Abstract
To develop a gene therapy strategy for treating bovine mastitis, a new mammary-specific vector containing human lysozyme (hLYZ) cDNA and kanamycin resistance gene was constructed for intramammary expression and clinical studies. After one time acupuncture or intracisternal infusion of healthy cows with 400 μg of the p215C3LYZ vector, over 2.0 μg/ml of rhLYZ could be detected by enzymatic assay for about 3 weeks in the milk samples. Western blotting showed that rhLYZ secreted into milk samples from the vector-injected cows had molecular weight similar to that of the natural hLYZ in human colostrums. Twenty days after the primary injection, the quarters were re-injected with the same vector by quarter acupuncture and even higher concentrations of rhLYZ could be detected. Indirect competitive ELISA of milk samples showed that the vector injection did not induce detectable humoral immune response against hLYZ. Clinical studies showed that twice acupuncture of quarters with the p215C3LYZ vector had overt therapeutic effect on clinical and subclinical mastitis previously treated with antibiotics, including disappearance of clinical symptoms and relatively high microbiological cure rates. These data provide a solid rationale for using the vector to develop gene therapy for treating bovine mastitis.
Keywords: Human lysozyme cDNA, Mammary-specific vector, Expression, Mastitis, Gene therapy
INTRODUCTION
Bovine mastitis causes significant economic losses due to lowered production, rejected milk and drug costs. Although significant progress in mastitis control has been made, many dairy herds continue to be plagued by this disease due to the complexities of bovine udder anatomy and physiopathology (Gruet et al., 2001). The present strategies for mastitis control include systemic or intramammary administration of antibiotics during lactation and at drying off. One of the main problems associated with the antimicrobial therapy is increasing prevalence of antibiotics-resistant bacteria, which not only compromises the effectiveness of the treatment of the disease, but also poses potential risk of transmission of resistant bacteria to human beings. Therefore, considerable international pressure has been applied to limit antimicrobial therapy and to develop new therapeutic strategies for mastitis control (Erskine et al., 2004).
Lysozyme (LYZ) is a well-known muramidase with potent antibacterial activities against a variety of microorganisms (Fleming, 1922). The lytic mechanisms for bacterial killing by LYZ include enzymic peptidoglycan hydrolysis of bacterial wall and induction of autolysins that can cause bacterial autolysis. The non-lytic mechanisms include induction of membrane perturbation of its targets through binding of a certain domain to and interaction with bacterial surface. In addition, both heat-denatured and recombinant LYZ devoid of enzymic activity also has been shown to have strong bactericidal activities independent of catalytic functions (Chen et al., 2005).
Our initial interest was to generate mammary gland bioreactors expressing hLYZ. To this end, a mammary-specific expression vector, called p205C3LYZ, was constructed using the regulatory regions of the β-lactoglobulin gene and the first intron of the β-casein gene of dairy goat for mammary-specific expression of hLYZ cDNA (Li et al., 2004). Our previous studies showed that injection of quarters of lactating dairy cows with the construct has overt therapeutic effect on naturally-occurring mastitis (Sun et al., 2004), which warrants us to further investigate the feasibility of the strategy for treating bovine mastitis. In those experiments, however, the rationale behind the therapeutic effect was not defined and the vector used the ampicillin resistance-conferring β-lactamase (bla) gene as the selection marker, which is discouraged in clinical studies (Murphy and Epstein, 1998). In this experiment we modified the mammary-specific expression vector by replacing its bla gene with the more acceptable kanamycin (kan) resistance gene (Norman et al., 1997). Following intramammary injection with the new vector, a relatively long-lasting and high level expression of rhLYZ was detected in milk from healthy lactating cows and a clear therapeutic effect was demonstrated in the cows with clinical and subclinical mastitis. These data provide a solid rationale for using the construct to develop gene therapy strategy for treating bovine mastitis.
