Abstract
The Defense Medical Surveillance System (DMSS) is the central repository of medical surveillance data for the US armed forces. The DMSS integrates data from sources worldwide in a continuouslyexpanding relational database that documents the military and medical experiences of servicemembers throughout their careers.
The Department of Defense Serum Repository (DoDSR) is a central archive of sera drawn from servicemembers for medical surveillance purposes.
Currently, the DMSS contains data relevant to more than 7 million individuals who have served in the armed forces since 1990, and the DoDSR contains more than 27 million specimens that are linkable to data in the DMSS. Recent applications of the DMSS and DoDSR provide glimpses of the capabilities and uses of comprehensive public health surveillance systems.
PUBLIC HEALTH SURVEILLANCE is the routine and systematic collection, analysis, interpretation, and reporting of population-based data for the purposes of detecting, characterizing, and countering threats to the health, fitness, well-being, and performance of members of defined populations. Many “surveillance systems” have characteristics inconsistent with this definition. For example, many systems are registries of cases or repositories of data relevant to conditions or exposures of a priori interest. In many systems, the data are not routinely or systematically collected or do not refer to defined populations. Some systems have no inherent capabilities to analyze data, interpret results, or disseminate findings in timely ways. Finally, “special interest” systems, such as those targeted at specific diseases, exposures, or subgroups, have narrow focuses and thus limited applications in general public health practice.
In contrast, the potential values of comprehensive public health surveillance are numerous and well recognized.1–8 For example, if population-based demographic, exposure, and medical outcomes data were routinely and systematically collected from various sources; and if such data were integrated in a database and easily analyzed, interpreted, and results reported, then public health officials could detect new and emerging hazards; track rates and trends of illnesses and injuries of concern; prioritize and focus prevention programs (and allocate resources appropriately); document effects of policies and programs; justify requirements for personnel and other resources; project the natures, distributions, and magnitudes of future health care needs; and support health education and medical research activities.
Comprehensive public health surveillance is sometimes considered infeasible or impractical for general use. For example, sources of relevant data are often difficult to identify and may not be automated, and there is often variability across sources in database structures, record definitions, and coding schemes.9,10 Computer networks and data transmission capabilities are often unreliable or nonexistent, and the hardware, software, and personnel required to collect, integrate, and analyze large quantities of data are often too expensive or too easily overwhelmed by the demands of operating the system.6,10 Finally, there is often weak institutional support for public health surveillance.11
A number of authors have articulated visions of the future of epidemiological practice and public health surveillance.2,4,6,7,12-21 However, while technological advances have significantly enhanced current capabilities, there are few published descriptions of functioning systems that employ new approaches, exploit modern technologies, or preview capabilities of future systems.
MILITARY PUBLIC HEALTH SURVEILLANCE
There are characteristics of servicemembers, their activities, and the locations and settings in which they operate that make comprehensive medical surveillance uniquely challenging in the US armed forces. First, the military is a dynamic cohort whose membership changes daily. Second, servicemembers are widely dispersed and extremely mobile. Third, there are unique hazards associated with military occupations and activities and the locations and settings in which they are conducted. Fourth, adverse effects of exposures that are unique to the military may have long lead times and variable clinical expressions that are hard to detect and characterize. Finally, concerns about potential adverse effects often have political and emotional dimensions that complicate the timely and accurate assessment of these effects.
However, there are characteristics of the US armed forces that enhance opportunities to conduct comprehensive public health surveillance. First, all individuals are closely tracked from the day they enter service until the day they leave. Second, all individuals have free and open access to medical care in the military health system, and nearly every encounter is documented with a standardized record. Third, records of demographic characteristics, military experiences, and inpatient and outpatient encounters of all servicemembers are regularly transmitted to and maintained in centralized data archives. Fourth, the armed forces have telecommunications networks that can securely link data archives and medical institutions with a centralized data system. Fifth, post–cold war strategic circumstances and managed health care initiatives have stimulated support for health promotion and morbidity prevention programs in the armed forces. Finally, sera are routinely collected from servicemembers for medical surveillance purposes, and these specimens are available for archiving in a central repository.
RECENT BACKGROUND
Following the Persian Gulf War, investigations of medical complaints of Gulf war veterans were hindered because relevant records were often inaccessible or nonexistent, lacked uniformity and accuracy, and were generally not automated.22,23 At least partly in response, “deployment medical surveillance” became a DoD priority.
