Abstract
Campylobacter jejuni 81-176 lipooligosaccharide (LOS) is composed of two covalently linked domains: lipid A, a hydrophobic anchor, and a nonrepeating core oligosaccharide, consisting of an inner and outer core region. We report the isolation and characterization of the deepest rough C. jejuni 81-176 mutant by insertional mutagenesis into the waaC gene, encoding heptosyltransferase I that catalyzes the transfer of the first l-glycero-d-manno-heptose residue to 3-deoxy-d-manno-octulosonic residue (Kdo)-lipid A. Tricine gel electrophoresis, followed by silver staining, showed that site-specific mutation in the waaC gene resulted in the expression of a severely truncated LOS compared to wild-type strain 81-176. Gas-liquid chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy showed that the waaC LOS species lacked all sugars distal to Kdo-lipid A. Parallel structural studies of the capsular polysaccharides of the wild-type strain 81-176 and waaC mutant revealed loss of the 3-O-methyl group in the waaC mutant. Complementation of the C. jejuni mutant by insertion of the wild-type C. jejuni waaC gene into a chromosomal locus resulted in LOS and capsular structures identical to those expressed in the parent strain. We also report here the presence of O-methyl phosphoramidate in wild-type strain 81-176 capsular polysaccharide.
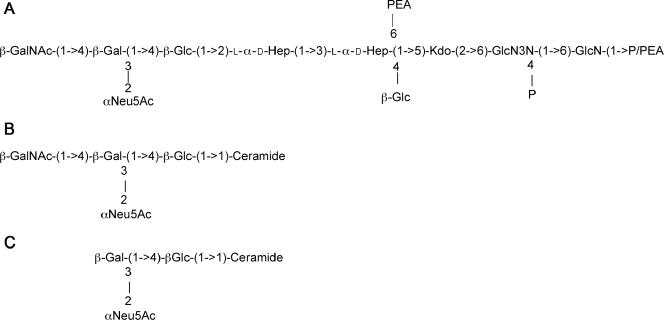
The food-borne pathogen Campylobacter jejuni is one of the principal causes of human gastroenteritis worldwide (6, 24, 25). In addition, Campylobacter infection is linked to the development of a serious neurological disorder, Guillain-Barré syndrome (GBS) (8, 24). It is believed that GBS is triggered as a result of molecular mimicry between the Campylobacter lipooligosaccharide (LOS) and the carbohydrate moiety of human gangliosides. The Campylobacter strain 81-176 LOS is an outer membrane glycolipid composed of two covalently linked domains: lipid A, a hydrophobic anchor, and a nonrepeating core oligosaccharide, consisting of an inner and outer core region. The carbohydrate structure of the outer core region of C. jejuni 81-176 has been shown to mimic primarily GM2 (Fig. 1) and GM3 (12, 17) human gangliosides, although minor forms of GD2 and GD1b ganglioside mimics have been reported (12). The variation between GM2 and GM3 mimicry is due to the presence of a phase-variable homopolymeric G tract in the cgtA gene, which encodes an N-acetylgalactosaminyltransferase (12). The C. jejuni 81-176 inner core consists of a single 3-deoxy-d-manno-octulosonic residue (Kdo) attached to lipid A and two l-glycero-d-manno-heptose (heptose) residues attached to Kdo. The inner core region also contains a glucose residue and phosphoethanolamine moiety attached to the first heptose residue (12).
FIG. 1.
(A) Core oligosaccharide of C. jejuni 81-176 (serogroups O:23/O:36) and the phosphorylated disaccharide backbone of lipid A (determined for strain CCUG 10936) (23). The structure of 81-176 LOS has been reported to vary between the structure shown in panel A, which mimics GM3 ganglioside (B), and a structure lacking the terminal GalNAc, which mimics GM2 ganglioside (C). This variation is due to slip strand mismatch repair of the cgtA gene, encoding a GalNAc transferase (12).
To better understand the function of the core LOS and its role in the development of bacterial gastroenteritis and GBS, we have initiated a study of the isolation of mutants involved in the biosynthesis of LOS from C. jejuni strain 81-176. WaaC (heptosyltransferase I) catalyzes the transfer of the first l-glycero-d-manno-heptose residue to the inner core oligosaccharide moiety of LOS (18, 29, 31, 32, 33). Although the enzymatic function of the product of waaC has been inferred by the ability of the C. jejuni gene to complement the corresponding mutation in Salmonella enterica serovar Typhimurium (18), mutation of waaC in C. jejuni has been suggested to be a lethal event (26). However, here we report the isolation and characterization of a waaC mutant of C. jejuni strain 81-176. The 81-176 waaC mutant produces a severely truncated LOS that is deficient in all sugars distal to Kdo-lipid A. Unexpectedly, mutation of waaC also resulted in a modified capsule polysaccharide (CPS) structure that lacked a 3-O-methyl (3-O-Me) group.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni strain 81-176 (Penner serotype) (23, 36) has been previously described (1-3, 9, 11, 15-17). C. jejuni strains were grown in Mueller-Hinton (MH) broth under microaerophilic conditions at 37°C. When necessary, medium was supplemented with kanamycin (30 μg/ml), ampicillin (100 μg/ml), or chloramphenicol (30 μg/ml).