MATERIALS AND METHODS
Vector construction
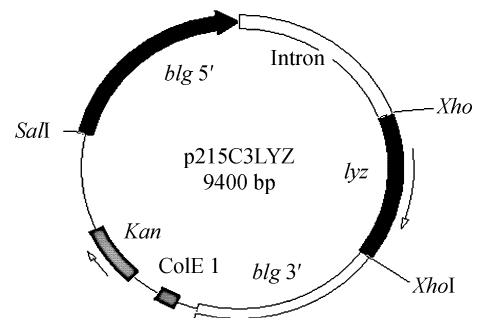
Construction of the mammary-specific expression vector p205C3LYZ described by Li et al.(2004), used the 5′- and 3′-regulatory elements of goat β-lactoglobulin (blg) gene and the first intron of the β-casein gene of dairy goat to control hLYZ cDNA expression and bla gene as the selection marker. To construct a new vector more acceptable for clinical use, eukaryotic expression vector pcDNA3 (invitrogen) was digested with restriction enzymes EcoV and PvuII to remove its neo gene and other unnecessary sequences and the bla gene was replaced with kan resistance gene amplified by PCR from pET-3a vector (Bio-Rad). The hLYZ cDNA expression cassette from the p205C3LYZ vector was inserted into the modified pcDNA3 vector as a SalI/HindIII fragment. The effectiveness of the new mammary-specific vector, designated p215C3LYZ (Fig.1), was confirmed by restriction digestion and PCR analysis. The hLYZ cDNA was also sub-cloned into eukaryotic expression vector pcDNA3 (under the control of cytomegalovirus or CMT promoter) and the recombinant vector pcDNAKLYZ was used as the control.
Fig. 1.
Schematic representation of the mammary-specific expression vector p215C3LYZ
Vector preparation
The single colonies of DH5α E. coli transformants of p215C3LYZ, the control vector pcDNAKLYZ and empty vector p215C3 were cultured overnight at 37 °C with shaking in Loria broth (LB) containing 50 μg/ml kanamycin or ampicillin. The plasmid DNAs were prepared by standard alkaline lysis (Sambrook and Russell, 2002), purified by cetyltrimethylammonium bromide (CTAB) precipitation (Lander et al., 2002), and dissolved in 0.15 mol/L sodium phosphate solution at a final concentration of 1 mg/ml before use.
Vector injection
For expression studies, milk samples were collected from healthy lactating cows from the Yangzhou University Dairy Farm and somatic cell counts (SCC) were determined using the standard California mastitis test (CMT) (Middleton et al., 2004). Twenty-four quarters of clinically healthy cows with negative CMT score (SCC≤100 000 cells/ml) were chosen. Before intracisternal injection, 25 ml of phosphate buffered saline (PBS) was infused into each cistern, disposed following gentle massage, and then 400 μl (400 μg) of the p215C3LYZ vector (5 quarters), control vector pcDNAKLYZ (5 quarters) or empty vector p215C3 (2 quarters) was injected into each quarter cistern through the teat canal. For quarter acupuncture, 400 μl (400 μg) of the p215C3LYZ vector (5 quarters), pcDNAKLYZ vector (5 quarters) or empty vector (2 quarters) was injected aseptically through skin into each quarter using a syringe with a 10-cm needle. For p215C3LYZ-injected (by acupuncture) cows, the secondary vector injection was performed 20 d after the primary injection using the same method.
For gene therapy studies, 7 quarters with clinical mastitis (characterized by udder redness, swelling, hardness, pain and grossly abnormal milk) and 9 quarters with subclinical mastitis (without visual symptoms but SCC≥2 700 000 cells/ml) were chosen based on clinical examination and somatic cell counting of milk samples by CMT. The retrospective data showed that these cows had a long mastitis history and were treated at least once with 750 000 U ampicillin plus 250 000 μg streptomycin per quarter. After milking, 400 μl (400 μg) of the p215C3LYZ vector was injected aseptically into each quarter by acupuncture and the injection was repeated once on the following day.
Enzymatic assay
About 10 ml of milk samples were aseptically collected from each quarter 0, 2, 4, 6, 8 10, 15 and 20 d after vector injection and defatted by twice centrifugation at 4 000 r/min for 5 min following dilution with one-fold of distilled water. LYZ concentrations were measured using remazol brilliant blue R (RBB-R)-labelled colorimetric assay (Ito et al., 1992). Briefly, 700 μl of RBB-R-labelled Micrococcus lysodeikticus in KH2PO3-NaOH solution, pH 7.0 was transferred to each Eppendorf tube and pre-warmed to 40 °C. One hundred microlitres of purified hLYZ (Calbiochem-Novabiochem Corporation) of different concentrations (for generation of standard curve) or defatted milk sample was added and incubated at 40 °C for 30 min. Finally, 50 μl of 1 mol/L NaOH was added to stop the reaction. Following centrifugation at 1500 r/min for 5 min, OD 600 values of the supernatants were read on spectrometer. Averaged concentrations of LYZ were calculated from the standard curve and expressed as μg/ml.