About the same time, largely in response to the end of the cold war, national security policies, structures, and priorities fundamentally changed.24 As military forces were downsized, overseas operations became more frequent and more geographically dispersed; were more likely to be conducted in places with nonfunctional public health and public safety infrastructures; and were generally peacekeeping, counterterrorist, humanitarian, or drug interdictive, rather than conventional combat, operations.
The maximization of the health, fitness, and medical preparedness of forces being deployed and the minimization of disease and injury risks during deployments became cornerstones of post–cold war military medical support strategy.21,25 There is now a broad understanding that the successful execution of this strategy depends on the effective conduct of comprehensive medical surveillance.26
THE DEFENSE MEDICAL SURVEILLANCE SYSTEM
In 1986, the Army established a data center to support its HIV-related screening, clinical care, and epidemiological research programs. In 1993, the Army’s HIV-1 data system transitioned to the Army Medical Surveillance System. In so doing, it expanded its scope to include all illnesses and injuries of public health or military operational importance. In 1997, the Army Medical Surveillance System transitioned to the Defense Medical Surveillance System (DMSS), and the Army Medical Surveillance Activity (AMSA) was assigned responsibility for its operation.
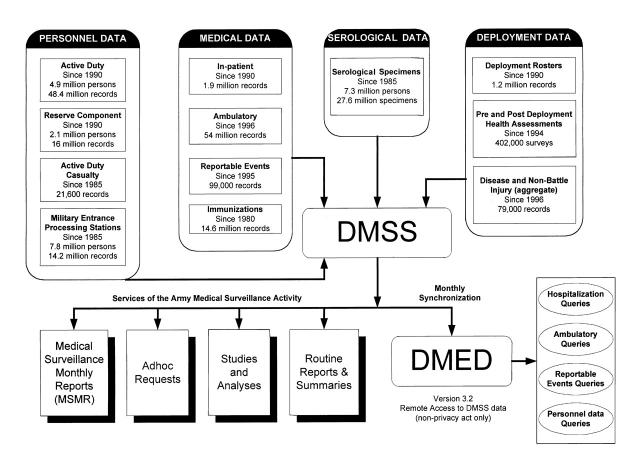
The DMSS (Figure 1 ▶) now serves as the central repository of medical surveillance data for the US armed forces. The DMSS receives data from many sources, including more than 100 field sites. Upon receipt, the data are processed through “edit check” programs that ensure completeness (e.g., that all essential fields have entries), consistency (e.g., birth dates are unchanged from previous entries), and accuracy (e.g., compliance with specified formats and within acceptable ranges). After processing, relevant data are integrated into the DMSS database.
FIGURE 1.
—Overview of the Defense Medical Surveillance System architecture, with key data sources and functional interrelationships.
Note. DMSS=Defense Medical Surveillance System; DMED=Defense Medical Epidemiology Database.
Longitudinal records are established and continuously updated for all individuals who have served in the armed forces since 1990. DMSS records document statuses of and changes in demographic and military characteristics as well as military and medical experiences of servicemembers throughout their military careers. Records are maintained in person, place, and time frames of reference. The maintenance of person, place, and time relationships permits, for example, nearly instantaneous assessments of the morbidity experiences of servicemembers who shared characteristics, were in specific locations, or had similar experiences on specific days or during periods of interest since 1990.
All data in the DMSS are maintained in a relational database (Oracle; Oracle Inc; Redwood City, Calif.). Tools for accessing and managing data in the DMSS have been customized with commercial off-the-shelf relational database management systems development software. Extensive physical and electronic security measures are used to restrict access to the DMSS and protect its integrity. For example, multiple layers of password protection are used to restrict access to information linkable to specific individuals.
In summary, the DMSS is a continuously updated, fully integrated, easily accessed relational database system. As of October 2001, the DMSS consisted of more than 200 million rows of data related to more than 7 million servicemembers.
THE DEPARTMENT OF DEFENSE SERUM REPOSITORY
Servicemembers are routinely screened for antibodies to HIV-1 during preinduction and periodic medical examinations, prior to overseas assignments, and before and after major overseas deployments. Since approximately 1990, sera remaining after routine HIV-1 antibody testing and sera collected before and after major deployments have been forwarded to the DoD Serum Repository (DoDSR).
