Abstract
Notch signaling is an evolutionarily conserved pathway involved in intercellular communication and is essential for proper cell fate choices. Numerous genes participate in the modulation of the Notch signaling pathway activity. Among them, Notchless (Nle) is a direct regulator of the Notch activity identified in Drosophila melanogaster. Here, we characterized the murine ortholog of Nle and demonstrated that it has conserved the ability to modulate Notch signaling. We also generated mice deficient for mouse Nle (mNle) and showed that its disruption resulted in embryonic lethality shortly after implantation. In late mNle−/− blastocysts, inner cell mass (ICM) cells died through a caspase 3-dependent apoptotic process. Most deficient embryos exhibited a delay in the temporal down-regulation of Oct4 expression in the trophectoderm (TE). However, mNle-deficient TE was able to induce decidual swelling in vivo and properly differentiated in vitro. Hence, our results indicate that mNle is mainly required in ICM cells, being instrumental for their survival, and raise the possibility that the death of mNle-deficient embryos might result from abnormal Notch signaling during the first steps of development.
The Notch signaling pathway plays a central role in the control of cell fate decisions in a wide variety of cell lineages during invertebrate and vertebrate development (1, 2, 24). Basically, this is achieved in two different ways, lateral inhibition and inductive signaling, to create either a group of highly similar cells or, in contrast, a mosaic of different cell types from a group of initially equivalent cells. Hence, the Notch signaling pathway is involved in the development of many cell types, and modification of its activity can dramatically affect proliferation, differentiation, and apoptotic events. The genes of the Notch family encode large single-spanning transmembrane receptors that interact with membrane-bound ligands of the Delta and Serrate/Jagged family. After activation by a ligand, the Notch receptor is proteolytically cleaved, releasing the Notch intracellular domain (NICD) from the membrane. The NICD then translocates to the nucleus and interacts with the CSL DNA-binding protein (CBF1 or RBP-Jκ in vertebrates, Suppressor of hairless in Drosophila melanogaster, and Lag-1 in Caenorhabditis elegans) and the Mastermind/Lag-3 coactivator to activate target gene expression (27, 37). Spatiotemporal control of Notch signaling activity is achieved by numerous accessory proteins first identified in Drosophila and C. elegans (1, 37). Among them, a novel direct regulator of the Notch signaling pathway, called Notchless (Nle), has been identified in Drosophila during a genetic screen for suppressors of the notchoid mutant (36). Nle is composed of a well-conserved amino-terminal region, named Nle, and belongs to the WD40 repeat family of proteins. Members of this family interact with several protein partners and are involved in many cellular functions (40). An Nle-like amino-terminal domain was also found in another protein, named Wdr12, recently identified during a genetic screen for modulators of T-lymphocyte differentiation (29). The nuclear Wdr12 protein is composed of seven WD40 repeat domains, which, however, are not related to the WD40 domains of mouse Nle (mNle).
In Drosophila, a decrease of Nle expression suppresses the phenotype observed in the wings of the notchoid mutants, indicating that Nle reduces Notch activity (36). Furthermore, overexpression of Nle enhances the phenotype observed in Drosophila carrying Notch gain-of-function mutations and inhibits neuronal differentiation in Xenopus laevis. Hence, depending on the context, Nle either negatively or positively regulates the Notch pathway. Biochemical experiments showed that Nle binds to the NICD, but the molecular mechanism of action of Nle has yet to be clarified. Previously, Royet et al. also showed that Nle genetically interacts with other genes of the Notch pathway such as Deltex, suppressor of hairless, and groucho but not with Serrate, Delta, Hairless, or strawberry notch (36).
To obtain insights into the in vivo function of Nle in mammals, we genetically disrupted the mNle gene. Interestingly, we find that the absence of mNle results in peri-implantation lethality and demonstrate that mNle is a novel gene essential for the survival of inner cell mass (ICM) cells in mammals.
MATERIALS AND METHODS
Northern blot.
A specific probe corresponding to the open reading frame (ORF) of mNle was labeled with 32P and used for hybridization in ExpressHyb buffer (Clontech) on adult and embryonic mouse multiple tissue Northern blots (Clontech) using standard procedures. After stripping, membranes were rehybridized with a 32P-labeled full-length murine actine cDNA probe.
Xenopus laevis embryo injections.
Synthetic capped RNAs for microinjection were synthesized by in vitro transcription using mMessage mMachine kit (Ambion) and purified according to the manufacturer's instructions. mNle and green fluorescent protein (GFP) RNAs were prepared from pRN3 derivatives, and NICD RNAs were prepared from pCS2 (kindly provided by A. Israël). X. laevis embryos were obtained by in vitro fertilization. Dorsal blastomeres of four-cell-stage embryos were injected with 100 pg of GFP capped RNAs alone or in combination with 100 pg of mNle or NICD RNAs. Expression of N-tubulin at the neural plate stage was analyzed by whole-mount in situ hybridization using digoxigenin-labeled probes, as described previously (6). Images were acquired using an SMZ1500 stereomicroscope (Nikon) equipped with an axioCam color camera (Zeiss).
Targeted disruption of the mNle gene.
A 10.5-kb NdeI-HincII 129/Sv genomic DNA fragment, containing exons 1 to 13, was subcloned from clone 4B7 of the Caltech CITB-BAC library (GenBank accession no. AL713882) in pBKS(+). The BglI-BsrGI fragment was subcloned, and the PstI-EcoRV fragment was replaced by nlsLacZ (isolated from the plasmid pSKTNLSLACZ, a gift from S. Tajbakhsh) and floxed pgk-Neo cassettes (provided by P. Soriano). The targeted allele, mNle, and the pgk-Neo cassette are transcribed in the opposite orientation. The BsrGI-HincII fragment was then subcloned 3′ to the pgk-Neo cassette. A pgk-DTA cassette encoding the A subunit of the diphtheria toxin gene (provided by P. Soriano) was inserted at the 5′ end of the construct to allow for negative selection in embryonic stem (ES) cells (50). The resulting targeting vector was linearized at a unique XhoI site, gel purified, and electroporated (20 μg) (1 pulse of 0.23 kV and 950 μF, Gene PulserII; Bio-Rad) into 1.6 × 106 exponentially growing 129/Sv CK35 ES cells (22). ES cells were maintained, as described previously (34), in high-glucose Dulbecco's modified Eagle's medium supplemented with a solution containing 15% fetal calf serum, 0.1 mM β-mercaptoethanol, and 1 mM sodium pyruvate in the presence of 103 U/ml murine leukemia inhibitory factor (LIF). The cells were cultured on mitomycin C-treated Neor primary fibroblasts. Twenty hours after electroporation, ES cells were selected for G418 resistance (300 μg/ml; Invitrogen) for 10 days and genotyped. Genomic DNA was purified from G418-resistant ES cell clones, digested with BsrGI or EcoRV, transferred onto nitrocellulose membranes (Hybond N+; Amersham), and hybridized with the 5′ and 3′ probes according to standard procedures. Southern blot analysis detected products of 12 kb and 7 kb using a 5′ probe and 16 kb and 12 kb using a 3′ probe for the targeted and wild-type (WT) alleles, respectively. ES cells exhibiting the correct targeting event were injected into C57BL/6N blastocysts which were transferred into (C57BL/6N × CBA)F1 pseudopregnant females. Resulting chimeric males were mated with C57BL/6N or 129/Sv females. Germ line transmission of the targeted mNle locus was confirmed by PCR that amplified a 269-bp fragment for the WT allele with primers a (5′-CTG GCG TTC TAT GTC CAC GAT G-3′) and b (5′-GGA TGG TCC TCT CCA CCT GTC-3′) and a 460-bp fragment for the mutant mNle allele with primers c (5′-GAC TAG GGG AGG AGT AGA AGG T-3′) and b. The PCR amplification protocol was as follows: 94°C for 5 min followed by 33 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s.
