Abstract
Starvation-induced development of Myxococcus xanthus is an excellent model for biofilm formation because it involves cell-cell signaling to coordinate formation of multicellular mounds, gene expression, and cellular differentiation into spores. The role of σD, an alternative σ factor important for viability in stationary phase and for stress responses, was investigated during development by measuring signal production, gene expression, and sporulation of a sigD null mutant alone and upon codevelopment with wild-type cells or signaling mutants. The sigD mutant responded to starvation by inducing (p)ppGpp synthesis normally but was impaired for production of A-signal, an early cell density signal, and for production of the morphogenetic C-signal. Induction of early developmental genes was greatly reduced, and expression of those that depend on A-signal was not restored by codevelopment with wild-type cells, indicating that σD is needed for cellular responses to A-signal. Despite these early developmental defects, the sigD mutant responded to C-signal supplied by codeveloping wild-type cells by inducing a subset of late developmental genes. σD RNA polymerase is dispensable for transcription of this subset, but a distinct regulatory class, which includes genes essential for sporulation, requires σD RNA polymerase or a gene under its control, cell autonomously. The level of sigD transcript in a relA mutant during growth is much lower than in wild-type cells, suggesting that (p)ppGpp positively regulates sigD transcription in growing cells. The sigD transcript level drops in wild-type cells after 20 min of starvation and remains low after 40 min but rises in a relA mutant after 40 min, suggesting that (p)ppGpp negatively regulates sigD transcription early in development. We conclude that σD synthesized during growth occupies a position near the top of a regulatory hierarchy governing M. xanthus development, analogous to σ factors that control biofilm formation of other bacteria.
In nature, many bacteria exist in biofilms, within which cells send signals to each other and respond by changing expression of certain genes (67, 68). This not only alters the physiology of individual cells but also often causes macroscopic change in the morphology of the biofilm. Understanding how cells interact to bring about such change is a fundamental challenge of broad practical significance (48).
Myxococcus xanthus is a gram-negative soil bacterium that provides an excellent experimental model for biofilm research (53). Upon sensing nutrient limitation, certain genes are induced and signals are produced (29, 31, 64). Cells respond by altering their pattern of gliding movements and piling on top of one another to form mounds. Within the mounds further signaling and gene expression cause cells to differentiate from metabolically active rods into dormant, spherical spores.
Decades of research on M. xanthus have elucidated numerous molecular events that occur during the developmental process (13). The events tracked in this study are depicted in Fig. 1. Starvation induces a stringent response that involves RelA-dependent synthesis of (p)ppGpp (23, 49, 50, 63). This intracellular signal induces early developmental genes such as csgA (10) and sdeK (23, 63). CsgA is a 25-kDa protein that helps maintain the stringent response within cells (11) and appears to serve as an extracellular signal (C-signal) after being cleaved to a 17-kDa form at the cell surface (34, 47, 62). SdeK is a histidine kinase essential for expression of many developmental genes (18, 56), but neither its input nor its substrate is known. (p)ppGpp also initiates production of A-signal (23), a mixture of peptides and amino acids generated by extracellular protease activity (41, 55). If cells are at a high enough density (42), sufficient A-signal is made to trigger expression of genes such as Ω4521 (also known as spi), Ω4514 (40), and fruA (52). The genes Ω4521 and Ω4514 were identified by insertion of Tn5 lac (38) (as is the case for other numbered genes and operons described below). Ω4521 expression has been used to develop an assay for A-signal (39, 40) and to facilitate genetic screens that have elucidated the A-signal transduction pathway (21, 76, 77). The Ω4514 operon is expressed slightly later during development than Ω4521 due to negative autoregulation by the product of the first gene in the operon (22). FruA is a response regulator necessary for responses to C-signal (14, 52), but its putative kinase has not been found. C-signaling is needed for progression beyond the morphological stage of forming broad, low mounds and for full expression of most genes that begin to be transcribed after about 6 h into development (36). Some genes, such as Ω4400 and the Ω4499 and dev operons, are expressed weakly in the absence of C-signaling but require C-signaling for full expression (4, 36). Of these, only dev is important for sporulation (37, 38, 71). Genes expressed later, such as Ω4403 and the Ω7536 operon, fail to be expressed in the absence of C-signaling, and Ω7536 is required for sporulation (36, 46).
FIG. 1.
Timeline of events during M. xanthus development. Signal inputs are below the line and genes induced are above.
In this study, we used the molecular markers described above to investigate the role of σD in regulating genes and signals during M. xanthus development. σD is somewhat similar to M. xanthus σA, the major vegetative σ factor in the σ70 family, and to three alternative M. xanthus σ factors (σB, σC, and σE), although σD and the other alternative σ factors are much smaller than σA (73). σD is essential for viability in stationary phase, helps cells resist oxidative and osmotic stress, and is required for normal development (73). A sigD null mutant exhibits altered patterns of protein synthesis compared to wild-type cells during the late-log phase of growth and early in development (73); however, none of the differentially expressed proteins was identified. Here, we employ well-characterized markers to examine the role of σD in development. Under the conditions we used, the sigD null mutant failed to form mounds containing spores (fruiting bodies) within 72 h, whereas wild-type cells formed mounds by 12 h and some spores by 24 h, with the spore number increasing over the next 2 days. We found that the sigD mutant undergoes a stringent response to starvation but fails to induce developmental genes and signals. Surprisingly, the sigD mutant was able to respond to codeveloping wild-type cells by inducing a subset of C-signal-dependent genes; however, the results suggest that σD RNA polymerase or a gene under its control is required cell autonomously to increase transcription of the dev operon in response to C-signal, an event that is important for sporulation. We also discovered that the level of sigD transcripts in cells decreases more than 100-fold after 20 min of starvation, so it appears that σD synthesized during growth fulfills its early role in development.
MATERIALS AND METHODS
Bacterial strains and plasmids.
M. xanthus strains that were used in this work are listed in Table 1. Plasmids were introduced into M. xanthus by electroporation (32). The plasmid pPVsigD was used as a control for quantitative PCR (QPCR; see below). To construct pPVsigD, a 951-bp DNA fragment including the sigD gene and 200 bp of upstream DNA was amplified from M. xanthus DK1622 chromosomal DNA using the primer pair 5′-CCGAAGCTTTCGTGGCCGACGCGGCTC-3′ and 5′-GCCGGATCCTCACGCCGCGTCGGCGG-3′. The DNA fragment was purified by agarose gel extraction and cloned into pCR2.1-TOPO according to the manufacturer's instructions. The DNA insert was sequenced at the Michigan State University Genomics Technology Support Facility to ensure that the correct sequence was obtained. Tn5 lac fusions were introduced into M. xanthus by generalized transduction; Mx4 phage stocks (6, 19, 28) were prepared on donor strains, and phages were mixed with recipients (108 cells) at multiplicities of infection of 0.1, 0.5, 1.0, and 2.0.
