Abstract
Cytoskeletal rearrangements are central to endothelial cell physiology and are controlled by soluble factors, matrix proteins, cell-cell interactions, and mechanical forces. We previously reported that aortic endothelial cells can rearrange their cytoskeletons into complex actin-based structures called podosomes when a constitutively active mutant of Cdc42 is expressed. We now report that transforming growth factor beta (TGF-β) promotes podosome formation in primary aortic endothelial cells. TGF-β-induced podosomes assembled together into large ring- or crescent-shaped structures. Their formation was dependent on protein synthesis and required functional Src, phosphatidylinositide 3-kinase, Cdc42, RhoA, and Smad signaling. MT1-MMP and metalloprotease 9 (MMP9), both upregulated by TGF-β, were detected at sites of podosome formation, and MT1-MMP was found to be involved in the local degradation of extracellular matrix proteins beneath the podosomes and required for the invasion of collagen gels by endothelial cells. We propose that TGF-β plays an important role in endothelial cell physiology by inducing the formation of podosomal structures endowed with metalloprotease activity that may contribute to arterial remodeling.
Transforming growth factor beta (TGF-β) is a multifunctional cytokine that modulates the growth and differentiation of many cells (45). It plays an important role in development and cell differentiation and regulates the epithelium-to-mesenchyme transition (16, 55). TGF-β is associated with invasive behavior by its ability to regulate the expression and activation of metalloproteases, endopeptidases that cleave virtually all components of the extracellular matrix (38). In the vascular system, TGF-β regulates the processes of angiogenesis (27), vasculogenesis (23), and arteriogenesis (57, 59).
TGF-β signals through a family of receptor-activated transcription factors, the Smads. Following binding of TGF-β, the TGF-β type II receptor recruits and phosphorylates the TGF-β type I receptor (activin-like receptor 5 [Alk5]). The latter phosphorylates regulatory Smads (R-Smad), Smad2/3, which in turn undergo homotrimerization, associate with Smad4 (common Smad), and translocate to the nucleus, where the complex binds to the promoters of TGF-β-responsive genes. Importantly, certain endothelial cells display an additional type I receptor named Alk1, which upon TGF-β binding regulates another subset of R-Smads, Smad1/5. However, Alk1 signaling is dependent on Alk5 kinase activity, as Alk5 is essential for efficient Alk1 activation and subsequent Smad1/5 phosphorylation (22). Smad4 is also required for Smad1/5 nuclear translocation and transcriptional regulation of an Alk1-specific subset of genes. Gain- and loss-of-function studies in mice demonstrated that TGF-β is essential for proper blood vessel formation and maintenance and that this function is mediated by Alk1, Alk5, and Smad5 (10).
Besides these canonical pathways, noncanonical TGF-β-activated pathways involving the Rho family of GTPases have been described for epithelial models, and they account for cytoskeletal remodeling and morphological changes associated with cell differentiation. Rho and phosphatidylinositide 3-kinase (PI3K) have been shown to be regulated by TGF-β in the context of the epithelium-to-mesenchyme transition (3), and Cdc42 and RhoA were found to be involved in TGF-β-induced cytoskeletal remodeling in carcinomas (17, 18) and skeletal muscle cells (34). Previous studies to explore the role of RhoGTPases in endothelial cytoskeletal reorganization revealed that constitutive activation of Cdc42 by the expression of V12Cdc42 triggered the formation of podosomes (37). These actin-based attachment structures, first described for Rous sarcoma virus-transformed fibroblasts, are located at the ventral membranes of cells (20, 54). A core of actin filaments and actin-associated proteins is surrounded by a ring of vinculin, talin, and paxillin (20), together with podosomal markers not found in focal adhesions, such as gelsolin, cortactin, dynamin, WASP/NWASP, and Arp2/3 proteins associated with the actin polymerization machinery (8, 29). Podosomes have also been found in highly metastatic cells such as melanoma and breast cancer cells (8, 39, 48). In physiological settings, podosomes form spontaneously in certain cells such as macrophages (13), immature dendritic cells (9), and osteoclasts (42, 61), which share the common feature of traveling across tissues. Podosomes also differ from focal adhesions by the presence of metalloproteases (MMPs). MT1-MMP (47) and MMP9 (15) are found at podosomes, supporting the concept that podosomes, also known in invasive tumor cells as invadopodia, may serve to spatially restrict sites of matrix degradation.
To further characterize endothelial podosomes, several factors known to act on the vascular endothelium were tested for the ability to induce these actin-based structures. In the present study, we report that TGF-β is able to induce the formation of large rosettes of podosomes in aortic endothelial cells. This is a novel function of TGF-β in these cells which integrates multiple signaling pathways downstream of TGF-β receptors.
MATERIALS AND METHODS
Cells.
BAE cells (Cambrex Bioscience) were maintained in complete endothelial cell growth medium (EGM-MV; Promocell) at 37°C in a 5% CO2 humidified atmosphere and used between passages 3 and 6. Cells were plated onto type I collagen-coated substrata, dishes, or coverslips in all experiments.
Reagents.
Recombinant human TGF-β1 (used at 5 ng/ml in all experiments) was obtained from R&D Systems, and type I collagen was obtained from BD Biosciences. GM6001 and NC-GM6001 were purchased from Calbiochem, and Fluoromount mounting medium and anti-p-Y418Src were purchased from Biosource International. Glutathione-Sepharose beads, PP1, and antibodies against vinculin (hVIN-1), Von-Willebrand factor, α-actinin, tropomyosin, and α-tubulin (clone DM1A) were purchased from Sigma. Other antibodies against the following proteins were obtained as indicated: cortactin (clone 4F11) and p-Smad2 (Ser465/467), Upstate Biotechnology International; Cdc42, paxillin, β3 integrin, FAK, p-FAK(Y397), Smad4, and p-Smad1/5 (Ser463/465, Ser426/428), Cell Signaling Technologies; RhoA (clone 26C4), Santa Cruz Biotechnologies; αVβ3 integrin (LM609), Chemicon; glutathione S-transferase (GST), Oncogene; MT1-MMP and MMP9, Biomol; Myc (9E10), phosphotyrosine (4G10), and p85α (U5), D. Cantrell (University of Dundee, United Kingdom); gelsolin, C. Chaponnier (University of Geneva, Geneva, Switzerland); Arp3, M. Welch (University of California, Berkeley); dynamin 2, M. McNiven (Mayo Clinic, Rochester, Minn.); and N-WASP, M. Way (London Research Institute, London, United Kingdom). The WIP-Myc tag construct was obtained from D. Stewart (NCI/NIH, Bethesda, Md.). Alexa 546-phalloidin, Alexa 488-labeled secondary antibodies, fluorescein isothiocyanate (FITC), pinocytic cell loading reagent, and Hoescht 33342 were purchased from Molecular Probes.
Immunofluorescence staining.
Subconfluent cells grown on collagen (0.2 mg/ml)-coated glass coverslips were fixed with 3% paraformaldehyde prepared in cytoskeletal buffer (37). The coverslips were washed in water and mounted on microscope slides with Fluoromount mounting medium. The same protocol was used for the invasion assays.
Microscopy and image analysis.