Early embryo isolation and in vitro cultures.
Heterozygous mNle+/− females, obtained from crosses between WT (C57BL/6 × SJL/J) females and mNle+/− 129/Sv males, were superovulated by injection of 5 units of pregnant mare serum gonadotrophin (Calbiochem) followed by injection of 5 units of human chorionic gonadotrophin (Intervet) 48 h later and were then mated with heterozygous mNle+/− 129/Sv males. The next day, successful matings were detected by the presence of a vaginal plug. Embryos were collected from different stages of development (embryonic day 1.5 [E1.5] through E2.5) by either dissecting ampullae or flushing oviducts with PB1 medium (48). E3.5 blastocysts were collected by flushing uteri with ES cell medium without LIF and supplemented with 20 mM HEPES. When mentioned, zona pellucida was removed from E3.5 blastocysts with acid Tyrode's solution. E3.5 blastocysts from mNle+/− intercrosses were transferred in ES cell medium without LIF onto 0.1% gelatin-coated chambered slides (Lab-Tek) at 37°C with 8% CO2 during 24 h and for up to 7 days and photographed every 24 h (DMIL; Leica) (Coolpix990; Nikon). Immunosurgical isolation of inner cell populations was carried out, as previously described (41), on E3.5 blastocysts cultured in vitro during 24 h. Inner cell clumps were cultured in ES cell medium without LIF for 7 days. Preimplantation embryos or TE lysates were incubated in lysis buffer (10 mM Tris, pH 8.5, 50 mM KCl, 0.01% gelatin, 300 μg/ml proteinase K) at 55°C for 60 min and 95°C for 15 min, and the genotype was assessed by nested PCR. The first round of PCR amplification used primers b, c, and d (5′-GAC GTG CAG CGG CTG CTC GTA-3′) for 25 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 40 s. The second round of PCR amplification for 25 cycles used primers a, e (5′-GGG CTG CTA AAG CGC ATG CT), and f (5′-CTT CGC TGT GAC CCT CCA ATG-3′).
Immunofluorescence and TUNEL assay.
Immunofluorescence and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay were performed as described previously (3) on fully expanded E3.5 blastocysts cultured in vitro during 24 h. The antibodies used were as follows: anti-cytokeratin-Endo-A (TROMA-1; 1:100; DSHB clone SP2/0), anti-CDX2 (1/50; BioGenex), anti-Oct-3/4 (1:300; BD Biosciences), anti-phospho-histone H3 (1:100; Upstate), anti-active human caspase 3 (1:200; Pharmingen), Alexa 488-nm-anti-rabbit antibody (1:100; Molecular Probes), and Alexa 594-nm-anti-mouse antibody (1:200; Molecular Probes).
X-Gal staining.
After fixation of embryos, β-galactosidase expression was visualized by staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal for embryos; Life Technologies).
Histological analysis.
Uteri containing E5.5 and E6.5 decidual swellings were fixed for 24 h in Bouin's solution (Sigma) and then dehydrated and embedded in paraffin. Next, 5-μm sections were stained with hematoxylin and eosin.
RESULTS
Characterization of the mNle gene.
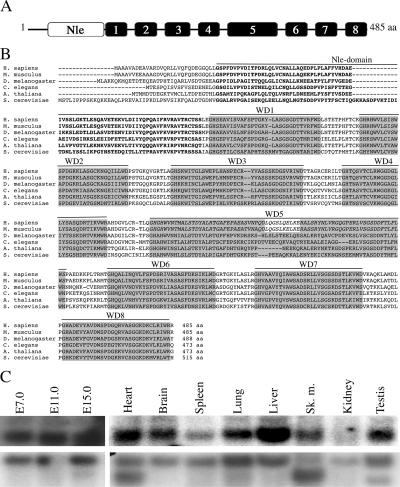
We isolated the mNle gene (GenBank accession no. NM_145431) during the positional cloning of the Ovum mutant locus responsible for a conditional preimplantation lethal syndrome, called DDK syndrome (4, 7, 25). The mNle gene contains 13 exons and encodes a protein of 485 amino acids. mNle consists of the Nle amino-terminal domain followed by WD40 repeat domains (Fig. 1A and B) and presents 51% and 96% identity with yeast and human Nle proteins, respectively. In Drosophila, Royet et al. proposed previously that Nle contains nine WD40 repeat domains (36). However, our analysis suggests that all Nle orthologous proteins actually contain eight WD40 domains (Fig. 1B). Northern blot experiments revealed that a single mNle-specific transcript of approximately 2 kb was present in embryos and in all adult tissues tested (Fig. 1C).
FIG. 1.
Structure and amino acid alignments of mNle protein and expression pattern of mNle transcripts. (A) Schematic representation of the mNle protein containing a single Nle domain (white box) at its amino-terminal region and eight WD40 domains (gray boxes). (B) Alignments of sequences of Nle orthologs by clustalW. Using the website at http://bmerc-www.bu.edu/wdrepeat to search for WD40 domains, eight domains were predicted for Nle in Saccharomyces, Xenopus, and Drosophila, whereas nine domains were predicted for mouse and human Nle proteins. Based on amino acid sequence comparisons and on the fact that, in mouse and human, the fifth domain does not end by a consensus WD motif and that the sixth domain does not begin by a consensus GH sequence, we propose that the fifth and sixth predicted WD40 domains of the human and mouse Nle proteins correspond to a single WD40 domain (in italics), as predicted for other species. GenBank/EMBL accession numbers for the Nle orthologous sequences are as follows: Homo sapiens, NP_060566; Mus musculus, NP_663406; D. melanogaster, NP_477294.1; C. elegans, NP_493745; Arabidopsis thaliana, NP_200094.1; and S. cerevisiae, NP_009997. Amino acids corresponding to the Nle domain are in boldface type. WD40 repeat domains are highlighted in gray boxes. The numbers of amino acids (aa) are indicated at the end of the sequences. (C) Northern blot analysis of poly(A)+ mRNAs of embryos at E7.0 to E15.0 and various adult tissues hybridized with an mNle-specific probe (upper panel) or a β-actin probe (lower panel).
mNle has conserved its ability to modulate Notch activity.