Antibody assay
After primary injection with p215C3LYZ vector, milk samples were collected from three different cows at day 1 and weeks 1, 2, 3, 5 and 7, and defatted by centrifugation at 4000 r/min for 5 min. The milk samples were then assayed for hLYZ-specific antibody by indirect competitive ELISA according to published method (Hurley et al., 2004) using purified human LYZ as the coating antigen, rabbit anti-hLYZ serum as the indicator antibody, and HRP-labelled goat anti-rabbit IgG as the second antibody.
Western blotting
At day 4 after vector injection, milk samples were collected from the healthy cow quarters and centrifuged for 10 min at 4000 r/min. One microlitre of the defatted milk sample was adjusted to pH 4.3 with 1 mol/L HCl and incubated for 20 min at 4 °C to precipitate down most of the milk proteins. After additional centrifugation for 10 min at 4000 r/min, the supernatant was adjusted to pH 8.0 with 1 mol/L NaOH and 40 μl of the sample was mixed with 10 μl of 5×SDS-PAGE loading buffer. Normal cow milk, human colostrums containing natural hLYZ and mouse milk before and after p215C3LYZ injection were treated similarly and used as the controls. Following boiling for 5 min and brief centrifugation, 20 μl of each sample was loaded and separated by 12% SDS-PAGE. Following transfer onto nitrocellulose membrane, Western blotting was performed using rabbit anti-human lysozyme serum as the first antibody (1:50 in blocking buffer) and IRDye™ conjugated goat anti-rabbit IgG (Rockland, USA) as the secondary antibody (1:10 000 in blocking buffer) according to the manufacturer’s instructions. The signals were scanned at 800 nm on Odyssey Infrared Imaging System (LI-COR, Biosciences) according to the manufacturer’s instructions.
Bacteriological test
One day before and 10 d after vector injection, milk samples were collected from the p215C3LYZ-injected quarters and 50 μl of each milk sample was spread on blood agar plate immediately upon return to the laboratory. The plates were incubated for 24 h at 37 °C and bacteria were initially identified by colony characteristics. Gram-negative organisms were further identified by biochemical tests, streptococcal organisms by catalase, Gram-stain, esculin and CAMP reactions, and staphylococcal organisms by the tube-coagulase test and haemolytic patterns.
Cure definitions
Outcomes examined included CMT score, clinical cure and microbiological cure. A clinical cure was defined as no visual mastitis symptoms and clots or flakes in the milk 10 d after vector injection. A microbiological cure was defined as no growth of the originally isolated pathogen 10 d after vector injection. CMT score decreased from “+++” or “++” to “+” was defined as improved and to “−” as cured 10 d after vector injection.
RESULTS
Vector preparation
The purified plasmid DNAs were analyzed by spectrometry with an OD 260/OD 280 ratio of 1.75. Agarose gel electrophoresis showed that 95% of the plasmid DNAs was supercoiled without visible contamination of bacterial genomic DNA and RNA (data not shown). After primary and secondary vector injection, all cows had no visual clinical and milk abnormalities.
Expression of p215C3LYZ after primary injection by acupuncture
After one time injection by quarter acupuncture with the mammary-specific vector p215C3LYZ and the control vectors p215C3 and pcDNAKLYZ, milk samples were collected at different days for enzymatic assay. As Table 1 shows, following injection with the empty vector p215C3, no increase in enzymatic activity could be detected. After injection with p215C3LYZ vector, however, concentrations of LYZ in the milk samples started to increase from day 2 and reached the highest-level at day 4, followed by a gradual decrease to the prior injection level by day 20 post-injection. Both duration and highest levels of rhLYZ expression after p215C3LYZ injection were similar to that after pcDNAKLYZ injection.
Table 1.