TABLE 1.
M. xanthus strains used in this study
| Strain | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| DK101 | pilQ1 | 25 |
| DK1622 | Wild type | 30 |
| DK4322 | Tn5 lac (Kmr) Ω4521 | 40 |
| DK4324 | Tn5 lac (Kmr) Ω4521 asgB480 | 40 |
| DK4368 | Tn5 lac (Kmr) Ω4403 | 38 |
| DK4408 | Tn5 lac (Kmr) Ω4408 (insertion in sdeK) | 40 |
| DK5057 | Tn5 (Kmr) Ω4560 asgA476 | 39 |
| DK5208 | csgA::Tn5-132 (Tcr) Ω205 | 61 |
| DK5270 | Tn5 lac (Kmr) Ω4403 csgA::Tn5-132 (Tcr) Ω205 | 36 |
| DK5279 | Tn5 lac (Kmr) Ω4414 (insertion in devR) | 36 |
| DK5287 | Tn5 lac (Kmr) Ω4414 csgA::Tn5-132 (Tcr) Ω205 | 36 |
| DK10524 | Tn5 lac (Kmr) Ω7536 | 46 |
| JPB40030 | attB::pJB40030 (Kmr) (Ω4400-lacZ fusion) | 78 |
| MDS12 | attB::pDS12.P (Kmr) (fruA-lacZ fusion) | 65 |
| MDS200 | csgA::pJM200 (Kmr) (csgA-lacZ fusion) | 59 |
| MDY101 | attB::pDY101 (Kmr) (Ω4499-lacZ fusion) | 79 |
| MDY4400.SD | ΔsigD Tn5 lac (Kmr) Ω4400 | 78 |
| MDY4499.SD | ΔsigD attB::pDY101 (Kmr) | 79 |
| MS1000 | DK101 ΔrelA1 | 12 |
| MTH1-3 | attB::pTH1-6 (Kmr) (Ω4514-lacZ fusion) | 22 |
| ΔsigD | sigD deletion | 73 |
| sigDcsgA | ΔsigD csgA::pJM200 (Kmr) | This study |
| sigD4408 | ΔsigD Tn5 lac (Kmr) Ω4408 | This study |
| sigD4521 | ΔsigD Tn5 lac (Kmr) Ω4521 | This study |
| sigDfruA | ΔsigD attB::pDS12.P (Kmr) | This study |
| sigD4514 | ΔsigD attB::pTH1-6 (Kmr) | This study |
| sigDdev | ΔsigD Tn5 lac (Kmr) Ω4414 | This study |
| sigD7536 | ΔsigD Tn5 lac (Kmr) Ω7536 | This study |
| sigD4403 | ΔsigD Tn5 lac (Kmr) Ω4403 | This study |
| ΔfruA | fruA deletion/replacement (Smr) | T. Ueki and S. Inouye |
| sigD4400fruA | ΔsigD Tn5 lac (Kmr) Ω4400 ΔfruA (Smr) | This study |
| sigD4403fruA | ΔsigD Tn5 lac (Kmr) Ω4403 ΔfruA (Smr) | This study |
| sigD4499fruA | ΔsigD attB::pDY101 (Kmr) ΔfruA (Smr) | This study |
Kmr, kanamycin resistant; Tcr, oxytetracycline resistant; Smr, streptomycin resistant.
Bacterial growth and development.
Escherichia coli was grown at 37°C in Luria-Bertani medium (60) containing 50 μg/ml of ampicillin. M. xanthus was grown at 32°C in CTT medium (24) (1% casitone, 10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4 · K2HPO4, 8 mM MgSO4 [final pH 7.6]) in liquid cultures or on agar (1.5%) plates. Forty micrograms of kanamycin per milliliter, 12.5 μg/ml of oxytetracycline, or 200 μg/ml of streptomycin was used when required for selective growth. For starvation induction of M. xanthus in liquid cultures, cells were grown to a density of approximately 5 × 108 cells/ml, the culture was centrifuged, and the cell pellet was resuspended in prewarmed TPM (10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4 · K2HPO4, 8 mM MgSO4 [final pH 7.6]) liquid medium to a density of approximately 5 × 108 cells/ml, as described previously (63). Fruiting body development was performed on TPM agar (1.5%) plates as described previously (38). Developmental β-galactosidase activity was measured as described previously (38). Codevelopment (also called extracellular complementation) was performed by mixing equal optical density units of cells as described previously (36). For mutants alone or upon codevelopment with wild-type cells, developmental β-galactosidase activity was measured for three isolates, and points on the graphs show the average while error bars show one standard deviation of the data (in many cases the error bar is so small that it is hidden by the symbol representing the point on the graph). In each experiment, developmental β-galactosidase activity from the corresponding lacZ fusion in wild type (one isolate) was measured as a positive control (which agreed with previously published results) and is plotted on the graph. Extracellular complementation of sporulation was performed as described previously (36), except the production of heat- and sonication-resistant spores was assayed as follows. Cells from spots of five 20-μl aliquots were scraped from TPM agar and suspended in 500 μl of sterile distilled water. The suspension was sonicated four times for 10 s with intermittent cooling and then incubated in a water bath at 50°C for 2 h. Tenfold dilutions of the resulting suspension in distilled water were plated in CTT soft agar and incubated at 32°C for 4 days before colonies were counted.
Quantification of guanosine nucleotides.
In vivo analysis of guanosine nucleotides was performed as described previously (49) with the following modifications. Cells were grown in CTT medium without the addition of 1 mM KH2PO4 · K2HPO4. The concentration of inorganic phosphate in this medium was determined to be 0.75 mM using a method described previously (2). After 6 h of growth in the presence of 100 μCi/ml [32P]orthophosphate (NEX053H carrier free, 285.6 Ci/mg; Perkin Elmer) to equilibrate the phosphate pools at a cell density of 100 Klett units, a 1-ml aliquot was removed as the time zero reference sample, and nucleotides were extracted as described previously (49). The remaining culture was centrifuged, and the cell pellet was resuspended in TPM medium modified to contain 0.75 mM KH2PO4 · K2HPO4 and 100 μCi/ml [32P]orthophosphate to maintain the same specific activity. One-milliliter aliquots were removed at 15, 30, 45, and 60 min, and nucleotides were extracted and analyzed by thin-layer chromatography as described previously (49). The amount of radioactivity in each spot was then quantified on a PhosphorImager 400S (Molecular Dynamics, Sunnyvale, CA) equipped with the ImageQuant, version 5, program (Molecular Dynamics, Sunnyvale, CA).