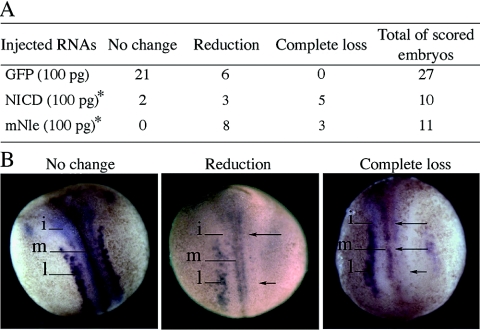
The Xenopus neuronal specification assay (6) was used to test whether the described activity of Xenopus and Drosophila Nle proteins as modulators of the Notch signaling pathway (36) was conserved in mouse. Indeed, the formation of primary neurons can be used as a readout of Notch signaling in Xenopus: inhibition of Notch signaling results in an overproduction of primary neurons, while an increase in Notch signaling leads to a marked reduction in the differentiation of primary neurons (6). Thus, we injected a single dorsal blastomere of four-cell-stage embryos with GFP RNAs alone or in combination with mNle or NICD RNAs. Correctly injected embryos were identified based on GFP expression at the neurula stage (stage 16) and processed for N-tubulin, which marks primary neurons in medial, intermediate, and lateral stripes (Fig. 2B). When embryos were injected with mNle mRNAs, a significantly higher proportion of embryos showed a reduced or complete loss of primary neurons in the three neural stripes compared to GFP-injected embryos (Fig. 2A). This reduction is indicative of activated Notch signaling (6) and is similar to that observed when embryos were injected with NICD (Fig. 2). This result strongly suggests that the murine Nle protein has the capacity to modulate the Notch signaling activity, as it is the case for its fly and frog orthologs (36).
FIG. 2.
Effect of GFP, NICD, and mNle RNA injections on the production of primary neurons in Xenopus embryos. Injected embryos (stage 16) were monitored for the N-tubulin expression pattern. (A) Number of embryos that present, in the injected side compared to the noninjected side, no change, reduction, or complete loss of N-tubulin-positive neurons. *, analysis of distribution of the three N-tubulin expression profiles between NICD and GFP (χ2, 0.001 < P < 0.01) and between mNle and GFP (χ2, P < 0.001). (B) Examples of the three types of N-tubulin expression patterns obtained after RNA injections. Dorsal views are shown with the anterior end up. The injected side is shown on the right side of the images. N-tubulin expression was detected in primary neurons of medial (m), intermediate (i), and lateral (l) stripes in the noninjected side. Arrows indicate the reduction or complete loss of N-tubulin expression.
Targeted disruption of the mNle gene.
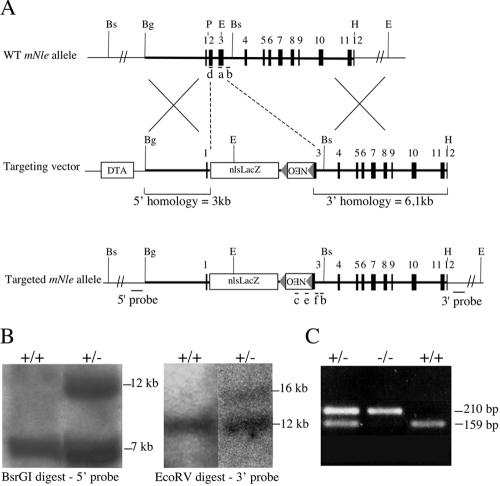
To elucidate the in vivo function of mNle, we disrupted it via homologous recombination in 129/Sv ES cells by an in-frame insertion of the nlsLacZ ORF (Fig. 3A). This insertion was expected to result in a null allele, since it deletes exons 2 and 3 and results in the fusion of the first six amino acids of the Nle protein to the ORF of nlsLacZ. Correctly targeted clones were identified by Southern blot analysis (Fig. 3B). Modified cells were injected into C57BL/6 blastocysts, and germ line transmission of the mNle-targeted allele was obtained. The phenotype described below was fully penetrant on a mixed 129/Sv × (C57BL/6 × SJL/J) genetic background.
FIG. 3.
Targeted disruption of the mNle gene. (A) Structure of the WT mNle allele, the targeting vector, and the targeted allele. Exons (solid boxes), LoxP sequences (triangles), and positions of primers used for genotype analysis (a to f, bars), probes used for Southern blot analysis (bars), and pgk-DTA and pgk-Neo cassettes used for negative and positive selection are indicated. Restriction sites relevant to the targeting construct and to the screening strategies are as follows: BglI (Bg), BsrGI (Bs), EcoRV (E), HincII (H), and PstI (P). (B) Southern blot analysis of genomic DNA obtained from wild-type and heterozygous ES cells. (C) Genotype analysis of early embryos from mNle+/− intercrosses. The first round of PCR used primers b, c, and d. The second round of PCR used primers a, e, and f, yielding amplification products of 159 bp and 210 bp for the wild-type and mutant alleles, respectively.
mNle gene disruption results in peri-implantation lethality and ICM cell failure.
Males and females heterozygous for the mNle-null allele were not phenotypically different from the wild-type mice. When mNle+/− mice were intercrossed, no homozygous progeny were found among 68 newborns (Table 1), indicating that homozygosity for the mNle-null allele is lethal during embryonic development. Similarly, no homozygous embryos could be found from E12.5 to as early as E6.5. By contrast, a Mendelian distribution was observed at E3.5 for mNle+/+, mNle+/−, and mNle−/− embryos (Table 1 and Fig. 3C). To further precise the time of embryonic death, we performed a histological analysis of serial sections of E5.5 and E6.5 decidual swellings from mNle+/− intercrosses. For both gestation times, abnormal implants that contained very few cells and no discernible embryonic tissue were observed (Table 2 and data not shown). As the frequency of such abnormal deciduae was much lower in control crosses, they were considered to be elicited from mNle−/− embryos. We conclude from these results that mNle−/− embryos do implant but rapidly degenerate thereafter.
TABLE 1.
Viability analysis of mNle−/− mouse and embryosa
| Time of analysis | No. (%) of live births and dissected embryos from mNle+/− intercrosses from genotype:
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| Postnatal | 22 (32) | 46 (68) | 0 |
| E10.5-E12.5 | 14 (29) | 34 (71) | 0 |
| E6.5 | 9 (29) | 22 (71) | 0 |
| E3.5 | 13 (20) | 39 (59) | 14 (21) |
Mice and E6.5 to E12.5 embryos were genotyped by PCR, and E3.5 embryos were genotyped by nested PCR (see Fig. 3C).
TABLE 2.