DNA cloning and sequence analysis.
Two overlapping plasmids were identified in an ordered library of Sau3A1 partially digested 81-176 DNA cloned into λ-ZAPII that encoded part of the LOS locus. DNA sequencing was performed on a Perkin-Elmer Applied Biosystems model 3100 automated DNA sequencer. Custom primers were synthesized on a Perkin-Elmer Applied Biosystems model 292 DNA synthesizer.
Generation of the waaC mutant.
The waaC mutant was constructed using a Tn5-based in vitro transposition system (Epicenter, Madison, Wis.), in which the Cmr cassette from pRY109 (38) was cloned into pEZ::TN pMOD as previously described (10). The in vitro reaction was performed according to the manufacturer's instructions, with an Escherichia coli plasmid clone of the 81-176 LOS region as the target DNA. The reaction product was transformed into E. coli DH5α by electroporation. The plasmid DNAs from individual transformants were sequenced using primers that read out from within the Cmr cassette to determine the insertion point and the orientation within the gene. An insertion was selected in which the Cmr cassette had been inserted in the same orientation that the target gene had been transcribed to minimize polarity on downstream genes. Plasmids were used to transform C. jejuni 81-176, with selection on MH agar supplemented with chloramphenicol (15 μg/ml) (36). The successful mutation of waaC was verified by PCR, with primers bracketing the Cmr insertion point to confirm that the DNA had been inserted by a double crossover. The primers used to verify the waaC mutation had the following sequences: forward, 5′-CGTTTGTCAGCACTTGGTGAT-3′; and reverse, 5′-AAGTAAGCCTTTGGCAAGTTTGAA-3′.
Complementation of the 81-176 waaC mutant by insertion of a wild-type allele into arylsulfatase.
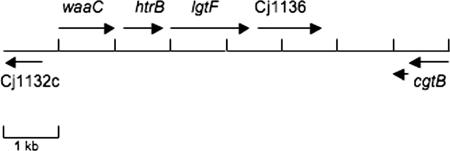
The waaC mutation was complemented by insertion of a wild-type allele into the arylsulfatase (astA) gene on the chromosome of 81-176, as previously described (9, 11). Inactivation of astA does not affect adherence or invasion in vitro or virulence in vivo (9, 11). The waaC gene was PCR amplified using the following primers: forward, 5′-CGGGATCCCCCACTTTGTTCTTTTTGCCAC-3′; and reverse, 5′-GGAATTCCGCTTTCGTTTTCTACGCAGTC-3′. The forward primer, which is found 178 bp from the translational start site of Cj1132, included a BamHI site, and the reverse primer, which bound 587 bp into the Cj1134 (htrB) gene, included an EcoRI site. The resulting 1,828-bp PCR product, which was presumed to include the promoter of waaC (Fig. 2), was digested with BamHI and EcoRI and cloned into BamHI-EcoRI-digested pBluescript. An aph3 cassette from pILL600 (19) was then cloned into the unique EcoRI site to generate pCPE2266. The waaC-aph3 construct was moved from pCPE2266 as an XbaI-XhoI fragment, blunted with Klenow I, and cloned into a unique EcoRV site within the 81-176 astA gene cloned in pYG660 (38) to generate pCPE2268. Plasmid pCPE2268 was used to electroporate 81-176 waaC::Cm to kanamycin resistance, and resulting colonies were screened for loss of AstA activity using a chromogenic substrate (39). A double crossover into astA was subsequently confirmed by PCR.
FIG. 2.
Schematic representation of the C. jejuni 81-176 chromosomal region encoding relevant LOS genes. The overall genomic organization is similar to that of class B loci described by Gilbert and colleagues and Parker et al. (7, 28). The ORF designated waaC showed 87% identity and 88% similarity to WaaC (Cj1133) from the C. jejuni genome strain NCTC11168. The cat transposon insertion that was used to generate the mutation was at 677 bp from the translational start of the 1,029-bp waaC gene. The second ORF encoded a predicted protein with 99% identity to HtrB, a lipid A biosynthesis lauroyl acyltransferase, from C. jejuni strain GB11. The third ORF encoded a protein that showed 88% identity and 94% similarity to a putative two-domain glycosyltransferase of C. jejuni NCTC11168 (Cj1135). The fourth ORF exhibited 75% identity and 86% similarity to a putative β-1,3-galactosyltransferase from C. jejuni NCTC1168 (Cj1136). The 81-176 homolog of cgtB was slip stranded out of frame as cloned. This gene encodes a galactosyltransferase that has been shown to undergo phase variation in another strain to convert GM2 to GM1 mimics (20). Between Cj1136 and the cgtB homolog, there was no ORF of >174 bp, but BLASTX analysis revealed homology to a β-1,4-GalNAc transferase, similar to what has been reported for other class B LOS loci (7, 28).
Extraction of lipooligosaccharide and preparation of core oligosaccharide.