A-signal production and assay.
A-factor was harvested from developing cells as described previously (39, 40). A-factor was assayed by measuring the restored expression of Tn5 lac Ω4521 in the asgB480 mutant background (M. xanthus DK4324) as described previously (39). One unit of A-factor produced 1 nmol of o-nitrophenyl-β-d-galactopyranoside per min, for an entire test well containing 1.25 × 108 cells, above the background. To compare the activities of A-factor harvested from different bacterial strains, dilutions of each cell-free supernatant were assayed, and the linear portion of an activity-versus-volume curve was used to estimate A-factor activity (39).
Measurement of sigD transcript levels by QPCR.
Total RNA samples were extracted by the hot phenol method (60) from approximately 1010 quick-frozen M. xanthus cells. These cells were harvested either during vegetative growth at a cell density of approximately 5 × 108 cells/ml or after 20 or 40 min poststarvation as described above. Analysis was performed as described by Diodati et al. (12). Expression of the sigD gene was calculated from DNA quantities obtained for concurrent standard curve reactions using pPVsigD. Typically, a standard curve was generated by reacting a 10-fold dilution series of plasmid DNA, ranging from 1010 to 101 copies/μl, with the following sigD forward and reverse primer pair: 5′-CATGGCCAATTCGACGAAGT-3′ and 5′-GCCTTCATGAGACCGACGTT-3′, respectively. The DyNAmo HS SYBR Green QPCR Master Mix (Finnzymes, Espoo, Finland) was used as described by the manufacturer. Each sample was assayed in triplicate, and at least two biological samples were assayed.
RESULTS
σD is required for normal expression of early, A-signal-independent genes.
The csgA and sdeK genes are induced at the onset of development, and their expression does not depend on A-signaling (10, 40, 45). Developmental expression of both genes was severely impaired in a sigD null mutant (Fig. 2). The defect in expression was cell autonomous; it could not be rescued extracellularly by codevelopment of the sigD mutant with wild-type cells (Fig. 2).
FIG. 2.
Expression of A-signal-independent genes in a sigD mutant. β-Galactosidase specific activity during development was measured for lacZ fusions to csgA (A) and sdeK (B) in wild type (▪) or in a sigD mutant alone (•) or upon codevelopment of the fusion-containing sigD mutant with an equal number of fusionless wild-type cells (○).
A sigD mutant responds normally to starvation by synthesizing ppGpp.
Developmental induction of csgA and sdeK depends on RelA (10, 23), which mediates the stringent response to amino acid limitation by synthesizing (p)ppGpp (23). To determine whether σD is required for the stringent response, ppGpp and GTP (from which ppGpp is synthesized) levels were measured during nutritional downshift. The sigD null mutant was indistinguishable from wild type (Fig. 3). We conclude that σD RNA polymerase is not required for the stringent response to starvation but is directly or indirectly needed for increased transcription of csgA and sdeK early in development (Fig. 2).
FIG. 3.
ppGpp and GTP levels during nutritional downshift of wild type and a sigD mutant. Guanosine nucleotides were measured in extracts of wild-type cells (squares) or a sigD mutant (circles) as described in Materials and Methods during growth (time zero) or after resuspension in starvation buffer. ppGpp (closed symbols) and GTP (open symbols) levels are expressed relative to the time zero level in a representative experiment.
σD is important for expression of A-signal-dependent genes.
Three reporter fusions, Ω4521, fruA, and Ω4514, that are normally induced early in development and whose expression depends on A-signaling (40, 52), also failed to be expressed normally in the sigD null mutant (Fig. 4). Supplying A-signal by mixing wild-type cells with the reporter fusion-bearing sigD mutants and allowing them to codevelop did not restore developmental expression (Fig. 4). These results suggest that σD RNA polymerase is required in a cell autonomous fashion for normal expression of A-signal-dependent genes.
FIG. 4.
Expression of A-signal-dependent genes in a sigD mutant. β-Galactosidase specific activity during development was measured for lacZ fusions to Ω4521 (A), fruA (B), and Ω4514 (C) in wild type (▪) or in a sigD mutant alone (•) or upon codevelopment of the fusion-containing sigD mutant with an equal number of fusionless wild-type cells (○).
A sigD mutant is defective in producing A-signal.
Although the sigD mutant did not respond properly to A-signal (Fig. 4), it was possible that the sigD mutant could produce this signal. We tested this possibility in two ways. First, the sigD mutant was codeveloped with an asgA mutant that is unable to make A-signal. Alone, the asgA mutant is unable to sporulate (Table 2). Upon mixing with the sigD mutant and codevelopment, spores formed, and all the spores were derived from the asgA mutant, but the number was about 104-fold less than the number of asgA-derived spores produced upon codevelopment with wild-type cells (Table 2). This suggests that the sigD mutant makes A-signal at a reduced level. In agreement, the sigD mutant released about fourfold less A-signal than wild-type cells (after the background value observed for the asgA mutant was subtracted), as measured by the ability of medium from starved cells to stimulate Ω4521 expression in asgA mutant cells (Table 3). We conclude that σD is required both to produce the normal level of A-signal and to respond to it by directly or indirectly increasing transcription of A-signal-dependent genes.
TABLE 2.
Extracellular complementation of sporulation
| Strain or mixture (description)a | Sporulation (% wild type)b | Rescue (%)c |
|---|---|---|
| DK1622 (wild type) | 100 | |
| ΔsigD (sigD mutant) | <0.001 | |
| DK5057 (asgA mutant) | <0.001 | |
| DK1622+DK5057 | 140 ± 38 | 18 |
| ΔsigD+DK5057 | 0.0023 ± 0.0007 | 100 |
| DK5208 (csgA mutant) | <0.001 | |
| DK1622+DK5208 | 130 ± 18 | 100 |
| ΔsigD+DK5208 | 15 ± 11 | 100 |
For strain mixtures (+), the second listed strain was unable to sporulate alone and contained a marker gene that confers resistance to kanamycin (DK5057) or oxytetracycline (DK5208).
