greater than among those who were not so exposed (24.72% vs 2.75%).21 Upon reaching the age of 20–24 years, Pima offspring of diabetic pregnancies were 32 times more likely to develop type 2 diabetes than were counterpart offspring of nondiabetic pregnancies.22 Whether control of diabetes during pregnancy could delay or prevent the development of diabetes in the offspring is unknown.23
The prevalence of diabetes among American Indians and Alaska Natives younger than 35 years increased substantially in all regions. The largest relative increase was seen in the Alaska region, even though this region had the lowest age-adjusted prevalence of diabetes in 1990. This increase in diabetes prevalence among young Alaska Natives was two times the increase among all Alaska Natives4 and may signal the acceleration of a diabetes epidemic in this population.
The reasons for the increasing prevalence of diabetes among young American Indians and Alaska Natives cannot be determined from these cross-sectional data. It is possible that the increase is due in part to better case ascertainment. Population-based studies that include regular screening for diabetes, such as studies among the Pima Indians, however, indicate that the increase in prevalence may be attributable to a true increase in incidence.21
The risk of developing type 2 diabetes, the most common form of the disease, is associated with modifiable risk factors such as obesity and inactivity.5 Over recent decades, the prevalence of overweight and obesity has increased among children and adolescents in the US general population as well as among American Indian children.21,24,25 One study suggested that the increases in weight and in the frequency of exposure to diabetes in utero among Pima Indian children together account for most of the recent increase in diabetes prevalence in this population.21 Physical inactivity is also a problem among American Indian children.26 Because most of the diabetes cases among American Indians and Alaska Natives are type 2,16 the prevalence of diabetes is likely to continue to grow as the prevalence of risk factors increases.
This analysis of the trends in diabetes prevalence has several limitations. First, the data underestimate the true prevalence of diabetes because we were unable to include persons with undiagnosed diabetes. Studies estimate that 1 in 3 persons with diabetes has not received a diabetes diagnosis.19,27–29 Moreover, no guidelines exist within IHS for selective or systematic screening at the national or regional level. Second, our data did not include young American Indians and Alaska Natives with diabetes who visited IHS or tribal health facilities that incompletely reported data to the IHS outpatient database, or those who did not visit IHS or tribal health facilities at least once during each of the years studied. Third, population numbers based on US census estimates may be inaccurate, and undercounts could result in an overestimation of diabetes prevalence. In the past, census counts have underreported American Indians and Alaska Natives, and they do not account for migration between IHS or tribal health facilities between census years.11 Despite these limitations, the IHS outpatient database has been used effectively to estimate diabetes prevalence among American Indians and Alaska Natives, and it is sufficiently consistent to allow the observation of trends.30
Because young American Indians and Alaska Natives who did not reside in IHS contract health service delivery areas and therefore did not receive care from IHS or tribal health facilities were not included in our analysis, our data do not represent this segment of the population. The prevalence of diabetes is likely to be comparable, however, if the levels of risk factors for developing diabetes—such as genetic susceptibility, obesity, and inactivity—in this segment of the population are similar to the levels among those who receive services from IHS.
In 1979, Congress established the IHS National Diabetes Program to address the burden of diabetes among American Indians and Alaska Natives. Significant strides have been made since then in improving diabetes care, treatment, and surveillance,30–33 and now American Indian and Alaska Native adults with diabetes receive care that is as good as or better than that received by their counterparts in the US general population.34–36
Young American Indians and Alaska Natives now have disproportionately high rates of diabetes, and the prevalence appears to be increasing substantially. Because young persons with diabetes will have more years of disease burden and a higher probability of developing diabetes-related complications early in life,16 young American Indians and Alaska Natives should be the target of efforts to prevent diabetes, and those with diabetes should be the target of efforts to prevent or delay the development of complications. Diabetes care and management for youths, however, present unique challenges, such as a lack of symptoms, absence of family support, and denial about the disease.8 Other challenges may include a lack of appropriate role models for youth, insufficient motivation to adopt healthy eating habits, and inadequate coping skills to adapt to life with diabetes.
Moreover, the quality of diabetes care and management among American Indian and Alaska Native youths with the disease is an area of heightened concern that has not been well studied. A functional and supportive environment is critical to the successful management of diabetes among children and adolescents, and clinical trials of behavioral and treatment interventions for youth are needed.