Cells were analyzed by confocal imaging using a Zeiss LSM 510 Meta inverted laser scanning fluorescence microscope equipped with acquisition software (LSM 510 acquisition software; Zeiss) and a 63× (numerical aperture [NA], 1.4) oil immersion objective. Triple-color imaging using Hoescht 33342, Alexa 488-labeled secondary antibodies, and Alexa 546-phalloidin was obtained using selective laser excitation at 350 nm, 488 nm, and 543 nm, respectively. Phase-contrast and fluorescence imaging was performed using a Nikon TE2000 inverted fluorescence microscope (4× objective with an optical magnification lens of 1.5× and oil immersion objectives of 40× [NA, 1.30] and 60× [NA, 1.40]) and digitally acquired using a Nikon DXM 1200F camera (LUCIA 5.0 acquisition software; Nikon). Fluorescent images were processed with Adobe Photoshop 7.0. Quantification of cells showing podosome rosettes was done by counting 500 cells for each coverslip. Quantification of degradation areas on FITC-labeled gelatin was performed for at least 70 fields (40× objective) for each coverslip. Invasion was quantified by the number of sprouts invading collagen gels per field for each well (4× objective with magnification lens of 1.5×).
Western blotting.
Cells were collected in reducing Laemmli sample buffer. Lysates were sonicated, boiled, and subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Proteins were transferred from gels to Immobilon polyvinylidene difluoride membranes (Millipore). Proteins were detected by chemiluminescence (ECL+; Amersham Pharmacia Biotech) using horseradish peroxidase-coupled secondary antibodies (DAKO). The amounts of proteins detected by Western blotting were determined by scanning the autoradiograph, followed by processing of the data with NIH Image software.
Rho and Cdc42 activity assay.
RhoA and Cdc42 total activities were measured by pull-down assays using GST-Rho-binding domain (RBD)-rhotekin and GST-Cdc42/Rac-interactive binding domain (CRIB)-PAK (37, 46). Cdc42 activity was quantitated as the ratio of the measured staining intensity of the GTP-Cdc42 band pulled down with GST-PAK divided by that measured for tubulin, which was used to normalize protein loading. A Rho in situ activity assay was performed using a purified GST-RBD protein as a probe to assess GTP-loaded Rho protein subcellular localization. Cells were incubated with GST-RBD (10 μg/ml) for 2 h and processed by immunofluorescence staining with anti-GST primary and Alexa 488-labeled secondary antibodies.
Fractionation protocol.
Cells were treated with TGF-β for various times, washed, and lysed in 5 mM Tris-HCl, pH 7, 5 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 2 mM dithiothreitol, 1 mM Na3VO4, 10 mM NaF, 0.1 mM phenylmethylsulfonyl fluoride, and protease inhibitors for 30 min on ice. Samples were then centrifuged at 100,000 × g (1 h, 4°C). Pellets (membrane/cytoskeletal fractions) were solubilized in 1% Triton-containing buffer and run in parallel with supernatants (cytosol).
Preparation of endothelial cells from aortic explants.
Endothelial cells were isolated from freshly explanted human aorta fragments from patients undergoing surgery by using a modified version of a published protocol (2). The vessel was cut off under sterile conditions and cleaned free of connective tissue. The adventitia was peeled from the medium, and the fragment was incubated with collagenase. Endothelial cells were then harvested from the subendothelial bed, seeded onto collagen-coated coverslips in culture medium, and processed for immunofluorescence.
siRNA and plasmid transfection.
Small interfering RNA (siRNA; QIAGEN) transfection into BAE cells was performed by two rounds of transfection with double-stranded RNA (50 nM) (58). Plasmids were transfected in a single round of transfection (4 μg for a 35-mm dish), and 24 h after transfection, cells were stimulated with TGF-β for the indicated times and analyzed. Smad4 sense siRNA was designed against a homologous target sequence between human, rat, mouse, pig, and mink species (CACUGCAGAGTAATGCTCC). For the other siRNAs, the gene target sequence was homologous between the human and bovine species (RhoA sense siRNA [GAAGUCAAGCAUUUCUGUC], Cdc42 sense siRNA [GAUAACUCACCACUGUCCA], and MT1-MMP sense siRNA [GGCCAAUGUUCGAAGGAAG]). As a negative control, a nonsilencing siRNA labeled with Alexa fluor 488 which has no known homology to mammalian genes was used.
Gelatinase activity.
MMP activities were measured in supernatants (serum-free) of cells plated in 60-mm-diameter culture dishes. Gelatinolytic activity was assayed by SDS-polyacrylamide gel electrophoresis with gelatin (53). Secreted MMP activity was analyzed in 40-μl aliquots of conditioned medium supplemented with Laemmli sample buffer. Proteins were separated in 10% acrylamide gels containing 1 mg/ml gelatin run at 20 mA/gel. SDS was removed from the gels by incubation in 2.5% Triton X-100 for 60 min, and gelatinolytic activity was allowed to develop overnight in 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij 35, 50 mM Tris, pH 7.6. Gels were stained using standard procedures.
Matrix degradation assay.
BAE cells were seeded on FITC-gelatin-coated coverslips (6). Colocalization between dark areas and podosome rosettes was visualized after staining with Alexa 546-phalloidin.
Invasion assays.
Collagen gels were prepared in 96-well culture plates with 50 μl type I collagen used at a final concentration of 2.2 mg/ml in minimum essential medium with 2.27 mg/ml Na2CO3 and 20 mM HEPES. After gelling of the medium, 4.5 × 104 cells in complete medium were added to the substrate gel. When indicated, gels and media were supplemented with GM6001 or NC-GM6001. Once cell adhesion was achieved, TGF-β was added and invasive activity was quantified by counting the number of sprouts by phase-contrast microscopy at 48 h. To visualize the cytoskeletons of invading cells, 105 cells in complete medium were seeded on a thin collagen layer on the microporous membranes of transwell culture plates (3-μm pore size; Corning, Inc.). After cell adhesion, TGF-β was added to the upper compartment of the transwell chamber. Actin/vinculin staining was performed to visualize invasive cellular protrusions inside the pores by confocal scanning microscopy.
Statistics.
Each experiment was performed in triplicate, and quantification values represent the means of three independent experiments ± standard deviations. Significance was determined using Student's t test.
RESULTS
TGF-β induces rings of podosomes in aortic endothelial cells.
Primary bovine endothelial cells (BAE cells; CD31+ VE cadherin+ Von Willebrand factor+ [data not shown]) were grown on type I collagen-coated glass coverslips to 70% confluence in complete medium to analyze the cytoskeletal organization after the addition of TGF-β. Untreated adherent cells displayed thin stress fibers and few lamellipodial extensions, as most of the polymerized actin was seen along the cell cortex (Fig. 1A). After overnight treatment, cells had adopted an elongated morphology. However, in 10% to 40% of the cells, ring-like structures, highly reminiscent of the rosettes observed in v-src-transformed fibroblasts (54), were observed in well-formed lamellipodial extensions (Fig. 1A; see Fig. S1 in the supplemental material). At higher magnification, arrays of podosomes were identified by their typical cores of F-actin surrounded by a vinculin rim (Fig. 1B). In a time course experiment, podosomes were found to arise directly organized in clusters and never appeared as isolated podosomes (data not shown). In a dose-response experiment, TGF-β could induce podosomes at doses as low as 0.1 ng/ml, at the low end of the physiological range (from 0.1 to 4 ng/ml in blood plasma) (data not shown). Once established, this phenotype was stable and could be observed for up to 48 h.
FIG. 1.