Production of heat- and sonication-resistant spores was assayed as described in Materials and Methods after 4 days of development at 32°C. Wild type produced 5 × 106 spores. Numbers indicate averages and standard deviations of three experiments.
The ability of the first strain to rescue sporulation of the second was assessed by transferring cells from spore-derived colonies onto CTT agar containing the appropriate antibiotic. The number that grew after 3 days at 32°C is expressed as a percentage of the number tested, which was 200.
TABLE 3.
A-factor assay
| Strain | A-factor (U)a |
|---|---|
| DK1622 (wild type) | 11.5 ± 0.1 |
| DK5057 (asgA mutant) | 3.3 ± 0.1 |
| sigD4403 (sigD mutant) | 5.2 ± 0.2 |
A-factor released from starved cells was measured as described in Materials and Methods. Numbers are the average and standard deviation of three experiments.
σD is required for expression of C-signal-dependent genes, but for some genes the requirement is not cell autonomous.
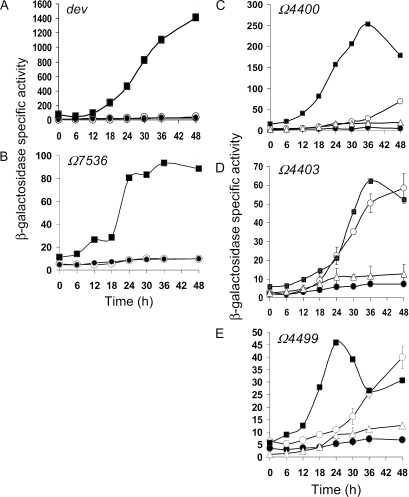
Five genes that depend not only on A-signaling (40) but also on C-signaling (36, 46) failed to be expressed normally during development in the sigD null mutant (Fig. 5). Expression of two, dev (37, 38, 71) and Ω7536 (46), is important for development and was not rescued by codeveloping the sigD mutant with wild-type cells (Fig. 5). Surprisingly, expression of the other three, Ω4400, Ω4403, and Ω4499, increased significantly in the sigD mutant upon codevelopment with wild-type cells (Fig. 5). These results indicate that σD is needed in a cell autonomous fashion for expression of some C-signal-dependent genes (dev and Ω7536), but others (Ω4400, Ω4403, and Ω4499) do not require σD within the cell for their expression if extracellular signals are supplied by codeveloping wild-type cells.
FIG. 5.
Expression of C-signal-dependent genes in a sigD mutant. β-Galactosidase specific activity during development was measured for lacZ fusions to dev (A), Ω7536 (B), Ω4400 (C), Ω4403 (D), and Ω4499 (E) in wild type (▪) or in a sigD mutant alone (•) or upon codevelopment of the fusion-containing sigD mutant with an equal number of fusionless wild-type (○) or csgA mutant (▵) cells.
Expression of at least five essential developmental genes (csgA, sdeK, fruA, dev, and Ω7536) depends on σD cell autonomously (Fig. 2, 4, and 5), so it is not surprising that wild-type cells failed to rescue sporulation of the sigD null mutant (data not shown). On the other hand, the sigD mutant was capable of rescuing a csgA mutant that does not make C-signal, although the number of csgA-derived spores was about eightfold less than the number in mixtures with wild-type cells (Table 2). A reduced level of C-signal from the sigD mutant is expected because csgA expression is reduced (Fig. 2). It is worth noting that even in the mixture with wild-type cells, all the spores were derived from the csgA mutant (Table 2). This behavior, called developmental cheating, in which the mutant sporulates disproportionately, has been observed for the csgA mutant previously (75).
Rescue of gene expression in the sigD mutant depends on csgA and fruA.
To determine whether the rescue of expression of certain C-signal-dependent genes in the sigD mutant upon codevelopment with wild-type cells (Fig. 5) depends on the normal C-signal transduction pathway, we performed two experiments. First, we substituted a csgA mutant for wild type in the codevelopment experiments with the sigD mutants containing the Ω4400, Ω4403, or Ω4499 reporter. There was no rescue of gene expression (Fig. 5, triangles), demonstrating that rescue depends on csgA, which is needed to produce C-signal (34, 62). In the second experiment, we constructed sigD fruA double mutants bearing the Ω4400, Ω4403, or Ω4499 reporter. FruA mediates responses to C-signal during development (14, 52). The fruA mutation impaired rescue of Ω4400 expression and prevented rescue of Ω4403 and Ω4499 expression when the double mutants were codeveloped with wild-type cells (Fig. 6; note the changes in the values along the y axes compared to Fig. 5). Taken together, the results suggest that the sigD mutant responds to C-signaling by wild-type cells (Fig. 5) via the normal pathway involving FruA. Expression of Ω4403 and Ω4499 relies completely on this pathway, and so does expression of Ω4400 for the most part, but the latter appears to be induced very weakly by another mechanism in the sigD fruA double mutant upon codevelopment with wild-type cells (Fig. 6).
FIG. 6.
Rescue of Ω4400, Ω4403, and Ω4499 expression in the sigD mutant depends on fruA. β-Galactosidase specific activity during development was measured for lacZ fusions to Ω4400 (A), Ω4403 (B), and Ω4499 (C) in the sigD fruA double mutant alone (•) or upon codevelopment of the fusion-containing sigD fruA mutant with an equal number of fusionless wild-type cells (○).
Inability to rescue dev expression in the sigD mutant is not due to a requirement for a high level of C-signaling.
Why was expression of only certain C-signal-dependent genes rescued in the sigD null mutant upon codevelopment with wild-type cells (Fig. 5)? Perhaps C-signaling in the mixture is suboptimal, and certain genes require a higher level of C-signaling than others in order to be induced. To test this hypothesis, we made use of our observations that csgA expression (Fig. 2) and C-signaling (Table 2) are reduced in the sigD mutant. The sigD mutant was used as a suboptimal donor of C-signal in mixing experiments with csgA mutants bearing reporters. Contrary to the hypothesis, we found that the sigD mutant partially rescued expression of dev, but not Ω4403, in the csgA mutant background (Fig. 7). Partial rescue of dev expression, which is important for sporulation, is consistent with the finding that the sigD mutant partially restored sporulation of the csgA mutant upon codevelopment (Table 2). Expression of Ω4403 is not required for sporulation (38). The failure of the sigD mutant to rescue expression of Ω4403 in the csgA mutant background suggests that Ω4403 expression requires a higher level of C-signaling than dev expression. Wild-type cells can supply sufficient C-signal to rescue expression of Ω4403 in the sigD mutant background, but even the high level of C-signal supplied by wild type is insufficient to rescue dev expression in sigD mutant cells (Fig. 5). Taken together, our results suggest that developing cells require σD RNA polymerase or a gene under its control to increase transcription of dev in response to C-signal.
