Persons born where tuberculosis (TB) is prevalent are at higher risk of contracting the disease. In Maryland, people born in countries where TB is prevalent comprised 43% of TB cases in 1998.1 In Anne Arundel County, Maryland, where only 3% of the population is foreign born,2 40% of active TB cases in 1998 were foreign born.3 The incidence rate for foreign-born county residents was 66 TB cases per 100 000 population (compared with a US rate of 6.8 per 100 000 for the general population and 28 per 100 000 for the foreign-born population).1 Among US-born county residents, incidence was only 3 TB cases per 100 000.
In 1987, active pulmonary TB occurred in 2 foreign-born students in the Anne Arundel County public school system, and 35 school contacts (i.e., people who came into contact with TB cases at school) developed latent TB infection (LTBI). In 1989, the Anne Arundel County Department of Health, in collaboration with the county’s public school system, instituted a policy of targeted screening of all foreign-born students entering public schools, using the tuberculin skin test (TST) and the guidelines of the American Academy of Pediatrics, the Advisory Committee for Elimination of Tuberculosis of the Centers for Disease Control and Prevention (CDC), and the American Thoracic Society.4,5 The policy was revised in 1996 to include any untested foreign-born students.
All those born outside the United States, including American citizens, are defined as foreign born. Such students must show TST documentation before enrolling in school. Students with a history of TB must show documentation of adequate therapy.4,5
In 1999, the county department of health evaluated the health and economic impact of targeted TB screening by retrospectively reviewing data on those that the department had screened from 1993 to 1998.
METHODS
TST report cards contain the ages and TST readings of students tested by the county department of health. TB clinic records contain more detailed data on students with positive TSTs, including age, sex, ethnicity, place of birth, TST reading, medical and radiological examination results, and medication, duration, outcome, and side effects of treatment for TB (either active or latent infection).
TSTs were administered and read by trained public health nurses. Results, recorded in millimeters, were classified as positive if induration size was 10 mm or larger. Reactor rate is the number of students who were TST positive divided by the number tested, and case finding rate is the number with active TB divided by the number tested. Adherence rate is the number of TST-positive students who accepted treatment and remained in Anne Arundel County until at least 6 months of treatment was completed.
For the economic analysis, intervention cost was defined as the cost of screening and medical follow-up, and disease cost averted as the potential cost of managing the TB cases prevented. Net cost (saving) was defined as intervention cost minus disease cost averted, and the dollar investment–saving ratio was net cost (saving) divided by intervention cost.6
The economic analysis involved the following assumptions: (1) all students with LTBI completed 9 months of treatment; (2) lifetime risk of active TB disease for a child with a positive TST was approximately 10%7,8 (all children were assumed to have normal immune systems and average reactivation risk); (3) the efficacy of 6 months of isoniazid in reducing the lifetime risk of active TB was considered to be 69%8; (4) the number of TB disease cases averted was calculated as the number of students remaining in Anne Arundel County who completed at least 6 months of LTBI treatment multiplied by 0.069 (lifetime risk of developing TB disease × isoniazid efficacy); (5) productivity losses and discounting of costs were ignored; (6) future costs of TB disease were conservatively assumed to remain the same as those reported in a 1995 study.9
RESULTS
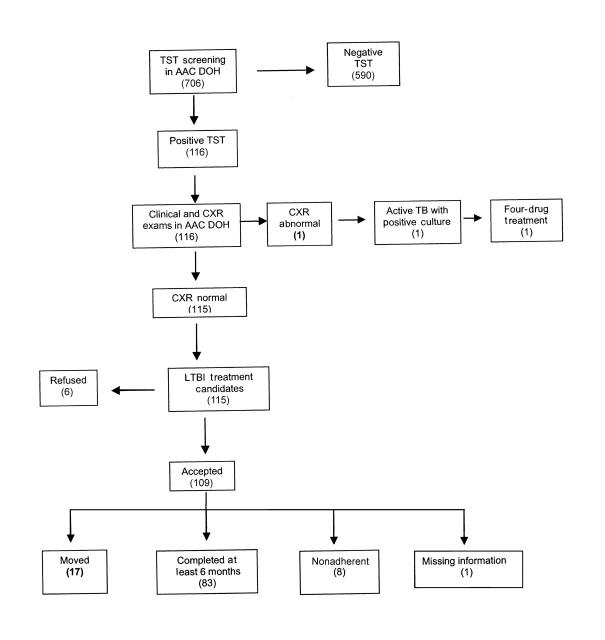
Between 1993 and 1998, a total of 706 foreign-born students referred by school registrars to the Anne Arundel County Department of Health, all without prior positive TSTs, received a TST and returned for a reading (adherence rate for TST reading = 100%) (Figure 1 ▶). Of these, 116 were positive (median age = 14 years) and 590 were negative (median age = 10 years), yielding a reactor rate of 16.4%. Students with positive TSTs tended to be older (for positive TST, mean = 13.0 years [SD = 3.9]; 95% confidence interval [CI] = 12.3, 13.7; P < .001; for negative TST, mean = 10.7 years [SD = 4.2]; 95% CI = 10.3, 11.0; P < .001). Ethnicity was 54% Asian, 23% Hispanic, 18% White, 4% Black, and 1% “Other.” Ninety-three percent of foreign-born students came from areas with high TB prevalence (Africa, Asia, Latin America, Middle East, parts of the former Soviet Union, and Eastern Europe).
