Abstract
The transmembrane adaptor molecule TRIM is strongly expressed within thymus and in peripheral CD4+ T cells. Previous studies suggested that TRIM is an integral component of the T-cell receptor (TCR)/CD3 complex and might be involved in regulating TCR cycling. To elucidate the in vivo function of TRIM, we generated TRIM-deficient mice by homologous recombination. TRIM−/− mice develop normally and are healthy and fertile. However, the animals show a mild reduction in body weight that appears to be due to a decrease in the size and/or cellularity of many organs. The morphology and anatomy of nonlymphoid as well as primary and secondary lymphoid organs is normal. The frequency of thymocyte and peripheral T-cell subsets does not differ from control littermates. In addition, a detailed analysis of lymphocyte development revealed that TRIM is not required for either positive or negative selection. Although TRIM−/− CD4+ T cells showed an augmented phosphorylation of the serine/threonine kinase Akt, the in vitro characterization of peripheral T cells indicated that proliferation, survival, activation-induced cell death, migration, adhesion, TCR internalization and recycling, TCR-mediated calcium fluxes, tyrosine phosphorylation, and mitogen-activated protein family kinase activation are not affected in the absence of TRIM. Similarly, the in vivo immune response to T-dependent and T-independent antigens as well as the clinical course of experimental autoimmune encephalomyelitis, a complex Th1-mediated autoimmune model, is comparable to that of wild-type animals. Collectively, these results demonstrate that TRIM is dispensable for T-cell development and peripheral immune functions. The lack of an evident phenotype could indicate that TRIM shares redundant functions with other transmembrane adaptors involved in regulating the immune response.
Upon ligation of the T-cell receptor (TCR) by peptide/major histocompatibility complex complexes, a plethora of signaling cascades are initiated within T cells that finally result in T-cell activation. It is well established that the TCR itself is not capable of transducing signals, as it possesses only a short intracellular tail that lacks any known signaling motif. Rather, signal transduction via the TCR is accomplished by the invariant CD3γ, CD3δ, CD3ɛ, and ζ subunits, which all possess particular amino acid motifs named ITAMs (immunoreceptor tyrosine-based activation motifs) in their cytoplasmic domains (17, 35). Overall, the TCR/CD3/ζ complex is organized in dimers (CD3ɛδ and CD3ɛγ dimers that noncovalently associate with the TCRαβ heterodimer and the TCRζ homodimer) and contains in total 10 ITAMs: 1 in each of the CD3γ, CD3δ, and CD3ɛ subunits and 3 in each of the two TCRζ chains. Upon phosphorylation by Src family kinases, the ITAMs are converted into high-affinity binding sites for the cytosolic protein tyrosine kinase ZAP-70, which is in turn recruited from the cytosol to the activated TCR by its tandem SH2 domains. After binding to the phosphorylated ITAMs, ZAP-70 serves as a substrate for Src kinases and becomes activated by phosphorylation. The biochemical cascade originating from the ligated TCR is then further propagated by the transmembrane adaptor protein LAT (linker for activation of T cells) which links the TCR to the mitogen-activated protein kinase (MAPK) and Ca2+ pathways after phosphorylation by ZAP-70 (12, 37).
In addition to being the signal transducing subunits of the TCR, the CD3 and TCRζ chains are also required for the correct expression of the TCR at the plasma membrane (for a review, see reference 1). TCR assembly begins in the endoplasmic reticulum with the pairing of CD3ɛ with either CD3γ or CD3δ. Once the ɛδ and ɛγ heterodimers are formed, they noncovalently associate with the TCRα/β heterodimer. The last component to be incorporated in the complex is the TCRζ homodimer, which overrides an endoplasmic reticulum retention signal within the CD3ɛ chain, thus allowing the complex to be transported to the plasma membrane (9).
Recent findings have indicated that the invariant chains of the TCR/CD3 complex might associate with a variety of additional molecules. For example, the TCRζ chain has been proposed to interact with SLAP-2 (26), TRIM (4, 20), CTLA4 (7), and Unc119 (5, 14), while CD3ɛ apparently complexes with CAST (36) and Nck (13). The physiological relevance of these interactions is so far not completely understood. However, it has been proposed that they could serve to integrate or regulate the signal capability of the TCR/CD3 complex or to modulate the expression levels of the T-cell receptor.
The nonraft transmembrane adaptor protein TRIM (T-cell receptor interacting molecule) is exclusively expressed in T lymphocytes. TRIM has been shown to coprecipitate with the TCR/CD3 complex under mild detergent conditions, and, similar to the TCR, its expression is downregulated after TCR triggering (4). A recent study demonstrated that TRIM preferentially interacts with the TCR complex via the TCRζ chain and that all three domains of TRIM (extracellular, transmembrane, and cytoplasmic domains) are required for this interaction (20).
The functional relevance of the association between TRIM and TCRζ has been addressed by overexpressing TRIM in the Jurkat T cell line (20). These experiments revealed that cells overexpressing TRIM show a considerable increase in cell surface expression of TCRαβ and CD3ɛ caused by TRIM-mediated inhibition of spontaneous TCR internalization (20). As expected, the enhanced expression levels of the TCR in TRIM transfectants concomitantly lead to an increased TCR-mediated Ca2+ flux. Based upon these data, it was proposed that TRIM regulates TCR-mediated signaling by modulating the expression levels of the TCR on the cell surface.
TRIM possesses three tyrosine-based signaling motifs (TBSMs) within its cytoplasmic domain that are potentially involved in regulating TCR-mediated signal transduction. One of these motifs (YEQM) represents a consensus motif for the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and, indeed, TRIM is capable of binding p85 via the YEQM motif after phosphorylation by Src kinases (4). Similarly, the two other TBSMs also become phosphorylated upon TCR engagement, but the molecules binding to these motifs are yet to be identified (20).
To study the function of TRIM in vivo, we generated TRIM-deficient mice by homologous recombination. Collectively, the functional and biochemical characterization of TRIM−/− mice suggest that TRIM is dispensable for the development and the function of the immune system.
MATERIALS AND METHODS
Mice.
TRIM−/− mice were generated by homologous recombination by replacing a part of exon 1 (including ATG) and exon 2 (coding for the entire transmembrane region) with a NEO cassette. The linearized targeting construct was transfected into SVJ129-derived embryonic stem cells, and G418-selected clones were transferred into C57BL/6 blastocysts as previously described (2). Germ line mutated animals were backcrossed onto the C57BL/6 background for more than 10 generations in a conventional animal facility. Animals were genotyped by PCR using the following primers: 5′TRIM (CGT CTC TGC TTC TCT ACA TAG TGG), 3′TRIM (GCT CTG GAT GCC CCT TCT TCC), and 3′Neo (GAC GTG CTA CTT CCA TTT GTC ACG TCC) (BioTez GmbH).
OT-I and OT-II TCR transgenic mice were kindly provided by Percy Knolle, P14 mice were provided by Thomas Kammerthoens, and H-Y TCR transgenic mice were provided by Gary Koreztky. For TCR transgenic studies, TRIM−/− mice crossed with TCR transgenic mice were obtained by crossing TRIM−/− mice with TRIM+/− mice with TCR heterozygote transgenics.
Flow cytometry analysis.