Expression of the hLYZ cDNA in mammary gland after primary injection by acupuncture
| Vector* | LYZ concentration after vector injection (μg/ml) |
|||||||
| 0 d | 2 d | 4 d | 6 d | 8 d | 10 d | 15 d | 20 d | |
| p215C3 | 0.6±0.1 | 0.7±0.1 | 0.7±0.0 | 0.7±0.2 | 0.7±0.0 | 0.7±0.0 | 0.8±0.1 | 0.6±0.1 |
| p215C3LYZ | 0.3±0.1 | 1.8±0.5 | 2.6±0.7 | 2.3±0.8 | 1.6±0.3 | 1.6±0.4 | 0.9±0.2 | 0.5±0.1 |
| pcDNAKLYZ | 0.2±0.0 | 1.9±0.6 | 2.8±0.7 | 2.3±0.6 | 1.6±0.3 | 1.0±0.3 | 1.0±0.2 | 0.6±0.2 |
n=2 for p215C3; n=5 for p215C3LYZ and pcDNAKLYZ
Expression of p215C3LYZ after primary intracisternal injection
After one time intracisternal injection with the mammary-specific expression vector p215C3LYZ and the control vectors p215C3 and pcDNAKLYZ, milk samples were collected at different days for enzymatic assay. As Table 2 shows, concentrations of LYZ in the milk samples from p215C3LYZ-injected quarters started to increase from day 2 and reached the highest-level at day 4, followed by a gradual decrease to the prior injection level by day 20 post-injection. Both duration and highest level of rhLYZ expression after p215C3LYZ injection were also similar to those after pcDNAKLYZ injection.
Table 2.
Expression of the hLYZ cDNA in mammary gland after primary intracisternal injection
| Vector* | LYZ concentration after vector injection (μg/ml) |
|||||||
| 0 d | 2 d | 4 d | 6 d | 8 d | 10 d | 15 d | 20 d | |
| p215C3 | 0.6±0.1 | 0.7±0.1 | 0.7±0.0 | 0.7±0.2 | 0.7±0.0 | 0.7±0.0 | 0.8±0.1 | 0.6±0.1 |
| p215C3LYZ | 0.2±0.1 | 1.6±0.7 | 2.0±0.5 | 1.2±0.2 | 1.1±0.3 | 1.0±0.3 | 0.8±0.5 | 0.4±0.0 |
| pcDNAKLYZ | 0.2±0.0 | 2.2±0.1 | 2.3±0.4 | 1.6±0.1 | 1.4±0.0 | 1.4±0.1 | 1.1±0.0 | 0.7±0.1 |
n=2 for p215C3; n=5 for p215C3LYZ and pcDNAKLYZ
Dose-dependent expression of p215C3LYZ in mammary gland
To investigate whether the expression of p215C3LYZ vector in mammary gland was dose-dependent, 200 or 400 μg of the vector DNA was injected into each quarter by acupuncture and milk samples were collected at different days after vector injection. Enzymatic assay of milk samples showed that the concentrations of rhLYZ secreted into milk after injection with 200 μg of p215C3LYZ vector were slightly lower than those with 400 μg of the vector (Table 3).
Table 3.
Injection dose-dependent expression of p215C3LYZ vector in mammary gland after primary injection by acupuncture (n=5)
| Injection dose | LYZ concentration after vector injection (μg/ml) |
|||||||
| 0 d | 2 d | 4 d | 6 d | 8 d | 10 d | 15 d | 20 d | |
| 200 μg | 0.3±0.1 | 1.5±0.2 | 1.6±0.6 | 2.3±0.8 | 1.3±0.3 | 1.0±0.9 | 1.0±0.6 | 0.8±0.2 |
| 400 μg | 0.3±0.1 | 1.7±0.5 | 2.9±0.7 | 2.3±0.8 | 1.6±0.3 | 1.2±0.4 | 0.9±0.2 | 0.6±0.1 |
Expression of p215C3LYZ vector after secondary injection
Since the primary vector injection resulted in a relatively high level of rhLYZ expression in milk and hLYZ was different from bovine LYZ at amino acid sequence level, primary vector injection, in principle, could induce immune responses with inhibitory effect on rhLYZ expression after secondary vector injection. To address this issue, 20 d after primary vector injection, the quarters were re-injected by acupuncture with the same dose of the p215C3LYZ vector and milk samples were collected for enzymatic assay. As Table 4 shows, after the secondary vector injection, the concentrations of rhLYZ secreted into milk were even higher than those after the primary injection.
Table 4.