FIG. 7.
Rescue of dev but not Ω4403 expression in a csgA mutant upon codevelopment with the sigD mutant. β-Galactosidase specific activity during development was measured for lacZ fusions to dev (A) and Ω4403 (B) in a csgA mutant upon codevelopment with an equal number of fusionless wild-type (○), sigD mutant (□), or csgA mutant (▵) cells.
The level of sigD transcript drops rapidly during starvation, and sigD transcript levels are altered in a relA mutant during growth and starvation.
The foregoing results demonstrate that a sigD mutant responds to starvation by synthesizing ppGpp normally but is severely defective for developmental gene expression, including expression of early, A-signal-independent genes such as csgA and sdeK. Expression of these genes also depends on the relA gene, whose product synthesizes (p)ppGpp in response to starvation (10, 23). Does expression of sigD depend on relA? To investigate this question, we compared sigD transcript levels in wild-type and relA mutant cells during growth and shortly after starvation. The level of sigD transcript in growing cells was greatly decreased in the relA mutant compared to wild type (Table 4, time zero). This suggests that the low level of (p)ppGpp synthesized by RelA in growing cells (Fig. 3) positively regulates sigD transcription. Upon starvation, the level of sigD transcript decreased more than 100-fold in wild type by 20 min and remained low after 40 min (Table 4). The sigD transcript level decreased to a similar low level in the relA mutant after 20 min of starvation but then rose to an intermediate level by 40 min. The intermediate level was about 10-fold lower than in growing wild-type cells but about 10-fold higher than in wild-type cells that had been starving for 40 min. The latter observation suggests that RelA-dependent synthesis of (p)ppGpp in starving wild-type cells (Fig. 3) negatively regulates transcription of sigD. Taken together, these results suggest that σD synthesized during growth plays a critical role early in development.
TABLE 4.
sigD transcript levels
| Time (min)a |
sigD transcript level (%)b
|
|
|---|---|---|
| DK101 (wild type) | MS1000 (ΔrelA1) | |
| 0 | 100 ± 28 | 4 ± 4 |
| 20 | 0.3 ± 0.2 | 0.3 ± 0.1 |
| 40 | 0.7 ± 0.2 | 12 ± 3 |
Amount of time cells were in starvation buffer, as described in Materials and Methods.
Numbers indicate the average and standard deviation of three biological replicates, expressed as a percentage of the DK101 vegetative (time 0) value, for which six biological replicates were performed.
DISCUSSION
Our results provide several new insights into the role of σD in regulating genes and signals during M. xanthus development. σD is required very early during development for full expression of key genes such as csgA and sdeK that depend on the intracellular signal (p)ppGpp but not the extracellular A-signal. This requirement for σD does not appear to reflect a role in the stringent response to starvation, because a sigD null mutant accumulates (p)ppGpp normally when starved. On the other hand, the low level of (p)ppGpp synthesized in growing cells appears to be necessary for sigD transcription, and σD made during growth appears to fulfill its early role in development. σD is required both for normal production of A-signal and for cellular responses to A-signal. Supplying A-signal by codeveloping the sigD mutant with wild-type cells did not restore normal expression of A-signal-dependent genes in the sigD mutant. Another insight was quite surprising in light of the requirement of σD for so many early events in development; we discovered that the sigD mutant can respond to C-signal from codeveloping wild-type cells by inducing a subset of late developmental genes. This subset of genes apparently does not require σD RNA polymerase for expression. On the other hand, a second class of C-signal-dependent genes, including the dev operon that is important for sporulation, was not induced. In the case of the dev operon, this is not due to a requirement for a higher level of C-signaling, but rather a cell autonomous requirement for σD. Below, we discuss the implications of these new insights and present a model that depicts the role of σD in regulating genes and signals during M. xanthus development.
Role of σD early in development.
Fig. 8 shows the position of σD near the top of a regulatory hierarchy built from the results of this study and others cited below. Accumulation of sigD transcripts in growing cells depends strongly on relA (Table 4). In E. coli, RelA is responsible for ribosome-dependent synthesis of (p)ppGpp (reviewed in reference 7). The M. xanthus relA gene complements an E. coli relA mutant for production of ppGpp, suggesting that it has a similar function (23). Only a low level of ppGpp is detected in growing M. xanthus (49). Presumably, this depends on RelA activity, and the resulting low concentration of (p)ppGpp positively regulates sigD transcript accumulation. We suggest that (p)ppGpp positively regulates sigD transcription, by analogy with the effect of (p)ppGpp on rpoS transcription in E. coli, which appears to be at the level of elongation rather than initiation (44). Also, (p)ppGpp affects transcription of many genes in E. coli (reviewed in references 51 and 54). Alternatively, (p)ppGpp might influence sigD mRNA stability.
FIG. 8.
Model of regulatory events governing M. xanthus development. Arrows indicate positive regulation, which may be direct or indirect. The dashed arrows indicate regulatory inputs that this study suggests might be different for dev than for Ω4400, Ω4403, and Ω4499.
The level of sigD transcripts in wild-type cells decreased more than 100-fold after 20 min of starvation (Table 4). Starvation induces (p)ppGpp synthesis in M. xanthus (49, 50), and this depends on RelA (23) but not on σD (Fig. 3 and 8). The resulting high concentration of (p)ppGpp appears to be at least partly responsible for negatively regulating sigD transcript accumulation after starvation, as depicted in Fig. 8, because the sigD transcript level was higher in relA mutant cells than in wild-type cells after 40 min of starvation (Table 4). Whether (p)ppGpp alone negatively regulates sigD transcript accumulation or works in combination with other starvation-induced signals is an open question, as is the mechanism(s) of regulation. Previously, sigD expression was investigated with transcriptional and translational fusions to lacZ (73). β-Galactosidase activity was observed during growth and increased about twofold as cells entered stationary phase or were placed under conditions that induce development. The stability of β-galactosidase, the longer times prior to sampling, and/or a difference in the starvation conditions might explain why a decrease in sigD expression was not detected previously. It will be interesting to measure sigD transcript and σD protein levels under different starvation conditions and after longer periods of starvation. The rapid drop in sigD transcript levels after starvation suggests that σD made during growth fulfills its early role in development.