FIGURE 1.
—Flowchart of tuberculin skin test (TST) screening and clinical follow-up among foreign-born students: Anne Arundel County, Maryland, 1993–1998.
Note. Numbers of students are given in parentheses. AAC DOH=Anne Arundel County Department of Health; CXR=chest x-ray; TB=tuberculosis; LTBI=latent TB infection.
All students with positive TSTs (n = 116) received physician evaluations and chest x-rays. The median interval between TST reading and chest x-ray examination was 26 days (95% CI = 24, 27). One student was diagnosed with active TB, for a case finding rate of 0.14%. The infection was asymptomatic, with an abnormal chest x-ray result consistent with TB, a negative smear, and a positive culture.
Of the 115 candidates for LTBI treatment (TST positive, age < 35 years, no active disease), 6 (5%) refused. All but 2 of the 109 who agreed to treatment received self-administered isoniazid under parental supervision; the 2 exceptions, who were contacts of a child with isoniazid-resistant TB, received rifampin. Seventeen of these 109 students moved away from Anne Arundel County before completing treatment; of the remaining 92, 83 received at least 6 months of treatment, 8 were nonadherent (< 6 months of treatment), and 1 had missing information, for an adherence rate of 90%. No side effects were reported.
Without treatment, an estimated 11 cases of active TB disease would have occurred during the lifetimes of those with LTBI. An estimated 6 to 8 cases were averted with isoniazid treatment (assuming an isoniazid efficacy of 69%–99% in children).4,8,10,11
Staff salaries and benefits (physician, nurse, and radiology technician) accounted for 85% of the intervention cost, an estimated $32 617 for 6 years. The potential cost of lifetime disease averted was estimated at $98 350 (Table 1 ▶). Net cost (saving) was $65 733. From the societal long-term perspective, $2 was saved for every $1 invested.
TABLE 1.
—Economic Analysis of Targeted Tuberculosis Screening in Public Schools: Anne Arundel County, Maryland, 1999
| Cost category | Cost per individual, $ | No. of students | Total cost, $ | Source |
| Intervention cost | ||||
| One TST and reading | 11.70 | 706 | 8 260.20 | |
| One physician evaluation, 1 CXR and reading, 8 follow-up visits, 9 months of isoniazid | 211.80 | 115 | 24 357.00 | |
| Total: screening, diagnosis, and treatment | 32 617.20 | AAC DOHc | ||
| Disease cost averted: outpatient/inpatient treatment and contact tracing | 16 391.66a | 6b | 98 349.96 | Mohle-Boetani et al.9 |
Note. Preventive treatment with rifampin would add additional cost of $540.00 per individual. AAC DOH = Anne Arundel County Department of Health; TST = tuberculin skin test; CXR = chest x-ray.
aCost per tuberculosis case.
bNumber of tuberculosis cases prevented.
cUnpublished data.
DISCUSSION
This study shows the large public health and economic impacts of targeted TB screening in public schools. The adherence rate was 100% for TST reading and 90% for LTBI treatment, which exceeded the Healthy People 2010 objective of 85% and the US baseline of 62% for those treated for LTBI in 1997.12 The return on investment for TB prevention was 200%.