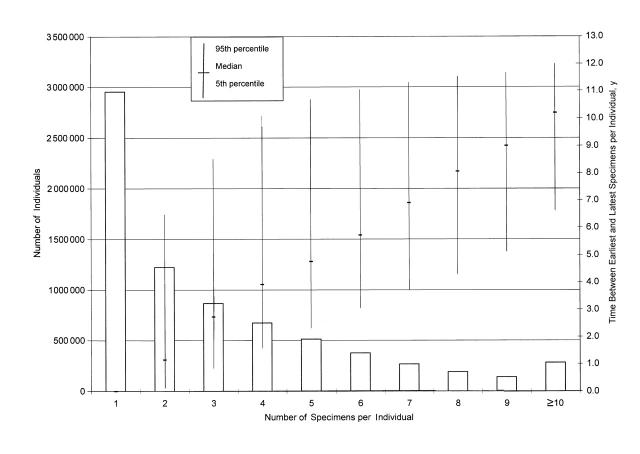
At the repository, specimens are stored in precisely documented locations in walk-in freezers at –30 °C. In the DMSS, serum identification numbers and repository locations are linked to dates of specimen collection and personal identifiers of donors. As of October 2001, more than 27 million specimens related to nearly 7.5 million individuals were stored in the DoDSR. Approximately 4.5 million individuals had at least 2 specimens in the repository, and among servicemembers with 2 or more archived specimens, the median time between the earliest and latest was 4.0 years (Figure 2 ▶). The DoDSR adds a powerful seroepidemiological capability to the overall surveillance program.
FIGURE 2.
—Numbers of individuals with serum specimens stored in the Department of Defense Serum Repository as of June 2001, summarized by number of specimens and distribution of times between earliest and latest collection dates.
ACCESS
Direct access to DMSS data is restricted to on-site members of the AMSA staff. Other users access the DMSS through telephonic and written requests, hard-copy and on-line publications and reports, and the Defense Medical Epidemiology Database (DMED), a Web-based application that provides remote and rapid (within seconds) access to data summaries in response to user-defined queries.
Personal identifier information is not available through remote access. On rare occasions, information linked to individuals is provided to enhance individual patient care, protect individual or community health, increase military operational preparedness, or support the conduct of research (contingent on prior approval by cognizant scientific and human subjects review committees).
Since 1995, the Medical Surveillance Monthly Report (MSMR) has been the principal tool for disseminating results of DMSS data analyses. MSMR reports frequencies, rates, and trends of ambulatory visits, hospitalizations, and reportable medical events among active servicemembers. MSMR also publishes reports of cases and outbreaks of illnesses, injuries, and exposures with broad military or medical relevance. MSMR is mailed to designated recipients and posted on the AMSA Web site (http://amsa.army.mil).
Finally, the DMSS enables rapid access to sera in the DoDSR. Specimens are retrieved from the repository to support the care of individual patients, outbreak investigations, assessments of deployment-related health threats, and seroepidemiological studies. Guidelines for accessing specimens are posted on the AMSA Web site.
USE
Since 1998, the AMSA has responded to an average of 350 requests per year for tailored data sets, data summaries, and epidemiological analyses. In addition, remote users have accessed the DMSS an average of 239 sessions per month (average of 11 queries per session) through the Web-based DMED application.