LOS samples were prepared from whole-cell lysates that were subjected to complete digestion with proteinase K as described by Hitchcock and Brown (14). LOS extracts were separated on 16% Tricine gels (Invitrogen, Carlsbad, Calif.) and then silver stained (Bio-Rad, Hercules, Calif.). Crude LOS and capsule (CPS) for gas-liquid chromatography-mass spectrometry (GLC-MS) and 1H nuclear magnetic resonance (1H NMR) spectroscopy were isolated by hot phenol-water extraction (37). The water layer was dialyzed overnight with distilled water, concentrated, and applied to a column (1.5 m by 1.5 cm) of Bio-Gel P2 for purification. In both instances, one carbohydrate fraction was obtained at the void volume (CPS) and another at a lower-molecular-weight range (LOS), as detected by the phenol-sulfuric acid assay (5). The fractions were eluted with water, lyophilized, and assayed with 1D 1H and 1D 31P NMR spectroscopy.
Immunoblotting of capsular polysaccharides.
Since Campylobacter capsular polysaccharides cannot be visualized by silver staining (22), the immunoblotting procedure described by Bacon et al. (2) was used for detection. Proteinase K-digested whole-cell preparations of C. jejuni were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% SDS-PAGE) gels and immunoblotted with a rabbit polyclonal serum generated against formalin-treated whole cells of 81-176 (CPE1) at a final dilution of 1:500. The secondary antibody was goat anti-rabbit antiserum conjugated to alkaline phosphatase (Caltag, Burlingame, CA) at a dilution of 1:5,000. The immunoblot was detected with the 5-bromo-4-chloro-3-indolylphosphate (BCIP)/Nitro Blue Tetrazolium detection system (Bio-Rad).
Sugar composition analysis and NMR spectroscopy.
For sugar composition analysis, the monosaccharide components of CPSs and LOS were derivatized into the alditol acetate derivatives according to Sawardeker and Sloneker (30). For sugar linkage analysis, the CPS was methylated with methyl iodide in dimethyl sulfoxide and NaOH (4). The methylated core was hydrolyzed in 4 M trifluoroacetic acid at 100°C for 4 h, followed by reduction in H2O with NaBD4. The permethylated alditols were then acetylated in acetic anhydride (100°C for 2 h), with residual sodium acetate as the catalyst. The permethylated alditol acetate derivatives were then characterized by GLC (DB-5; 30 m, isothermally at 200°C) and GLC-MS (DB-5; 30 m, 190°C [60 min]). The NMR experiments were performed in D2O on a Bruker 400 MHz at 21°C, with the water resonance as a reference (δH 4.824) in 1H NMR experiments. Orthophosphoric acid was used as an external reference in 31P NMR experiments.
FAB-MS.
A fraction of the methylated sample was used for positive-ion fast atom bombardment-mass spectrometry (FAB-MS), which was carried out on a Jeol JMS-AX505H mass spectrometer with glycerol-thioglycerol (1:3) as the matrix. A 6-kV xenon beam was used to produce pseudomolecular ions that were accelerated to 3 kV, and their mass was analyzed.
Nucleotide sequence accession number.
The DNA sequence shown in Fig. 2 has been deposited in GenBank under accession number AY862985.
RESULTS
Isolation and characterization of the C. jejuni 81-176 waaC mutant.
The region of the 81-176 chromosome corresponding to Cj1132c through Cj1139c is shown schematically in Fig. 2. The genes from strain 81-176, which serotypes as both HS:23 and HS:36 (3), are most similar to the class B loci found in the serotype strains of HS:23 and HS:36 of a recently described LOS genotyping method (7, 28). One of the open reading frames (ORFs) encoded a predicted protein of 342 amino acids, with a predicted molecular mass of 61 kDa, that showed 88% identity to Cj1133 of C. jejuni NCTC11168, annotated as waaC or heptosyltransferase I, and 24% identity and 40% similarity to WaaC of Salmonella enterica serovar Typhimurium (32).
The C. jejuni 81-176 waaC gene was disrupted by insertional mutagenesis of cloned DNA in E. coli, as described in Materials and Methods. The position and orientation of the random insertions of the cat gene within the clone were determined by sequence analysis. One insertion that occurred 677 bp from the translational start site of waaC in a nonpolar orientation was selected to generate mutants in C. jejuni. Plasmid DNA from this cat insertion was used to electroporate C. jejuni 81-176 with selection for Cmr. Several Cmr transformants were confirmed by PCR in order to verify that the insert had integrated via a double crossover. The primers used (see Materials and Methods) resulted in an amplicon of 998 bp using 81-176 DNA as a template and an amplicon of approximately 2 kb using the waaC::cat mutant DNA as a template (data not shown). One such mutant was further characterized.
The LOS from the waaC mutant migrated more quickly on 16% Tricine gels than the parental strain 81-176 (Fig. 3, lane 2 versus 1), consistent with a defect in the synthesis of the inner core region of LOS. The presence of an LOS similar to that of the wild type was restored when the waaC mutant strain was complemented by insertion of a copy of the C. jejuni waaC gene into the arylsulfatase gene of the chromosome of the mutant (Fig. 3, lane 3) (9, 11, 39). Immunoblotting of the cores separated on SDS-PAGE gels using anti-81-176 whole-cell antibody also indicated reduced immunoreactivity of the truncated waaC core compared to that of the core of wild-type 81-176 (data not shown).
FIG. 3.