The total number of foreign-born students in the county public schools, as well as the number of students screened by private physicians, is unknown. Despite the fact that its evaluation of school-based, targeted TB screening was limited to those students screened in Anne Arundel County Department of Health clinics, the results of this study should be viewed in terms of relevance to public health, the benefits and drawbacks of the targeted screening policy, and potential duplication of the program.
Although transmission of TB from children with active TB is rare, several instances of school transmission, most involving foreign-born, older children, have been reported.9,13–15 Ideally, the school health system should ensure that students with active TB are identified and treated before attending school to avoid potential transmission, but such a requirement would be difficult to implement, given a mean time of 26 days between screening and clinical evaluation.
The targeted screening policy had limitations. The sensitivity and specificity of the TST for detecting TB depend on the epidemiological risk of the target population, and predictive values are influenced by the size of indurations considered to be positive.4,16,17 False-positive results occur owing to nontuberculous mycobacteria or bacille Calmette-Guérin, especially within 3 to 5 years of vaccination.18,19 False-negative results occur in about 10% of immunecompetent children with culture-documented TB.20–22 However, the TST is the best screen available.4,5,22
Birthplace was checked at school registration. Individual risk assessment would be ideal, since only those from countries with high TB prevalence need screening. Individual risk assessment was too difficult for the admissions staff to administer, however, so all foreign-born students were asked for documentation of TST, regardless of citizenship or country of origin. US-born students with high-risk household members or extensive travel to TB-endemic areas were not screened, although they were also at risk.23
Variation in reactor rates, treatment adherence, local school structure, origins and migration rates of foreign-born persons, parental cooperation, and local TB control program strength could all affect attempts to duplicate the results described here.
Universal TB screening is contraindicated in low-risk populations.4,5,9,24 Targeted screening of high-risk groups for TST and treatment of LTBI are recommended by the American Academy of Pediatrics, the Advisory Committee for Elimination of Tuberculosis of the CDC, and the American Thoracic Society4,5 and have been proven cost-effective.9,25–29 School TB screening practices, both in Maryland and in the United States as a whole, vary by state statutes, local policies, screening criteria, type of test, and follow-up.30,31
As US rates decline, the treatment of LTBI and the control of imported TB become priorities. Despite its limitations, targeted school screening followed by LTBI treatment can be cost-effective against TB in recent immigrants (< 5 years).9,14,30,32,33 This study offers further support for the recommendations of the American Academy of Pediatrics, the CDC, and the American Thoracic Society.
Acknowledgments
We thank Loretta Gossett, RN, of the Communicable Disease Program, Anne Arundel County Department of Health, for assistance in providing information about the tuberculosis program and the data for this study.
Human Participant Protection No institutional review board approval was needed for this study.
S. Chang was responsible for the design of the study and for data entry and analysis. L. S. M. Wheeler and K. P. Farrell were responsible for the conception of the study. All authors were involved in interpreting the results and drafting and revising the brief.
Peer Reviewed
References
- 1.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 1998. MMWR Morb Mortal Wkly Rep. August 1999:1–51.
- 2.Anne Arundel County Census Tract Profiles: 1990 Census Social and Economic Data From Summary Tape File 3A. Annapolis, Md: Anne Arundel County Dept of Planning and Code Enforcement; 1994.
- 3.Phillips FB, Farrell KP, Curtis JJ, et al. Toward a Healthier Anne Arundel County. Community Health Indicators Report Card. Annapolis, Md: Anne Arundel County Dept of Health; 1999:15.
- 4.Tuberculosis. In: Pickering LK, ed. 2000 Red Book: Report of the Committee on Infectious Diseases. 25th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2000:593–613.
- 5.American Thoracic Society and Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. [DOI] [PubMed] [Google Scholar]
- 6.Haddix AC, Teutsch SM, Shaffer PA, Dunet DO. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. 1st ed. New York, NY: Oxford University Press; 1996.
- 7.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99:131–138. [DOI] [PubMed] [Google Scholar]
- 8.Core Curriculum on Tuberculosis: What the Clinician Should Know. 4th ed. Atlanta, Ga: Centers for Disease Control and Prevention; 2000.
- 9.Mohle-Boetani JC, Miller B, Halpern M, et al. School-based screening for tuberculous infection: a cost-benefit analysis. JAMA. 1995;274:613–619. [PubMed] [Google Scholar]
- 10.International Union Against Tuberculosis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis. Bull World Health Organ. 1982;60:555. [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu KH. Thirty years after isoniazid: its impact on tuberculosis in children and adolescents. JAMA. 1984;251:1283–1285. [DOI] [PubMed] [Google Scholar]
- 12.Healthy People 2010. Conference ed, 2 vol. Washington, DC: US Dept of Health and Human Services; January 2000.