σD is needed for full expression of csgA and sdeK (Fig. 2) and to produce the wild-type level of A-signal (Tables 2 and 3). As depicted in Fig. 8, induction of (p)ppGpp is also required for these events (10, 23). This could mean that (p)ppGpp and σD RNA polymerase affect the same step(s) very early in development. A simple model would be that (p)ppGpp stimulates σD RNA polymerase to transcribe one or more genes, analogous to stimulation of σS RNA polymerase activity by (p)ppGpp in stationary phase E. coli (43). However, based on studies in E. coli, the effects of (p)ppGpp on RNA polymerase are likely to be complex (reviewed in references 51 and 54). We think it is unlikely that σD RNA polymerase transcribes sdeK, because the promoter of this gene has sequences in the −24 and −12 regions similar to the consensus for σ54 (18). Since σ54 RNA polymerase requires an activator protein to initiate transcription, perhaps σD RNA polymerase transcribes the gene encoding the activator. Likewise, it seems likely that σD RNA polymerase transcribes an activator of csgA, rather than directly transcribing csgA, for several reasons. First, the csgA promoter has sequences centered at −35 and −10 similar to the consensus for σA (3, 45). Second, deletion analysis of the csgA promoter region suggests the involvement of one or more activator proteins (45). Third, csgA is expressed, albeit poorly, in the sigD null mutant (Fig. 2). With respect to the role of σD in producing A-signal, at least five genes are known to be involved in A-signal production (8, 17, 39), but transcription of only one, asgE, has been studied in detail (16). asgE appears to be transcribed from a promoter similar to the σ54 consensus during growth and from two promoters during development. One of the putative developmental promoters is similar to the σ54 consensus, but the other is not similar to the σ54 or σA consensus; so it is possible that σD RNA polymerase transcribes asgE from this promoter.
Our investigation also revealed that σD is important for cellular responses to A-signal (Fig. 4), which include induction of Ω4521, fruA, and Ω4514 early in development. Ω4521 is very likely to be transcribed by σ54 RNA polymerase, based on mutational analysis of its promoter (33). Also, an activator protein appears to bind somewhere between −146 and −90 relative to the transcriptional start site (20). A candidate for the activator protein is SasR, an NtrC-like response regulator identified in a genetic screen as a positive regulator of Ω4521 (21). Transcription of sasR and/or several other genes involved in Ω4521 regulation (21) might be the direct target(s) of σD RNA polymerase activity that explain the failure of the sigD mutant to induce Ω4521 expression upon codevelopment with wild-type cells that supply A-signal (Fig. 4). Transcription of fruA appears to be regulated differently than that of Ω4521. It involves MrpC, a cyclic AMP receptor protein-like activator (70), which binds upstream of the fruA promoter (72). Expression of mrpC is reduced in the absence of A-signaling (69), which might explain the dependence of fruA expression on A-signaling (14, 52). Since expression of mrpC depends on MrpB, an NtrC-like response regulator (70), transcription of the mrpAB operon might be the direct target of σD RNA polymerase that explains the cell autonomous requirement of σD for fruA induction in response to A-signal. Alternatively or in addition, the fruA promoter might be a target of σD RNA polymerase activity, since mutational analysis indicates that the −35 and −10 regions are important (65), but the sequences do not match the σA consensus very well. In contrast, the promoter of the Ω4514 operon is similar to the σA consensus and can be transcribed in vitro by σA RNA polymerase (22). Multiple upstream DNA elements are necessary for full induction of the Ω4514 operon, suggesting the involvement or one or more transcriptional activator proteins, whose transcription might be the direct target(s) of σD RNA polymerase. Identification of the direct targets of σD RNA polymerase activity is an important future goal in order to better understand the role of σD early in M. xanthus development.
Role of σD late in development.
Given the impaired expression of csgA (Fig. 2), whose product appears to be the C-signal (34, 47, 62), and fruA (Fig. 4), whose product mediates responses to C-signal (14, 52), it is not surprising that the sigD mutant failed to express all five C-signal-dependent genes tested (Fig. 5). Moreover, expression of three of these, Ω4400, Ω4403, and dev, has been shown to be affected by a mutation in sdeK (56) (Ω4499 and Ω7536 were not tested), and sdeK expression is impaired in the sigD mutant (Fig. 2).
Despite the impaired early developmental gene expression, the sigD mutant expressed Ω4403 normally, Ω4499 in a delayed fashion, and Ω4400 partially, upon codevelopment with wild-type cells (Fig. 5). This rescue depends on the normal C-signal transduction pathway, since it required the donor cells to be csgA+ (Fig. 5) and the recipient sigD mutant cells to be fruA+ (Fig. 6). We infer that sigD mutant cells synthesize enough FruA to mediate some responses to C-signal supplied by wild-type cells during codevelopment. The amount of FruA in the sigD mutant is likely to be low, since very little expression of a fruA-lacZ fusion was detected (Fig. 4). We also infer that a small amount of SdeK is made in the sigD mutant, since sdeK-lacZ expression is low (Fig. 2). Apparently, this amount is sufficient for expression of Ω4400, Ω4403, and Ω4499, and σD RNA polymerase is dispensable for transcription of these genes (Fig. 5).
No rescue of dev expression in the sigD mutant was observed upon codevelopment with wild-type cells (Fig. 5). This is not due to a requirement for a higher level of C-signaling, since dev, but not W4403, was expressed in csgA mutant cells codeveloped with sigD mutant cells (serving as donor of a low level of C-signal) (Fig. 7). Rather, the developmental increase in dev transcription appears to require σD RNA polymerase or a gene under its control for a regulatory input distinct from that required by Ω4400, Ω4403, and Ω4499. Several possibilities are depicted by dashed lines in Fig. 8. One possibility is that dev transcription requires a higher level of the SdeK histidine kinase and/or a downstream component in the SdeK signal transduction pathway. Another possibility is that dev transcription requires a higher level of phosphorylated FruA (FruA-P). The idea that a lower level of FruA-P might suffice for expression of C-signal-independent genes and a higher level might be needed for expression of C-signal-dependent genes has been proposed previously (26, 72). Likewise, different levels of FruA-P might be necessary to activate different C-signal-dependent genes. A third intriguing possibility is that σD RNA polymerase transcribes dev, or that an unknown activator protein under σD control (Fig. 8, question mark) is needed for dev transcription. In any case, our analysis of late developmental gene expression in the sigD mutant separated C-signal-dependent genes into two distinct regulatory classes. The Ω7536 operon is in the class with the dev operon, as expected, because expression of Ω7536 depends on dev (46).