TGF-β induces rosettes of podosomes in primary aortic endothelial cells. (A) Fluorescence staining of BAE cells either left untreated (top panels) or treated with TGF-β for 20 h (bottom panels). Cells were processed for staining to detect F-actin (with fluorescent phalloidin [red] or with antivinculin antibodies and fluorescent secondary antibodies [green]) and the nucleus (Hoescht). Bar, 10 μm. (B) Higher magnification of the podosome rosette depicted in panel A, showing the core of F-actin surrounded by the vinculin ring. Bar, 1 μm. (C) Podosomes were induced by either TGF-β or conditioned medium prepared from BAE cell cultures treated with TGF-β for 20 h, and rosettes were detected as described above. The graph shows the quantification of induced rosettes counted as described in Materials and Methods. (D) Quantification of podosome rosettes formed in response to a 20-h exposure to either TGF-β or conditioned medium in the presence of either control immunoglobulin G (100 μg/ml), TGF-β-blocking antibodies (100 μg/ml), or SB431542 (5 μM). (E) Human endothelial cells prepared from a freshly explanted aorta fragment were directly seeded on type I collagen-coated coverslips and stimulated with TGF-β for 20 h. Fluorescence staining was performed as described for panel A for F-actin/vinculin detection, and Von-Willebrand factor (vWF) staining was performed to identify endothelial cells. Rosettes of podosomes visualized by F-actin and vinculin colocalization are shown with white arrows in endothelial (vWF positive) cells. Bar, 10 μm.
The contribution of serum to this process was minimal, since podosomes formed to the same extent when the serum concentration was reduced to 0.2% (data not shown). Interestingly, a brief (3 min) incubation with the cytokine was sufficient to induce these ring-like structures, which persisted for the same duration as those in cultures where TGF-β had not been washed out (data not shown). Podosomes were observed only after a lag period of 6 h following the addition of TGF-β (Fig. 1C). This cytokine is known to induce profound changes in the pattern of protein expression and the production of extracellular components during the process of epithelium-to-mesenchyme transition (55). The production of such factors or matrix constituents could potentially contribute to podosome formation. To explore whether podosomes form in response to the release of a TGF-β-induced factor(s), the supernatant from a culture of 20-h TGF-β-treated cells was applied to untreated BAE cells. In these cells, podosomes formed in response to the conditioned medium to the same extent and with the same time course as those in TGF-β-treated cells (Fig. 1C). Thus, the formation of podosomes was not induced by a soluble intermediate released by TGF-β-treated cells, and the 6-h delay preceding the onset of podosome appearance was likely to reflect the activation of intracellular events. Furthermore, no other active substances examined, including vasoconstrictors (endothelin 1 and ethanol), vasodilators (bradykinin and NO), angiogenic factors (vascular endothelial growth factor [VEGF] and basic fibroblast growth factor), growth factors, cytokines and chemokines (interleukin-1α [IL-1α] or -β, IL-8, tumor necrosis factor alpha, macrophage colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, epidermal growth factor, platelet-derived growth factor BB, IL-6, insulin, and thrombin), and other factors (collagen I, osteopontin, albumin, ApoA1, Wnt3a, sFRP1, and reactive oxygen species), could induce podosomes (data not shown). Similar results were obtained when cells were plated on fibronectin-, vitronectin-, osteopontin-, or gelatin-coated coverslips or uncoated glass surfaces (data not shown), indicating that the observed phenotype was entirely and directly dependent on TGF-β. Finally, preventing TGF-β signals by neutralizing active TGF-β with blocking antibodies or by direct pharmacological inhibition of the TGF-β receptors Alk5/Alk1 with the pharmacological compound SB431542 (22) ablated podosome formation (Fig. 1D). Similar results were obtained when induction was obtained by the addition of conditioned medium (Fig. 1D).
To examine the ability of freshly excised aortic endothelial cells to form podosomes, a fragment of a human aorta was processed shortly after its surgical resection. Pieces of intact endothelium were quickly isolated, and endothelial cells were stained shortly after. In the Von Willebrand factor-positive endothelial cell population, some cells clearly formed podosomes upon overnight TGF-β exposure (Fig. 1E). Taken together, these results demonstrate that TGF-β is a direct inducer of podosome rosettes in both freshly isolated and cultured aortic endothelial cells from different species.
Molecular characterization of TGF-β-induced endothelial podosomes.
To further define the subcellular localization of TGF-β-induced podosomes, confocal microscopy was performed. As shown in the stack of optical sections pictured in Fig. 2A, podosomes were exclusively located at the ventral surfaces of the cells, indicating that these structures are used by endothelial cells to adhere to the matrix and not to interact with circulating cells (1, 25, 33). A detailed list of podosome components has been previously established (29), and when investigated, these proteins were found in TGF-β-induced structures (Table 1). αVβ3 integrin is found at osteoclastic podosomes (44). This integrin is upregulated on activated endothelial cells engaged in the angiogenic program and plays a decisive role in cell survival and invasiveness (7). In our model, TGF-β upregulated β3 subunit expression in BAE cells (Fig. 2B). Furthermore, whereas αVβ3 integrin staining showed a diffuse pattern throughout the cell surface in untreated BAE cells, a fraction of αVβ3 redistributed to the rim of podosomes forming the rosettes upon TGF-β addition (Fig. 2C).
FIG. 2.
TGF-β-induced rosettes of podosomes are localized at the ventral surfaces of endothelial cells and contain αVβ3 integrin. (A) BAE cells were treated with TGF-β for 20 h, processed for fluorescence staining as described in the legend to Fig. 1, and analyzed by confocal microscopy. Serial optical sections (0.31 μm) are shown and presented from the basal (bottom) to the apical (top) regions of the cells. Bar, 10 μm. (B) Western blot analysis of β3 integrin expression of BAE cells treated or not treated with TGF-β for 20 h. The similar tubulin levels seen in both lanes demonstrate equal protein loading. (C) Fluorescence staining for αVβ3 integrin together with F-actin in BAE cells cultured on type I collagen. Bar, 10 μm.
TABLE 1.
Components of TGF-β-induced rosettes of podosomes
| Componenta | Function |
|---|---|
| Actin-associated proteins and regulators | |
| F-actin | Structural element |
| Arp 2/3 | Actin nucleation |
| N-WASP | Arp 2/3 complex activation |
| VASP | Enhancer of N-WASP activity |
| WIP (WIP-GFP transfected) | WASP interacting protein |
| Cortactin | F-actin linker, Arp 2/3 activator |
| α-Actinin | F-actin linker |
| Gelsolin | Uncapping and Severing of F-actin |
| Tropomyosin | Actin filament-associated protein |
| Integrins and integrin-associated proteins | |
| αVβ3 integrin | Cell-matrix contact |
| Vinculin | Integrin/actin linker |
| Paxillin | Integrin/actin linker |
| Kinases | |
| Src (phosphorylated on Y418) | Tyrosine kinase |
| FAK | Adhesion regulator |
| p85α of PI3K | Inositol lipid kinase |
| Phosphotyrosines | Regulatory signals |
| Others | |
| Dynamin2 | Formation of canaliculi? |
| RhoA | Cytoskeleton regulator |
| Metalloproteases | |
| MT1-MMP | ECM degradationb |
| MMP9 | ECM degradationb |
Proteins were detected at podosomes by immunofluorescence using specific antibodies, except in the case of WIP, where cells were transfected with a plasmid encoding a GFP-WIP fusion protein.
ECM, extracellular matrix.
Signaling pathways involved in TGF-β-mediated podosome formation.