Histological analysis of embryos in uteroa
| Cross | Stage | No. of embryos
|
||
|---|---|---|---|---|
| Total | Normal | Resorbed | ||
| mNle+/− × mNle+/− | E5.5 | 9 | 6 | 3 |
| mNle+/− × mNle+/− | E6.5 | 18 | 11 | 7 |
| mNle+/+ × mNle+/− | E5.5 | 6 | 6 | 0 |
| mNle+/+ × mNle+/− | E6.5 | 16 | 15 | 1 |
For histological analysis, uteri were fixed, sectioned and stained with hematoxylin and eosin, and scored as normal or resorbed based on presence or absence of embryos and general morphology.
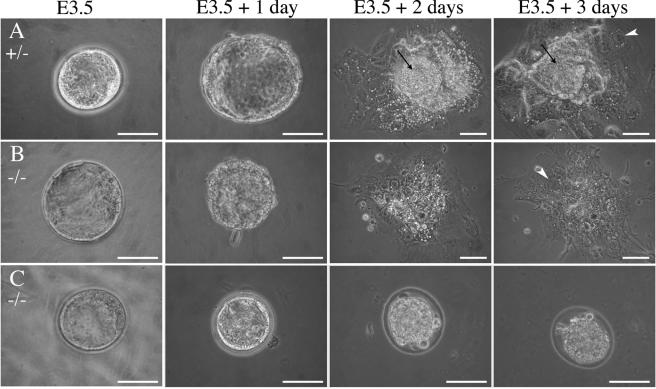
The developmental status of mutant blastocysts was then examined by in vitro culture experiments. At the time of harvest, homozygous E3.5 blastocysts were indistinguishable from their heterozygous and wild-type counterparts (Fig. 4). After 3 days in culture, mNle+/+ and mNle+/− blastocysts had hatched from the zona, ICMs had increased in size, and TE had proliferated and differentiated into giant cells (Fig. 4A). In contrast, the null blastocysts behaved differently in this assay. The majority of null blastocysts (17 out of 21) failed to hatch and degenerated rapidly inside the zona pellucida (Fig. 4C). The remaining null embryos hatched, adhered to the culture dish, and yielded apparently normal giant trophoblast cells but did not contain any recognizable ICM-derived structure after 2 days (Fig. 4B). This latter phenotype was observed for all mNle-deficient blastocysts when the zona pellucida was chemically removed at E3.5 (n = 21). After 7 days of culture, the giant trophoblast cells were still present in null outgrowths, but they occupied a threefold smaller area than in controls (data not shown). This is likely to be due to the absence of ICM, as interactions between ICM and TE are known to be essential for a harmonious development of the two lineages (35). We also tested whether degenerescence of ICM cells could be due to defective interactions with TE in mNle-deficient embryos. To do this, ICMs of E3.5 blastocysts harvested from mNle+/− intercrosses and cultured for 24 h (called E4.5 blastocysts hereafter) were isolated by immunosurgery and cultured for 7 days. Most of mNle+/+ and mNle+/− ICMs proliferated and gave rise to endodermal cells (Table 3). In contrast, inner cells from mNle−/− embryos did not survive after 24 h of culture. This result suggested that the absence of mNle causes ICM cell death in a cell-autonomous manner. Altogether, these results indicate that mNle disruption results in the absence of ICM derivatives and embryonic death shortly after implantation.
FIG. 4.
Cultures of embryos derived from mNle+/− intercrosses. E3.5 blastocysts were harvested and cultured for 3 days. mNle+/+ and mNle+/− blastocysts (A) hatched from the zona pellucida after 1 day and attached to the culture dish. During the following days, the ICM proliferated (arrows) and the trophectoderm differentiated (arrowheads). mNle−/− blastocysts were indistinguishable from controls at E3.5 (B and C). After 24 h in vitro, most of them were unable to hatch from the zona (C) and degenerated rapidly. The remaining mNle−/− embryos hatched (B) and attached to the dish, and the trophectoderm expanded and differentiated (arrowhead). However, after 2 days in culture, the ICM degenerated and was no longer discernible. Bars, 50 μm.
TABLE 3.
Culture of ICMs from mNle+/− intercrosses
| Phenotype | No. (%) with genotype:
|
||
|---|---|---|---|
| +/+ | +/− | −/−a | |
| Outgrowth | 16 (84) | 31 (89) | 0 |
| No survival | 3 (16) | 4 (11) | 20 (100)b |
χ2, P < 0.001.
One mNle−/− ICM gave rise to few endoderm-like cells.
Specification of early embryonic lineages in the absence of mNle.
The observation that ICM development is compromised in mNle−/− embryos to a greater extent than TE led us to assess mNle gene expression in early embryos. To do this, we took advantage of the nlsLacZ insertion and stained heterozygous embryos with X-Gal at various stages. We observed that zygotic transcription of mNle occurred at the two-cell stage, at the time of major zygotic gene activation (Fig. 5A). In blastocysts, X-Gal staining was found to be more intense in the ICM than in the TE (Fig. 5B). After implantation, β-galactosidase-expressing cells were observed mainly in the embryonic tissue derived from the ICM (Fig. 5C). Thus, the expression pattern of the mNle-targeted allele is consistent with mNle being preferentially required for the formation of ICM derivatives.
FIG. 5.
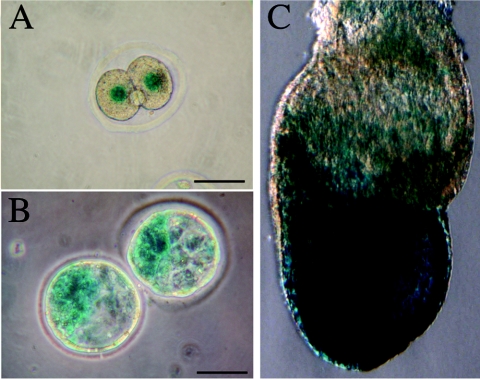
nlsLacZ expression pattern in mNle+/− heterozygous early embryos. X-Gal staining was performed on embryos harvested from crosses of wild-type females and heterozygous males at E1.5 (A), E3.5 (B), and at late bud stage (C). Scale bars, 50 μm.