Single-cell suspensions were obtained from thymus, lymph nodes, spleen, and bone marrow by dissociation of isolated tissues through a 70-μm mesh. Cells were stained with fluorescence-labeled monoclonal antibodies against CD3 (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8 (53-6.7), CD25 (7D4), CD44 (IM7), CD45RB (363.16A), CD62L (MEL-14), CD69 (H1.2F3), TCRβ (H57-597), TCRγδ (GL3), B220 (RA3-6B2), and TCRVα2 (B20.1), all obtained from BD Biosciences, or with fluorescein isothiocyanate (FITC)-labeled HY-TCR (T3.70) (eBiosciences) for 30 min at 4°C. Cell-associated fluorescence was analyzed by a FACSCalibur and Cell Quest Pro software (BD Biosciences).
Immunoblotting.
For all biochemical analyses, CD4+ T cells were purified by an AutoMACS magnetic isolation system according to the manufacturer's instructions (Miltenyi). Cells were either left unstimulated, stimulated with 10 μg/ml of biotinylated CD3ɛ antibody (145-2C11; BD Biosciences), or stimulated with CD3 plus 10 μg/ml of biotinylated CD28 (BD Biosciences) monoclonal antibody (MAb) followed by cross-linking with 25 μg/ml of streptavidin (Dianova) at 37°C. Cells were lysed in lysis buffer containing 1% NP-40, 1% laurylmaltoside (N-dodecyl β-d-maltoside), 50 mM Tris, pH 7.5, 140 mM NaCl, 10 mM EDTA, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride or nitrocellulose membranes, and blotted with the following antibodies: anti-phosphotyrosine (4G10), anti-ERK1/2 (pT202/pT204), anti-JNK (pT183/pY185), anti-p38 (pT180/pT182), anti-phospho-Akt (S473), anti-Akt, all from Cell Signaling, and anti-ZAP70 (clone Z24820; Transduction Laboratories), anti-ERK1/2 (Cell Signaling), or β-actin (Sigma). Where PI3K inhibitor was used, CD4+ T cells were preincubated with 100 nM wortmannin (Calbiochem) for 30 min at 37°C prior to stimulation.
Ca2+ flux.
For Ca2+ measurement, cells were incubated for 30 min with 3.75 μg/ml indo-1-AM (Molecular Probes) in phenol red-free RPMI 1640 medium (GIBCO BRL) containing 10% fetal calf serum (FCS). After washing, the cells were incubated for 30 min at 37°C in RPMI 1640 and stained with CD4 and CD8 and incubated with 10 μg/ml biotinylated CD3 (145-2C11) MAbs at 4°C. Calcium fluxes were induced by cross-linking the TCR/CD3 complex with 75 μg/ml streptavidin and were measured using an LSR flow cytometer (BD Biosciences).
Proliferation assay.
CD4+ T cells were purified from single-cell suspensions of splenocytes by an AutoMACS magnetic isolation system according to the manufacturer's instructions (Miltenyi). Cells were stimulated for 72 h with either the indicated concentrations of plate-bound CD3 MAb (145-2C11), 4 μg/ml concanavalin A (ConA) (Calbiochem), or 2.5 μg/ml staphylococcal enterotoxin B (SEB; Sigma), or with 2 × 10−9 M phorbol-12-myristate-13-acetate (PMA) (Calbiochem) and 0.5 μg/ml ionomycin (Calbiochem) in 96-well U-bottomed plates (Corning Costar) at a density of 25 × 104 cells per well in 200 μl of RPMI medium supplemented with penicillin, streptomycin, β-mercaptoethanol, and 10% FCS. Cells were pulsed with 1 μCi [3H]thymidine per well during the last 8 h.
Cell survival assays.
Thymocytes or purified splenic CD4+ T cells were incubated for 24 h in the presence of the following stimuli: plate-bound CD3 MAb (145-2C11), CD3+ CD28 MAb (37.51), 1 μM etoposide (Sigma), 1 μg/ml Fas MAb (BD Biosciences) plus 30 μg/ml cycloheximide (Calbiochem), or 2 nM dexamethasone (Sigma). Cells were harvested and stained with annexin V and propidium iodide (BenderMedSystem) according to the manufacturer's instructions. Apoptotic cells were defined as annexin V-positive and propidium iodide-negative cells.
For activation-induced cell death (AICD), 2 × 106 CD4+ lymphocytes were stimulated with 5 μg/ml plate-bound CD3 MAb in RPMI in flat-bottomed 24-well plates (Corning Costar) for 2 days. Cells were then grown in medium containing 5 IU/ml recombinant human interleukin-2 (rIL-2) (PeproTech) for an additional 2 days in flat-bottomed 24-well plates, and 1 × 106 cells were restimulated overnight with 5 μg/ml plate-bound CD3 MAb in new 24-well plates with medium containing 5 IU/ml rIL-2. Apoptosis was measured as described above.
Migration assay.
Cell migration was performed in a 24-well Transwell plate (Corning Costar) with 3-μm-pore polycarbonate filters. Freshly isolated lymph node cells were resuspended at a density of 4 × 107 cells/ml in RPMI containing 0.5% bovine serum albumin (BSA). One hundred microliters of cell suspension was placed in the upper chamber, and 600 μl of medium was placed in the lower chamber. After equilibration at 37°C for 1 h, 50 nM of mouse recombinant SDF1β (PeproTech) was added to the wells, and the plates were incubated for an additional 3 h. Cells were then harvested, stained with anti-CD4 and anti-CD8, and counted by fluorescence-activated cell sorting (FACS). Duplicates were used for each condition.
Mouse immunization and ELISA.
Two- to 3-month-old mice were immunized intraperitoneally with 20 μg/animal 2,4-dinitrophenyl (DNP)-keyhole limpet hemocyanin (KLH) (Calbiochem) in 200 μl complete Freund's adjuvant (Sigma) and were boosted 10 days later with 20 μg/animal DNP-KLH in 200 μl incomplete Freund's adjuvant (Sigma). Before and after immunization, mice were bled and the amount of hapten-specific immunoglobulins was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (31). Briefly, plates were coated with 3 μg/ml DNP-BSA in bicarbonate buffer overnight at 4°C. Nonspecific binding was prevented by blocking the plates with 1% BSA for 2 h at room temperature. Serial dilutions of mouse sera were incubated overnight at 4°C, and the specific binding was detected using alkaline-phosphatase-labeled goat anti-mouse immunoglobulin M (IgM; Serotec) or goat anti-mouse IgG (subclasses 1, 2a, 2b, 3; Dianova).
Basal levels of immunoglobulins were measured as previously described (31).
TCR internalization and recycling.
For TCR internalization assays, 2 × 106 lymph node cells were precoated for 30 min at 4°C with phycoerythrin (PE)-labeled TCRβ (H57-597) MAb in RPMI containing 10% FCS, 50 μM β-mercaptoethanol, penicillin, and streptomycin. After washing, cells were plated in 96-well plates at a density of 1 × 106 cells per 200 μl of medium and incubated at 37°C for the indicated times. TCR internalization was measured as previously described (10). Briefly, TCR internalization was stopped by washing the cells in ice-cold phosphate-buffered saline (PBS) containing 0.1% sodium azide and 1% BSA. The surface-bound antibody was removed by washing lymphocytes in stripping buffer (100 mM Glycin, 100 mM NaCl, pH 2.5). The intracellular fluorescence was measured by FACS. To distinguish the different lymphocyte subsets, cells were stained with CD4-FITC and CD8-CyChrome MAbs after stripping. Internalization of the TCR was calculated according to the following equation, as previously published (10, 16, 32): % internalization = 100 × [(acid-resistant fluorescence at t − cellular autofluorescence)/(fluorescence of cells stained with TCRβ-PE MAb − cellular autofluorescence)], where t is time.