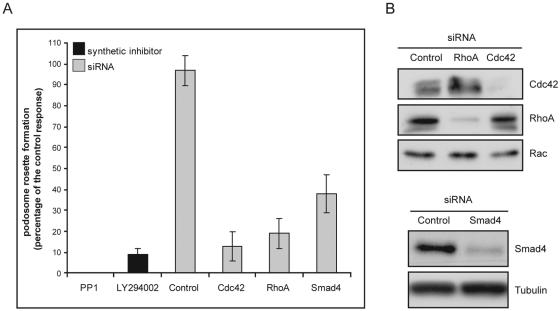
Podosome formation occurs in a limited number of cell types, in response to different inducers (including fibronectin for immature dendritic cells [9] and IL-5 and tumor necrosis factor alpha for eosinophils [25]), and via distinct signaling pathways. Src, PI3K, and RhoGTPases are key proteins always involved in the complicated process of podosome formation (29). Since these three pathways can be regulated by TGF-β in other models (5, 17, 18, 26, 34, 60), we examined their contributions to TGF-β-induced podosome formation in endothelial cells. Src and PI3K catalytic activities were inhibited using a pharmacological approach, whereas RhoGTPases and Smad expression were suppressed using siRNAs. The data presented in Fig. 3A established that podosome assembly was completely ablated by the Src-like kinase inhibitors PP1 and PP2 (data not shown) or the PI3K inhibitor LY294002. Likewise, TGF-β-induced podosome formation was prevented in the absence of Cdc42. Silencing of RhoA expression also caused an inhibitory effect (Fig. 3A). The essential role of RhoA was confirmed in cells following transduction of the C3 toxin to inactivate Rho proteins by covalent modification (data not shown). Smad signaling pathways were also investigated to explore TGF-β-specific responses. Smad signaling was found to be required, as reducing Smad4 expression strongly inhibited the process of podosome formation (Fig. 3A). The extinction of protein expression was controlled by Western blot experiments (Fig. 3B).
FIG. 3.
Inhibition of signaling through Src family kinase, PI3K, Smads, and the RhoGTPases RhoA and Cdc42 impairs the formation of podosome rings in response to TGF-β. (A) BAE cells were treated with a Src (PP1; 5 μM) or PI3K (LY294002; 10 μM) inhibitor 1 h prior to 20 h of TGF-β stimulation. Smad4, Cdc42, and RhoA protein expression was inhibited using specific siRNAs (50 nM), and TGF-β stimulation was performed 24 h after the second siRNA transfection. Cells with podosome rosettes after pharmacological inhibition or siRNA transfection were recorded. The result obtained with cells transfected in the absence of siRNA and treated with TGF-β was fixed arbitrarily at 100%. The control response was measured in cells transfected with siRNA-488 and treated with TGF-β in the absence of an inhibitory treatment. (B) Western blot analysis of Cdc42, RhoA, and Smad4 expression in cells subjected to siRNA transfection as described above. Similar Rac or tubulin levels seen in all lanes demonstrate equal protein loading.
To further assess the roles of these proteins, we examined whether TGF-β regulated their activities by analyzing their phosphorylation states and subcellular distribution. During the course of stimulation, Src phosphorylated on tyrosine 418 (Y-418), a site known to be regulated by TGF-β and indicative of Src kinase activity (41), was found associated with the cytoskeletal/membrane fraction at 30 min (Fig. 4A). By 20 h of TGF-β stimulation, immunofluorescence experiments showed intense Y-418 Src phosphorylation at podosomes (Fig. 4B). We detected FAK at endothelial podosomes, as in human umbilical vein endothelial cells (43). The protein was found to be phosphorylated at position 407 (tyrosine) (data not shown), providing a binding site for p85α, which was also visualized at the podosomal rim, in agreement with the proposed spatial organization of the proteins inside the complex (29, 30). Although a constitutively active mutant of Cdc42 induced podosomes in these cells (data not shown), in agreement with our previous report (37), endogenous Cdc42 was not detected at TGF-β-induced podosomes (data not shown). In contrast, RhoA was clearly present at these structures (Fig. 4C). To verify the status of RhoA at this site, a GST-RBD-rhotekin fusion protein was used as an immunolocalization probe. Immunofluorescence experiments showed specific binding and revealed that the fraction of RhoA localized at podosomes was active (Fig. 4D). Not surprisingly, RhoGDI was found to be excluded from these areas (data not shown).
FIG. 4.
Src, Smad, RhoA, and Cdc42 activities are regulated by TGF-β during podosome rosette formation. (A) Western blot analysis of BAE cell extracts after fractionation, showing cytosolic and membrane/cytoskeleton-bound Y418-phosphorylated Src in the course of TGF-β stimulation in 0.3% serum. (B) Fluorescence staining of Y418-phosphorylated Src (green), F-actin (red), and nuclei (blue) in BAE cells. (C) Cells were prepared as described for panel B, and subcellular localization of RhoA was performed. (D) Cells were prepared as described for panel B, except that subcellular localization of active RhoA was performed using a soluble GST-RBD fusion protein as a probe, which was in turn visualized by GST immunofluorescence staining. Bar, 10 μm. (E) Modulation of Cdc42 and RhoA activities by TGF-β was assessed in pull-down assays using GST-CRIB-PAK and GST-RBD fusion proteins, respectively, and the amounts of precipitated proteins were determined by Western blotting using specific antibodies. A fraction of whole-cell lysate was harvested to assess total Cdc42 and RhoA protein contents. (F) Western blot analysis of Smad1/5 and Smad2 phosphorylation levels in whole-cell lysates from BAE cells treated with TGF-β in medium containing 0.3% serum. The similar tubulin levels in all lanes demonstrate equal protein loading.
To explore the alterations in Rho family GTPase activities that occurred in BAE cells during the induction of podosome rosettes by TGF-β, pull-down experiments were performed. A small but significant increase in Cdc42 activity was induced by 30 min (1.40- ± 0.13-fold increase; P < 0.05), which persisted for several hours (1.20- ± 0.09-fold increase [P < 0.05] at 2 h) before being downregulated at 20 h of treatment (Fig. 4E). This initial phase was followed by the reappearance of active Cdc42 at 28 h. In contrast, RhoA activity progressively increased and was high when podosomes became visible, in agreement with the in situ RhoA activity assay (Fig. 4D).
Smad proteins are specific mediators of TGF-β signals. Western blots carried out with specific phospho-Smad antibodies revealed that both families of R-Smad, Smad2/3 and Smad1/5, were rapidly phosphorylated in response to TGF-β, strongly suggesting that TGF-β signals were transduced through both Alk1 and Alk5 in our model (Fig. 4F). The phosphorylation of Smad1/5 (Alk1) was more robust than that of Smad2/3 (Alk5). Furthermore, the addition of cycloheximide during TGF-β stimulation prevented podosome formation (data not shown), indicating that Smad-regulated transcription could be required for podosome formation in response to TGF-β. However, expression levels of key proteins involved in podosome formation, such as Arp3, gelsolin, cortactin, and RhoGTPases, were not significantly regulated (see Fig. S2 in the supplemental material). Taken together, our data show that Cdc42/RhoA, Src, PI3K, and Smad proteins are regulated in response to TGF-β and required for podosome formation.
MMP activity is associated with podosomes in endothelial cells.