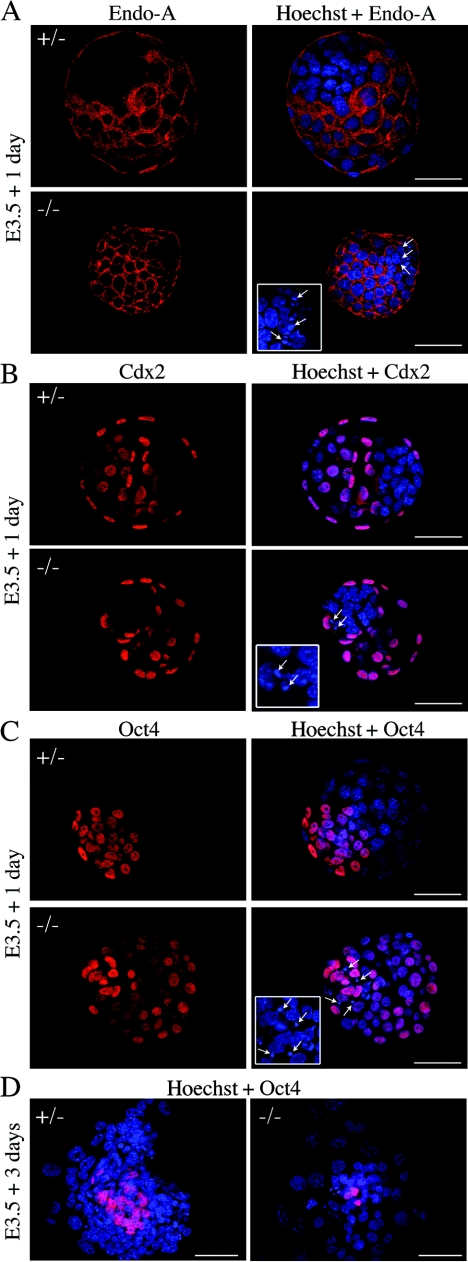
We then examined TE and ICM specification in mNle-deficient embryos using specific markers. Analysis was performed on E4.5 blastocysts, i.e., 1 day before degenerescence of mNle-deficient ICMs (Fig. 4). To examine TE specification, we analyzed the expression pattern of Endo-A cytokeratin, an intermediate filament of the type II cytokeratin class, and Cdx2, a transcription factor required for establishment and function of the TE (42). Both Endo-A and Cdx2 can be detected in the eight-cell morulae, and their expression became tightly restricted to the TE at the blastocyst stage (5, 42). We observed that Endo-A and Cdx2 expression was restricted to the TE of mNle+/+, mNle+/−, and mNle−/− E4.5 embryos (Fig. 6A), suggesting that correct specification of TE occurred in mNle-deficient embryos. To monitor ICM specification, we looked at the expression pattern of Oct4, a transcription factor essential for the maintenance of pluripotency in ICM (30). Initially present in the nuclei of all cells, Oct4 protein becomes progressively restricted to the ICM during blastocyst differentiation (26, 32). When mNle+/−-intercrossed embryos were immunostained with a specific anti-Oct4 antibody, we observed that the majority of control embryos (72%; n = 53) exhibited ICM-restricted expression (Fig. 6C, upper panel). In the remaining mNle+/+ and mNle+/− embryos, staining in all the cells (ICM and TE) was observed; nevertheless, staining in TE cells was less intense than that in ICM cells (data not shown). This is likely to reflect a developmental asynchrony between embryos. Interestingly, the reverse situation was observed for mNle-null embryos. Indeed, 35% of mNle−/− embryos exhibited ICM-restricted expression of Oct4, while 65% exhibited expression in all the cells (n = 26; P < 0.01, χ2 test) (Fig. 6C, lower panel). After 3 days in culture, only the few remaining ICM cells of mNle−/− blastocyst outgrowths stained positively for Oct4, indicating that Oct4 gene expression has been turned off in trophoblastic cells (Fig. 6D). These data suggest that mNle deficiency does not impair ICM specification but results in a delay of down-regulation of Oct4 in the TE.
FIG. 6.
TE and ICM specification in E4.5 blastocysts and outgrowths from mNle+/− intercrosses. Immunohistochemical detection of cytoplasmic Endo-A cytokeratin (red) (A) and nuclear Cdx2 (red) (B), specific for TE, and nuclear Oct4 (red) (C), specific for ICM, in mNle+/− and mNle−/− embryos is shown. Representative control embryos (upper panel) and mNle−/− embryos (lower panel) are shown. (D) Oct4 expression in mNle+/− and mNle−/− outgrowths from blastocysts cultured for 3 days. In mNle+/− outgrowths, Oct4-positive immunostaining was detected in the ICM. In contrast, very few Oct4-positive cells were observed in mNle−/− outgrowths. Nuclei were counterstained with Hoechst stain (blue). One optical section is shown for panels A to C (flattened blastocyst morphology was due to mounting on one slide with a glass coverslip placed over it). Micronuclei were detected in the ICM of the mNle−/− blastocysts (white arrows and insets, panels A to C). Bars, 50 μm (panels A to C) and 200 μm (panel D).
The absence of mNle results in ICM cell apoptosis.
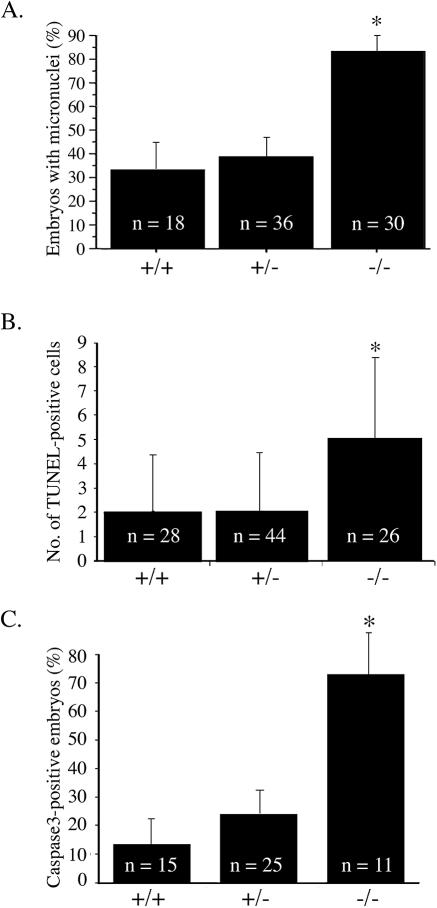
The observation that mNle−/− ICMs degenerate in culture led us to analyze cell proliferation and death in E4.5 blastocysts in more detail. First, the number of TE cells (external layer of cells) and ICM cells (internal cells with strong Oct4-positive signal) was determined by counting the number of nuclei stained with Hoechst stain in serial optical sections. No difference in the number of TE cells between the three genotypes was observed (Table 4). In contrast, the number of ICM cells in mNle-deficient embryos was significantly lower than that in control embryos (Table 4) (P < 0.0001, analysis of variance [ANOVA] test) (this reduction can also be visualized in Fig. 6). We next examined cell proliferation and cell death status. The histone H3 phosphorylation on serine residue 10 was examined to determine the number of cells in M phase (16). No difference was observed in E4.5 mNle−/− embryos compared with controls, indicating that the absence of mNle does not impair proliferation. To address a possible effect of the absence of mNle on apoptosis, we then performed microscopic examination of nuclear staining with Hoechst stain as well as TUNEL analysis. As shown in Fig. 6 (insets), mNle−/− E4.5 blastocysts exhibited an abnormally high number of condensed micronuclei. Fragmented DNA was observed essentially in the ICM and more rarely in the TE. The percentage of mNle−/− blastocysts yielding micronuclei was significantly higher than that observed for mNle+/− and mNle+/+ blastocysts (83% for mNle−/− blastocysts compared to 39% and 33% for mNle+/− and mNle+/+ blastocysts, respectively) (Fig. 7A). Next, a TUNEL assay was performed, which showed a twofold increase of TUNEL-positive cells in mNle−/− embryos compared to mNle+/− and mNle+/+ embryos (5.3 ± 0.6 compared to 2.2 ± 0.4 and 2.4 ± 0.4, respectively) (Fig. 7B). Apoptosis was further analyzed by evaluating the caspase 3-dependent apoptotic process. To do this, we performed immunostaining of E4.5 mNle+/−-intercrossed embryos using a specific antibody recognizing the active form of caspase 3. The percentage of caspase 3-positive mNle−/− embryos was significantly greater than those of the mNle+/− and mNle+/+ embryos (73% compared with 24% and 13%, respectively) (Fig. 7C). It is noteworthy that no significant difference could be found between the three genotypes regarding the percentage of micronucleus-containing and caspase 3-positive embryos a day earlier (E3.5 blastocysts) (not shown). Altogether, these data indicate that caspase 3-dependent apoptotic cell death largely contributes to embryonic lethality in mNle−/− embryos.