The pair-wise comparison of p215C3LYZ expression in mammary gland after primary and secondary vector injection (n=5)
| Vector injection | LYZ concentration after vector injection (μg/ml) |
|||||||
| 0 d | 2 d | 4 d | 6 d | 8 d | 10 d | 15 d | 20 d | |
| Primary | 0.2±0.0 | 1.5±0.1 | 2.6±0.2 | 2.0±0.2 | 1.7±0.4 | 1.1±0.3 | 0.7±0.0 | 0.6±0.1 |
| Secondary | 0.3±0.0 | 2.1±0.8 | 3.3±0.1 | 2.9±0.2 | 2.0±0.1 | 1.6±0.3 | 0.7±0.1 | 0.5±0.0 |
Detection of hLYZ by Western blotting
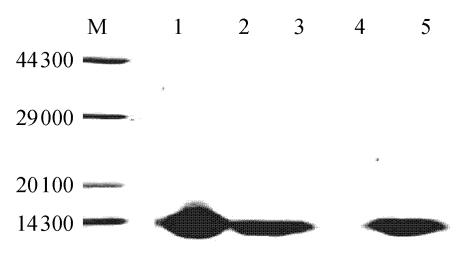
To test the identity of the rhLYZ, milk samples were collected at day 4 from p215C3LYZ-injected cow quarters and analyzed by Western blotting using rabbit anti-human lysozyme serum as the probe. As Fig.2 shows, a specific protein band was detected in human colostrums and milk from p215C3LYZ-injected cow or mouse, but not in normal cow and mouse milk. The molecular weight (about 14 000) of the rhLYZ expressed in cow or mouse mammary gland was similar to the natural hLYZ in human colostrums.
Fig. 2.
The Western blotting of milk samples
1: Woman colostrums; 2: Milk from p215C3LYZ-injected cow; 3: Cow milk prior to vector-injection; 4: Milk from p215C3LYZ-injected mouse; 5: Mouse milk prior to vector injection; M: Protein molecular markers
Antibody response after p215C3LYZ injection
To investigate the immunogenicity of p215C3LYZ vector, milk samples were collected at different time points after primary and secondary vector injection for antibody assay by indirect competitive ELISA. As Table 5 shows, compared to the milk samples prior to vector injection, no clear difference in OD 490 value was found in milk samples from p215C3LYZ-injected cows 3 weeks after primary vector injection and 2 additional weeks after secondary vector injection.
Table 5.
Detection of hLYZ-specific antibody in milk of p215C3LYZ-injected cows by indirect competitive ELISA
| Milk sample |
OD490 |
||||||
| Prior injection | Post injection |
||||||
| 1 d | 1 W | 2 W | 3 W* | 5 W | 7 W | ||
| A | 3.398 | 3.312 | 3.552 | 3.554 | 3.644 | 2.874 | 3.130 |
| B | 3.280 | 3.813 | 3.120 | 3.091 | 3.680 | 3.025 | 3.340 |
| C | 3.080 | 3.365 | 2.997 | 3.820 | 3.384 | 3.516 | 3.097 |
The time when the secondary vector injection was performed
Therapeutic effect of vector injection on bovine mastitis
Clinical examination showed that all cows (7/7) had no visual mastitis symptoms and milk abnormalities 10 d after twice quarter acupuncture with p215C3LYZ vector. Compared to prior vector injection (Table 6), 86% (6/7) and 78% (7/9) of milk samples (quarters) from cows with clinical and subclinical mastitis, respectively, had lowered CMT scores and 57% (4/7) of quarters with clinical mastitis had a normal SCC. Microbiological tests showed that most milk samples from cows with clinical disease had a negative growth of originally identified bacteria, but similar therapeutic effect was not seen in cows with subclinical mastitis.
Table 6.
