Abstract
The tropomyosin fraction of shrimp proteins is potentially responsible for allergic reaction in individuals with genetic predisposition to allergy. However, there are no efficient and safe methods to reduce its allergenicity. High intensity ultrasound is known to change the structure of proteins. This study is aimed at assessing high intensity ultrasound’s effect on the allergenicity of shrimp allergen. Shrimp and purified shrimp allergen were treated with high intensity ultrasound for 30~180 min. Extracts of treated samples were analyzed by enzyme-linked immunosorbent assay (ELISA) with pool serum of shrimp allergy patients and polyclonal anti-allergen antibodies and by immunoblotting after polyacrylamide gel electrophoresis. Shrimp treated with high intensity ultrasound showed a decrease in allergenicity measured with ELISA. A linear relationship between the immune response induced by treated shrimp allergen and the applied treatment time was observed. The decrease in allergenicity was confirmed by immunoblot assays with shrimp allergic patients serum. Allergenicity of shrimp allergen extracted from treated shrimp was higher than that of purified shrimp allergen with the same treatment time. Gel-filtration HPLC was applied for analysis of shrimp allergen after treatment with high intensity ultrasound. Some fractions were appeared with increasing treatment time. The results suggested that high intensity ultrasound could be used to reduce the allergenicity of shrimp.
Keywords: Shrimp, Allergen, Allergenicity, High intensity ultrasound
INTRODUCTION
Food allergies are common symptoms in most parts of the world. Allergy induced by ingestion of food causing severe hypersensitive reactions in humans, was frequently reported (Daul et al., 1990; 1993). Among allergy causing foods, shrimp widely consumed as nutritional food is one of the most important (Schafer et al., 2001). Reducing the allergenicity of shrimp allergen will help decrease individuals’ sensitivity to shrimp.
Among several shrimp allergens reported (Daul et al., 1994), the major shrimp allergen (Pen a 1) was identified as tropomyosin, a regulatory protein in muscle (Bailey, 1946). At least 80% of shrimp-allergy subjects reacted to the major allergen, which is bound to approximately 85% of shrimp-specific IgE from shrimp-allergy subjects (Leung et al., 1994). Several studies showed that the major shrimp allergen (Besler et al., 2001; Hoffman et al., 1981; Hefle, 1996) is stable and keeps its activity even after being boiled in water.
Since Hoffman et al.(1981) first isolated and partially characterized a major shrimp allergen, many methods have been tried to reduce the allergenicity of shrimp (Lee and Song, 2002; Byun et al., 2002). Among these methods, gamma irradiation was regarded as an effective way to reduce the allergenicity of shrimp allergens. But this technology needs large investment and encounters some consumer resist and to its use in the food industry. Some researchers reported that shrimp allergen was liable to enzyme hydrolysis (Shimakura et al., 2005). It is important to explore efficient ways to reduce the allergenicity of shrimp allergens.
High-intensity ultrasound is an efficient food processing and preservation technology, used successfully for homogenizing emulsions, deactivating enzyme, enhancing extraction processes, accelerating dehydration, ageing, and ripening processes (Villamiel and Jong, 2000; Graham and Phillips, 1979). However little known about the effect of high-intensity ultrasound on food allergenicity. Application of high-intensity ultrasound causes chemical and physical changes in a viscous medium by cyclic generation and collapse of cavities (Gunasekaran, 1994). Increased pressure and temperature in the vicinity of these cavities cause the observed chemical and mechanical effects, resulting in changing the native protein structure into a molten globule state and even degradation (Fukase et al., 1994). This paper postulates that protein structure changes induced by high-intensity ultrasound might affect the allergenicity of shrimp allergens. This research was aimed at determining if high-intensity ultrasound can be used to reduce the allergenicity of shrimp.
MATERIALS AND METHODS
Reagents
Shrimp (Penaeus vannamei) were purchased in the local market. Unless otherwise stated, all reagents were of analytical grade. Buffers and reagents used for Western blotting were as follows: PBS: 10 mmol/L phosphate buffer, pH 7.4, 0.15 mol/L NaCl; PBST: 10 mmol/L phosphate buffer, pH 7.4, 0.15 mol/L NaCl, 0.05% Tween 20. Buffers and reagents used for indirect ELISA were as follows: Blocking buffer: 0.01 mol/L phosphate buffer, pH 7.4, containing 0.1% BSA (bovine serum albumin), 0.15 mol/L NaCl; washing buffer (PBST): 0.01 mol/L phosphate buffer, pH 7.4, containing 0.05% Tween 20. Goat anti-human IgE antibody conjugated with peroxidase and goat anti-rabbit IgG antibody conjugated with peroxidase (Sigma, Missouri, USA) was used in ELISA assay. Solid-phase enzyme immunoassays were performed in 96-well microtiter plates (Nunc, Denmark) using Multiskan MK3 ELISA reader (Thermo Labsystems).