TABLE 4.
Differential count of ICM and TE nuclei in E3.5 blastocysts from mNle+/− intercrosses cultured for 24 h
| Type | No. ± SEM of nuclei (no. of embryos) with genotype:
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| ICM | 29.5 ± 3.0 (8) | 27.7 ± 1.5 (20) | 14.4 ± 1.2a (11) |
| TE | 58.9 ± 2.2 (8) | 60.6 ± 2.0 (17) | 56.1 ± 5.2 (9) |
n = 39; P < 0.0001, ANOVA test.
FIG. 7.
Apoptosis in E3.5 blastocysts from mNle+/− intercrosses cultured for 24 h. (A) Percentage of mNle+/+, mNle+/−, and mNle−/− embryos yielding micronuclei detected by Hoechst reagent staining (n = 84; P < 0.001, χ2 test). Data ± standard errors of the means (bars) are shown. (B) A twofold increase of TUNEL-positive cells was observed in mNle−/− blastocysts compared with control counterparts (n = 90; P < 0.0001, ANOVA test). Data ± standard deviations (bars) are shown. (C) Percentage of mNle+/+, mNle+/−, and mNle−/− embryos positive for the active form of caspase 3 protein (n = 51; P < 0.01, χ2 test). Data ± standard errors of the means (bars) are shown. Asterisks indicate statistical difference.
DISCUSSION
Nle is a well-conserved protein found from yeast to humans. In Drosophila, Nle binds to the intracellular domain of the Notch receptor and has been demonstrated to be either a positive or a negative regulator of the Notch signaling pathway (36). There are very few reports concerning the role of Nle in species other than Drosophila and Xenopus. In Saccharomyces cerevisiae, large-scale deletion studies suggest that the loss of Nle, referred to as YCR072C, is lethal (11). Because genes encoding the Notch receptors and their ligands are absent in yeast, this phenotype might account for an ancestral function of Nle. In C. elegans, large-scale RNA interference experiments indicate that the absence of Nle does not alter embryonic development but leads to slow growth and patchy coloration, larval arrest, or egg-laying defects (20, 39). However, it is not known whether these phenotypes are due to a dysregulation of the Notch pathway. Importantly, our study is the first to define the role of Nle in mammals.
Disruption of the mNle gene leads to embryonic lethality shortly after implantation. mNle-null blastocysts implant into the uterine wall but do not progress much further. In particular, no epiblast or extraembryonic ectoderm could be recognized in E5.5 implantation sites elicited by mNle−/− embryos, suggesting early failure of ICM derivatives. Consistent with this, ICM cells did not survive in mNle−/− blastocyst outgrowths. Thus, the absence of mNle prevents the ICM cells from thriving both in vivo and in vitro, indicating that mNle is instrumental for their survival. In mutant embryos, ICM cells degenerate, while TE is able to induce a decidual reaction and to differentiate normally during the initial steps of blastocyst outgrowth, suggesting that mNle function is required mainly in ICM cells. Supporting this idea is the observation that zygotic mNle expression, monitored via the nlslacZ knock-in allele, is stronger in the ICM than in the TE. However, we cannot formally exclude that mNle-null ICM failure is a consequence of abnormal interactions between inner and outer cells earlier in development. It should nevertheless be noted that E3.5 mNle-deficient blastocysts cannot be distinguished from their wild-type or heterozygous counterparts either by gross morphological examination or through counting inner and outer cell numbers. It should also be noted that mNle is expressed in oocytes (25), and therefore, maternally supplied mNle products might have obscured an eventual requirement for mNle function before the blastocyst stage.
mNle-deficient ICM failure does not seem to result from lineage specification defects. Indeed, we showed that these cells express, before they degenerate, high levels of Oct4 protein and are negative for Cdx2 and Endo-A immunoreactivity. Interestingly, the persistence of the Oct4 protein in the TE was observed in an abnormally high proportion of E4.5 mNle-deficient embryos, suggesting a delay in the temporal down-regulation of Oct4 in the trophectoderm lineage. As Oct4 and Cdx2 have recently been shown to form a repressor complex in ES cells (31), this observation raises the possibility that the TE would not be correctly specified in the absence of mNle. However, this is not supported by our observation that the mNle-deficient TE exhibited robust differentiation and functional capabilities. Altogether, these results indicate that mNle is dispensable for ICM and TE specification but is essential for the survival of ICM cells.
Apoptosis is an essential feature of many normal and pathological processes and takes place in normal mammalian preimplantation embryos. It has been proposed that apoptosis allows the elimination of ICM cells that retain trophectodermal potential and contributes to the threshold number of ICM cells compatible with a correct development of the embryo (15, 19, 33). In E4.5 mNle−/− blastocysts, ICM cells were shown to degenerate through a caspase 3-dependent apoptotic process. Selective apoptosis of ICM at the peri-implantation period has been observed in mice disrupted for various genes, including genes involved in the control of the cell cycle (10, 43), the proteasome, and the ubiquitin-like conjugation pathway (28, 44, 45) or a novel gene of unknown function (47). Whether mNle is a direct regulator of apoptosis or mNle-deficient embryos died of apoptosis as a consequence of some severe defect directly caused by the absence of mNle remains to be clarified.