The therapeutic effect of p215C3LYZ vector injection on bovine mastitis*
| Mastitis form |
CMT |
Microbiological cure |
Clinical cure | |||
| Approved | Cured | Staphylococci | Streptococci | E. coli | ||
| Clinical | 86 (6/7) | 57 (4/7) | 75 (3/4) | 60 (3/5) | 100 (4/4) | 100 (7/7) |
| Sunclinical | 78 (7/9) | 0 (0/9) | 67 (6/9) | 0 (0/5) | 29 (2/7) | |
All the experimental cows were submitted to antibiotics treatment without improvement before vector injection
DISCUSSION
Mastitis is the most devastating disease affecting adult dairy cows and the associated economic losses continue to present a serious burden to producers. Although current practices have reduced its occurrence, the disease remains prevalent in many dairy herds and thus innovative control approaches are needed. The new alternative approaches under investigation include cytokine immunotherapy, recombinant mucolytic protein therapy, viral-mediated gene transfer and genetic breeding of disease-resistant cows. Although these new approaches are promising, much work remains to be done to confirm their clinical usefulness and to evaluate their potential side effects (Sordillo et al., 1997; Gruet et al., 2001; Fan et al., 2002; Wall et al., 2005).
LYZ is one of the important soluble factors associated with the innate or non-specific defense against intramammary infection (IMI) (Sordillo et al., 1997). Bovine milk, however, contains only residual concentrations of LYZ (Maga et al., 1995), which may be one of many reasons for the prevalence of bovine IMI. Our previous studies showed that intramammary injection of the mammary-specific vector p205C3LYZ has therapeutic effect on naturally-occurring mastitis (Sun et al., 2004). However, the vector contained ampicillin resistance gene as the selection marker, which is discouraged or prohibited in clinical use due to unnecessary risk of spread of antibiotic resistance traits to environmental microbes (Murphy and Epstein, 1998). In addition, the expression of the therapeutic protein was not demonstrated in that experiment and thus the therapeutic effect of the vector injection on bovine mastitis remains to be defined. Here we constructed a new hLYZ-expressing vector with kan resistance gene as the selection marker, which is more acceptable for clinical studies (Norman et al., 1997). Intramammary expression studies showed that injection of the new vector p215C3LYZ led to a relatively high-level and long-lasting rhLYZ expression, which was comparable to that of the control vector pcDNAKLYZ. The control experiment using the empty vector p215C3 indicated that the increase in LYZ concentration was not due to non-specific stimulation by vector or its contaminants (such as endotoxin), which was further confirmed by Western blotting of milk samples from p215C3LYZ-injected cows.
The p215C3LYZ vector uses the mammary-specific blg promoter to control expression of transgenes and thus the expression of hLYZ cDNA should be restricted to mammary epithelial cells. This was consistent with the results of our intramammary expression studies using two different routes, where intracisternal infusion led to rhLYZ expression at lower levels to that of quarter acupuncture since there are generally not many mammary epithelial cells in the cistern. Possible explanations for relatively lower levels of rhLYZ expression after intracisternal vector injection include transfection of mammary epithelial cells in and the bilayered epithelium of the cistern by the phosphate-coated vector, penetration of a certain amount of the vector into the quarter, and/or diffusion of intracisternally expressed rhLYZ into the quarter.
At the amino acid sequence level, hLYZ is obviously different from bovine LYZ (Steinhoff et al., 1994) with a homology of about 85%. Therefore, rhLYZ expression after primary vector injection could induce immune responses with an inhibitory effect on rhLYZ expression after secondary vector injection. In our experiments, however, even higher amounts of rhLYZ were detected in milk samples after secondary vector injection. Indirect competitive ELISA showed that both primary and secondary vector injection did not induce detectable hLYZ-specific antibody. These data suggest that the immunogenicity of the p215C3LYZ vector is weak and that the repeated vector injection has no inhibitory effect on the expression of the therapeutic protein, which is generally needed for treating bovine mastitis.
The clinical studies showed that twice injection of the diseased quarters with the p215C3LYZ vector had overt therapeutic effect on bovine mastitis, clinical mastitis in particular, including complete disappearance of symptoms, significant improvement of CMT score and reasonably high microbiological cure rate. It is worth mentioning that all cows enrolled in the experiment had a long mastitis history and were previously treated at least once with antibiotics without clear improvement. Therefore, much higher cure rates can be achievable for treating newly occurring mastitis using the p215C3LYZ vector.