Purification of shrimp allergen
Shrimp allergen was separated and purified by a combination of ammonium sulfate and isoelectric precipitation (Asturias et al., 1999). In brief, shrimp muscle (5 g) was mashed and incubated in 50 ml extraction buffer (1 mol/L KCl and 0.5 mmol/L DTT (1,4-dithiothreitol), pH 7.0) for 16 h at 4 °C with constant stirring. After centrifugation at 12000×g for 15 min at 4 °C, the supernatant was dialyzed (12 000~14 000, Union Carbide Corporation) for 48 h at 4 °C against 10 mmol/L PBS, pH 7.0, and then the resultant was precipitated with 35%~60% saturated ammonium sulfate; the precipitation was dissolved in 0.01 mol/L PBS, pH 7.0 and dialyzed (12 000~14 000, Union Carbide Corporation) against 0.01 mol/L PBS, pH 7.0. Further purification was obtained after isoelectric precipitation at pH 4.6 and dissolution of the precipitation in distilled water (pH 7.0) and dialyzed (12 000~14 000, Union Carbide Corporation) against distilled water (pH 7.0); The solution was lyophilized and stored at −20 °C.
Shrimp allergen antibodies
Fifteen patients were recruited at the Qingdao Municipal Hospital (Qingdao, China). All the patients had confirmed shrimp allergy on the basis of extensive history, physical examination, skin prick testing, and objective manifestations observed after shrimp ingestion. The study population was all 18 years old. Three healthy subjects without shrimp-allergy were used as negative controls. All 15 shrimp allergy patients had a history of atopic disease, with 67% having a history of asthma, 60% of allergic rhinitis, and 20% of atopic dermatitis. All patients are volunteers.
Anti-allergen (shrimp) polyclonal antibodies were produced by giving an initial injection of 0.5 mg antigen in Freund’s complete adjuvant to the rabbits. After boosting three times (at 0.5 mg per boost in incomplete Freund’s adjuvant at 2~3 week intervals), the rabbits were bled and the resultant sera were stored at −20 °C until further use.
Treated with high intensity ultrasound
Broken shrimp (Penaeus vannamei) muscle and purified shrimp allergen (2 mg/ml in PBS) were sealed in a plastic bag and subjected to high intensity ultrasound treatments (30 Hz, 800 W) for 30~180 min. After treatment, 2 g shrimp muscle was extracted with 0.01 mol/L PBS for 30 min. The extraction was centrifuged at 5000×g and the supernatant was freeze-dried for further use. Concentration of protein in the extracts was determined using Bio-Rad (Bradford, 1976).
ELISA for protein extracts
Microtiter plates were coated overnight at 4 °C with 100 μl of protein extracts in 0.1 mol/L carbonate buffer (pH 9.6), containing about 2.0 μg protein. The plates were washed and free binding sites were blocked with blocking buffer for 2 h at 37 °C. This was followed by removal of buffer solution, rinsing of the plates three times and further incubation with pool serum (1:5 dilution with blocking buffer) or polyclonal antibodies (1:104 dilution with blocking buffer) overnight at 4 °C. The plates were washed again and 100 μl of 2000-fold diluted solution of antibody (goat anti-human IgE antibody or goat anti-rabbit IgG antibody) conjugated with peroxidase was added. After incubation of the plates for 2 h and rinsing with PBST, the bound peroxidase activity was determined by reaction with TMB (3,3′,5,5′-tetramethylbenzidine). All ELISA experiments were performed in triplicate, with and the data given in mean values.
Electrophoresis and immunoblotting
Denaturing protein electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (1970). Samples (50 μg per well) were mixed 4:1 with loading buffer (2% SDS, 25% glycerol, 14.4 mmol/L β-mercaptoethanol, and 0.1% bromphenol blue in 1 mol/L Tris-HCl, pH 6.8), heated at 100 °C for 6 min, electrophoresed in 12% analytical SDS-polyacryl-amide gels employing a vertical electrophoresis system (Bio-Rad) according to the manufacturer’s recommendations, and either stained with Coomassie Brilliant Blue R-250 (Smith et al., 1988) or transferred to NC (nitrocellulose membrane, 450 nm, Pall-Gelman, USA). For immunoblot, proteins were electrophoretically transferred from the gels to NC by applying a constant current of 8.0 mA/cm2 for 2 h at room temperature, essentially according to the method of Towbin and Gordon (1984). A piece of membrane was stained with amido-black to verify protein transfer. Blots were blocked with a 0.5% BSA in PBST (pH 7.4) for 1.5 h at 37 °C, and then incubated with pooled sera (1:5 dilution with blocking buffer) overnight at 4 °C. A polyclonal goat anti-human IgE antibody conjugated with peroxidase was used as second antibody (diluted 1:2000 with blocking buffer). Finally, immunoreactive bands were visualized using DAB (Diaminobenzidine). Nonspecific binding of the anti-IgE antibody conjugate was measured in a similar blotting procedure, omitting the incubation step with patient sera.