Role of σD in M. xanthus development compared with that of σ factors in other bacteria that form biofilms.
Like σS in E. coli, M. xanthus σD is important for cell viability in stationary phase (73). Also, the amino acid sequence of σD region 2 is similar to region 2 of stationary-phase σ factors (73). However, whereas σD clearly plays an overall positive role in M. xanthus development, the overall role of σS in E. coli biofilm formation can be positive (1) or negative (9), depending on the experimental conditions. This probably reflects the involvement of σS in a complex regulatory network governing E. coli biofilm formation (57).
σD also mediates the responses of M. xanthus to osmotic and oxidative stress (73). Osmotic stress induced biofilm formation by a Staphylococcus aureus mucosal isolate, and σB was essential for the response (58); however, in another study, σB was not essential for S. aureus biofilm formation (74). Here again, a complex regulatory network is involved (27). In Staphylococcus epidermidis, σB plays an overall positive role in biofilm formation, but this appears to involve production of a repressor of icaR, which encodes a negative regulator of genes required for biofilm formation (35). Likewise, in Bacillus subtilis, σH regulates many genes during the transition from stationary phase to sporulation or biofilm formation via Spo0A-mediated removal of AbrB repression (5, 15, 66, 67). It would not be surprising if the overall positive role of σD in M. xanthus development also involves removal of repression, as this appears to be a common theme in the regulation of genes required for biofilm formation. In any case, our results demonstrate that σD plays a key role in the complex regulatory network governing M. xanthus development.
Acknowledgments
We thank Y. Cheng, D. Kaiser, T. Ueki, S. Inouye, and L. Sogaard-Andersen for providing bacterial strains. The authors also thank M. E. Diodati and I. R. Jose for helpful discussions and technical assistance.
This research was supported by National Science Foundation grant MCB-0416456 to L.K., by National Institutes of Health grant GM54592 to M.S., and by the Michigan Agricultural Experiment Station.
REFERENCES
- 1.Adams, J. L., and R. J. McLean. 1999. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 65:4285-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aimes, B., and B. Dubin. 1960. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J. Biol. Chem. 235:769-775. [PubMed] [Google Scholar]
- 3.Biran, D., and L. Kroos. 1997. In vitro transcription of Myxococcus xanthus genes with RNA polymerase containing σA, the major sigma factor in growing cells. Mol. Microbiol. 25:463-472. [DOI] [PubMed] [Google Scholar]
- 4.Brandner, J. P., and L. Kroos. 1998. Identification of the Ω4400 regulatory region, a developmental promoter of Myxococcus xanthus. J. Bacteriol. 180:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton, R. A., P. Eichenberger, J. E. Gonzalez-Pastor, P. Fawcett, R. Monson, R. Losick, and A. D. Grossman. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 184:4881-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos, J. M., J. Geisselsoder, and D. R. Zusman. 1975. Isolation of bacteriophage Mx4, a generalized transducing phage of Myxococcus xanthus. J. Mol. Biol. 119:167-178. [DOI] [PubMed] [Google Scholar]
- 7.Cashel, M., and K. Rudd. 1987. The stringent response, p. 1410-1438. In F. C. Neidhardt, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 8.Cho, K., and D. R. Zusman. 1999. AsgD, a new two-component regulator required for A-signalling and nutrient sensing during early development of Myxococcus xanthus. Mol. Microbiol. 34:268-281. [DOI] [PubMed] [Google Scholar]
- 9.Corona-Izquierdo, F. P., and J. Membrillo-Hernandez. 2002. A mutation in rpoS enhances biofilm formation in Escherichia coli during exponential phase of growth. FEMS Microbiol. Lett. 211:105-110. [DOI] [PubMed] [Google Scholar]
- 10.Crawford, E. W., Jr., and L. J. Shimkets. 2000. The Myxococcus xanthus socE and csgA genes are regulated by the stringent response. Mol. Microbiol. 37:788-799. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, E. W., Jr., and L. J. Shimkets. 2000. The stringent response in Myxococcus xanthus is regulated by SocE and the CsgA C-signaling protein. Genes Dev. 14:483-492. [PMC free article] [PubMed] [Google Scholar]
- 12.Diodati, M. E., F. Ossa, N. B. Caberoy, I. R. Jose, W. Hiraiwa, M. M. Igo, M. Singer, and A. G. Garza. 2006. Nla18, a key regulatory protein required for normal growth and development of Myxococcus xanthus. J. Bacteriol. 188:1733-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworkin, M., and D. Kaiser. 1993. Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 14.Ellehauge, E., M. Norregaard-Madsen, and L. Sogaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30:807-817. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett, P., P. Eichenberger, R. Losick, and P. Youngman. 2000. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97:8063-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza, A. G., B. Z. Harris, B. M. Greenberg, and M. Singer. 2000. Control of asgE expression during growth and development of Myxococcus xanthus. J. Bacteriol. 182:6622-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garza, A. G., B. Z. Harris, J. S. Pollack, and M. Singer. 2000. The asgE locus is required for cell-cell signalling during Myxococcus xanthus development. Mol. Microbiol. 35:812-824. [DOI] [PubMed] [Google Scholar]
- 18.Garza, A. G., J. S. Pollack, B. Z. Harris, A. Lee, I. M. Keseler, E. F. Licking, and M. Singer. 1998. SdeK is required for early fruiting body development in Myxococcus xanthus. J. Bacteriol. 180:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisselsoder, J., J. M. Campos, and D. R. Zusman. 1978. Physical characterization of bacteriophage Mx4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:179-189. [DOI] [PubMed] [Google Scholar]
- 20.Gulati, P., D. Xu, and H. Kaplan. 1995. Identification of the minimum regulatory region of a Myxococcus xanthus A-signal-dependent developmental gene. J. Bacteriol. 177:4645-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, D., Y. Wu, and H. B. Kaplan. 2000. Identification and characterization of genes required for early Myxococcus xanthus developmental gene expression. J. Bacteriol. 182:4564-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao, T., D. Biran, G. J. Velicer, and L. Kroos. 2002. Identification of the Ω4514 regulatory region, a developmental promoter of Myxococcus xanthus that is transcribed in vitro by the major vegetative RNA polymerase. J. Bacteriol. 184:3348-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgkin, J., and D. Kaiser. 1977. Cell-to-cell stimulation of motility in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 74:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodgkin, J., and D. Kaiser. 1979. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol. Gen. Genet. 171:177-191. [Google Scholar]
- 26.Horiuchi, T., M. Taoka, T. Isobe, T. Komano, and S. Inouye. 2002. Role of fruA and csgA genes in gene expression during development of Myxococcus xanthus: analysis by two-dimensional gel electrophoresis. J. Biol. Chem. 277:26753-26760. [DOI] [PubMed] [Google Scholar]
- 27.Jefferson, K. K., D. B. Pier, D. A. Goldmann, and G. B. Pier. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 186:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaiser, D. 1984. Genetics of myxobacteria, p. 163-184. In E. Rosenberg (ed.), Myxobacteria, development and cell interactions. Springer-Verlag, N.Y.