- 13.Sacks JJ, Brenner ER, Breeden DC, Anders HM, Parker RL. Epidemiology of a tuberculosis outbreak in a South Carolina junior high school. Am J Public Health. 1985;75:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry MA, Shirley L, Grady MT, et al. Tuberculosis infection in urban adolescents: results of a school-based testing program. Am J Public Health. 1990;80:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reves R, Blakey D, Snider DE Jr, Farer LS. Transmission of multiple drug-resistant tuberculosis: report of a school and community outbreak. Am J Epidemiol. 1981;113:423–435. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Diagnostic standards and classification of TB in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 17.Rose DN, Schechter CB, Adler JJ. Interpretation of the tuberculin skin test. J Gen Intern Med. 1995;10:635–642. [DOI] [PubMed] [Google Scholar]
- 18.Lockman S, Tappero JW, Kenyon TA, Rumisha D, Huebner RE, Binkin NJ. Tuberculin reactivity in a pediatric population with high BCG vaccination coverage. Int J Tuberc Lung Dis. 1999;3:23–30. [PubMed] [Google Scholar]
- 19.Joe L, Hall E. Mycobacterium marinum disease in Anne Arundel County: 1995 update. Md Med J. 1995;44:1043–1046. [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics, Committee on Infectious Diseases. Tuberculosis. Screening for tuberculosis in infants and children. Pediatrics. 1994;93:131–134. [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics, Committee on Infectious Diseases. Tuberculosis. Update on tuberculosis skin testing of children. Pediatrics. 1996;97:282–284. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Screening for tuberculosis and tuberculosis infection in high-risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44(RR-11):19–34. [PubMed] [Google Scholar]
- 23.Lobato MN, Hopewell PC. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158:1871–1875. [DOI] [PubMed] [Google Scholar]
- 24.Serwint JR, Hall BS, Baldwin RM, Virden JM. Outcomes of annual tuberculosis screening by Mantoux test in children considered to be at high risk: results from one urban clinic. Pediatrics. 1997;99:529–533. [DOI] [PubMed] [Google Scholar]
- 25.Adhikari N, Menzies R. Community-based tuberculin screening in Montreal: a cost-outcome description. Am J Public Health. 1995;85:786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colice GL. Decision analysis, public health policy, and isoniazid chemoprophylaxis for young adult tuberculin skin reactors. Arch Intern Med. 1990;150:2517–2522. [PubMed] [Google Scholar]
- 27.Snider DE Jr, Caras GJ, Koplan JP. Preventive therapy with isoniazid. Cost-effectiveness of different durations of therapy. JAMA. 1986;255:1579–1583. [PubMed] [Google Scholar]
- 28.Rose DN, Schechter CB, Fahs MC, Silver AL. Tuberculosis prevention: cost-effectiveness analysis of isoniazid chemoprophylaxis. Am J Prev Med. 1988;4:102–109. [PubMed] [Google Scholar]
- 29.Fitzgerald JM, Gafni A. A cost-effectiveness analysis of the routine use of isoniazid prophylaxis in patients with a positive Mantoux skin test. Am Rev Respir Dis. 1990;142:848–853. [DOI] [PubMed] [Google Scholar]
- 30.Driver CR, Valway SE, Cantwell MF, Onorato IM. Tuberculin skin test screening in schoolchildren in the United States. Pediatrics. 1996;98:97–102. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Tuberculosis control laws—United States, 1993: recommendations of the Advisory Council for the Elimination of Tuberculosis (ACET). MMWR Morb Mortal Wkly Rep. 1993;42:1–28. [PubMed] [Google Scholar]
- 32.Pong AL, Anders BJ, Moser KS, Starkey M, Gassmann A, Besser RE. Tuberculosis screening at 2 San Diego high schools with high-risk populations. Arch Pediatr Adolesc Med. 1998;152:646–650. [DOI] [PubMed] [Google Scholar]
- 33.Scholten JN, Fujiwara PI, Frieden TR. Prevalence and factors associated with tuberculosis infection among new school entrants, New York City, 1991–1993. Int J Tuberc Lung Dis. 1999;3:31–41. [PubMed] [Google Scholar]