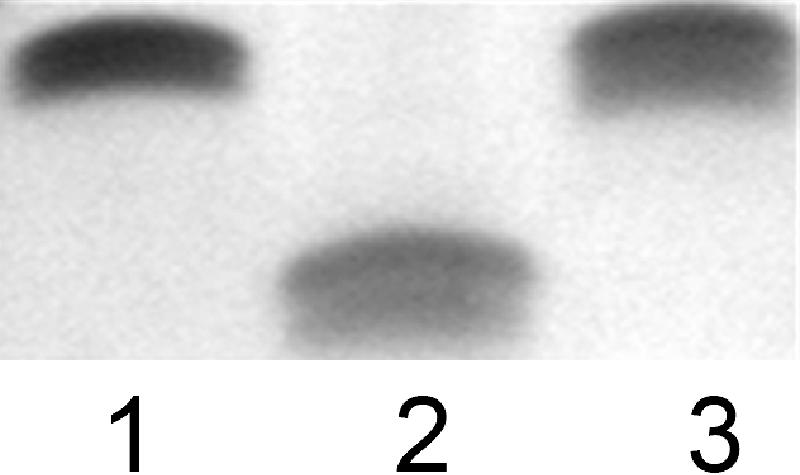
LOS core mobility. Proteinase K-digested whole-cell preparations were electrophoresed on 16% Tricine gels and silver stained. Lane 1, 81-176; lane 2, waaC mutant; and lane 3, complement of waaC mutant.
The core region of LOS plays an important role in the integrity of the outer membrane, and deep rough mutants have been shown to display sensitivity to hydrophobic antibiotics, detergents, and bile salts and have decreased levels of outer membrane proteins (33, 35). Indeed, the 81-176 waaC mutant showed greater sensitivity to antibiotics and detergents (data not shown) than the wild-type strain 81-176 and a waaF mutant of 81-176 (17). Furthermore, examination of the morphology of the waaC mutant by transmission electron microscopy revealed cells that were often shorter than those from the wild-type strain 81-176 (data not shown). However, there was no significant difference in growth of the waaC mutant, as determined by an optical density at 600 nm in MH broth, or motility in comparison to that of the wild-type strain 81-176 (data not shown).
GLC-MS and NMR spectroscopy of LOS from the C. jejuni 81-176 waaC mutant.
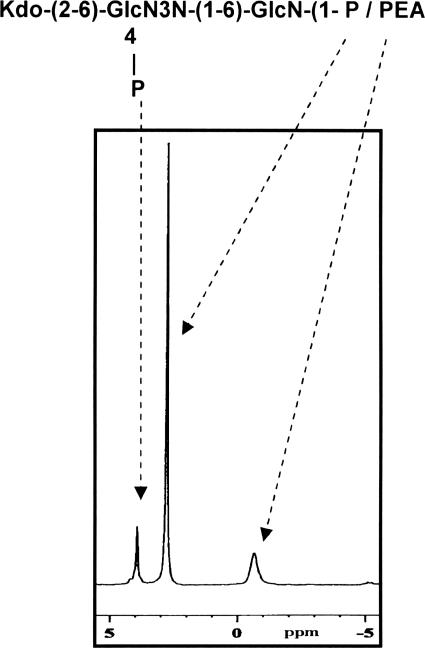
The cell surface carbohydrate material from the waaC mutant was extracted and analyzed by GLC-MS and NMR spectroscopy as described in Materials and Methods. Sugar analysis by the alditol acetate method on the truncated waaC LOS revealed the presence of only GlcNAc and GlcNAc3NAc from lipid A. No units representative of the core oligosaccharide (Fig. 1), such as Glc, Gal, Hep, and GalNAc, were detected. 1H NMR and 2D 1H-13C heteronuclear single quantum correlation of the truncated waaC LOS showed deoxy resonances at δH-3,3′ 1.80/2.02 and at δC-3 34.2, which confirmed the presence of 3-deoxy-d-manno-2-octulosonic acid (Kdo). The 31P NMR spectrum (Fig. 4) of the truncated waaC LOS showed three distinct resonances at δ 4.0 ppm for an ester-bound phosphomonoester (O-4 position of GlcN3N) (Fig. 1), at δ 2.7 for a glycosidic phosphomonoester (O-1 position of GlcN), and at δ −0.8 for a glycosidic phosphodiester (PEA) (Fig. 1). These values were in agreement with those previously reported for C. jejuni lipid A moieties (23).
FIG. 4.
31P NMR spectrum of the truncated waaC LOS showing phosphorus resonances for ester-bound phosphate and for glycosidic-linked phosphate residues.
Effect of the waaC mutation on capsule expression and structure.