EXAMPLES OF APPLICATIONS
Population-Based Morbidity
In April of each year, the AMSA summarizes the morbidity experiences of active duty servicemembers during the previous calendar year. Hospitalizations and ambulatory visits are summarized in age- and sexdefined subgroups—overall, in major diagnostic categories, and at 3-digit levels of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).27
Risk Assessments
At the request of Army policymakers, respiratory illness hospitalization rates were compared between soldiers who had served at a military installation in a unique desert environment and closely matched “unexposed” soldiers. The exposed cohort consisted of more than 20 000 soldiers who had been assigned to the subject installation at any time during a 10-year surveillance period. The control cohort consisted of more than 80 000 soldiers who were randomly selected from among all soldiers who were contemporaries of and closely matched to index exposed soldiers. Compared with their counterparts, exposed soldiers had higher hospitalization rates for respiratory illnesses after they had left (but not before or during) their desert assignments.28
Vaccine Adverse Effects
In 1997, the Department of Defense began a program to immunize all servicemembers against anthrax.29,30 To monitor the vaccine’s safety, the AMSA compared rates of hospitalizations and ambulatory visits for specific diagnoses between servicemembers who had received anthrax vaccine and those who had not. Diagnoses that were statistically significantly overrepresented in the vaccinated cohort were referred for more detailed assessments.31
Emerging Threats
In 1993, after South Korea had been considered malaria-free for decades, vivax malaria reemerged in the country.32,33 Many of the cases had long incubation periods; thus, many servicemembers who acquired Plasmodium vivax infection in Korea had clinical presentations during subsequent assignments.34 For disease control purposes, vivax malaria among US soldiers was tracked in relation to time and location of infection acquisition, rather than clinical presentation. From 1993 to 1999, 147 cases of vivax malaria that were considered to have been acquired in Korea were found among soldiers at 34 locations in 16 states and on 4 continents.35
Deployment Surveillance
Since December 1995, the United States has deployed servicemembers to Bosnia-Herzegovina. Hospitalization rates were compared among deployed and contemporaneous nondeployed servicemembers; adjustment was made for potentially confounding differences between them. In general, crude hospitalization rates were lower among deployed than nondeployed personnel, and among the deployed, adjusted hospitalization rates were higher during and after deployment than before.36 The strongest predictor of hospitalization risk during deployment was a hospitalization prior to deployment, and the more recent a hospitalization was at the time of deployment, the greater the risk of hospitalization during deployment.37
Policy Effects
In April 1998, the Army revised its fluid replacement guidelines to lessen the risk of overhydration/hyponatremia during training in heat-stressful conditions.38,39 In the next 2 years, there were 65 cases of overhydration/hyponatremia among soldiers, and compared with 1997, there were 12% and 28% fewer cases in 1998 and 1999, respectively.40
Serological Surveys
In 1997, a serological survey was conducted to assess the nature, distribution, and magnitude of hepatitis C in US servicemembers. The AMSA randomly selected 21 000 members of the US armed forces who had serum specimens archived in the DoDSR. Some subgroups were oversampled to increase the precision of subgroup-specific estimates. Assays were conducted to detect antibodies to hepatitis C. Seroprevalences were lower in current servicemembers than in comparable groups of civilians and military veterans.41
Seroepidemiological Research
In collaboration with civilian and military researchers, the DMSS identified all servicemembers who were diagnosed with Hodgkin’s disease during a specified period and who had archived sera that was drawn prior to their diagnoses. A referent group was selected from among all noncases who had contemporaneous periods of service, were matched on potentially confounding factors, and had sera drawn at similar times to index cases. Sera from cases and noncases were assayed for markers of Epstein-Barr virus infection. Similar designs were used to study prostate,42 testicular, and cervical cancers; systemic lupus erythematosis; acute myocardial infarction; and postwar syndromes.43
CONCLUSION
The DMSS is a continuously expanding, fully integrated, comprehensive public health surveillance system. In effect, the DMSS has completed the data collection and database management phases of every epidemiological study that could be conceived using data from sources that contribute to the DMSS. The DMSS eliminates most of the administrative downtime and associated costs from the timelines and budgets of epidemiological studies. As a result, studies that used to require months to years (if feasible and affordable at all) can now be conducted in days to weeks by in-house epidemiologists.
The linkages of data relevant to individual characteristics, exposure states, medical events, and specimens in the DoDSR provide powerful seroepidemiological capabilities. Over time, the serum repository will increase in its value as new etiologic hypotheses are developed, as technologies for detecting biological markers in sera are improved, and as medical events accrue among aging cohorts of contributors.
The DMSS and DoDSR do not obviate the need for thoughtful and informed study designs, analysis methods, and interpretation of results. On the contrary, the DMSS and DoDSR enable researchers to focus time and attention on solving methodological problems, interpreting results, and producing summaries and reports.
In summary, the DMSS and DoDSR provide unprecedented capabilities for conducting comprehensive population-based surveillance of the US armed forces while protecting the privacy and confidentiality of servicemembers. They provide glimpses of the capabilities and potential uses of public health surveillance systems of the future.
Acknowledgments
The authors thank Richard N. Miller, MD, MPH, for constructive criticism and helpful suggestions.