- 29.Kaiser, D. 2004. Signaling in Myxobacteria. Annu. Rev. Microbiol. 58:75-98. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan, H. 2003. Multicellular development and gliding motility in Myxococcus xanthus. Curr. Opin. Microbiol. 6:572-577. [DOI] [PubMed] [Google Scholar]
- 32.Kashefi, K., and P. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthux frzF− defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 33.Keseler, I., and D. Kaiser. 1995. An early A-signal-dependent gene in Myxococcus xanthus has a σ54-like promoter. J. Bacteriol. 177:4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, S. K., and D. Kaiser. 1990. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell 61:19-26. [DOI] [PubMed] [Google Scholar]
- 35.Knobloch, J. K., S. Jager, M. A. Horstkotte, H. Rohde, and D. Mack. 2004. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σB by repression of the negative regulator gene icaR. Infect. Immun. 72:3838-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 37.Kroos, L., A. Kuspa, and D. Kaiser. 1990. Defects in fruiting body development caused by Tn5 lac insertions in Myxococcus xanthus. J. Bacteriol. 172:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 39.Kuspa, A., and D. Kaiser. 1989. Genes required for developmental signaling in Myxococcus xanthus: three asg loci. J. Bacteriol. 171:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 41.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signaling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kvint, K., A. Farewell, and T. Nystrom. 2000. RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of σS. J. Biol. Chem. 275:14795-14798. [DOI] [PubMed] [Google Scholar]
- 44.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, S.-F., B. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 46.Licking, E., L. Gorski, and D. Kaiser. 2000. A common step for changing cell shape in fruiting body and starvation-independent sporulation of Myxococcus xanthus. J. Bacteriol. 182:3553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobedanz, S., and L. Sogaard-Andersen. 2003. Identification of the C-signal, a contact-dependent morphogen coordinating multiple developmental responses in Myxococcus xanthus. Genes Dev. 17:2151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 49.Manoil, C., and D. Kaiser. 1980. Accumulation of guanosine tetraphosphate and guanosine pentaphosphate in Myxococcus xanthus during starvation and myxospore formation. J. Bacteriol. 141:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manoil, C., and D. Kaiser. 1980. Guanosine pentaphosphate and guanosine tetraphosphate accumulation and induction of Myxococcus xanthus fruiting body development. J. Bacteriol. 141:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nystrom, T. 2004. Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol. Microbiol. 54:855-862. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 53.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 54.Paul, B. J., W. Ross, T. Gaal, and R. L. Gourse. 2004. rRNA transcription in Escherichia coli. Annu. Rev. Genet. 38:749-770. [DOI] [PubMed] [Google Scholar]
- 55.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174:3311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollack, J. S., and M. Singer. 2001. SdeK, a histidine kinase required for Myxococcus xanthus development. J. Bacteriol. 183:3589-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen, A. A., and L. Sogaard-Andersen. 2003. TodK, a putative histidine protein kinase, regulates timing of fruiting body morphogenesis in Myxococcus xanthus. J. Bacteriol. 185:5452-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 61.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:63-71. [DOI] [PubMed] [Google Scholar]
- 62.Shimkets, L. J., and H. Rafiee. 1990. CsgA, an extracellular protein essential for Myxococcus xanthus development. J. Bacteriol. 172:5299-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singer, M., and D. Kaiser. 1995. Ectopic production of guanosine penta-and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 9:1633-1644. [DOI] [PubMed] [Google Scholar]
- 64.Sogaard-Andersen, L., M. Overgaard, S. Lobedanz, E. Ellehauge, L. Jelsbak, and A. A. Rasmussen. 2003. Coupling gene expression and multicellular morphogenesis during fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 48:1-8. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasan, D., and L. Kroos. 2004. Mutational analysis of the fruA promoter region demonstrates that C-box and 5-base-pair elements are important for expression of an essential developmental gene of Myxococcus xanthus. J. Bacteriol. 186:5961-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanley, N. R., R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley, N. R., and B. A. Lazazzera. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. 52:917-924. [DOI] [PubMed] [Google Scholar]
- 68.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 69.Sun, H., and W. Shi. 2001. Analyses of mrp genes during Myxococcus xanthus development. J. Bacteriol. 183:6733-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun, H., and W. Shi. 2001. Genetic studies of mrp, a locus essential for cellular aggregation and sporulation of Myxococcus xanthus. J. Bacteriol. 183:4786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thony-Meyer, L., and D. Kaiser. 1993. devRS, an autoregulated and essential genetic locus for fruiting body development in Myxococcus xanthus. J. Bacteriol. 175:7450-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueki, T., and S. Inouye. 2003. Identification of an activator protein required for the induction of fruA, a gene essential for fruiting body development in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100:8782-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ueki, T., and S. Inouye. 1998. A new sigma factor, SigD, essential for stationary phase is also required for multicellular differentiation in Myxococcus xanthus. Genes Cells 3:371-385. [DOI] [PubMed] [Google Scholar]
- 74.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 75.Velicer, G. J., L. Kroos, and R. E. Lenski. 2000. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404:598-601. [DOI] [PubMed] [Google Scholar]
- 76.Xu, D., C. Yang, and H. B. Kaplan. 1998. Myxococcus xanthus sasN encodes a regulator that prevents developmental gene expression during growth. J. Bacteriol. 180:6215-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, C., and H. B. Kaplan. 1997. Myxococcus xanthus sasS encodes a sensor histidine kinase required for early developmental gene expression. J. Bacteriol. 179:7759-7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoder, D., and L. Kroos. 2004. Mutational analysis of the Myxococcus xanthus Ω4400 promoter region provides insight into developmental gene regulation by C signaling. J. Bacteriol. 186:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoder, D., and L. Kroos. 2004. Mutational analysis of the Myxococcus xanthus Ω4499 promoter region reveals shared and unique properties in comparison with other C-signal-dependent promoters. J. Bacteriol. 186:3766-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]