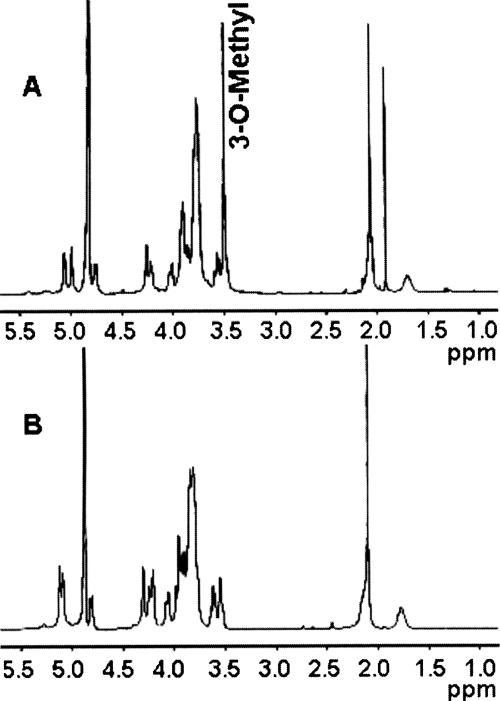
Proteinase K-digested whole cells of the wild-type strain 81-176 and the waaC mutant were immunoblotted with rabbit polyclonal antiserum to whole cells of 81-176 to detect capsular polysaccharides, as previously described (2). Figure 5 shows that there was a change in the immunoreactivity of the waaC mutant capsule (CPS) compared to that of the wild-type strain (lane 3 versus 2). Based on these results, we were interested in determining whether there were any changes in the structure of the waaC CPS compared to that of the wild type (Fig. 6) (27, 34). Sugar composition and linkage analysis (GLC-MS) and 1H NMR spectroscopy (Fig. 7) performed on the waaC CPS revealed a structure composed of a 3-substituted Gal and a 3-substituted GlcNAc, similar to that of the wild-type 81-176 CPS. In addition, the CPS of the wild-type strain was observed to be present as a 2-substituted 3-O-methyl-6-deoxy-altro-heptose while the C. jejuni 81-176 waaC CPS was present as a 2-substituted 6-deoxy-altro-heptose (Fig. 7), revealing the loss of the 3-O-methyl group from the 81-176 waaC CPS (Fig. 6). Complementation of the waaC mutant as mentioned earlier restored the LOS defect, and there was also partial complementation (65%) of the methyl defect in the capsule (data not shown).
FIG. 5.
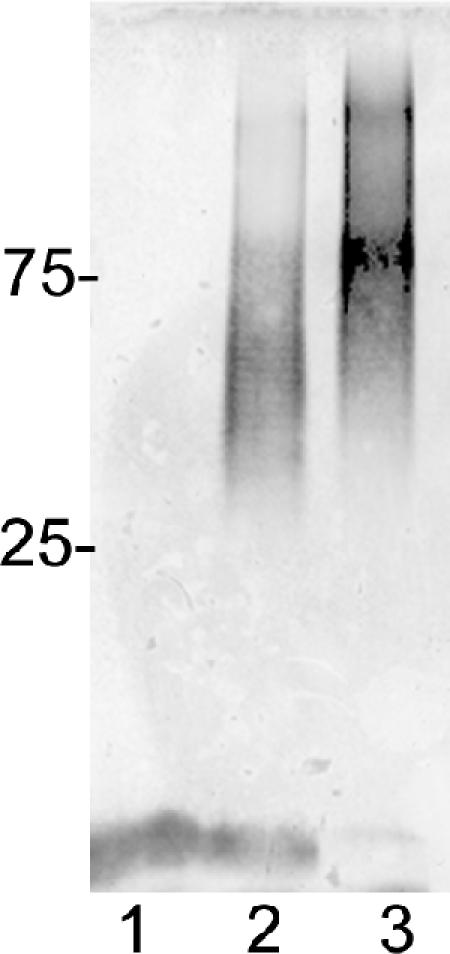
Immunoblot of C. jejuni waaC capsular material from a 12% SDS-PAGE gel. Lane 1, 81-176 kpsM mutant (2); lane 2, 81-176; and lane 3, 81-176 waaC mutant. The positions of protein markers in kDa are shown at the left. The LOS cores of the 81-176 kpsM mutant and the wild type are visible at the bottom of the immunoblot; the core of the waaC mutant is not visible.
FIG. 6.
The reported CPS structures of C. jejuni O:23/O:36 and 81-176 strains (32) and the 81-176 waaC mutant. Abbreviations: 6d-3-O-Me-altro-Hep, 6-deoxy-3-O-methyl-altro-heptose; 6d-altro-Hep, 6-deoxy-altro-heptose.
FIG. 7.
NMR analyses. (A) 1H NMR of C. jejuni 81-176 wild-type CPS (1% acetic acid treated) showing (i) the anomeric resonances at δ 5.06 for α-Gal, δ 4.99 for 3-O-Me-6-deoxy-α-altro-Hep, and δ 4.75 for β-GlcNAc, (ii) the methyl signal at δ 3.50 for 3-O-Me-6-deoxy-α-altro-Hep, (iii) the methyl resonance at δ 2.09 for 3-O-Me-6-deoxy-α-altro-Hep, and (iv) the deoxy resonance at δ 1.70 for 3-O-Me-6-deoxy-α-altro-Hep. (B) 1H NMR of C. jejuni 81-176 waaC CPS showing (i) the anomeric resonances at δ 5.12 for 6-deoxy-α-altro-Hep, δ 4.98 for α-Gal, and δ 4.75 for β-GlcNAc, (ii) the methyl signal at δ 2.09 for GlcNAc, and (iii) the deoxy resonance at δ 1.75 for GlcNAc. No 3-O-methyl signal (3-O-Me-6-deoxy-α-altro-Hep) was observed in the waaC CPS 1H NMR spectrum. Also of note, the α-anomeric signal of 6-deoxy-α-altro-Hep in the waaC CPS resonated at δ 5.12, whereas the α-anomeric signal of 3-O-Me-6-deoxy-α-altro-Hep in wild-type CPS resonated at δ 4.99. The peak at δ 1.9 in panel B represents contaminating sodium acetate. All the results described were assigned by 2D 1H-1H NMR spectroscopy (data not shown).