Coomassie-stained gels and immunostained membranes were scanned using a GIS 2009 densitometer (Tanon), and the generated files were analyzed with Quantity One software (Tanon) using the low-range prestained SDS-PAGE protein mixture (Tanon) as standard.
HPLC analysis
The HPLC analyses (Shimakura et al., 2005) were performed on a gel filtration HPLC with a TSKgel G3000SW (0.75 cm×30 cm; Tosoh, Tokyo, Japan). The elution system was 0.01 mol/L PBS (pH 7.4) containing 0.15 mol/L NaCl. The flow rate was 0.5 ml/min and the analysis was conducted at 20 °C. Protein was monitored at 220 nm with a UV detector. Shrimp allergen was prepared by dissolving 1 mg protein in 1 ml 0.01 mol/L PBS (pH 7.4) and injected on to the column (20 μl). Data acquisition was implemented using Agilent 1100 software.
RESULTS AND DISCUSSION
Allergenicity of treated shrimp allergen
The effect of high intensity ultrasound on allergenicity of shrimp allergen was measured by ELISA using specific IgE from pool serum of shrimp allergic patients. For comparison, the allergenicity of allergen extracted from treated shrimp was also investigated. Pure allergen and shrimp muscle were treated for 130 min to 180 min with high intensity ultrasound. Before extraction, the treated shrimp was stored at 4 °C. The allergenicity of treated allergen was calculated in relation to the immune response of untreated allergen and shrimp protein extracts. The results are expressed as a percentage of the immune response of untreated allergen and are presented in Table 1.
Table 1.
Effect of high intensity ultrasound on shrimp allergen allergenicity determined by ELISA with pool serum of shrimp allergic patients and with polyclonal antibodies against shrimp allergen
| Treatment time (min) | Allergenicity of shrimp allergen determined by ELISA with pool serum of shrimp allergic patients (%) |
Allergenicity of shrimp allergen determined by ELISA with polyantibodies (%) | |
| Shrimp allergen | Allergen from shrimp muscle | ||
| 0 | 100±4.7 | 100±5.2 | 100±5.7 |
| 30 | 87.1±4.5 | 91.8±4.7 | 89.2±4.1 |
| 60 | 62.3±3.6 | 82.4±4.2 | 71.4±3.9 |
| 120 | 32.2±4.3 | 63.9±5.9 | 42.9±3.8 |
| 180 | 18.7±5.1 | 31.1±2.7 | 25.3±3.1 |
Note: Data are expressed as ratio of IC50 of processed/IC50 to unprocessed samples (mean±SD)
Elisco showed that the treated shrimp allergen had lower allergenicity ELISA to pool serum from shrimp allergic patients than that of untreated samples. The decrease in allergenicity was closely related to the applied time. Furthermore, the decrease rate of treated pure allergen was higher than that of treated shrimp muscle. This phenomenon may be explained by the protection provided by other shrimp components to the allergen. The decrease in allergenicity of treated allergen was also confirmed by ELISA using polyclonal anti-allergen antibodies (Table 1). For all methods applied, a linear relationship between allergen allergenicity and treated time was observed. The standard deviations of immune-response measurements results were the 2.7%~5.9%.