Both TGF-β and members of the Rho family of GTPases have been shown to regulate the expression of MMPs, suggesting that TGF-β may regulate MMP activity during podosome formation in endothelial cells. Transmembrane MT1-MMP (39) is found at podosomes in osteoclasts and endothelial cells (43, 47), whereas the presence of membrane-associated MMP9 is less well established (15, 43). In resting cells, MT1-MMP staining showed a diffuse pattern throughout the cell surface. MMP9 staining was low and cytosolic (nuclear staining was also observed). Upon TGF-β treatment, MT1-MMP became colocalized with podosomes in BAE cells (Fig. 5A). MMP9 was also partly recruited to these sites, but the enzyme was detected throughout the cell body as well (Fig. 5A). Concentrated serum-free supernatants were analyzed in gelatin zymography assays to assess secreted levels of MMP2, an MMP activated by MT1-MMP in complex with αVβ3 integrin, and MMP9. Figure 5B shows that MMPs were secreted as latent proenzymes: pro-MMP2 and pro-MMP9 were identified by their molecular masses of 72 and 92 kDa, respectively. A selective increase in MMP9 expression was observed after 36 h of TGF-β treatment, whereas the expression of the 72-kDa protein, MMP2, remained essentially unchanged. To determine if the expression of MMP9 could be uncoupled from podosome formation, the same experiment was carried out in the presence of siRNAs designed to knock down the expression of proteins critically involved in endothelial podosome assembly, including Smad4 and Cdc42. siRNA against Smad4 prevented MMP9 expression and podosome formation (Fig. 5C). In contrast, in the absence of Cdc42 and podosome formation, MMP9 was induced normally in response to TGF-β (Fig. 5C). These results indicate that MMP9 induction can be uncoupled from podosome formation in the TGF-β response.
FIG. 5.
MMP9 and MT1-MMP are regulated by TGF-β and localize at TGF-β-induced podosomes. (A) Fluorescence staining of BAE cells for MT1-MMP and MMP9 subcellular localization, determined together with F-actin (MMP, green; F-actin, red; nucleus, blue). Bar, 10 μm. (B) Serum-starved cells were stimulated for various times with TGF-β, and supernatants were subjected to gelatinolytic analysis by zymography. The migration of pro- and active forms of MMP9 and MMP2 is shown. (C) Serum-starved cells transfected with the indicated siRNAs were stimulated for 36 h with TGF-β, and supernatants were subjected to gelatinolytic analysis by zymography.
To determine if proteolytic activity was associated with the podosomal structures, gelatin degradation was investigated in situ by plating cells on a fluorescent matrix made of gelatin conjugated with a fluorescent dye (FITC). Substrate degradation was visualized as punctate areas devoid of staining that colocalized to podosomes and corresponded in size to individual podosomes in the rings. As can be seen in Fig. 6A (upper panels), rosettes and the dark circles formed by degradation of the gelatin-FITC substrate were superimposable, reflecting localized proteolytic degradation of the extracellular matrix. Other areas of degradation did not match the podosome rosettes, likely due to turnover of the rosettes and the motility of the cells (Fig. 6A, lower panels). To determine if the proteolytic activities could be ascribed to MMPs recruited to podosomes, these experiments were carried out in the presence of pharmacological inhibitors of podosome formation or specific inhibitors of MMP activity. PP1/PP2 and LY294002 fully prevented local matrix degradation, as expected (data not shown). The synthetic metalloproteinase inhibitor GM6001, which targets a restricted subset of metalloproteases (MMP1, -2, -3, -8, -9, and -14 [MT1-MMP] [52]), completely ablated proteolytic degradation of the extracellular matrix (Fig. 6B), leaving podosome formation unaltered. The transfection of siRNA targeting MT1-MMP also produced a total inhibition of proteolytic degradation, indicating that MT1-MMP plays a major role in this process. Zymograms showed that silencing of MT1-MMP did not reduce pro-MMP9 expression (Fig. 6C). Western analyses confirmed that MT1-MMP expression was efficiently suppressed by MT1-MMP siRNA transfection (Fig. 6D). In addition, they revealed a strong upregulation of MT1-MMP in response to TGF-β stimulation. Taking the zymography and matrix degradation assays together, our results show that proteolytic activity can be selectively inhibited by MMP inhibitors without altering podosome formation. In contrast, the disruption of podosomes always leads to the inhibition of local matrix degradation.
FIG. 6.
Proteolytic activities associated with TGF-β-induced podosome rosettes are dependent on MT1-MMP expression and activity. BAE cells were seeded on glass coverslips coated with cross-linked FITC-labeled gelatin and were either left untreated or treated with TGF-β for 20 h. Fluorescence staining for F-actin to visualize podosome rosettes was then performed. (A) Degradation areas were visualized as dark holes in the FITC-labeled gelatin matrix. Merging F-actin with FITC-gelatin images revealed the overlap between podosome structures and matrix degradation areas. Bar, 10 μm. (B) Cells were prepared as described above, and MMP inhibition was initiated 1 h before TGF-β stimulation by adding either the synthetic broad-spectrum MMP inhibitor GM6001 (5 μM) or its inactive derivative (negative control), NCGM6001 (5 μM). MT1-MMP expression was inhibited by siRNA transfection (50 nM) before plating of cells on FITC-labeled gelatin-coated coverslips. Cells with residual podosome rosette formation after pharmacological inhibition or siRNA transfection were recorded, and results are presented as percentages of the control response obtained in the presence of TGF-β without inhibitory treatment (taken as 100%). (C) Serum-starved cells transfected with the indicated siRNAs were stimulated for 36 h with TGF-β, and supernatants were subjected to gelatinolytic analysis by zymography. (D) Western blot analysis of MT1-MMP expression in whole-cell lysates prepared from the cultures used for panel C.
BAE cells bearing podosomes are invasive cells.
Collagen proteolysis is essential for endothelial cell invasiveness (49), and the presence of MT1-MMP at endothelial podosomes is consistent with a role for this enzyme in this process. TGF-β promotes the formation of tube-like structures in collagen gels (32, 35). To explore the invasive potential conferred to BAE cells by podosomes, cells were plated on top of collagen gels and treated with TGF-β for 48 h (a period allowing podosome formation in cells attached to collagen-coated coverslips) to assess endothelial tube-like structures in an in vitro angiogenic assay (36). Quantitative measurements of endothelial sprouting are presented in Fig. 7A, which shows that TGF-β promoted the formation of capillary-like structures at 48 h. When the signals emanating from TGF-β receptors were blocked with SB431542, the process of tube-like formation was completely inhibited. Further downstream, when the activity or expression of MT1-MMP was blocked with GM6001 or siRNA against MT1-MMP, respectively, invasion was equally suppressed (Fig. 7A).
FIG. 7.
BAE cells bearing podosomes are invasive cells. (A) BAE cells were seeded atop a collagen I gel in a 96-well plate and treated or not treated with GM6001 (5 μM), NCGM6001 (5 μM), or SB431542 (5 μM), followed by TGF-β stimulation. Alternatively, cells transfected with control or MT1-MMP siRNA were seeded atop the gel 24 h after the last siRNA transfection and then treated with TGF-β. Invasion into collagen gels was quantified by counting the number of sprouts per field at a ×6 magnification by phase-contrast microscopy after 48 h, and the results are presented as described in the legend to Fig. 3. (B) BAE cells were seeded atop a collagen gel poured into transwell filters fitted with a microporous membrane. Cells were stimulated with TGF-β for 20 h and then processed for fluorescence staining for F-actin (red) and vinculin (green). Confocal sections (0.5 μm) were taken from the top of the cell to the bottom of the microporous membrane, and orthogonal z sections from the red and green axes on the x-y view are shown. Arrows indicate invasive protrusions from the podosome rosettes inside the pores. Bar, 10 μm.
To correlate the presence of podosomes with the ability of TGF-β-treated cells to invade the collagen matrix, experiments were designed to visualize endothelial cells bearing podosomes inside collagen gels. To this end, cells were plated on top of collagen gels in a transwell device and treated for 24 h to induce the rosettes of podosomes and allow matrix invasion. The data presented in Fig. 7B (also see Fig. S3 in the supplemental material) show confocal sections of cells exhibiting podosomes on top of the porous membrane. When podosomes were formed on flat membrane surfaces, they appeared with their usual actin/vinculin configuration, as routinely seen on collagen-coated glass coverslips. In contrast, at pore locations, the podosomes forming the rosettes appeared to coalesce to form an invading tubular structure protruding through the pore of the membrane beneath the plane of the cells. This technical approach revealed that depending on the matrix configuration, podosomes can organize themselves into a distinct conformation reminiscent of invasive structures described for transformed cells (8, 40, 48).