The ideas expressed in this article are those of the authors and do not necessarily reflect the official views of the US Department of Defense or the US Army.
M. V. Rubertone has been chief of the AMSA since its inception. He has been involved in every aspect of planning, developing, and operating the DMSS. He is also responsible for the management and operations of the DoDSR. J. F. Brundage participated in the development of the AMSA, the DMSS, and the DoDSR. Both authors participated in every phase of the planning, writing, and editing of this article.
Peer Reviewed
References
- 1.Thacker SB, Berkelman RL, Stroup DF. The science of public health surveillance. J Public Health Policy. 1989;10:187–203. [PubMed] [Google Scholar]
- 2.Thacker SB, Stroup DF. The future of national public health surveillance in the United States. J Public Health Manag Pract. 1996;2(4):1–3. [DOI] [PubMed] [Google Scholar]
- 3.Thacker SB, Stroup DF, Rothenberg RB. Public health surveillance for chronic conditions: a scientific basis for decisions. Stat Med. 1995;14:629–641. [DOI] [PubMed] [Google Scholar]
- 4.Thacker SB, Stroup DF. Future directions for comprehensive public health surveillance and health information systems in the United States. Am J Epidemiol. 1994;140:383–397. [DOI] [PubMed] [Google Scholar]
- 5.Thacker SB, Koplan JP, Taylor WR, Hinman AR, Katz MF, Roper WL. Assessing prevention effectiveness using data to drive program decisions. Public Health Rep. 1994;109:187–194. [PMC free article] [PubMed] [Google Scholar]
- 6.Yasnoff WA, O’Carroll PW, Koo D, Linkins RW, Kilbourne EM. Public health informatics: improving and transforming public health in the information age. J Public Health Manag Pract. 2000;6(6):67–75. [DOI] [PubMed] [Google Scholar]
- 7.Committee for the Study of the Future of Public Health, Division of Health Care Services, Institute of Medicine. The Future of Public Health. Washington, DC: National Academy Press; 1988.
- 8.Virnig BA, McBean M. Administrative data for public health surveillance and planning. Annu Rev Public Health. 2001;22:213–230. [DOI] [PubMed] [Google Scholar]
- 9.Morris G, Snider D, Katz M. Integrating public health information and surveillance systems. J Public Health Manag Pract. 1996;2(4):24–27. [DOI] [PubMed] [Google Scholar]
- 10.Burwen DR, Seawright MF. Challenges in electronic importing of health data. J Public Health Manag Pract. 2000;6(1):87–94. [DOI] [PubMed] [Google Scholar]
- 11.Osterholm MT, Birkhead GS, Meriwether RA. Impediments to public health surveillance in the 1990s: the lack of resources and the need for priorities. J Public Health Manag Pract. 1996;2(4):11–15. [DOI] [PubMed] [Google Scholar]
- 12.Madans JH, Hunter EL. Improving and integrating data systems for public health surveillance. J Public Health Manag Pract. 1996;2(4):42–44. [DOI] [PubMed] [Google Scholar]
- 13.Harman J. Topics of our times: new health care data—new horizons for public health. Am J Public Health. 1998;88:1019–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meriwether RA. Blueprint for a national public health surveillance system for the 21st century. J Public Health Manag Pract. 1996;2(4):16–23. [DOI] [PubMed] [Google Scholar]
- 15.Koplan JP, Thacker SB, Lezin NA. Epidemiology in the 21st century: calculation, communication, and intervention. Am J Public Health. 1999;89:1153–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce N. Traditional epidemiology, modern epidemiology, and public health. Am J Public Health. 1996;86:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz S, Susser E, Susser M. A future for epidemiology? Annu Rev Public Health. 1999;20:15–33. [DOI] [PubMed] [Google Scholar]
- 18.Susser M. Does risk factor epidemiology put epidemiology at risk? Peering into the future. J Epidemiol Community Health. 1998;52:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susser M, Susser E. Choosing a future for epidemiology, I: eras and paradigms. Am J Public Health. 1996;86:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi BC. Perspectives on epidemiologic surveillance in the 21st century. Chronic Dis Can. 1998;19(4):145–151. [PubMed] [Google Scholar]
- 21.Brundage JF. Military preventive medicine and medical surveillance in the post-cold war era. Mil Med. 1998;163:272–277. [PubMed] [Google Scholar]
- 22.Presidential Advisory Committee on Gulf War Related Illnesses. Final Report. Washington, DC: US Government Printing Office; December 1996.