A TCR recycling assay was performed as reported previously (24). Briefly, 4 × 106 freshly isolated lymph node cells were stained at 37°C with 1 μg/ml PE-labeled anti-TCRβ for 1 h. After washing, surface-bound antibody was stripped as reported above. To allow reexpression of the internalized receptor, cells were then incubated at 37°C for different times, washed in ice-cold PBS containing 0.1% sodium azide and 1% BSA, and again stripped in low-pH buffer. Lymphocytes were stained for CD4 and CD8 and analyzed by FACS. Recycled TCR was calculated using the following formula: % recycled = 100 × [(acid-resistant fluorescence after second low-pH buffer treatment − autofluorescence)/(acid-resistant fluorescence after first low-pH buffer treatment)].
EAE.
Experimental autoimmune encephalomyelitis (EAE) was induced and scored as previously described (28, 31).
RESULTS AND DISCUSSION
Generation of TRIM-deficient mice.
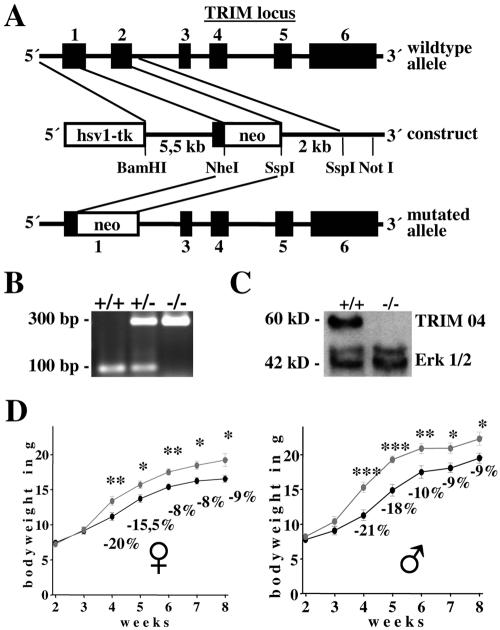
To assess the role of TRIM within the immune system, the TRIM gene was disrupted by homologous recombination in embryonic stem (ES) cells (Fig. 1A). The targeting vector replaced part of exon 1 and exon 2 of the TRIM gene with a neomycin selection cassette. The deleted region contained the translation start site as well as the entire extracellular and transmembrane regions of TRIM. The linearized targeting vector was introduced into ES cells by electroporation, and cells were selected with G418 and ganciclovir. Successfully targeted ES clones were injected into C57BL/6 blastocysts. To avoid potential phenotypic bias due to inbred genetics, germ line-competent chimeric mice were backcrossed on a C57BL/6 genetic background for more than 10 generations. Mutation of the TRIM gene was confirmed by genomic PCR (Fig. 1B). To ensure that the targeted allele results in loss of protein, lysates were prepared from thymocytes of null and wild-type mice and analyzed by anti-TRIM Western blotting with two different monoclonal or two polyclonal anti-TRIM reagents, all directed at particular regions of the cytoplasmic tail of TRIM. Under all conditions tested, we did not detect any anti-TRIM-reactive product, corresponding either to the full-length or to a truncated (cytoplasmic) form in TRIM−/− thymocytes (Fig. 1C and data not shown). These data demonstrate that the TRIM gene was indeed successfully inactivated. Analysis of genotypes at weaning revealed that the mutated allele segregates with the expected Mendelian frequency, thus indicating that TRIM does not cause embryonic lethality. Indeed, among the 608 viable offspring obtained by breeding TRIM+/− mice, 149 (24.5%) were TRIM+/+, 290 (47.7%) were TRIM+/−, and 169 (27.8%) were TRIM−/−.
FIG. 1.
Generation of TRIM-deficient mice. (A) Partial restriction map of the TRIM locus and the targeting construct. Exons are represented by filled boxes. Neo, neomycin resistance cassette; Tk, thymidine kinase. (B) PCR analysis of TRIM wild type, heterozygous, and knockout mice. (C) Western blot analysis of cell lysates prepared from wild-type and TRIM−/− thymocytes. The blot was probed with the monoclonal anti-TRIM antibody TRIM04 and with the polyclonal anti-ERK1/2 antibody to show equal loading. (D) TRIM-deficient mice display lower body weights. Shown are growth curves of female and male TRIM+/+ or TRIM−/− animals (n = 15 mice per group). Statistically significant differences are indicated: *, P < 0.05; **, P < 0.005; ***, P < 0.001.
TRIM−/− mice are viable and fertile but display a smaller body size.
Anatomical and histological analysis of hematoxylin-eosin sections of liver, muscle, smooth muscle from heart, brain, testis, gut, and primary and secondary lymphoid organs such as thymus, spleen, and lymph nodes from TRIM-deficient mice did not reveal any detectable abnormalities (data not shown). Moreover, TRIM−/− mice were viable and fertile. However, compared to sex-matched wild-type littermates, TRIM-deficient mice displayed a mild reduction (about 10 to 20%) in body weight which became evident at 3 weeks of age and remained constant throughout the aging process (Fig. 1D). Accordingly, we found a decrease (about 10 to 20%) in the size of several organs, such as thymus, spleen, lymph nodes, liver, kidney, and heart (data not shown). Because TRIM−/− and TRIM+/− mice maintain constant organ-to-body-mass ratios, the difference in body weight could be due to an overall reduction in cell size or in cellularity.
Analysis of lymphoid organs (thymus, lymph nodes, spleen, and bone marrow) revealed that cell sizes were normal, whereas cell numbers within the organs were significantly reduced (about 20% [Tables 1 and 2 and data not shown]). This indicates that loss of TRIM results in a reduction of organ cellularity and consequently in body mass. Since TRIM appears to be exclusively expressed in T cells, the reason for this unexpected observation remains elusive. At present, it is not known whether the immune system is directly involved in the regulation of body weight (particularly in healthy animals such as TRIM-deficient mice, which also do not show any histological signs of chronic inflammation that could be responsible for reduced body weight). A decrease in body mass has been observed in several knockout strains of mice in which molecules that are expressed in T cells (3, 6, 8, 15, 22, 23) were eliminated. However, in these models, the reduction in body weight is caused by alterations of organ development or by altered hormone action (as the molecules under investigation are also expressed outside of the hematopoietic system) but not by impaired immune functions. One possible explanation for our observation, therefore, could be that TRIM is not only expressed in T cells but also in other types of cells, e.g., hormone-producing cells. In this regard it is worth mentioning that Ikaros, a well-known lymphoid transcription factor, has recently been found to be expressed in cells of the pituitary gland (11). It appears that Ikaros is required not only for the development of the immune system but also for the regulation of the endocrine pituitary-adrenocortical system and body weight (11). Possibly a similar situation holds true for TRIM. Experiments are in progress to assess this possibility.
TABLE 1.
T-cell subsets in thymusa
| TRIM status | n | Total no. of cells (106) | No. of cells with indicated phenotype
|
CD4/CD8 ratio | |||
|---|---|---|---|---|---|---|---|
| CD4− CD8− | CD4+ | CD8+ | CD4+ CD8+ | ||||
| TRIM+/+ | 19 | 119.8 (9.7) | 3.9 (0.4) | 11.8 (1.1) | 3.4 (0.4) | 100.6 (8.3) | 3.8 (0.2) |
| TRIM−/− | 20 | 92.0 (9.2) | 2.8 (0.3) | 8.9 (1.2) | 2.7 (0.3) | 77.5 (8.0) | 3.5 (0.2) |
The numbers of lymphocyte subsets were determined on the basis of the total cell count and flow cytometric analysis shown in Fig. 2. Data represent means and standard errors of the means (in parentheses).