In the present study, we show that the murine Nle protein has the ability to modulate Notch signaling and that its deficiency results in lethality at the peri-implantation period. Together with the recent demonstration that various components of the Notch pathway are expressed in oocytes and preimplantation embryos (9), these data raise the possibility that the death of mNle-deficient embryos might result from abnormal Notch signaling during the first steps of development. Interestingly, disruption of the murine ortholog of brainiac, which interacts with the Notch pathway and plays a role in follicular epithelium morphogenesis in Drosophila (12, 13), results in embryonic lethality by the time of implantation (46), which also might signify an implication of Notch signaling in peri-implantation development. However, the phenotypes of mNle- and brainiac-null mice are very different from phenotypes of mice knocked out for core components of the Notch signaling cascade (for examples, see references 8, 14, 17, 18, 21, 23, 38, and 49). The absence of phenotypes during preimplantation development for some of the latter mutants could be due to maternally inherited products that might have overcome zygotic gene disruption (9). However, normal preimplantation development of embryos lacking both maternal and zygotic expression of either RBP-Jκ (C. Souilhol, S. Cormier, S. Vandormael-Pournin, C. Babinet, and M. Cohen-Tannoudji, unpublished data) or O-fut (37a), two essential components of the Notch pathway, strongly indicates that the canonical RBP-Jκ-dependent Notch pathway is dispensable for preimplantation development. Therefore, decreased Notch signaling activity cannot be the cause of mNle-deficient embryo lethality. Alternatively, a loss of mNle might result in an increased RBP-Jκ-dependent Notch signaling activity, as shown in Drosophila wing imaginal disks (36), which would in turn lead to compromised development. Another possibility would be that mNle is acting on an RBP-Jκ-independent Notch pathway during mouse preimplantation development. Future work will be necessary to determine whether either of these two possibilities might account for the death of mNle-null embryos at the time of implantation.
Acknowledgments
We thank J. Artus, S. Tajbakhsh, J. Hadchouel, F. Relaix, and C. Brou for helpful discussions, C. Kress for her invaluable help and instruction with culture and manipulation of ES cells, and S. Lecorre for technical help. We acknowledge the dynamic imaging platform, Institut Pasteur, for helpful assistance.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Association pour la Recherche contre le Cancer (ARC), the Institut Pasteur GPH07 on stem cells, and the Action concertée incitative Biologie du Développement et Physiologie Intégrative from the Ministère de l'Education Nationale, de la Recherche, et de la Technologie. S.C. received grants from the Pasteur-Negri-Weizmann Council and the ARC. S.L.B. received funding from the Ministere de l'Education Nationale and was a recipient of funding from the ARC. C.S. received grants from CNRS (Bourse de Doctorat pour les Ingénieurs).
REFERENCES
- 1.Artavanis-Tsakonas, S., K. Matsuno, and M. E. Fortini. 1995. Notch signaling. Science 268:225-232. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284:770-776. [DOI] [PubMed] [Google Scholar]
- 3.Artus, J., S. Vandormael-Pournin, M. Frodin, K. Nacerddine, C. Babinet, and M. Cohen-Tannoudji. 2005. Impaired mitotic progression and preimplantation lethality in mice lacking OMCG1, a new evolutionarily conserved nuclear protein. Mol. Cell. Biol. 25:6289-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babinet, C., V. Richoux, J. L. Guenet, and J. P. Renard. 1990. The DDK inbred strain as a model for the study of interactions between parental genomes and egg cytoplasm in mouse preimplantation development. Dev. Suppl. 1990:81-87. [PubMed] [Google Scholar]
- 5.Chisholm, J. C., and E. Houliston. 1987. Cytokeratin filament assembly in the preimplantation mouse embryo. Development 101:565-582. [DOI] [PubMed] [Google Scholar]
- 6.Chitnis, A. B. 1995. The role of Notch in lateral inhibition and cell fate specification. Mol. Cell. Neurosci. 6:311-321. [PubMed] [Google Scholar]
- 7.Cohen-Tannoudji, M., S. Vandormael-Pournin, S. Le Bras, F. Coumailleau, C. Babinet, and P. Baldacci. 2000. A 2-Mb YAC/BAC-based physical map of the ovum mutant (Om) locus region on mouse chromosome 11. Genomics 68:273-282. [DOI] [PubMed] [Google Scholar]
- 8.Conlon, R. A., A. G. Reaume, and J. Rossant. 1995. Notch1 is required for the coordinate segmentation of somites. Development 121:1533-1545. [DOI] [PubMed] [Google Scholar]
- 9.Cormier, S., S. Vandormael-Pournin, C. Babinet, and M. Cohen-Tannoudji. 2004. Developmental expression of the Notch signaling pathway genes during mouse preimplantation development. Gene Expr. Patterns 4:713-717. [DOI] [PubMed] [Google Scholar]
- 10.Dobles, M., V. Liberal, M. L. Scott, R. Benezra, and P. K. Sorger. 2000. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101:635-645. [DOI] [PubMed] [Google Scholar]
- 11.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 12.Goode, S., M. Melnick, T. B. Chou, and N. Perrimon. 1996. The neurogenic genes egghead and brainiac define a novel signaling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development 122:3863-3879. [DOI] [PubMed] [Google Scholar]
- 13.Goode, S., M. Morgan, Y. P. Liang, and A. P. Mahowald. 1996. Brainiac encodes a novel, putative secreted protein that cooperates with Grk TGF alpha in the genesis of the follicular epithelium. Dev. Biol. 178:35-50. [DOI] [PubMed] [Google Scholar]
- 14.Hamada, Y., Y. Kadokawa, M. Okabe, M. Ikawa, J. R. Coleman, and Y. Tsujimoto. 1999. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development 126:3415-3424. [DOI] [PubMed] [Google Scholar]
- 15.Hardy, K. 1997. Cell death in the mammalian blastocyst. Mol. Hum. Reprod. 3:919-925. [DOI] [PubMed] [Google Scholar]
- 16.Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli, B. R. Brinkley, D. P. Bazett-Jones, and C. D. Allis. 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348-360. [DOI] [PubMed] [Google Scholar]
- 17.Hrabe de Angelis, M., J. McIntyre II, and A. Gossler. 1997. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386:717-721. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, R., Y. Lan, H. D. Chapman, C. Shawber, C. R. Norton, D. V. Serreze, G. Weinmaster, and T. Gridley. 1998. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 12:1046-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurisicova, A., and B. M. Acton. 2004. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction 128:281-291. [DOI] [PubMed] [Google Scholar]
