Abstract
To reveal the possible mechanism of silica deposition in higher plants, lignin was isolated from rice straw following a modified method to conduct a simulation experiment in vitro. UV and infrared absorption spectra showed that the substance had the unique characteristics of pure lignin. The presence of silicon in the precipitation was revealed by TEM (transmission electron microscopy) with EDXA (energy dispersive X-ray analysis) device. It was found that in the borax solution where lignin precipitation occurred silica-lignin co-precipitation was produced but not in the DMSO solution where lignin was broken into its composition compounds and did not precipitate. This means that it is macromolecular lignin itself but not its compounds that could induce silica deposition in higher plants.
Keywords: Lignin, Silica precipitation, Higher plants
INTRODUCTION
Many scientists have confirmed that monocotyledon like grasses deposit opaline silica to regulate biochemical reactions, support the plant and enhance its tolerance to salt, heavy metals and pathogenic fungi (Epstein, 1994; Liang, 1999; Parry and Smithson, 1964; Kaufman et al., 1970; Xing and Zhang, 1998; Inanaga et al., 2002; Datnoff et al., 2005; Datnoff and Rutherford, 2004; Rodrigues et al., 2003). Evidence has shown that silica accumulates mainly in a species’ epidermis of the upper part, in the central sclerenchyma tissue of the stele, in the vessel walls, the endodermal walls and the epidermis and subepidermal cells of the proximal end of the thick cord roots (Parry and Kelso, 1975). The common characteristic of the silica deposition in these tissues is that it begins at the time when the cell stops growth to synthesize and accumulate lignin in the secondary wall. Montgomery and Parry (1979) agreed that during this procedure, lignin is permeable to water and some solutes, but it is highly unlikely that it is permeable to a relatively large molecule such as monosilicic acid, resulting in the deposition of silica. Inanaga et al.(1995) also observed that silica was present in the rice cell wall along with phenol-polysaccharide compound or lignin-polysaccharide compound. They presumed that silica might be associated with phenol or lignin such as the element calcium (Inanaga and Okasaka, 1995). We also showed in a simulation experiment that nanostructural silica deposition can be induced by the polymerization of phenols (Fang et al., 2003). To obtain direct evidence of the relationship between silica depositions and lignification, a simulation experiment including silicon and lignin from rice, one of the heavy silica accumulation plants, was carried out. The simplest and most credible method to extract lignin from grass was established by Sun et al.(2000). In the isolation, ball-milling process was cancelled due to the very long time and the demand for special equipment, and the characteristics of the extracted substance were examined with UV and infrared absorption. Transmission electron microscopy (TEM) with energy dispersive X-ray analysis (EDXA) device was used to detect the presence of silica in the deposition powder.
EXPERIMENTAL DETAILS
Materials
Mature rice straw (Oryza sativar L., cultivar upland 297, provided by the School of Agronomy and Biotechnology, China Agricultural University) was ground with a laboratory mill to pass a 60-mesh sieve. The grease contained in the straw powder was removed by toluene-methanol (2:1, v/v) solution and evaporated in a Soxhlet apparatus for 5 h.
Lignin isolation
Alkali lignin was prepared from the extractive grease-free powder according to modified Sun et al.(2000)’s method, omitting the procedure of ultra grinding by ball milling. The powder was subjected to evaporation under a fume hood to remove the organic solvent. Then, it was stirred in 1.5% NaOH aqueous solution (40 ml solution for 1 g straw powder) at room temperature for 6 d. After filtration, the supernatant was acidified to pH 5.5 with 20% HCl aqueous solution, precipitated with 4 volumes of ethanol to isolate crude hemicelluloses and hemicellulosic-lignin complexes. The supernatant was concentrated by an evaporator (Labconco, Centrivap Cold Trap & Labconco, Centrivap Concentrator) with reduced pressure at 40 °C to obtain the alkali-soluble lignin fraction and then re-precipitated at pH 1.5 with 20% HCl solution. Again after filtration, the isolated lignin fraction was rinsed with pH 2.0 H2O and then air-dried. To remove the possible associated silicon, the sample was soaked in 2% HF aqueous solution buffered with 3% NH4F and shaken on a gyratory shaker (80 r/min) at 28 °C for 24 h. Centrifugation was employed to wash the sample for 5 times.