As the prevalence of diabetes has continued to grow, prevention of the disease has become an increasingly important goal for American Indian and Alaska Native communities. Through university-based research, some American Indian communities have already developed school programs, such as the Pathways and Quest programs, to increase physical activity, improve diet, and reduce obesity among children.37,38 In 1997, the Balanced Budget Act provided $150 million in grants to the IHS over 5 years for programs to “prevent and treat” diabetes among American Indians and Alaska Natives.39 These grants have helped to establish more than 300 new diabetes programs in American Indian and Alaska Native communities. A significant number of these programs target children and adolescents through school activities that focus on diabetes prevention and health promotion.39 Ongoing evaluation of Pathways, Quest, and community prevention interventions may provide examples of successful strategies and best-practice models to guide future widespread diabetes prevention efforts in American Indian and Alaska Native communities. Positive results from clinical trials aimed at prevention of diabetes among adults at risk of developing the disease40,41 suggest that lifestyle interventions to reduce overweight and increase physical activity can be effective among children. As further research findings emerge from ongoing diabetes primary prevention trials and community-based interventions, rapid translation of successful strategies into public health action is an urgent priority for the IHS.
Peer Reviewed
K. J. Acton, M. M. Engelgau, and L. S. Geiss planned the study and contributed to the writing of the article. N. R. Burrows analyzed the data and wrote the article. K. Moore and L. Querec contributed to the writing of the article.
Footnotes
Human Participant Protection This study was reviewed by human subject officials at IHS and the Centers for Disease Control and Prevention and was determined to be exempt from institutional review under federal regulation 45 CFR 46.
References
- 1.Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet. 1971;2(7716):125–128. [DOI] [PubMed] [Google Scholar]
- 2.Gohdes D. Diabetes in American Indians: a growing problem. Diabetes Care. 1986;9:609–613. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505. [DOI] [PubMed] [Google Scholar]
- 4.Burrows NR, Geiss LS, Engelgau MM, Acton KJ. Prevalence of diabetes among Native Americans and Alaska Natives, 1990–1997: an increasing burden. Diabetes Care. 2000;23:1786–1790. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI. Summary. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, eds. Diabetes in America. 2nd ed. Washington, DC: US Dept of Health and Human Services, Public Health Service, National Institutes of Health; 1995:3–4. DHHS publication NIH95–-1468.
- 6.Ríos Burrows N, Acton K, Geiss L, Venkat Narayan KM. Trends in diabetes prevalence in American Indians and Alaska Natives: an increasing burden among younger people. Diabetes. 1998;47(suppl 1):A187. [Google Scholar]
- 7.Savage PJ, Bennett PH, Senter RG, Miller M. High prevalence of diabetes in young Pima Indians: evidence of phenotypic variation in a genetically isolated population. Diabetes. 1979;28:937–942. [DOI] [PubMed] [Google Scholar]
- 8.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–672. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. [DOI] [PubMed] [Google Scholar]
- 10.Åberg A, Westbom L, Källén B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev. 2001;61:85–95. [DOI] [PubMed] [Google Scholar]
- 11.Indian Health Service. Trends in Indian health 1998–99. Rockville, MD: US Dept of Health and Human Services; 2000:1–14. Available at: http://www.ihs.gov/PublicInfo/Publications/trends98/trends98.asp. Accessed July 18, 2001.
- 12.US Census Bureau. Resident population estimates of the United States by sex, race, and Hispanic origin: April 1, 1990 to July 1, 1999, with short-term projection to November 1, 2000. Washington, DC: US Dept of Commerce; 1999. Available at: http://www.census.gov/population/estimates/nation/intfile3-1.txt. Accessed July 6, 2001.
- 13.Indian Health Service. IHS user population estimates by area and service unit—FY98. Rockville, MD: US Dept of Health and Human Services; 2001. Available at: http://www.ihs.gov/NonMedicalPrograms/IHS_Stats/files/FY98_UP.xls. Accessed July 18, 2001.
- 14.Wilson C, Susan L, Lynch A, Saria R, Peterson D. Patients with diagnosed diabetes mellitus can be accurately identified in an Indian Health Service patient registration database. Public Health Rep. 2001;116:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected US population. Healthy People 2010 Stat Notes. 2001;20:2. Available at: http://www.cdc.gov/nchs/data/statnt/statnt20.pdf. Accessed April 18, 2001. [PubMed] [Google Scholar]
- 16.Gohdes D. Diabetes in North American Indians and Alaska Natives. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, eds. Diabetes in America. 2nd ed. Washington, DC: US Dept of Health and Human Services, Public Health Service, National Institutes of Health; 1995:683–692. DHHSNo. NIH95=1468
- 17.Centers for Disease Control and Prevention. Diabetes surveillance, 1999. Available at: http://www.cdc.gov/diabetes/statistics/survl99/chap2/contents.htm. Accessed July 6, 2001.