- 20.Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin, M. Gotta, A. Kanapin, N. Le Bot, S. Moreno, M. Sohrmann, D. P. Welchman, P. Zipperlen, and J. Ahringer. 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421:231-237. [DOI] [PubMed] [Google Scholar]
- 21.Krebs, L. T., Y. Xue, C. R. Norton, J. R. Shutter, M. Maguire, J. P. Sundberg, D. Gallahan, V. Closson, J. Kitajewski, R. Callahan, G. H. Smith, K. L. Stark, and T. Gridley. 2000. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 14:1343-1352. [PMC free article] [PubMed] [Google Scholar]
- 22.Kress, C., S. Vandormael-Pournin, P. Baldacci, M. Cohen-Tannoudji, and C. Babinet. 1998. Nonpermissiveness for mouse embryonic stem (ES) cell derivation circumvented by a single backcross to 129/Sv strain: establishment of ES cell lines bearing the Omd conditional lethal mutation. Mamm. Genome 9:998-1001. [DOI] [PubMed] [Google Scholar]
- 23.Kusumi, K., E. S. Sun, A. W. Kerrebrock, R. T. Bronson, D. C. Chi, M. S. Bulotsky, J. B. Spencer, B. W. Birren, W. N. Frankel, and E. S. Lander. 1998. The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat. Genet. 19:274-278. [DOI] [PubMed] [Google Scholar]
- 24.Lai, E. C. 2004. Notch signaling: control of cell communication and cell fate. Development 131:965-973. [DOI] [PubMed] [Google Scholar]
- 25.Le Bras, S., M. Cohen-Tannoudji, V. Guyot, S. Vandormael-Pournin, F. Coumailleau, C. Babinet, and P. Baldacci. 2002. Transcript map of the Ovum mutant (Om) locus: isolation by exon trapping of new candidate genes for the DDK syndrome. Gene 296:75-86. [DOI] [PubMed] [Google Scholar]
- 26.Mitalipov, S. M., H. C. Kuo, J. D. Hennebold, and D. P. Wolf. 2003. Oct-4 expression in pluripotent cells of the rhesus monkey. Biol. Reprod. 69:1785-1792. [DOI] [PubMed] [Google Scholar]
- 27.Mumm, J. S., and R. Kopan. 2000. Notch signaling: from the outside in. Dev. Biol. 228:151-165. [DOI] [PubMed] [Google Scholar]
- 28.Nacerddine, K., F. Lehembre, M. Bhaumik, J. Artus, M. Cohen-Tannoudji, C. Babinet, P. P. Pandolfi, and A. Dejean. 2005. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell. 9:769-779. [DOI] [PubMed] [Google Scholar]
- 29.Nal, B., E. Mohr, M. I. Silva, R. Tagett, C. Navarro, P. Carroll, D. Depetris, C. Verthuy, B. R. Jordan, and P. Ferrier. 2002. Wdr12, a mouse gene encoding a novel WD-repeat protein with a notchless-like amino-terminal domain. Genomics 79:77-86. [DOI] [PubMed] [Google Scholar]
- 30.Nichols, J., B. Zevnik, K. Anastassiadis, H. Niwa, D. Klewe-Nebenius, I. Chambers, H. Scholer, and A. Smith. 1998. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379-391. [DOI] [PubMed] [Google Scholar]
- 31.Niwa, H., Y. Toyooka, D. Shimosato, D. Strumpf, K. Takahashi, R. Yagi, and J. Rossant. 2005. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123:917-929. [DOI] [PubMed] [Google Scholar]
- 32.Palmieri, S. L., W. Peter, H. Hess, and H. R. Scholer. 1994. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 166:259-267. [DOI] [PubMed] [Google Scholar]
- 33.Pierce, G. B., A. L. Lewellyn, and R. E. Parchment. 1989. Mechanism of programmed cell death in the blastocyst. Proc. Natl. Acad. Sci. USA 86:3654-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson, E. J., and A. Bradley. 1986. Production of permanent cell lines from early embryos and their use in developmental problems, p. 475-508. In J. R. R. A. Pedersen (ed.), Experimental approaches to mammalian embryonic development. IRL Press, Oxford, United Kingdom.
- 35.Rossant, J., and J. C. Cross. 2001. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2:538-548. [DOI] [PubMed] [Google Scholar]
- 36.Royet, J., T. Bouwmeester, and S. M. Cohen. 1998. Notchless encodes a novel WD40-repeat-containing protein that modulates Notch signaling activity. EMBO J. 17:7351-7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweisguth, F. 2004. Notch signaling activity. Curr. Biol. 14:R129-R138. [PubMed] [Google Scholar]
- 37a.Shi, S., M. Stahl, L. Lu, and P. Stanley. 2005. Canonical notch signaling is dispensable for early cell fate specification in mammals. Mol. Cell. Biol. 25:9503-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidow, A., M. S. Bulotsky, A. W. Kerrebrock, R. T. Bronson, M. J. Daly, M. P. Reeve, T. L. Hawkins, B. W. Birren, R. Jaenisch, and E. S. Lander. 1997. Serrate2 is disrupted in the mouse limb-development mutant syndactylism. Nature 389:722-725. [DOI] [PubMed] [Google Scholar]
- 39.Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. van den Berghe, R. S. Kamath, A. G. Fraser, J. Ahringer, and R. H. Plasterk. 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLOS Biol. 1:E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, T. F., C. Gaitatzes, K. Saxena, and E. J. Neer. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181-185. [DOI] [PubMed] [Google Scholar]
- 41.Solter, D., and B. B. Knowles. 1975. Immunosurgery of mouse blastocyst. Proc. Natl. Acad. Sci. USA 72:5099-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strumpf, D., C. A. Mao, Y. Yamanaka, A. Ralston, K. Chawengsaksophak, F. Beck, and J. Rossant. 2005. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132:2093-2102. [DOI] [PubMed] [Google Scholar]
- 43.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, and M. Nakanishi. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 44.Tateishi, K., M. Omata, K. Tanaka, and T. Chiba. 2001. The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J. Cell Biol. 155:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomoda, K., N. Yoneda-Kato, A. Fukumoto, S. Yamanaka, and J. Y. Kato. 2004. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J. Biol. Chem. 279:43013-43018. [DOI] [PubMed] [Google Scholar]
- 46.Vollrath, B., K. J. Fitzgerald, and P. Leder. 2001. A murine homologue of the Drosophila brainiac gene shows homology to glycosyltransferases and is required for preimplantation development of the mouse. Mol. Cell. Biol. 21:5688-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voss, A. K., T. Thomas, P. Petrou, K. Anastassiadis, H. Scholer, and P. Gruss. 2000. Taube nuss is a novel gene essential for the survival of pluripotent cells of early mouse embryos. Development 127:5449-5461. [DOI] [PubMed] [Google Scholar]
- 48.Whittingham, D. G., and R. G. Wales. 1969. Storage of two-cell mouse embryos in vitro. Aust. J. Biol. Sci. 22:1065-1068. [DOI] [PubMed] [Google Scholar]
- 49.Xue, Y., X. Gao, C. E. Lindsell, C. R. Norton, B. Chang, C. Hicks, M. Gendron-Maguire, E. B. Rand, G. Weinmaster, and T. Gridley. 1999. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8:723-730. [DOI] [PubMed] [Google Scholar]
- 50.Yagi, T., Y. Ikawa, K. Yoshida, Y. Shigetani, N. Takeda, I. Mabuchi, T. Yamamoto, and S. Aizawa. 1990. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc. Natl. Acad. Sci. USA 87:9918-9922. [DOI] [PMC free article] [PubMed] [Google Scholar]