TABLE 2.
T-cell subsets in peripheral lymph nodesa
| TRIM status | n | Total no. of cells (106) | No. of cells with indicated phenotype
|
CD4/CD8 ratio | CD3/B220 ratio | |||
|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD3+ | B220+ | |||||
| TRIM+/+ | 11 | 28.3 (3.5) | 10.8 (1.3) | 10.4 (1.7) | 21.1 (2.7) | 6.2 (0.7) | 1.12 (0.1) | 3.38 (0.2) |
| TRIM−/− | 12 | 20.3 (2.5) | 7.0 (0.7) | 7.9 (1.1) | 15.5 (2.0) | 4.1 (0.5) | 0.92 (0.04) | 3.78 (0.3) |
The numbers of lymphocyte subsets were determined on the basis of the total cell count and flow cytometric analysis shown in Fig. 2. Data represent means and standard errors of the means (in parentheses).
TRIM-deficient T cells show normal TCR/CD3 surface levels and normal TCR dynamics.
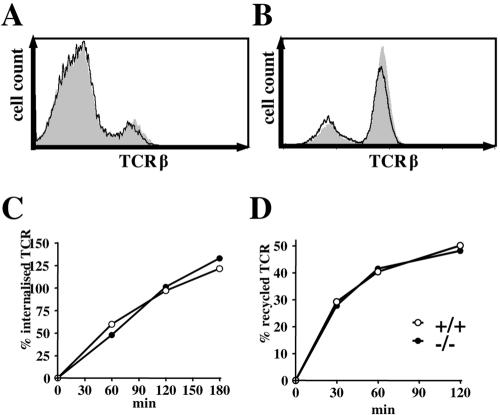
Previously, we had shown that overexpression of TRIM in Jurkat T cells enhances the expression levels of the TCR on the cell surface by altering spontaneous TCR internalization (4, 20). To address the question of whether TRIM exerts a similar function in nontransformed primary mouse T cells, we investigated the expression levels of the TCR on thymocytes and peripheral T cells of wild-type and TRIM-deficient mice by flow cytometry. As shown in Fig. 2A and B, the absence of TRIM had no impact on the expression levels of the TCR (or CD3ɛ [data not shown]). We next investigated whether internalization of the TCR or TCR recycling after antibody-mediated internalization was affected in TRIM−/− T lymphocytes. Figure 2C shows that the internalization of the TCR in peripheral lymph node T cells and in thymocytes (data not shown) occurred at normal rates in the absence of TRIM. In addition, the reexpression kinetics of the internalized TCR pool at the cell surface was comparable in wild-type and TRIM-deficient T cells (Fig. 2D). These data suggest that in contrast to Jurkat T cells, TRIM is dispensable for both the internalization and the recycling of the TCR in primary mouse T cells. A possible explanation could be that the regulation of TCR expression-downregulation-internalization by TRIM can only be observed in an overexpression system or in transformed cell lines, such as Jurkat. Alternatively, it might be that primary T cells possess other molecules that compensate for the absence of TRIM.
FIG. 2.
TCR expression, internalization, and recycling are normal in TRIM-deficient mice. Thymocytes (A) and lymph node cells (B) were stained with anti-TCRβ MAb and analyzed by flow cytometry. The histograms represent profiles of cells from representative TRIM+/+ (shaded) and TRIM−/− (thick lines) mice. (C) Measurement of TCR internalization. Lymph node T cells were incubated with PE-labeled anti-TCRβ antibody (H57-597) for 30 min on ice. Cells were then washed to remove unbound antibody and placed at 37°C for 0, 30, 60, 90, and 180 min. Surface-bound antibody was removed by low-pH washes, and internalized antibody in CD4+ cells was quantified by flow cytometry. (D) Reexpression of previously internalized anti-TCRβ antibody. To label an internalized pool of TCRβ, lymph node T cells were stained with PE-labeled anti-TCRβ antibody, cultured for 90 min at 37°C (to allow optimal TCR internalization), and stripped at low pH to remove cell surface TCR-bound antibody as described for panel C. Cells were then incubated at 37°C for various time points. TCR/anti-TCRβ-PE complexes recycling to the cell surface of CD4+ cells were quantified as the fluorescence lost after a second low-pH wash at the indicated time point and the fluorescence at time zero.
Normal T-cell development in TRIM−/− mice.
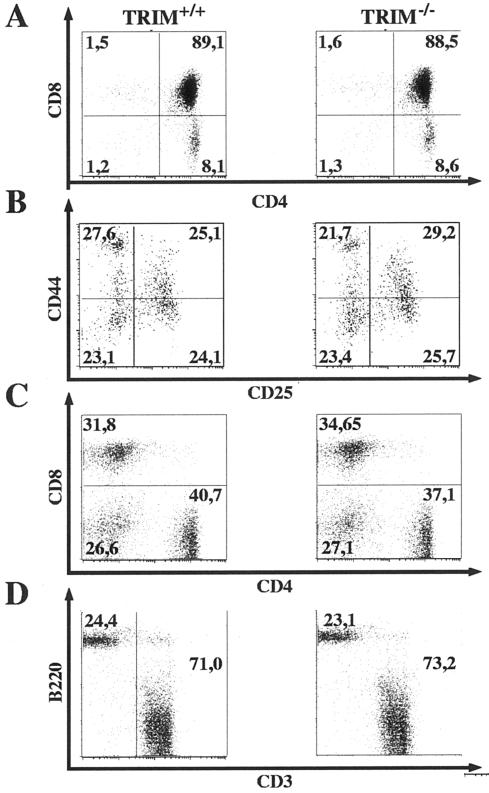
To assess the role of TRIM during T-cell development, flow cytometric analyses of lymphocyte suspensions prepared from thymus, spleen, and lymph nodes (Tables 1 and 2 and Fig. 3) were performed. As depicted in Table 1, the total cellularity of the thymus was found to be slightly reduced in TRIM−/− mice. However, the distribution of thymic subpopulations, as defined by CD4 and CD8 coreceptor expression, was similar between TRIM−/− animals and control littermates Fig. 3A. Moreover, we did not observe a significant alteration in the CD4/CD8 ratio (Table 1), and there was also no difference in CD25 and CD44 expression within the double negative thymocyte population (Fig. 3B). Collectively, these findings suggest that TRIM is dispensable for thymic development.
FIG. 3.
Development of lymphoid organs in TRIM-deficient mice. Thymocytes (A), double negative thymocytes (B), and peripheral lymph node cells (C and D) from 6- to 10-week-old mice were stained with MAbs for CD3, CD4, CD8, CD25, CD44, and B220 and analyzed by flow cytometry. Numbers represent percentages of cells falling into the indicated quadrant of total living cells.