Lignin identification
UV spectra were recorded on a UV-VIS recording spectrophotometer (UV-2201). The lignin sample was dissolved in ethonal or 4% NaOH in ethonal solution. The absorbance at 190 to 500 nm was estimated.
Micro IR spectra were recorded on an IR microscope (Nicolet, NIC-PLAN) attached to an IR spectrometer (Nicolet, MAGNA-IR 750), with a BaF2 slide to support the lignin powder. The measurement range was 800~4000 cm−1, scanning 5 min and 526 times.
Co-precipitation of Si and lignin
Method 1: Citrate-phosphate pH 6.4 and 5.4 buffers were prepared to make 20 mmol/L Na2SiO3 solution and pH was maintained with 0.1 mmol/L citric acid solution. Lignin powder (2.5 mg) was added into 400 μl DMSO to get lignin solution. Then Na2SiO3 solution and lignin solution were mixed together according to the proportion in Table 1.
Table 1.
Proportion of Na2SiO3 solution to lignin solution
| pH | Proportion 1 | Proportion 2 |
| 5.4 | 1:1 | 4:6 |
| 6.4 | 1:1 | 4:6 |
Method 2: Na2SiO3 aqueous solution was prepared in the same way as in Method 1. In 1% borax aqueous solution, pH was adjusted from 9.11 to 10.05 with 1 mol/L NaOH. In 400 μl such borax solution, 26 mg of the lignin powder was slowly added to ensure a little lignin precipitation occurs. Then Na2SiO3 solution and lignin solution were mixed together in the same proportion. For each mixture, two contrasts were set. In CK1, Na2SiO3 solution was mixed with 1% borax solution (without lignin) and in CK2, citrate-phosphate buffer solution (without Na2SiO3) was mixed with lignin solution.
TEM and EDXA of the precipitation
Precipitation from each mixture was suspended in equal amount of water, stirred rapidly, dropped on Cu-grids and air-dried. The image of the precipitation was taken by a TEM (PHILIPS, EM400T) and the silicon composition was investigated by the same TEM with an EDXA detector (ECAMII-S), under 80 kV in 15 μA, with electron beam dot of 100 nm for 200 s.
RESULTS
Characteristics of lignin
The extracted powder dissolving in the ethanol solution had a distinctive absorption in UV area and red shifted when alkali was present, showing that the sample had phenolic groups, which was one of the characteristics of lignin (Sun et al., 2000; Harborne, 1991; Lewis and Yamamoto, 1990).
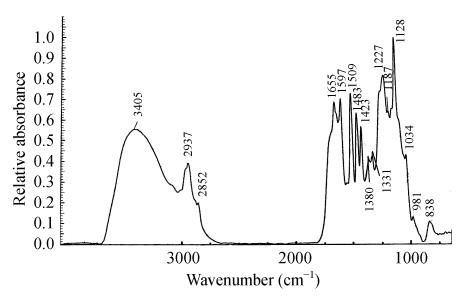
As in previously published data (Sun et al., 2000; Lawther et al., 1996), the sample showed typical infrared absorption of pure lignin from the grass (Fig.1). The details of the characteristic bands were analyzed (Table 2).
Fig. 1.
Infrared spectrum of lignin from rice straw
Table 2.