- 23.Institute of Medicine. Health Consequences of Service During the Persian Gulf War: Recommendations for Research and Information Systems. Washington, DC: National Academy Press; 1996. [PubMed]
- 24.Chairman of the Joint Chiefs of Staff. Joint Vision 2010: America’s Military Preparing for Tomorrow. Washington, DC: National Academy Press; 1996.
- 25.Mazzuchi JF, Claypool RG, Hyams KC, et al. Protecting the health of U.S. military forces: a national obligation. Aviat Space Environ Med. 2000;71:260–265. [PubMed] [Google Scholar]
- 26.Deputy Secretary of Defense. DoD Directive 6490.2: Joint Medical Surveillance. Washington, DC: The Pentagon; August 30, 1997.
- 27.Annual summary, US armed forces—2000. MSMR. 2001;7:4:2–23. [Google Scholar]
- 28.Lange JL, Campbell KE, Brundage JF. Respiratory morbidity associated with permanent duty assignment to Fort Irwin. Paper presented at: US Army Force Health Protection Conference; August 9, 2000; Baltimore, Md.
- 29.Memorandum from the Assistant Secretary of Defense (Health Affairs). Implementation of the anthrax vaccination program for the total force. Washington, DC: The Pentagon; May 18, 1998.
- 30.Friedlander AM, Pittman PR, Parker GW. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA. 1999;282:2104–2106. [DOI] [PubMed] [Google Scholar]
- 31.Lange J, Lesikar SE, Brundage JF, Rubertone MV. Quarterly Report: Surveillance Of Adverse Effects Of Anthrax Vaccine Adsorbed, January 1998–December 2000. Washington, DC: Army Medical Surveillance Activity; April 2001.
- 32.Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the republic of Korea. Emerg Infect Dis. 1998;4:295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petruccelli BP, Feighner BH, Craig SC, Kortepeter MG, Livingston R. Late presentations of vivax malaria of Korean origin, multiple geographic sites. MSMR. 1998;4(5):2–10. [Google Scholar]
- 35.Lange J. P. vivax malaria acquired by US soldiers in Korea: acquisition trends and incubation period characteristics, 1994–2000. MSMR. 2001;7(1):7–9. [Google Scholar]
- 36.Brundage JF, Kohlhase KF, Rubertone MV. Hospitalizations for all causes of U.S. military service members in relation to participation in Operations Joint Endeavor and Joint Guard, Bosnia-Herzegovina, January 1995 to December 1997. Mil Med. 2000;165:505–511. [PubMed] [Google Scholar]
- 37.Brundage JF, Kohlhase K, Gambel JM. Hospitalization experiences of US servicemembers before, during, and after participation in peacekeeping operations in Bosnia-Herzegovina. Am J Industrial Med. 2002;41(4):279–84. [DOI] [PubMed] [Google Scholar]
- 38.Garigan TP, Ristedt DE. Death from hyponatremia as a result of acute water intoxication in an Army basic trainee. Mil Med. 1999;164:234–238. [PubMed] [Google Scholar]
- 39.Montain SJ, Latzka WA, Sawka MN. Fluid replacement recommendations for training in hot weather. Mil Med. 1999;164:502–508. [PubMed] [Google Scholar]
- 40.Craig SC. Hyponatremia associated with heat stress and excessive water consumption: the impact of education and new Army fluid replacement policy. MSMR. 1999;5(2):2–9. [Google Scholar]
- 41.Hyams KC, Riddle J, Rubertone MV, et al. Prevalence and incidence of hepatitis C virus infection in the US military: a seroepidemiologic survey of 21,000 troops. Am J Epidemiol. 2001;153:764–770. [DOI] [PubMed] [Google Scholar]
- 42.Preston DM, Levin LI, Jacobson DJ, et al. Prostate-specific antigen levels in young white and black men 20 to 45 years old. Urology. 2000;56:812–816. [DOI] [PubMed] [Google Scholar]
- 43.Lo SC, Levin L, Ribas J, et al. Lack of serological evidence for Mycoplasma fermentans infection in army Gulf War veterans: a large scale case-control study. Epidemiol Infect. 2000;125:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]