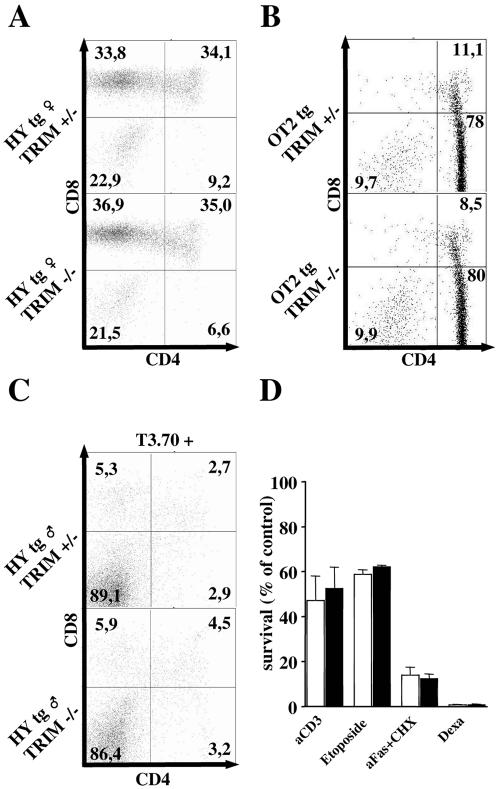
To corroborate this assumption and, in particular, to rule out subtle alterations in the selection processes in the absence of TRIM, we crossed TRIM−/− mice onto different TCR transgenic backgrounds. As shown in Fig. 4, the loss of TRIM did not influence positive selection in class I (HY)- or class II (OT-II)-restricted TCR transgenic models. We also tested positive selection using OT-I and P14 class I-restricted TCRs, which possess higher affinities for the selecting ligands than the HY TCR, and again we did not observe any alteration in TRIM-deficient animals (data not shown). Thus, TRIM appears to be dispensable for positive selection. Similarly, the loss of TRIM had no influence on the efficiency of negative selection in TRIM−/− HY male animals (Fig. 4C) or when negative selection was mimicked by intraperitoneal injection of the CD3ɛ MAb 145-2C11 (data not shown). In addition, the survival of TRIM−/− thymocytes after in vitro application of different apoptotic stimuli was found to be normal (Fig. 4D). Finally, membrane-proximal TCR-mediated signaling events (global tyrosine phosphorylation and calcium fluxes) in thymocytes were also unaffected in TRIM−/− thymocytes (data not shown). In summary, these data corroborate that TRIM is dispensable for both positive and negative selection as well as for thymocyte responses to either TCR-mediated or TCR-independent apoptotic stimuli.
FIG. 4.
Positive and negative selection in TRIM−/− mice. Normal positive selection in female HY (A) and OT-II (B) TCR transgenic (tg) mice. Thymocytes were stained with anti-HY (T3.70) and anti-Vα2 antibodies, and the CD4/CD8 profiles of transgenic TCR-gated cells are shown in panels A and B, respectively. (C) Normal negative selection in HY TCR tg TRIM−/− male mice. Profiles of thymocytes gated on T3.70+ cells and then examined for CD4 and CD8 expression. (D) Response to death-inducing signals within the thymocyte population. Freshly isolated thymocytes were incubated for 24 h in 24-well plates and were stimulated with 10 μg/ml plate-bound CD3 plus CD28 MAbs or incubated either with etoposide, anti-Fas plus cycloheximide (aFas+CHX), or dexamethasone (Dexa). Cell viability was measured by annexin V and propidium iodide staining.
We next analyzed peripheral lymphocyte populations prepared from secondary lymphoid organs. Similar to the situation within the thymus, TRIM−/− mice showed slightly reduced numbers of all lymphocyte subsets in the spleen and lymph nodes (Table 2 and data not shown), while the distributions of T- and B-cell subsets were normal (Fig. 3C and D and data not shown). In addition, we did not observe major alterations in the CD4/CD8 or the T-cell/B-cell ratios (Table 2) in TRIM−/− animals. A detailed expression analysis of a variety of lineage- and activation-specific markers (including CD5, CD25, CD44, CD45RB, CD62L, CD69, TCRαβ, TCRγδ, and NK1.1) also did not reveal any differences between TRIM−/− animals and control littermates. Similarly, TRIM deficiency had no influence on the expression levels of the accessory receptor CD28 or of the negative regulatory receptor CTLA4 (data not shown). Together, these results indicate a nonessential role for TRIM in the development, maturation, and distribution of peripheral T lymphocytes.
Analysis of in vitro and in vivo peripheral lymphocyte functions.
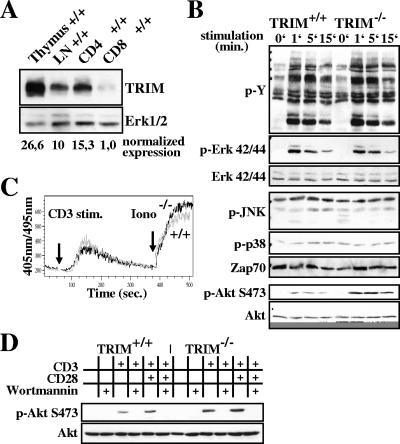
We have previously reported that TRIM is exclusively expressed in lymphoid organs (thymus, lymph node, and spleen) (4, 19). As shown in Fig. 5A, within the T-cell compartment TRIM is strongly expressed in thymocytes and in mature peripheral CD4+ T cells, whereas mature CD8+ cells express only minute amounts of the protein. These findings are in line with a recent report showing higher expression levels of TRIM mRNA in human and mouse CD4+ peripheral T lymphocytes than CD8+ T cells (29) (http://symatlas.gnf.org/SymAtlas/).
FIG. 5.
TCR-mediated signaling in TRIM-deficient CD4+ T cells. (A) TRIM expression in T cells. Postnuclear lysates were prepared from freshly isolated thymocytes, lymph node cells (LN), or purified CD4+ and CD8+ splenocytes, separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted with TRIM04 MAb. The blot was reprobed with anti-Erk1/2 to demonstrate equal loading. Numbers below the blot represent relative TRIM expression compared to the lowest. (B) Global tyrosine phosphorylation and MAP family kinase activation are normal, but Akt phosphorylation is enhanced in TRIM-deficient mice. Purified CD4+ splenic T cells were stimulated for the indicated periods of time with the CD3 MAb 145-2C11. The cells were lysed and processed for Western blot analysis. The blots were probed with anti-phosphospecific antibodies and then stripped and reprobed with anti-Erk1/2, anti-ZAP-70, and anti-Akt to show equal loading. Data are representative of five separate experiments. (C) Analysis of Ca2+ mobilization. Purified splenic CD4+ cells were loaded with indo-1-AM and incubated with 10 μg/ml of biotinylated anti-CD3 (145-2C11). The arrows indicate the addition of streptavidin and ionomycin (Iono), respectively. (D) TRIM regulation of Akt phosphorylation depends on PI3K activity. Purified CD4+ splenic T cells were stimulated for 2 min with CD3 MAb alone or with CD3 plus CD28 MAbs in the presence or absence of the PI3K inhibitor wortmannin. The level of Akt activation was measured by using a phosphospecific anti-Akt antibody and the equal loading by using an anti-Akt antibody. stim., stimulation.
To assess whether TRIM deficiency affects the function of peripheral CD4+ T cells, we investigated membrane-proximal TCR-mediated signaling events. Figures 5B and C demonstrate that TCR-mediated global tyrosine phosphorylation, the activation of MAPKs, and the elevation of intracellular calcium ions are all normal in TRIM−/− CD4+ T cells. Since TRIM has been shown to recruit the p85 regulatory subunit of PI3 kinase (4) and, therefore, may be involved in the regulation of PI3K activity, we also investigated TCR-mediated activation-phosphorylation of the serine/threonine kinase Akt/protein kinase B as a surrogate marker of PI3K activity. Surprisingly, TRIM-deficient T lymphocytes showed an augmented and prolonged phosphorylation of Akt compared to control cells. Since wortmannin treatment completely abolished Akt phosphorylation in both wild-type and TRIM-deficient CD4+ T cells (Fig. 5D), we conclude that PI3K activity is indeed augmented in TRIM−/− T lymphocytes.