DISCUSSION
The initial observation which gave rise to this study was the formation of podosomes in aortic endothelial cells expressing a constitutive form of Cdc42 (37). The data presented herein demonstrate that TGF-β can induce the formation of podosomes in endothelial cells of aortic origin. The organization of podosomes into rosettes in TGF-β-treated cells presents some distinctive features. First, podosomes do not form as rapidly as might be anticipated from the findings in other cellular models where protein synthesis is not required (54). Second, when podosomes appear 6 h after TGF-β stimulation, they arise directly organized in ring-shaped clusters. Interestingly, in serum-starved cultures, the assembly of similar rosettes in Src-transformed BHK cells takes at least 3 h after serum readdition (54). From these observations, it is tempting to speculate that in the TGF-β response, the spatial organization of podosomes is the rate-limiting step, not the assembly of individual podosomes. Another possibility is that a critical component for podosome assembly is synthesized in response to TGF-β. A protein like Tsk5/Fish is likely to fulfil this role, as in the Src/fibroblastic model, where podosomes induced in response to active Src will not form unless Tsk5/Fish is expressed (48).
The involvement of RhoGTPases in podosome formation has been demonstrated in all podosome models, with differences in the nature of the GTPase involved. While RhoA plays a predominant role in osteoclasts (12), Cdc42 is the key player in macrophage (31) and immature dendritic cell (9) models. The experiments depicted herein establish that Cdc42 and RhoA are both important in the aortic endothelial cell model. In BAE cells, TGF-β-induced podosomes are comprised of the same components as those formed in response to the active form of Cdc42 in porcine aortic endothelial cells (37). However, they present a distinct spatial organization, i.e., rosettes of podosomes versus individual podosomes. Exogenously expressed Cdc42 localizes to podosomes in V12Cdc42-expressing cells, whereas endogenous Cdc42 is required but not detected in TGF-β-induced podosome rosettes, suggesting that the rapid downregulation of Cdc42 which follows its activation or the proper cycling of Cdc42 might be involved in podosome patterning. Alternatively, TGF-β, in addition to the activation of Cdc42, could provide other signals which contribute to the spatial organization of podosomes. Cdc42 activity was found to be instrumental in podosome formation, and its downregulation at some point of the process may seem paradoxical. The recent demonstration that the phosphorylation/dephosphorylation of WASP/N-WASP effector proteins provides a mechanism of molecular memory may explain this observation (56). TGF-β quickly regulates Cdc42 but slowly increases RhoA activity, whereas ectopic expression of active Cdc42 in porcine aortic endothelial cells led to a sustained Cdc42 signal and to inhibition of RhoA (37). The increase in RhoA activity is likely a critical step for podosome ring assembly. Active RhoA was found at podosomes, and inhibiting RhoA prevented podosome formation. Our results are in agreement with those obtained by Berdeaux et al., who recently showed that Rho function is specifically required for podosome integrity, patterning, and function and that active Rho localizes to podosome rosettes in Src-transformed cells (4). Thus, upon TGF-β signaling in BAE cells, the events culminating in the formation of the actin rings are controlled by integration of the activities of Cdc42 and RhoA, each contributing in its own way to the process of actin ring formation. Src and PI3K have also been shown to play a central role in podosome assembly in osteoclasts (11), and inhibiting these activities disrupted podosome formation in endothelial cells. In osteoclasts, RhoA regulates podosome formation through PI3K activation (12), and the same mechanism could apply in endothelial cells. Further studies will determine the interplay between Src, PI3K, and RhoGTPases in this model.
TGF-β effects on endothelial cells appear to be highly dependent on the cellular context. Goumans and collaborators recently showed that the ratio of Alk1 and Alk5 receptors might explain, at least in part, why TGF-β effects are so diverse (21, 22, 28). The present report describes a novel role of TGF-β in endothelial cells in inducing podosome formation. Our data clearly show that Smad proteins are critically involved in this process, as silencing of Smad4 was inhibitory for podosome formation. The observed increase in Smad1/5 phosphorylation upon TGF-β stimulation suggests that BAE cells express and signal through Alk1. In addition to bovine cells, TGF-β was also found to induce podosomes in aortic endothelial cells of human (this study) and mouse (data not shown) origin but not in venous endothelial cells (C. Varon, unpublished data), suggesting that the ability of endothelial cells to form podosomes in response to TGF-β is dependent on the nature of the vascular beds. Consistent with this hypothesis, Alk1 is predominantly expressed in the arterial endothelium during development as well as during the process of arterial remodeling (50, 51).
A common feature of cells harboring podosomes, such as monocyte-derived cells and transformed fibroblasts, is their unique ability to travel across anatomical boundaries such as basement membranes. Our experiments were performed with type I collagen, a component of the interstitial extracellular matrix which is essential for endothelial cell invasiveness (49), and the finding that endothelial cell podosomes are endowed with matrix degradation activities supports their predicted role in invading tissues. TGF-β upregulates MT1-MMP, and its enzymatic activity appeared to be compulsory for collagen degradation and cellular invasion. MT1-MMP1 expression increased with TGF-β treatment, and the protein was redistributed to nascent podosomes. MMP9 was induced and partially localized in podosomes. Therefore, podosomes act as sites for concentrating MMPs for spatially restricted extracellular matrix protein degradation. Whereas MT1-MMP is brought to and retained at podosomes through its transmembrane domain, secreted MMP9 is likely recruited through binding to podosomal components, such as αVβ3 integrin localized at podosomes. By concentrating enzymatic activities, podosomes may pave a restricted path just beneath the endothelial cell, and rosettes may create the necessary space for endothelial sprouting. In this way, podosomes preclude diffuse degradation of the matrix, which would occur in the case of secreted MMPs. As shown in Fig. 6, degradation of the gelatin substrate is superimposable with the rosettes, a situation which suggests that the arrangement of individual podosomes into rosettes is rather stable. Consequently, matrix degradation is likely to be more efficient than that achieved by short-lived free podosomes, which are often associated with dynamic cell behavior in other models. Thus, the podosome rosette may represent a cellular device that allows arterial cell invasion. Such structures could be visualized in the transwell experiment described here, and the invasive behavior of these cells was further demonstrated in an in vitro angiogenesis assay, where TGF-β induced cells to reorganize into tube-like structures which penetrated collagen gels. Interestingly, podosomes have now been described as present in human endothelial cells isolated from umbilical veins and appear to be regulated by VEGF stimulation (43). VEGF is the major angiogenic factor that drives neovascularization through capillary tube formation from preexisting networks. It is tempting to speculate that TGF-β and VEGF regulate podosome formation, fulfilling analogous roles in the context of distinct vascular beds.
Our results show that podosomes can be formed independently of MT1-MMP and MMP9 expression or activity (this study; Varon, unpublished results). However, endothelial sprouting was completely inhibited by silencing MT1-MMP. The predominant role of MT1-MMP in collagen-mediated proteolysis associated with TGF-β-induced podosomes fits with the key role of MT1-MMP in collagen invasion described in vitro and in vivo (24). Interestingly, MMP9 is known to regulate the migration of smooth muscle cells and geometric arterial remodeling (14, 19) and could thus act in a paracrine manner in the vessel. These results suggest a scenario whereby podosomes form in endothelial cells in response to TGF-β in an adaptive response of the endothelium to restricted blood flow. This question is presently under investigation in our laboratory.