Assignments of IR absorption bands (cm−1) in lignin from rice straw
| Lit* | Wheat traw** | Rice straw | Assignment |
| 3400 | 3405 | 3405 | OH stretching |
| 2925 | 2923 | 2937 | CH stretching of methyl, methylene or methane group |
| 2850 | 2850 | 2852 | CH stretching of methyl, methylene or methane group |
| 1651 | 1655 | C=O conjugated ketone stretching | |
| 1600 | 1593 | 1596 | Aromatic skeletal vibrations |
| 1505 | 1507 | 1508 | Aromatic skeletal vibrations |
| 1461 | 1463 | Aromatic methyl group vibrations | |
| 1440 | 1422 | 1423 | Aromatic skeletal vibrations |
| 1325 | 1328 | 1331 | Syringyl ring breathing with CO stretching |
| 1280 | 1265 | 1290 | Guaiacyl ring breathing with CO stretching |
| 1220 | 1225 | 1227 | Guaiacyl ring breathing with CO stretching |
| 1155 | 1156 | 1153 | Aromatic CH in-plane deformation, guaiacyl type |
| 1120 | 1123 | 1126 | Aromatic CH in-plane deformation, syringyl type |
| 1015 | 1029 | 1034 | Aromatic CH in-plane deformation, guaiacyl type |
| 840 | 843 | 838 | Aromatic C-H out of plane bending |
Co-precipitation of Si and lignin
For the reaction between Si and lignin, precipitation was not observed in Method 1. Although no precipitation occurred in CK1, there was precipitation in all the other reactions including CK2 in Method 2. This result showed that Si without lignin failed to precipitate in the borax aqueous solution, but lignin could precipitate in the same solution.
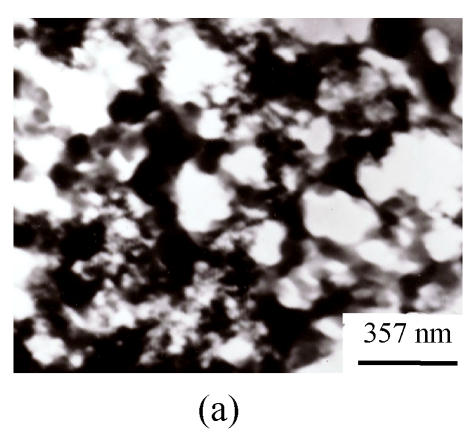
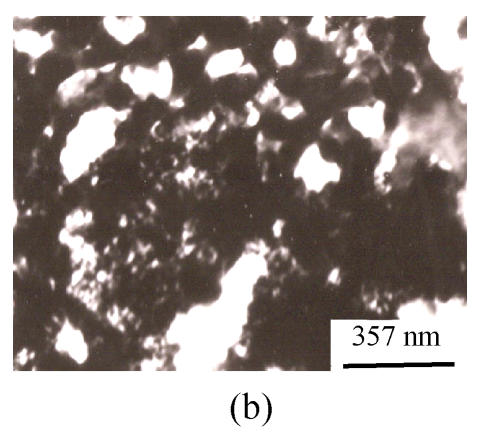
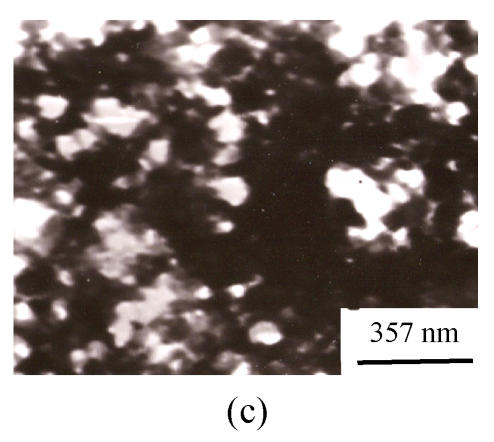
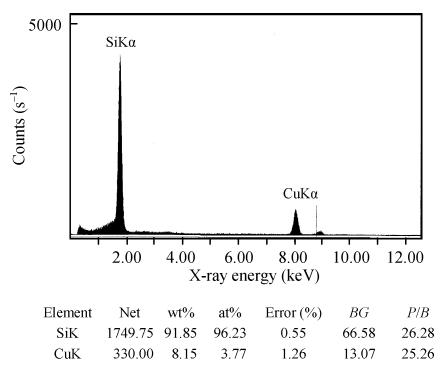
TEM photographs of the precipitation from lignin (CK2) and the mixture of Si and lignin are shown on Fig.2. It is distinctive that when Na2SiO3 was present in lignin aqueous solution, the precipitation is much more condensed than that in the lignin solution without silicon, showing that silica might be induced to precipitate by the precipitated lignin. The presence of silicon in the precipitation was directly confirmed by the EDXA (Fig.3).
Fig. 2.
TEM photographs of lignin ((a) pH 5.4) and the mixture of Si and lignin ((b) pH 5.4, 4:6; (c) pH 6.4, 4:6)
Fig. 3.