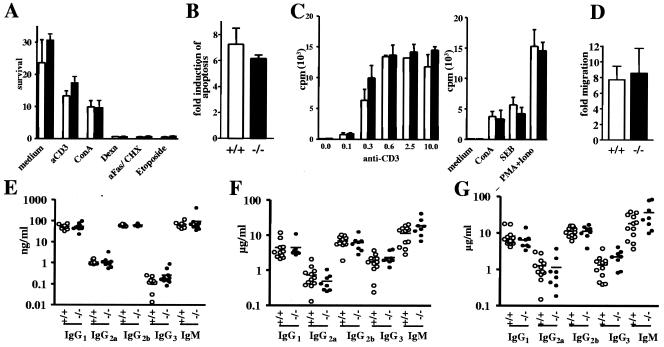
The PI3 kinase/Akt pathway is involved in many biological processes, including cell survival, proliferation, differentiation, and migration (25). To assess whether the augmented activity of Akt in the absence of TRIM would alter apoptotic responses, we stimulated mature peripheral CD4+ T cells with CD3 MAbs, corticosteroids, CD95 MAbs, ConA, or etoposide. Figure 6A shows that the survival of TRIM−/− T cells is indistinguishable from that of control T cells. To further investigate whether TRIM deficiency alters apoptosis of preactivated T cells (a phenomenon known as AICD), CD4+ T cells were stimulated in CD3 MAb-coated wells for 2 days. The activated cells were then grown in the presence of IL-2 for an additional 2 days and finally restimulated overnight with CD3 MAbs. The induction of apoptosis was subsequently measured by staining the cells with annexin V and propidium iodide. The data shown in Fig. 6B demonstrate that the absence of TRIM does not influence AICD.
FIG. 6.
T-cell responses in vitro and in vivo. (A) Normal survival of peripheral CD4+ T cells upon stimulation with CD3 MAb (aCD3), ConA, etoposide, anti-Fas plus cycloheximide (aFas/CHX), or dexamethasone (Dexa). One representative out of two experiments is shown. (B) Activation-induced cell death (AICD). CD4+ T cells were stimulated in CD3 MAb-coated plates, grown in IL-2, and restimulated overnight with CD3 antibody. Apoptosis was measured by annexin V and propidium iodide staining. The data are expressed as induction of apoptosis relative to control samples that were not restimulated. (C) Normal T-cell proliferation in TRIM-deficient mice. Purified CD4+ splenic T cells were stimulated in CD3 MAb-coated 96-well plates or with ConA, SEB, or PMA plus ionomycin (Iono) for 72 h, pulsed with [H3]thymidine, and processed for standard scintillation counting. One out of five independent experiments is shown. Three mice of each genotype were included in each experiment. (D) Migration assay. Freshly isolated lymph node cells were added to the insert of a transwell plate, with the lower chamber containing medium alone or medium supplemented with SDF1α. After 3 h, cells that had migrated into the lower wells were collected and counted by flow cytometry. The data shown are representative for three independently performed experiments. The migration is expressed as induction relative to that of control samples incubated with medium alone. (E) Serum immunoglobulin titers. The concentration of serum immunoglobulins was measured by ELISA. Bars represent mean values. (F and G) Humoral immune response. Mice were immunized with the T-dependent antigen DNP-KLH, and DNP-specific immunoglobulins were analyzed at day 10 (F), or they were reimmunized at day 14 with the same antigen and DNP-specific immunoglobulins were measured at day 28 (G) as described in Materials and Methods.
We next assessed the in vitro proliferative response after stimulation with a variety of mitogenic stimuli, such as CD3 MAbs, ConA, SEB, and CD3/CD28 costimulation. As shown in Fig. 6C, TRIM-deficient CD4+ T cells responded as efficiently as wild-type T cells to all applied stimuli. Similarly, the concentrations of IL-2, IL-4, tumor necrosis factor α, and gamma interferon in the culture supernatants were found to be normal (data not shown). Note that similar results were obtained when unfractionated splenic T cells were analyzed instead of purified CD4+ T cells (data not shown).
To investigate whether TRIM regulates the migration of peripheral T lymphocytes, we used a transwell system and allowed lymphocytes to migrate through fibronectin-coated polycarbonate meshes in response to the chemokine SDF1. Again, TRIM−/− T cells migrated with an efficiency similar to that of wild-type lymphocytes (Fig. 6D). Similarly, the constitutive and the CD3-mediated adhesion of β1 and β2 integrins to fibronectin or ICAM-I-coated plastic dishes were normal in TRIM−/− mice (data not shown). Collectively, these data suggest that the absence of TRIM does not influence T-cell proliferation, survival, migration, and adhesion.
In summary, the data shown in Fig. 6 demonstrate that despite the enhanced activity of PI3K in TRIM−/− mice, T-cell functions are unaffected under a variety of experimental conditions. A possible explanation for this somewhat unexpected observation could be that the slight augmentation of PI3K activity that occurs in the absence of TRIM is, per se, not sufficient to significantly alter downstream signaling cascades and thus cellular responses. It is also possible, however, that the augmented PI3K activity in TRIM−/− cells is partially compensated for by another nonraft transmembrane adaptor protein(s) exerting similar functions, the transmembrane adaptor protein LAX being a likely candidate. LAX also binds the p85 regulatory subunit of PI3K (40), and similar to the situation described here, LAX−/− T cells show a slightly augmented PI3K activity after TCR engagement (39). Thus, it appears as if both TRIM and LAX jointly regulate PI3K activity in peripheral T cells. Combined deletion of the TRIM and LAX genes will be required to assess whether both molecules indeed share redundant functions with regard to the TCR-mediated activation of PI3K or not.
One possibility to explain the enhanced activity of PI3K in the absence of either TRIM or LAX could be that in normal T cells both molecules are involved in sequestering the p85 subunit of PI3K from the lipid rafts to the nonraft fraction. Loss of TRIM or LAX might then cause a redistribution of PI3K from the nonraft to the raft fraction, thereby enhancing its activity. It is important to note that a similar “redistribution” phenomenon has recently been proposed to underlie the augmented response of mast cells that lack expression of the transmembrane adaptor protein NTAL/LAB (33).
The impact of TRIM deficiency on in vivo immune functions was further tested by challenging TRIM−/− mice and control littermates with the T-dependent antigen DNP-KLH at days 0 and 21. The levels of hapten-specific IgG1, IgG2a, IgG2b, IgG3, and IgM antibodies were measured 10 and 28 days after immunization. As shown in Fig. 6, both the primary (Fig. 6F) and the secondary (Fig. 6G) humoral responses of mutant and wild-type mice were comparable, indicating that T-helper functions are not affected in TRIM−/− mice.
To finally study the immune function of TRIM-deficient T cells in a disease model, we assessed the clinical course of experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis, in wild-type and TRIM-deficient animals. EAE is a well-established Th1-mediated disease that can be induced in C57BL/6 mice by immunization with MOG(35-55) peptide. The pathophysiology of EAE is characterized by T-cell-mediated demyelinization of axons in the central nervous system, which is followed by a progressive paralysis of the tail and the limbs (21). Due to the complex nature of the disease, which integrates many aspects of T-cell function, EAE represents a sensitive model system to detect even subtle alterations of immune functions. Indeed, we recently could show clear differences in the clinical course of EAE in knockout mice in which both T-dependent and T-independent immune responses were unaffected (28, 31).