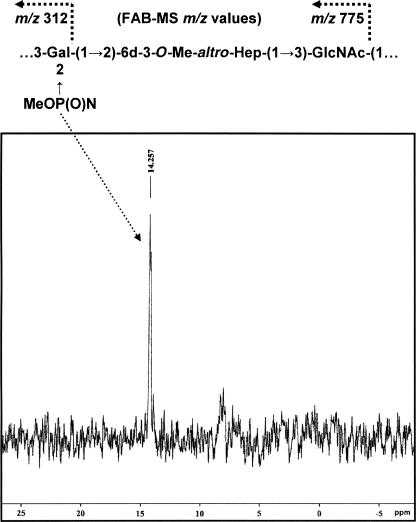
Similar to that described in our recent work (26), in addition to 3-substituted Gal, 2-substituted 6-deoxy-3-Me-altro-Hep, and 3-substituted GlcNAc, we also detected in this study by linkage analysis (GLC-MS) a quantity of 2,3-substituted Gal in wild-type 81-176 CPS (in this CPS preparation, the approximate molar ratio was 0.75:1:1:0.25, respectively) which we could not place within the framework of the reported CPS structure of serogroup HS:23/HS:36, which consists of trisaccharide-repeating blocks [(→3)-α-Gal-(1→2)-6-deoxy-3-Me-α-altro-Hep-(1→3)-β-GlcNAc-(1→)]. Smaller amounts of terminal and 2-substituted Gal were also detected. Knowing that McNally and coworkers (21) had recently shown that O-methyl phosphoramidate, CH3OP(O)NH2(OR), was present as a structural moiety in the C. jejuni HS:1 strain, we performed a 31P NMR experiment on wild-type 81-176 CPS to observe any phosphorous-containing residue. Figure 8 shows the 31P NMR spectrum of wild-type 81-176 CPS, obtained by hot water-phenol extraction, in that a resonance at 14.257 ppm can be observed and attributed to an O-methyl phosphoramidate unit comparable to that observed in the C. jejuni HS:1 strain (21). As stated above, linkage analysis revealed the presence of 2,3-disubstituted Gal and thus pointed to the fact that the O-methyl phosphoramidate may be attached at the O-2 position of Gal. A FAB-MS experiment yielded structural information which confirmed that the O-methyl phosphoramidate residue was indeed attached to the Gal unit, in that it yielded glycosyl oxonium ions of defined composition, emanating from the nonreducing end of the CPS, at m/z 312 for [MeOP(O)N→Gal+] (108 a.m.u. plus 204 a.m.u) and at m/z 775 for the complete trisaccharide repeat [MeOP(O)N→Gal→6-deoxy-3-Me-altro-Hep→GlcNAc+] (312 a.m.u. plus 218 a.m.u. plus 245 a.m.u.). As stated above, a small amount of 2-substituted Gal, representative of the nonreducing Gal carrying the O-methyl phosphoramidate, was also detected in the sugar linkage analysis. As expected, m/z ions not containing O-methyl phosphoramidate were also observed at m/z 219 for [Gal+] and at m/z 682 for [Gal→6-deoxy-3-Me-altro-Hep→GlcNAc+], in line with the previously reported C. jejuni HS:23/HS:36 CPS structure. We have yet to assign unambiguously the anomeric resonance that belongs to the 2,3-substituted Gal carrying the O-methyl phosphoramidate. Within the limits of detection, only traces of 2,3-substituted Gal were observed in the linkage analysis of waaC mutant CPS obtained from hot water-phenol extraction, and no O-methyl phosphoramidate was detected in the FAB-MS or 31P NMR spectrum of waaC mutant CPS, which pointed to the fact that this moiety may be present in very small amounts in waaC mutant CPS. Studies to evaluate the stability of O-methyl phosphoramidate during hot water-phenol extractions are being planned, but from the above results, it is clear that O-methyl phosphoramidate is to some extent stable during this extraction procedure.
FIG. 8.
The 31P NMR spectrum of C. jejuni wild-type 81-176 CPS showed a resonance at 14.257, attributable to an O-methyl phosphoramidate component, and the FAB-MS primary ions of the methylated wild-type 81-176 CPS indicated that O-methyl phosphoramidate is attached to the Gal unit in the CPS.