Supplementary Material
Acknowledgments
This work was supported by grants from INSERM, University of Bordeaux 1 and 2, the Association pour la Recherche sur le Cancer (grant 3549), La Ligue contre le Cancer (Comité Régionaux des Charentes et de la Dordogne), and Fondation de France. C.V. and F.T. are recipients of fellowships from Association pour la Recherche sur le Cancer and from Ligue Nationale contre le Cancer and Région Aquitaine, respectively.
We thank D. Cantrell (University of Dundee, United Kingdom) for Myc, phosphotyrosine, and p85α antibodies, C. Chaponnier (University of Geneva, Switzerland) for antibodies against gelsolin, M. Welch (University of California, Berkeley) for anti-Arp3, M. McNiven for anti-dynamin 2, M. Way (Cancer Research UK, London, United Kingdom) for anti-N-WASP, and D. Stewart (NCI/NIH, Bethesda, Md.) for the WIP-Myc construct. Jeff Rubin (NCI/CCR/LCMB, Bethesda, Md.) is acknowledged for the kind gift of sFRP1 and Wnt3a. We are also grateful to C. Goudonnet for help with the experiments, M. J. Goumans and F. Lebrin for helpful discussions, and K. Mizutani and T. Takenawa (Institute of Medical Science, University of Tokyo, Japan) for help with degradation assays.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allavena, P., C. Paganin, I. Martin-Padura, G. Peri, M. Gaboli, E. Dejana, P. C. Marchisio, and A. Mantovani. 1991. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J. Exp. Med. 173:439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonov, A. S., M. A. Nikolaeva, T. S. Klueva, A. Romanov Yu, V. R. Babaev, V. B. Bystrevskaya, N. A. Perov, V. S. Repin, and V. N. Smirnov. 1986. Primary culture of endothelial cells from atherosclerotic human aorta. 1. Identification, morphological and ultrastructural characteristics of two endothelial cell subpopulations. Atherosclerosis 59:1-19. [DOI] [PubMed] [Google Scholar]
- 3.Bakin, A. V., A. K. Tomlinson, N. A. Bhowmick, H. L. Moses, and C. L. Arteaga. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275:36803-36810. [DOI] [PubMed] [Google Scholar]
- 4.Berdeaux, R. L., B. Diaz, L. Kim, and G. S. Martin. 2004. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J. Cell Biol. 166:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhowmick, N. A., M. Ghiassi, A. Bakin, M. Aakre, C. A. Lundquist, M. E. Engel, C. L. Arteaga, and H. L. Moses. 2001. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden, E. T., P. J. Coopman, and S. C. Mueller. 2001. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 63:613-627. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, P. C., A. M. Montgomery, M. Rosenfeld, R. A. Reisfeld, T. Hu, G. Klier, and D. A. Cheresh. 1994. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157-1164. [DOI] [PubMed] [Google Scholar]
- 8.Buccione, R., J. D. Orth, and M. A. McNiven. 2004. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5:647-657. [DOI] [PubMed] [Google Scholar]
- 9.Burns, S., A. J. Thrasher, M. P. Blundell, L. Machesky, and G. E. Jones. 2001. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 98:1142-1149. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H., A. L. Lau, and M. M. Matzuk. 2001. Studying TGF-beta superfamily signaling by knockouts and knockins. Mol. Cell. Endocrinol. 180:39-46. [DOI] [PubMed] [Google Scholar]
- 11.Chellaiah, M. A., R. S. Biswas, D. Yuen, U. M. Alvarez, and K. A. Hruska. 2001. Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J. Biol. Chem. 276:47434-47444. [DOI] [PubMed] [Google Scholar]
- 12.Chellaiah, M. A., N. Soga, S. Swanson, S. McAllister, U. Alvarez, D. Wang, S. F. Dowdy, and K. A. Hruska. 2000. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275:11993-12002. [DOI] [PubMed] [Google Scholar]
- 13.Davies, W. A., and T. P. Stossel. 1977. Peripheral hyaline blebs (podosomes) of macrophages. J. Cell Biol. 75:941-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defawe, O. D., R. D. Kenagy, C. Choi, S. Y. Wan, C. Deroanne, B. Nusgens, N. Sakalihasan, A. Colige, and A. W. Clowes. 2005. MMP-9 regulates both positively and negatively collagen gel contraction: a nonproteolytic function of MMP-9. Cardiovasc. Res. 66:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaisse, J. M., M. T. Engsig, V. Everts, M. del Carmen Ovejero, M. Ferreras, L. Lund, T. H. Vu, Z. Werb, B. Winding, A. Lochter, M. A. Karsdal, T. Troen, T. Kirkegaard, T. Lenhard, A. M. Heegaard, L. Neff, R. Baron, and N. T. Foged. 2000. Proteinases in bone resorption: obvious and less obvious roles. Clin. Chim. Acta 291:223-234. [DOI] [PubMed] [Google Scholar]
- 16.Derynck, R., R. J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29:117-129. [DOI] [PubMed] [Google Scholar]
- 17.Edlund, S., M. Landstrom, C. H. Heldin, and P. Aspenstrom. 2004. Smad7 is required for TGF-beta-induced activation of the small GTPase Cdc42. J. Cell Sci. 117:1835-1847. [DOI] [PubMed] [Google Scholar]
- 18.Edlund, S., M. Landstrom, C. H. Heldin, and P. Aspenstrom. 2002. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell 13:902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galis, Z. S., C. Johnson, D. Godin, R. Magid, J. M. Shipley, R. M. Senior, and E. Ivan. 2002. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 91:852-859. [DOI] [PubMed] [Google Scholar]
- 20.Gavazzi, I., M. V. Nermut, and P. C. Marchisio. 1989. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells. J. Cell Sci. 94:85-99. [DOI] [PubMed] [Google Scholar]
- 21.Goumans, M. J., F. Lebrin, and G. Valdimarsdottir. 2003. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc. Med. 13:301-307. [DOI] [PubMed] [Google Scholar]
- 22.Goumans, M. J., G. Valdimarsdottir, S. Itoh, F. Lebrin, J. Larsson, C. Mummery, S. Karlsson, and P. ten Dijke. 2003. Activin receptor-like kinase (ALK) 1 is an antagonistic mediator of lateral TGF-beta/ALK5 signaling. Mol. Cell 12:817-828. [DOI] [PubMed] [Google Scholar]
- 23.Goumans, M. J., A. Zwijsen, M. A. van Rooijen, D. Huylebroeck, B. A. Roelen, and C. L. Mummery. 1999. Transforming growth factor-beta signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development 126:3473-3483. [DOI] [PubMed] [Google Scholar]
- 24.Hotary, K. B., E. D. Allen, P. C. Brooks, N. S. Datta, M. W. Long, and S. J. Weiss. 2003. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114:33-45. [DOI] [PubMed] [Google Scholar]
- 25.Johansson, M. W., M. H. Lye, S. R. Barthel, A. K. Duffy, D. S. Annis, and D. F. Mosher. 2004. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am. J. Respir. Cell Mol. Biol. 31:413-422. [DOI] [PubMed] [Google Scholar]
- 26.Kim, J. T., and C. K. Joo. 2002. Involvement of cell-cell interactions in the rapid stimulation of Cas tyrosine phosphorylation and Src kinase activity by transforming growth factor-beta 1. J. Biol. Chem. 277:31938-31948. [DOI] [PubMed] [Google Scholar]