To induce EAE, matched groups of TRIM−/− and wild-type mice were immunized with MOG(35-55) peptide, and the clinical score of EAE was assessed over time in a blinded study. As shown in Table 3, TRIM-deficient mice showed a normal course of EAE both with regard to the onset of disease as well as with regard to the clinical score, which ranged between 1.7 and 1.9 in both TRIM−/− and TRIM+/+ mice. Together with the normal response after immunization with T-dependent antigens, these data suggest a nonessential role of TRIM within the immune system.
TABLE 3.
Clinical observations of MOG peptide-induced EAEa
| Group | Incidence of EAE | Avg score | No. of deaths |
|---|---|---|---|
| Wild type | 26/32 | 1.95 ± 0.2 | 10/32 |
| TRIM−/− | 25/33 | 1.75 ± 0.2 | 8/33 |
Disease severity was scored according to a scale from 0 to 5: 0, no signs; 0.5, partial tail weakness; 1, limp tail or slight slowing of righting from supine position; 2, paresis of hind limb or marked slowing of righting; 2.5, dragging of hind limb(s) without complete paralysis; 3, complete paralysis of hind limb; 4, severe forelimb paralysis; 5, moribund or dead. In respect of the animal protection laws, mice were killed when they reached a score of 3 and received this last score until the end of the experiment.
Normal B-cell development and B-cell functions in TRIM-deficient mice.
Although TRIM expression has been reported to be limited to T lymphocytes (4), we also investigated the generation and the functions of B cells in TRIM−/− animals. These experiments were aimed to exclude the possibility that TRIM is expressed as a functional protein in particular B-cell subsets or in B-cell precursors as had previously been shown for the transmembrane adaptor protein LAT and the tyrosine kinase ZAP-70 (27, 30). However, the development of B cells within the bone marrow as well as the in vitro proliferation of purified splenic B cells after anti-IgM, lipopolysaccharide, anti-CD40, and IL-4 stimulation was not affected by loss of TRIM (data not shown). Similarly, the serum Ig levels (Fig. 6E) and the humoral immune response after immunization with the T-independent antigen DNP-lipopolysaccharide was normal in TRIM−/− mice (data not shown). Thus, in contrast to LAT, TRIM is not involved in regulating B-cell development and B-cell functions both in vivo and in vitro.
Concluding remarks.
Among the seven TRAPs known to date, LAT clearly represents the master switch that critically regulates T-cell development and peripheral T-cell activation (12, 37, 38). In contrast, other transmembrane adaptors such as LAX, NTAL, and SIT rather appear to be involved in the fine-tuning of antigen receptor-mediated signaling within immune cells (28, 34, 39). The same seems to apply for the nonraft transmembrane adaptor protein investigated here, TRIM.
TRIM is strongly expressed in thymocytes and, as shown here, in peripheral CD4+ T cells. Previous reports suggested that TRIM might be involved in the regulation of TCR expression in Jurkat T cells (4, 20). In addition, TRIM was shown to undergo tyrosine phosphorylation after T-cell activation and to recruit the p85 regulatory subunit of PI3K to the plasma membrane (4, 20). Together, these observations suggested that TRIM might represent an important regulator of T-cell development and T-cell responses. However, the data presented in this report suggest that TRIM does not play an essential role within the (murine) immune system.
Although our study did not reveal major alterations of immune functions in the absence of TRIM, we certainly cannot exclude the possibility that TRIM regulates immunological processes that we have not investigated (for example, TRIM could be involved in pathways that emerge from signaling receptors distinct from the TCR). However, given the fact that several in vivo models (immunization with T-dependent antigens as well as induction of the autoimmune disease EAE) did not show abnormalities in TRIM-deficient animals, we believe this possibility to be unlikely.
To date, seven TRAPs have been identified: LAT, LAX, LIME, NTAL/LAB, PAG/CBP, SIT, and TRIM (for a review, see reference 18). Among them, LAX, SIT, and TRIM are localized in the nonraft fraction of the plasma membrane. Recently published data showed that both LAX and SIT function as negative regulators of antigen receptor-mediated signaling. Thus, LAX-deficient T and B lymphocytes are hyperresponsive to CD3- or anti-IgM-mediated stimuli (39). Similarly, we have most recently shown that SIT functions as a negative regulator of TCR-mediated signaling (28). The molecular mechanisms underlying LAX- and SIT-mediated negative regulation of TCR signaling are still unclear. One possibility is that LAX and SIT affect signaling within T cells by sequestering cytoplasmic effector molecules from the lipid rafts to the nonraft fraction or that they blunt T-cell responses by recruiting negative regulatory molecules to the plasma membrane.
The very mild phenotypes of the SIT and LAX knockout mice plus the almost nonexistent phenotype of the TRIM mouse described in this report indicate that each of the nonraft-associated TRAPs by itself plays a rather subtle role within the immune system. However, the orchestrated activities of all negative regulatory transmembrane adaptor proteins might play a major role in controlling cell growth and differentiation. The apparent redundancy among the different nonraft TRAPs may serve as a safety mechanism to guarantee the integrity of the system even when one component is missing or altered. In this regard, the analysis of TRIM/SIT double-deficient mice (note that TRIM and SIT share two tyrosine-based signaling motifs within their cytoplasmic tails, YGNL and YASV/L), of TRIM/LAX double-deficient mice (as both molecules are capable of binding PI3K and each of the single knockouts shows augmented activity of PI3K), or even of triple knockouts (as all three molecules carry YXN motifs that might bind the cytosolic adaptor protein Grb2) might shed further light onto the question of how TRAPs regulate homeostasis within the immune system.
Acknowledgments
We are grateful to Gary Koretzy, Thomas Kammerthoens, and Percy Knolle for TCR transgenic lines and to the employees of the animal facility for maintenance of the animals. The help of Robert Enders, Evi Schaller, Agnes Fütterer, and Jennifer Meinecke is also highly appreciated.
The work was supported by the Deutsche Forschungsgemeinschaft (DFG)-funded research group 521 (research grants to L.S. and B.S.) and by DFG grants to K.P.