- 18.Centers for Disease Control and Prevention. Prevalence of diagnosed diabetes among American Indians/Alaska Natives—United States, 1996. MMWR Morb Mortal Wkly Rep. 1998;47:901–904. [PubMed] [Google Scholar]
- 19.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. [DOI] [PubMed] [Google Scholar]
- 20.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes. 1991;40(suppl 2):126–130. [DOI] [PubMed] [Google Scholar]
- 21.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of Type II diabetes in American Indian children. Diabetologia. 1998;41:904–910. [DOI] [PubMed] [Google Scholar]
- 22.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–628. [DOI] [PubMed] [Google Scholar]
- 23.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breastfeeding in Pima Indians. Diabetes Care. 1998;21(suppl 2):B138–B141. [PubMed] [Google Scholar]
- 24.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149:1085–1091. [DOI] [PubMed] [Google Scholar]
- 25.Broussard BA, Sugarman JR, Bachman-Carter K, et al. Toward comprehensive obesity prevention programs in Native American communities. Obes Res. 1995;3(suppl 2):289s–297s. [DOI] [PubMed] [Google Scholar]
- 26.Salbe AD, Fontvieille AM, Harper IT, Ravussin E. Low levels of physical activity in 5-year-old children. J Pediatr. 1997;131:423–429. [DOI] [PubMed] [Google Scholar]
- 27.Will JC, Strauss KF, Mendlein JM, Ballew C, White LL, Peter DG. Diabetes mellitus among Navajo Indians: findings from the Navajo Health and Nutrition Survey. J Nutr. 1997;127(suppl 10):2106S–2113S. [DOI] [PubMed] [Google Scholar]
- 28.Lee ET, Howard BV, Savage PJ, et al. Diabetes and impaired glucose tolerance in three American Indian populations aged 45–74 years. The Strong Heart Study. Diabetes Care. 1995;18:599–610. [DOI] [PubMed] [Google Scholar]
- 29.Casper M, Rith-Najarian S, Groft J, Giles W, Donehoo R. Blood pressure, diabetes, and body mass index among Chippewa and Menominee Indians: the Inter-Tribal Heart Project Preliminary Data. Public Health Rep. 1996;111(suppl 2):37–39. [PMC free article] [PubMed] [Google Scholar]
- 30.Valway S, Freeman W, Kaufman S, Welty T, Helgerson SD, Gohdes D. Prevalence of diagnosed diabetes among American Indians and Alaska Natives, 1987: estimates from a national outpatient database. Diabetes Care. 1993;16:271–276. [DOI] [PubMed] [Google Scholar]
- 31.Acton K, Valway S, Helgerson S, et al. Improving diabetes care for American Indians. Diabetes Care. 1993;16:372–375. [DOI] [PubMed] [Google Scholar]
- 32.Mayfield JA, Rith-Najarian SJ, Acton KJ, et al. Assessment of diabetes care by medical record review: the Indian Health Service model. Diabetes Care. 1994;17:918–923. [DOI] [PubMed] [Google Scholar]
- 33.Gohdes D, Rith-Najarian S, Acton K, Shields R. Improving diabetes care in the primary health setting: the Indian Health Service experience. Ann Intern Med. 1996;124:149–152. [DOI] [PubMed] [Google Scholar]
- 34.Acton KJ, Shields R, Rith-Najarian S, et al. Applying the diabetes quality improvement project indicators in the Indian Health Service primary care setting. Diabetes Care. 2001;24:22–26. [DOI] [PubMed] [Google Scholar]
- 35.Acton K, Gohdes D. Comparison of benchmarks for diabetes care in elderly populations. Diabetes. 1998;47(suppl 1):A182. [Google Scholar]
- 36.Beckles GL, Engelgau MM, Narayan KM, Herman WH, Aubert RE, Williamson DF. Population-based assessment of the level of care among adults with diabetes in the US. Diabetes Care. 1998;21:1432–1438. [DOI] [PubMed] [Google Scholar]
- 37.Davis CE, Hunsberger S, Murray DM, et al. Design and statistical analysis for the Pathways study. Am J Clin Nutr. 1999;69(suppl 4):760S–763S. [DOI] [PubMed] [Google Scholar]
- 38.Cook VV, Hurley JS. Prevention of type 2 diabetes in childhood. Clin Pediatr. 1998;37:123–129. [DOI] [PubMed] [Google Scholar]
- 39.Indian Health Service. Special diabetes program for Indians: interim report to Congress. Rockville, MD: US Dept of Health and Human Services; 2000:1–3, 30. Available at: http://www.ihs.gov/MedicalPrograms/Diabetes. Accessed March 19, 2001.
- 40.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes mellitus. Diabetes Care. 1999;22:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]