Immunoblot analysis of reactivity of treated shrimp allergen
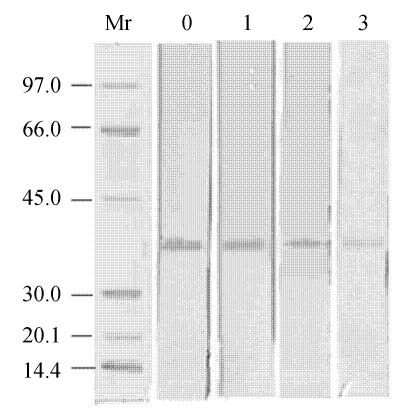
The decrease in allergenicity of treated allergen was also confirmed by immunoblotting analyses. Electrophoretically separated extracts from treated allergen samples were tested for their allergenicity with two human sera of shrimp allergy patients. Fig.1 shows immunoblotting results, supporting the conclusion that treated allergen exhibit decreased immune response. Each line shown corresponds to allergens treated for different time by the serum S199. Dried blots were then densitometrically scanned and the intensity of dark bands was measured. The results of these determinations are presented in Table 2. Although clearly different levels of integrated optical density (IOD) were obtained for both sera, e.g., for untreated allergen, IOD was equal to 250.3 for S199 and 112.4 for S250, respectively. The two sera’s percentage of allergenicity decrease rate was similar for both sera in the whole dose range. Further, the decrease in allergenicity tested by the immunoblot approach was evidently higher than the decrease obtained by ELISA for equal treatment time, because of the different immunological reactions to allergen due to the individual responses of allergic patients and to the different levels of reactive antibodies. Another reason was that some linear epitopes separated from the allergen when they treated with high intensity ultrasound. These epitopes cannot be detected by immunoblotting, but can be measured by ELISA (Lee et al., 2001).
Fig. 1.
Immunoblot analysis of the shrimp allergen treated with high intensity ultrasound determined using human scrum (S199) with allergy to shrimp
Treated time: 0, control shrimp allergen; 1, 60 min; 2, 120 min; 3, 180 min; Mr, molecular standard
Table 2.
Allergenicity of shrimp allergen treated with high intensity ultrasound determined by immunoblot assay with serum from two shrimp allergy patients (S199 and S250) respectively
| Treatment time (min) | S199 |
S250 |
||
| IODa | Percent | IOD | Percent | |
| 0 | 250.3 | 100 | 112.4 | 100 |
| 30 | 185.4 | 74.1 | 88.0 | 78.3 |
| 60 | 103.4 | 41.3 | 42.8 | 38.1 |
| 120 | 49.3 | 19.7 | 23.9 | 21.3 |
| 180 | 30.3 | 12.1 | 12.9 | 11.5 |
IOD: Integrated optical density; units obtained by scanning densitometry
HPLC separation of shrimp allergen
HPLC methods have been successfully applied for qualitative analysis of shrimp allergen (Ishikawa et al., 1997). It was interesting to evaluate the effects of high intensity ultrasound on the allergenicity of shrimp allergen.
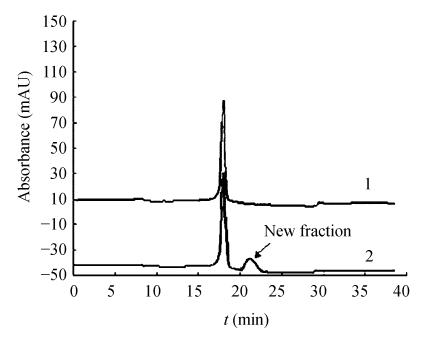
HPLC elution profiles of allergen treated with high intensity ultrasound for 180 min and of untreated allergen are presented in Fig.2. The chromatogram of control allergen, had only one peak representing the allergen (Rt. 18 min). The absorbance units at the wavelengths region from 210~220 nm are highly correlated with the amount of protein (Wieser et al., 1988), so the relative quantities of allergen could be derived from the areas of peaks in the chromatogram. After treatment with high intensity ultrasound, a new material with low molecular weight appeared, which increased with treatment time. The allergenicity of the new fraction is very low as determined with ELISA. The observed changes in the fraction of allergen may imply changes in the allergenicity of the treated allergen.
Fig. 2.
Gel filtration HPLC of shrimp allergen treated with high intensity ultrasound
1: Untreated shrimp allergen (control); 2: Treated with high intensity ultrasound (180 min)
In conclusion, the results of these experiments showed that shrimp allergen treated with high intensity ultrasound exhibited decreased allergenicity as measured by ELISA with pool serum of shrimp allergy patients and polyclonal antibodies. This decrease in allergenicity was confirmed by immunoblot assay with human sera from shrimp allergy patients. The decrease in allergenicity determined by the immunoblot approach was evidently higher than the decrease obtained by ELISA for equal treatment time. Allergenicity of allergen extracted from shrimp treated with high intensity ultrasound decreased less than that of treated pure allergen. A linear relationship between the decrease in allergenicity and treatment time was observed. The results obtained showed that high intensity ultrasound seem to be an efficient way to reduce the allergenicity of food, though further studies are needed to assess the clinical relevance of this finding.
Acknowledgments
We thank Dr. Zhao in the Qingdao Municipal Hospital for supplying the shrimp allergic sera.