DISCUSSION
We report for the first time the isolation and characterization of a waaC mutant in C. jejuni. The waaC gene encodes a heptosyltransferase I that catalyzes the transfer of the first heptose to the core oligosaccharide of the LOS or lipopolysaccharide in gram-negative bacteria (30, 31). Earlier studies by Klena et al. (18) showed that the waaC gene from C. jejuni F38011 was able to complement a Salmonella enterica serovar Typhimurium waaC mutant, and we have also made this observation with the 81-176 gene using the same S. enterica serovar Typhimurium mutant (data not shown). Our studies show that the C. jejuni waaC mutant possessed a truncated LOS that migrated more quickly than that of the wild-type strain 81-176 (Fig. 3) and the 81-176 waaF mutant (17 and data not shown). The truncation of the LOS was confirmed by GLC-MS analyses and NMR spectroscopy, showing the loss of the first heptose residue to the core oligosaccharide and therefore the loss of all sugars distal to Kdo (Fig. 1) and core mobility and structure were fully restored in the complement (Fig. 3 and data not shown).
The inner core region of lipopolysaccharide or LOS has been shown to play an important role in stabilizing the outer membrane (13). Deep rough mutants have been shown to result in decreased levels of outer membrane proteins and hypersensitivity to detergents, bile salts, and hydrophobic antibiotics (13, 29, 35). Like other deep rough mutants (29, 32, 33, 35), mutation of C. jejuni 81-176 waaC led to sensitivity to various antibiotics and detergents (data not shown), results that are consistent with the observed morphological changes.
Unlike the 81-176 waaF mutant (17), mutation of waaC resulted in a change in apparent immunoreactivity of the capsular polysaccharide, as evidenced by immunoblotting (Fig. 5), that led us to examine the structure of the capsular polysaccharide. Sugar composition and linkage analysis (GLC-MS) and 1H NMR spectroscopy (Fig. 7) performed on the 81-176 waaC CPS revealed a structure that lacked a 3-O-methyl group from the 6-deoxy-altro-heptose residue compared to that performed on wild-type strain 81-176. Phase variation in C. jejuni NCTC11168 has been shown to result in several different modifications, including loss of a 6-O-methyl group on the heptose of the CPS (34). However, complementation of the 81-176 waaC mutant restored not only the core structure but also the 3-O-methyl group on the capsule, indicating that the capsule changes observed here are not the result of phase variation. These studies clearly show that the loss of the entire core oligosaccharide distal to Kdo in the waaC mutant resulted in a concomitant loss of the ability to add the 3-O-methyl group, and possibly O-methyl phosphoramidate, to the capsular polysaccharide. These structural changes may have interfered with antibody binding or affected electrophoretic migration of the mutant capsular polysaccharide. The reasons for the observed changes in the chemical structure of the capsule from the waaC mutant require additional study. It may be that the WaaC heptosyltransferase is bifunctional. However, waaC mutants of E. coli K-12 are known to show changes in colanic acid capsule production that are the result of membrane perturbations that affect the membrane-bound sensor kinase RcsS, which activates the cytoplasmic response regulator of the capsule, RcsB (29). Thus, membrane alterations in the C. jejuni waaC mutant may also affect regulation of genes involved in capsule biosynthesis. Additional studies will be required to elucidate the regulatory pathway that links CPS and LOS biosynthesis in C. jejuni as well as the function of the 3-O-methyl modification of the capsule.
Acknowledgments
We thank Lisa Morrison for performing the FAB-MS and Robert Williams for electron microscopy. M.I.K. gives special thanks to the members of the Guerry Laboratory for technical assistance.
This work was supported by NMRC work unit no. 6000.RAD1.DA3.A0308 to P.G. from the Military Infectious Diseases Program. M.I.K. was a recipient of an ASEE-NAVY summer faculty internship. M.A.M. was supported in part by a grant from the National Sciences and Engineering Research Council of Canada.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government.
REFERENCES
- 1.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 68:4384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 3.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 4.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209-217. [Google Scholar]
- 5.Dubois, M., K. Giles, J. Hamilton, P. Rebers, and F. Smith. 1956. Colometric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 6.Friedman, C. R., J. Neiman, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 7.Gilbert, M., M. F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A. M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327-337. [DOI] [PubMed] [Google Scholar]
- 8.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goon, S., C. P. Ewing, M. Lorenzo, D. Pattarini, G. Majam, and P. Guerry. 2006. A σ28-regulated nonflagella gene contributes to virulence of Campylobacter jejuni 81-176. Infect. Immun. 74:769-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 68:6656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerry, P., C. P. Ewing, M. Schirm, M. Lorenzo, J. Kelly, D. Pattarini, G. Majam, P. Thibault, and S. M. Logan. 23February2006, posting date. Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. [Online] doi: 10.1111/j1365-2958.2006.05100.x. [DOI] [PMC free article] [PubMed]
- 12.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrichs, D. E., J. A. Yethon, and C. Whitfield. 1998. Molecular basis for structural diversity in the core regions of the lipooligosaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 30:221-232. [DOI] [PubMed] [Google Scholar]