- 27.Larsson, J., M. J. Goumans, L. J. Sjostrand, M. A. van Rooijen, D. Ward, P. Leveen, X. Xu, P. ten Dijke, C. L. Mummery, and S. Karlsson. 2001. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 20:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebrin, F., M. Deckers, P. Bertolino, and P. Ten Dijke. 2005. TGF-beta receptor function in the endothelium. Cardiovasc. Res. 65:599-608. [DOI] [PubMed] [Google Scholar]
- 29.Linder, S., and M. Aepfelbacher. 2003. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 13:376-385. [DOI] [PubMed] [Google Scholar]
- 30.Linder, S., K. Hufner, U. Wintergerst, and M. Aepfelbacher. 2000. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 113:4165-4176. [DOI] [PubMed] [Google Scholar]
- 31.Linder, S., D. Nelson, M. Weiss, and M. Aepfelbacher. 1999. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl. Acad. Sci. USA 96:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madri, J. A., B. M. Pratt, and A. M. Tucker. 1988. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 106:1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melder, R. J., E. R. Walker, R. B. Herberman, and T. L. Whiteside. 1990. Surface characteristics, morphology, and ultrastructure of human adherent lymphokine-activated killer cells. J. Leukoc. Biol. 48:163-173. [DOI] [PubMed] [Google Scholar]
- 34.Meriane, M., S. Charrasse, F. Comunale, A. Mery, P. Fort, P. Roux, and C. Gauthier-Rouviere. 2002. Participation of small GTPases Rac1 and Cdc42Hs in myoblast transformation. Oncogene 21:2901-2907. [DOI] [PubMed] [Google Scholar]
- 35.Merwin, J. R., J. M. Anderson, O. Kocher, C. M. Van Itallie, and J. A. Madri. 1990. Transforming growth factor beta 1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J. Cell Physiol. 142:117-128. [DOI] [PubMed] [Google Scholar]
- 36.Montesano, R., J. D. Vassalli, A. Baird, R. Guillemin, and L. Orci. 1986. Basic fibroblast growth factor induces angiogenesis in vitro. Proc. Natl. Acad. Sci. USA 83:7297-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau, V., F. Tatin, C. Varon, and E. Genot. 2003. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol. Cell. Biol. 23:6809-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mott, J. D., and Z. Werb. 2004. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakahara, H., L. Howard, E. W. Thompson, H. Sato, M. Seiki, Y. Yeh, and W. T. Chen. 1997. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl. Acad. Sci. USA 94:7959-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakahara, H., T. Otani, T. Sasaki, Y. Miura, Y. Takai, and M. Kogo. 2003. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells 8:1019-1027. [DOI] [PubMed] [Google Scholar]
- 41.Obergfell, A., K. Eto, A. Mocsai, C. Buensuceso, S. L. Moores, J. S. Brugge, C. A. Lowell, and S. J. Shattil. 2002. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 157:265-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oreffo, R. O., A. Teti, J. T. Triffitt, M. J. Francis, A. Carano, and A. Z. Zallone. 1988. Effect of vitamin A on bone resorption: evidence for direct stimulation of isolated chicken osteoclasts by retinol and retinoic acid. J. Bone Miner. Res. 3:203-210. [DOI] [PubMed] [Google Scholar]
- 43.Osiak, A. E., G. Zenner, and S. Linder. 2005. Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp. Cell Res. 307:342-353. [DOI] [PubMed] [Google Scholar]
- 44.Pfaff, M., and P. Jurdic. 2001. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J. Cell Sci. 114:2775-2786. [DOI] [PubMed] [Google Scholar]
- 45.Rifkin, D. B., S. Kojima, M. Abe, and J. G. Harpel. 1993. TGF-beta: structure, function, and formation. Thromb. Haemost. 70:177-179. [PubMed] [Google Scholar]
- 46.Sander, E. E., J. P. ten Klooster, S. van Delft, R. A. van der Kammen, and J. G. Collard. 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato, T., M. del Carmen Ovejero, P. Hou, A. M. Heegaard, M. Kumegawa, N. T. Foged, and J. M. Delaisse. 1997. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J. Cell Sci. 110:589-596. [DOI] [PubMed] [Google Scholar]
- 48.Seals, D. F., E. F. Azucena, Jr., I. Pass, L. Tesfay, R. Gordon, M. Woodrow, J. H. Resau, and S. A. Courtneidge. 2005. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7:155-165. [DOI] [PubMed] [Google Scholar]
- 49.Seandel, M., K. Noack-Kunnmann, D. Zhu, R. T. Aimes, and J. P. Quigley. 2001. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood 97:2323-2332. [DOI] [PubMed] [Google Scholar]
- 50.Seki, T., K. H. Hong, J. Yun, S. J. Kim, and S. P. Oh. 2004. Isolation of a regulatory region of activin receptor-like kinase 1 gene sufficient for arterial endothelium-specific expression. Circ. Res. 94:e72-e77. [DOI] [PubMed] [Google Scholar]
- 51.Seki, T., J. Yun, and S. P. Oh. 2003. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 93:682-689. [DOI] [PubMed] [Google Scholar]
- 52.Stawowy, P., C. Margeta, H. Kallisch, N. G. Seidah, M. Chretien, E. Fleck, and K. Graf. 2004. Regulation of matrix metalloproteinase MT1-MMP/MMP-2 in cardiac fibroblasts by TGF-beta1 involves furin-convertase. Cardiovasc. Res. 63:87-97. [DOI] [PubMed] [Google Scholar]
- 53.Stetler-Stevenson, M., A. Mansoor, M. Lim, P. Fukushima, J. Kehrl, G. Marti, K. Ptaszynski, J. Wang, and W. G. Stetler-Stevenson. 1997. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood 89:1708-1715. [PubMed] [Google Scholar]
- 54.Tarone, G., D. Cirillo, F. G. Giancotti, P. M. Comoglio, and P. C. Marchisio. 1985. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 159:141-157. [DOI] [PubMed] [Google Scholar]
- 55.Thiery, J. P. 2003. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15:740-746. [DOI] [PubMed] [Google Scholar]
- 56.Torres, E., and M. K. Rosen. 2003. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell 11:1215-1227. [DOI] [PubMed] [Google Scholar]
- 57.van Royen, N., I. Hoefer, I. Buschmann, M. Heil, S. Kostin, E. Deindl, S. Vogel, T. Korff, H. Augustin, C. Bode, J. J. Piek, and W. Schaper. 2002. Exogenous application of transforming growth factor beta 1 stimulates arteriogenesis in the peripheral circulation. FASEB J. 16:432-434. [DOI] [PubMed] [Google Scholar]
- 58.Vouret-Craviari, V., E. Boulter, D. Grall, C. Matthews, and E. Van Obberghen-Schilling. 2004. ILK is required for the assembly of matrix-forming adhesions and capillary morphogenesis in endothelial cells. J. Cell Sci. 117:4559-4569. [DOI] [PubMed] [Google Scholar]
- 59.Xu, C., S. Lee, C. Shu, H. Masuda, and C. K. Zarins. 2002. Expression of TGF-beta1 and beta3 but not apoptosis factors relates to flow-induced aortic enlargement. BMC Cardiovasc. Disord. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi, J. Y., I. Shin, and C. L. Arteaga. 2005. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 280:10870-10876. [DOI] [PubMed] [Google Scholar]
- 61.Zambonin-Zallone, A., A. Teti, A. Carano, and P. C. Marchisio. 1988. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J. Bone Miner. Res. 3:517-523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.