Footnotes
Project (No. 30471320) supported by the National Natural Science Foundation of China
References
- 1.Asturias JA, Gomez-Bayon N, Arilla MC, Martinez A, Palacios R, Sanchez-Gascon F, Martinez J. Molecular characterization of American cockroach tropomyosin (Periplaneta americana Allergen 7), a cross-reactive allergen. J Immunol. 1999;162:4342–4348. [PubMed] [Google Scholar]
- 2.Bailey K. Tropomyosin a new asymmetric protein component of muscle. Nature. 1946;157:368. doi: 10.1038/157368b0. [DOI] [PubMed] [Google Scholar]
- 3.Besler M, Steinhart H, Paschke A. Stability of food allergens and allergenicity of processed foods. J Chromatogr B. 2001;756(1-2):207–228. doi: 10.1016/s0378-4347(01)00110-4. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Byun MW, Lee JW, Yook HS, Jo CR, Kim HY. Application of gamma irradiation for inhibition of food allergy. Radiat Phys Chem. 2002;63(3-6):369–370. doi: 10.1016/S0969-806X(01)00528-X. [DOI] [Google Scholar]
- 6.Daul CB, Morgan JE, Lehrer SB. The natural history of shrimp hypersensitivity. J Allergy Clin Immunol. 1990;86(1):88–93. doi: 10.1016/s0091-6749(05)80127-7. [DOI] [PubMed] [Google Scholar]
- 7.Daul CB, Morgan JE, Lehrer SB. Hypersensitivity reactions to crustacea and mollusks. Clin Review Allergy. 1993;11(2):201–222. doi: 10.1007/BF02914471. [DOI] [PubMed] [Google Scholar]
- 8.Daul CB, Stattery M, Reese G, Lehrer SB. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994;105:49–55. doi: 10.1159/000236802. [DOI] [PubMed] [Google Scholar]
- 9.Fukase H, Ohdaira E, Masuzawa N, Ide M. Effect of ultrasound in soybean protein extraction. Japanese J Applied Physics Part 1—Regular Papers Short Notes and Review Papers. 1994;33(5B):3088–3090. [Google Scholar]
- 10.Graham DE, Phillips MC. Proteins at liquid interfaces. 1. Kinetics of adsorption and surface denaturation. J Colloid Interface Sci. 1979;70(3):403–415. doi: 10.1016/0021-9797(79)90048-1. [DOI] [Google Scholar]
- 11.Gunasekaran SCA. Evaluating milk coagulation with ultrasonic. Food Technol. 1994;48(12):74–78. [Google Scholar]
- 12.Hefle SL. The chemistry and biology of food allergens. Food Technol. 1996;50:86–92. [Google Scholar]
- 13.Hoffman DR, Day ED, Miller JS. The major heat stable allergen of shrimp. Ann Allergy. 1981;47:17–22. [PubMed] [Google Scholar]
- 14.Ishikawa M, Shimakura K, Nagashima Y, Shiomi K. Isolation and properties of allergenic proteins in the oyster (Crassostrea gigas) Fisheries Science. 1997;63:610–614. [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Song KB. Effect of gamma-irradiation on the molecular properties of myoglobin. J Biochem Mol Biol. 2002;35(6):590–594. doi: 10.5483/bmbrep.2002.35.6.590. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Kim JH, Yook HS, Kang KO, Lee SY, Hwang HJ, Byun MW. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J Food Prot. 2001;64:272–276. doi: 10.4315/0362-028x-64.2.272. [DOI] [PubMed] [Google Scholar]
- 18.Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, Nagy SM, Gershwin ME. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994;94(5):880–890. doi: 10.1016/0091-6749(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 19.Schafer T, Bohler E, Ruhdorfer S. Epidemiology of food allergy/food intolerance in adults: associations with other manifestations of atopy. Allergy. 2001;56(12):1172–1179. doi: 10.1034/j.1398-9995.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimakura K, Tonomura Y, Hamada Y, Shiomi K. Allergenicity of crustacean extractives and its reduction by protease digestion. Food Chemistry. 2005;91(2):247–253. doi: 10.1016/j.foodchem.2003.11.010. [DOI] [Google Scholar]
- 21.Smith I, Cromie R, Stainsby K. Seeing gel wells well. Anal Biochem. 1988;169(2):370–371. doi: 10.1016/0003-2697(88)90297-7. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Gordon J. Immunoblotting and dot immunobinding: current status and outlook. J Immunol Methods. 1984;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Villamiel M, Jong P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat proteins, and native enzymes of milk. J Agri Food Chem. 2000;48(2):472–478. doi: 10.1021/jf990181s. [DOI] [PubMed] [Google Scholar]
- 24.Wieser H, Antes S, Seilmeier W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem. 1988;75(5):644–650. [Google Scholar]