REFERENCES
- 1.Alarcon, B., D. Gil, P. Delgado, and W. W. Schamel. 2003. Initiation of TCR signaling: regulation within CD3 dimers. Immunol. Rev. 191:38-46. [DOI] [PubMed] [Google Scholar]
- 2.Alimzhanov, M. B., D. V. Kuprash, M. H. Kosco-Vilbois, A. Luz, R. L. Turetskaya, A. Tarakhovsky, K. Rajewsky, S. A. Nedospasov, and K. Pfeffer. 1997. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl. Acad. Sci. USA 94:9302-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, L. F., H. A. Runnels, T. D. Schell, Y. Cho, R. Gibbons, S. S. Tevethia, G. S. Deepe, Jr., and J. J. Monaco. 2004. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J. Immunol. 172:3948-3954. [DOI] [PubMed] [Google Scholar]
- 4.Bruyns, E., A. Marie-Cardine, H. Kirchgessner, K. Sagolla, A. Shevchenko, M. Mann, F. Autschbach, A. Bensussan, S. Meuer, and B. Schraven. 1998. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J. Exp. Med. 188:561-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cen, O., M. M. Gorska, S. J. Stafford, S. Sur, and R. Alam. 2003. Identification of UNC119 as a novel activator of SRC-type tyrosine kinases. J. Biol. Chem. 278:8837-8845. [DOI] [PubMed] [Google Scholar]
- 6.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikuma, S., J. B. Imboden, and J. A. Bluestone. 2003. Negative regulation of T cell receptor-lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 197:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, H., J. L. Thorvaldsen, Q. Chu, F. Feng, and M. J. Birnbaum. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 9.Delgado, P., and B. Alarcon. 2005. An orderly inactivation of intracellular retention signals controls surface expression of the T cell antigen receptor. J. Exp. Med. 201:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Oro, U., I. Munitic, G. Chacko, T. Karpova, J. McNally, and J. D. Ashwell. 2002. Regulation of constitutive TCR internalization by the zeta-chain. J. Immunol. 169:6269-6278. [DOI] [PubMed] [Google Scholar]
- 11.Ezzat, S., R. Mader, S. Yu, T. Ning, P. Poussier, and S. L. Asa. 2005. Ikaros integrates endocrine and immune system development. J. Clin. Investig. 115:1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finco, T. S., T. Kadlecek, W. Zhang, L. E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity 9:617-626. [DOI] [PubMed] [Google Scholar]
- 13.Gil, D., W. W. Schamel, M. Montoya, F. Sanchez-Madrid, and B. Alarcon. 2002. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109:901-912. [DOI] [PubMed] [Google Scholar]
- 14.Gorska, M. M., S. J. Stafford, O. Cen, S. Sur, and R. Alam. 2004. Unc119, a novel activator of Lck/Fyn, is essential for T cell activation. J. Exp. Med. 199:369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han, J., J. W. Shui, X. Zhang, B. Zheng, S. Han, and T. H. Tan. 2005. HIP-55 is important for T-cell proliferation, cytokine production, and immune responses. Mol. Cell. Biol. 25:6869-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding, S., P. Lipp, and D. R. Alexander. 2002. A therapeutic CD4 monoclonal antibody inhibits TCR-zeta chain phosphorylation, zeta-associated protein of 70-kDa Tyr319 phosphorylation, and TCR internalization in primary human T cells. J. Immunol. 169:230-238. [DOI] [PubMed] [Google Scholar]
- 17.Hombach, J., T. Tsubata, L. Leclercq, H. Stappert, and M. Reth. 1990. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343:760-762. [DOI] [PubMed] [Google Scholar]
- 18.Horejsi, V., W. Zhang, and B. Schraven. 2004. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4:603-616. [DOI] [PubMed] [Google Scholar]
- 19.Huynh, T., A. Wurch, E. Bruyns, V. Korinek, B. Schraven, and K. Eichmann. 2001. Developmentally regulated expression of the transmembrane adaptor protein trim in fetal and adult T cells. Scand. J. Immunol. 54:146-154. [DOI] [PubMed] [Google Scholar]
- 20.Kirchgessner, H., J. Dietrich, J. Scherer, P. Isomaki, V. Korinek, I. Hilgert, E. Bruyns, A. Leo, A. P. Cope, and B. Schraven. 2001. The transmembrane adaptor protein TRIM regulates T cell receptor (TCR) expression and TCR-mediated signaling via an association with the TCR zeta chain. J. Exp. Med. 193:1269-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linington, C. 1997. Actively induced models of central nervous system autoimmune disease, p. 1696-1700. In I. Lefkovits (ed.), Immunological methods - the comprehensive sourcebook of techniques, vol. 3. Harcourt Brace & Company, Basel, Switzerland. [Google Scholar]
- 22.Mikkers, H., M. Nawijn, J. Allen, C. Brouwers, E. Verhoeven, J. Jonkers, and A. Berns. 2004. Mice deficient for all PIM kinases display reduced body size and impaired responses to hematopoietic growth factors. Mol. Cell. Biol. 24:6104-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molero, J. C., T. E. Jensen, P. C. Withers, M. Couzens, H. Herzog, C. B. Thien, W. Y. Langdon, K. Walder, M. A. Murphy, D. D. Bowtell, D. E. James, and G. J. Cooney. 2004. c-Cbl-deficient mice have reduced adiposity, higher energy expenditure, and improved peripheral insulin action. J. Clin. Investig. 114:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers, M. D., L. L. Dragone, and A. Weiss. 2005. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCR{zeta} for degradation. J. Cell Biol. 170:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okkenhaug, K., and B. Vanhaesebroeck. 2003. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 3:317-330. [DOI] [PubMed] [Google Scholar]
- 26.Pandey, A., N. Ibarrola, I. Kratchmarova, M. M. Fernandez, S. N. Constantinescu, O. Ohara, S. Sawasdikosol, H. F. Lodish, and M. Mann. 2002. A novel Src homology 2 domain-containing molecule, Src-like adapter protein-2 (SLAP-2), which negatively regulates T cell receptor signaling. J. Biol. Chem. 277:19131-19138. [DOI] [PubMed] [Google Scholar]
- 27.Schweighoffer, E., L. Vanes, A. Mathiot, T. Nakamura, and V. L. Tybulewicz. 2003. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity 18:523-533. [DOI] [PubMed] [Google Scholar]
- 28.Simeoni, L., V. Posevitz, U. Kolsch, I. Meinert, E. Bruyns, K. Pfeffer, D. Reinhold, and B. Schraven. 2005. The transmembrane adapter protein SIT regulates thymic development and peripheral T-cell functions. Mol. Cell. Biol. 25:7557-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su, A. I., T. Wiltshire, S. Batalov, H. Lapp, K. A. Ching, D. Block, J. Zhang, R. Soden, M. Hayakawa, G. Kreiman, M. P. Cooke, J. R. Walker, and J. B. Hogenesch. 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101:6062-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su, Y. W., and H. Jumaa. 2003. LAT links the pre-BCR to calcium signaling. Immunity 19:295-305. [DOI] [PubMed] [Google Scholar]
- 31.Togni, M., K. D. Swanson, S. Reimann, S. Kliche, A. C. Pearce, L. Simeoni, D. Reinhold, J. Wienands, B. G. Neel, B. Schraven, and A. Gerber. 2005. Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 25:8052-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urba, W. J., C. Ewel, W. Kopp, J. W. Smith, R. G. Steis, J. D. Ashwell, S. P. Creekmore, J. Rossio, M. Sznol, and W. Sharfman. 1992. Anti-CD3 monoclonal antibody treatment of patients with CD3-negative tumors: a phase IA/B study. Cancer Res. 52:2394-2401. [PubMed] [Google Scholar]
- 33.Volna, P., P. Lebduska, L. Draberova, S. Simova, P. Heneberg, M. Boubelik, V. Bugajev, B. Malissen, B. S. Wilson, V. Horejsi, M. Malissen, and P. Draber. 2004. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 200:1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y., O. Horvath, A. Hamm-Baarke, M. Richelme, C. Gregoire, R. Guinamard, V. Horejsi, P. Angelisova, J. Spicka, B. Schraven, B. Malissen, and M. Malissen. 2005. Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol. Cell. Biol. 25:4455-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss, A. 1993. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell 73:209-212. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki, T., Y. Hamano, H. Tashiro, K. Itoh, H. Nakano, S. Miyatake, and T. Saito. 1999. CAST, a novel CD3epsilon-binding protein transducing activation signal for interleukin-2 production in T cells. J. Biol. Chem. 274:18173-18180. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323-332. [DOI] [PubMed] [Google Scholar]
- 39.Zhu, M., O. Granillo, R. Wen, K. Yang, X. Dai, D. Wang, and W. Zhang. 2005. Negative regulation of lymphocyte activation by the adaptor protein LAX. J. Immunol. 174:5612-5619. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, M., E. Janssen, K. Leung, and W. Zhang. 2002. Molecular cloning of a novel gene encoding a membrane-associated adaptor protein (LAX) in lymphocyte signaling. J. Biol. Chem. 277:46151-46158. [DOI] [PubMed] [Google Scholar]