- 14.Hitchcock, P., and T. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, L., and D. Kopecko. 1999. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect. Immun. 67:4171-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, L., and D. J. Kopecko. 2000. Interactions of Campylobacter with eukaryotic cells: gut luminal colonization and mucosal invasion mechanisms, p. 191-215. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 17.Kanipes, M. I., L. C. Holder, A. T. Corcoran, A. P. Moran, and P. Guerry. 2004. A deep-rough mutant of Campylobacter jejuni 81-176 is noninvasive for intestinal epithelial cells. Infect. Immun. 72:2452-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klena, J. D., S. A. Gray, and M. E. Konkel. 1998. Cloning, sequencing, and characterization of the lipopolysaccharide biosynthetic enzyme heptosyltransferase I gene (waaC) from Campylobacter jejuni and Campylobacter coli. Gene 222:177-185. [DOI] [PubMed] [Google Scholar]
- 19.Labigne-Roussel, A., P. Couroux, and L. S. Tompkins. 1998. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linton, D., M. Gilbert, P. G. Hitchen, A. Dell, H. R. Morris, W. W. Wakarchuk, N. A. Gregson, and B. W. Wren. 2000. Phase variation of a β-1,3 galactosyltransferase involved in generation of the GM1-like lipooligosaccharide of Campylobacter jejuni. Mol. Microbiol. 37:501-514. [DOI] [PubMed] [Google Scholar]
- 21.McNally, D. J., H. C. Jarrell, J. Li, N. Khieu, E. Vinogradov, C. M. Szymanski, and J.-R. Brisson. 2005. The HS:1 serostrain of Campylobacter jejuni has a complex teichoic acid-like capsular polysaccharide with nonstoichiometric fructofuranose branches and O-methyl phosphoramidate groups. FEBS J. 272:4407-4422. [DOI] [PubMed] [Google Scholar]
- 22.Mills, S. D., G. O. Aspinall, A. G. McDonald, T. S. Raju, L. A. Kurjanczyk, and J. L. Penner. 1992. Lipopolysaccharide antigens of Campylobacter jejuni, p. 223-229. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 23.Moran, A. P., U. Zahringer, U. Seydel, D. Scholz, P. Stutz, and E. T. Rietschel. 1991. Structural analysis of the lipid A component of Campylobacter jejuni CCUG 10936 (serotype O:2) lipopolysaccharide. Description of a lipid A containing a hybrid backbone of 2-amino-2-deoxy-d-glucose and 2,3-diamino-2,3-dideoxy-d-glucose. Eur. J. Biochem. 198:459-469. [DOI] [PubMed] [Google Scholar]
- 24.Nachamkin, I., B. M. Allos, and T. W. Ho. 2000. Campylobacter jejuni infection and association with Guillain Barré syndrome, p. 155-175. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 25.Oberhelman, R. A., and D. N. Taylor. 2000. Campylobacter infections in developing countries, p. 139-153. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 26.Oldfield, N. J., A. P. Moran, L. A. Millar, M. M. Prendergast, and J. M. Ketley. 2002. Characterization of the Campylobacter jejuni heptosyltransferase II gene, waaF, provides genetic evidence that extracellular polysaccharide is lipid A core independent. J. Bacteriol. 184:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papp-Szabó, E., M. I. Kanipes, P. Guerry, and M. A. Monteiro. 2005. Cell-surface alpha-glucan in Campylobacter jejuni 81-176. Carbohydr. Res. 340:2218-2221. [DOI] [PubMed] [Google Scholar]
- 28.Parker, C. T., S. T. Horn, M. Gilbert, W. G. Miller, D. L. Woodward, and R. E. Mandrell. 2005. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 43:2771-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker, C. T., A. W. Koser, C. A. Schnaitman, M. A. Stein, S. Gottesman, and B. W. Gibson. 1992. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J. Bacteriol. 174:2525-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawardeker, J., and J. Sloneker. 1967. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 37:1602-1604. [Google Scholar]
- 31.Schnaitman, C. A., and J. D. Klena. 1993. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol. Rev. 57:655-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirisena, D., K. Brozek, P. MacLachlan, K. Sanderson, and C. Raetz. 1992. The rfaC gene of Salmonella typhimurium. Cloning, sequencing, and enzymatic function in heptose transfer to lipopolysaccharide. J. Biol. Chem. 267:18874-18884. [PubMed] [Google Scholar]
- 33.Stojiljkovic, I., V. Hwa, J. Larson, L. Lin, M. So, and X. Nassif. 1997. Cloning and characterization of the Neisseria meningitidis rfaC gene encoding alpha-1,5 heptosyltransferase I. FEMS Microbiol. Lett. 151:41-49. [DOI] [PubMed] [Google Scholar]
- 34.Szymanski, C. M., F. S. Michael, H. C. Jarrell, J. Li, M. Gilbert, S. Larocque, E. Vinogradov, and J. R. Brisson. 2003. Detection of conserved N-linked glycans and phase-variable lipooligosaccharides and capsules from campylobacter cells by mass spectrometry and high resolution magic angle spinning NMR spectroscopy. J. Biol. Chem. 278:24509-24520. [DOI] [PubMed] [Google Scholar]
- 35.Tamaki, S., T. Sato, and M. Matsuhashi. 1971. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J. Bacteriol. 105:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, Y., and D. Taylor. 1990. Natural transformation in Campylobacter species. J. Bacteriol. 172:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westphal, O. K., and K. Jann. 1965. Bacterial lipopolysaccharide. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 38.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 39.Yao, R., and P. Guerry. 1996. Molecular cloning and site-specific mutagenesis of a gene involved in arylsulfatase production in Campylobacter jejuni. J. Bacteriol. 178:3335-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]