Footnotes
Project (Nos. BJ2001315 and BE2004611) supported by the Department of Science and Technology of Jiangsu Province, China
References
- 1.Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human β-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli . J Dermatol Sci. 2005;40(2):123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Erskine R, Cullor J, Schaellibaum M, et al. Bovine Matitis Pathogens and Trends in Resistance to Antibacterial Drugs. NMC Annual Meeting Proceedings; 2004. pp. 400–403. [Google Scholar]
- 3.Fan W, Plaut K, Bramley AJ, Barlow JW, Kerr DE. Adenoviral-mediated transfer of a lysostaphin gene into the goat mammary gland. J Dairy Sci. 2002;85(7):1709–1716. doi: 10.3168/jds.S0022-0302(02)74244-6. [DOI] [PubMed] [Google Scholar]
- 4.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proceedings of the Royal Society. 1922;B93:306. [Google Scholar]
- 5.Gruet P, Maincent P, Berthelot X, Kaltsatos V. Bovine mastitis and intramammary drug delivery: review and perspectives. Advanced Drug Delivery Reviews. 2001;50(3):245–259. doi: 10.1016/S0169-409X(01)00160-0. [DOI] [PubMed] [Google Scholar]
- 6.Hurley IP, Coleman RC, Ireland HE, Williams JHH. Measurement of bovine IgG by indirect competitive ELISA as a means of detecting milk adulteration. J Dairy Sci. 2004;87(3):543–549. doi: 10.3168/jds.S0022-0302(04)73195-1. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Yamada H, Imoto T. Colorimetric assay for lysozyme using Micrococcus luteus labeled with a blue dye, Remazol brilliant blue R, as a substrate. Chem and Pharm Bull. 1992;40(6):1523–1526. doi: 10.1248/cpb.40.1523. [DOI] [PubMed] [Google Scholar]
- 8.Lander RJ, Winters MA, Meacle FJ, Buckland BC, Lee AL. Fractional precipitation of plasmid DNA from lysate by CTAB. Biotechnol Bioeng. 2002;79(7):776–784. doi: 10.1002/bit.10335. [DOI] [PubMed] [Google Scholar]
- 9.Li GC, Sun HC, Sun Q, Chen G, Zhang Q, Shi WQ, Li HD. Transgenic mice expressing human lysozyme gene in milk. Chin J Microbiol Immunol. 2004;24(2):142–145. (in Chinese) [Google Scholar]
- 10.Maga EA, Anderson GB, Murray JD. The effect of mammary gland expression of human lysozyme on the properties of milk from transgenic mice. J Dairy Sci. 1995;78(12):2645–2652. doi: 10.3168/jds.S0022-0302(95)76894-1. [DOI] [PubMed] [Google Scholar]
- 11.Middleton JR, Hardin D, Steevens B, Randle R, Tyler JW. Use of somatic cell counts and california mastitis test results from individual quarter milk samples to detect subclinical intramammary infection in dairy cattle from a herd with a high bulk tank somatic cell count. J Am Vet Med Assoc. 2004;224(3):419–423. doi: 10.2460/javma.2004.224.419. [DOI] [PubMed] [Google Scholar]
- 12.Murphy DB, Epstein SL. Guidance for Human Somatic Cell Therapy and Gene Therapy. Rockville, MD: Food and Drug Administration; 1998. [Google Scholar]
- 13.Norman JA, Hobart P, Manthorp M, Felgner P. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine. 1997;15(8):801–803. doi: 10.1016/S0264-410X(96)00247-2. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Russell DW. Molecular Cloning, a Laboratory Manual. NY, USA: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 15.Sordillo LM, Shafer-Weaver K, DeRosa D. Immunology of the mammary gland. J Dairy Sci. 1997;80:1851–1865. doi: 10.3168/jds.S0022-0302(97)76121-6. [DOI] [PubMed] [Google Scholar]
- 16.Steinhoff UM, Senft B, Seyfert HM. Lysozyme-encoding bovine cDNAs from neutrophile granulocytes and mammary gland are derived from a different gene than stomach lysozyme. Gene. 1994;143(2):271–276. doi: 10.1016/0378-1119(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 17.Sun HC, Yu F, Su JH, Wu CH, Zhang Q, Li GC. Preliminary studies on gene therapy for dairy cow mastitis using human lysozyme gene. Chinese Journal of Animal and Veterinary Sciences. 2004;35(2):227–232. (in Chinese) [Google Scholar]
- 18.Wall RJ, Powell AM, Paape MJ, Kerr DE, Bannerman DD, Pursel VG, Wells KD, Talbot N, Hawk HW. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol. 2005;23(4):445–451. doi: 10.1038/nbt1078. [DOI] [PubMed] [Google Scholar]