EDXA of the precipitation of mixture of Si and lignin
BG is background absorbance value and P/B is the ratio of peak to background
DISCUSSION
Although great efforts have been made since a report on polycationic peptides from diatom cell walls directing silica nanosphere formation (Kröger et al., 1999), similar protein has not yet been found in higher plants such as grasses in which silicon is an important element for plant growth. Many scientists believe that silica deposition in higher plants is associated with lignification (Parry and Kelso, 1975; Montgomery and Parry, 1979; Wang and Naser, 1994; Chérif et al., 1994). In order to provide direct evidence to support this hypothesis, the first step was to isolate pure lignin from rice straw. UV and infrared spectra of the extracted substance indicated that pure lignin could be isolated following the modified Sun et al.(2000)’s method without ball milling for 6 d if the aim was just for qualitative analysis of the compound and not for quantity measurement. The next step was to mix Si with lignin in a proper solution. In both borax and DMSO aqueous solution, Si did not precipitate. However, with the presence of lignin precipitation in borax aqueous solution, Si precipitation occurred. Kinrade et al.(1999) reported that addition of aliphatic polyols to aqueous silicate solutions yielded high concentration of stable polyolate complexes containing five- or six-coordinated silicon. Lignin from the secondary cell wall of the rice has structure similar to that of aliphatic polyols. Therefore, it is estimated that five- and six-coordinated silicon and lignin could play an essential role during the absorption and transport of silicon in rice. Because the structure of the intact lignin in rice displays a net form, it cannot dissolve in any solution. Once lignin dissolves, it rapidly decomposed (Sun et al., 2000; Lewis and Yamamoto, 1990). In DMSO, lignin was decomposed into its components and failed to deposit (Labaj et al., 2004), it might not form coordinate bond with silica to co-precipitate. These results indicated that macro lignin could induce silica deposition, but the lignin residue did not have this function.
The monomer composition of lignin from plants can vary depending upon the plant family or morphological region of plant tissue under consideration (Lewis and Yamamoto, 1990). Only if more detailed information on the relationship between Si and lignification is obtained, will it be possible to reveal the mechanism of Si deposition in rice tissues.
Acknowledgments
We are grateful to Professor ZHANG Fu-suo for his valuable support and Dr. WANG He for his kind suggestions.
Footnotes
Projects supported by the National Natural Science Foundation of China (No. 30170550), the Natural Science Foundation of Anhui Provincial Education Department (No. 2005kj398zc) and the Culture Project for “the Tenth Five-year” Learning Leaders of Higher School of Anhui Province, China
References
- 1.Chérif M, Asselin A, Belanger RR. Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Mo Phytoopathol. 1994;84(3):236–242. [Google Scholar]
- 2.Datnoff LE, Rutherford BA. Effects of silicon on leaf spot and melting out in bermudagrass. Golf Course Manage. 2004;5(1):89–92. [Google Scholar]
- 3.Datnoff L, Brecht M, Kucharek T, Trenholm L, Nagata R, Snyder G, Unruh B, Cisar J. Influence of silicon (Si) on controlling gray leaf spot and more in St. Augustinegrass in Florida. TPI Turf News. 2005;3(2):30–32. [Google Scholar]
- 4.Epstein E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA. 1994;91(1):11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang JY, Wang H, Chen Y, Zhang FS. Silica nanosphere formation induced by peroxidase-catalyzed phenol polymerization. Progress in Natural Science. 2003;13(7):501–504. doi: 10.1360/03jz9088. [DOI] [Google Scholar]
- 6.Harborne JB. Phytochemical Methods, a Guide to Modern Techniques of Plant Analysis. Xiamen: Xiamen University Press; 1991. pp. 28–31. (in Chinese) [Google Scholar]
- 7.Inanaga S, Okasaka A. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Sci Plant Nutr. 1995;41(1):103–110. [Google Scholar]
- 8.Inanaga S, Okasaka A, Tanaka S. Does silicon exist in association with organic compounds in rice plants? Soil Sci Plant Nutr. 1995;41(1):111–117. [Google Scholar]
- 9.Inanaga S, Higuchi Y, Chishalci N. Effect of silicon application on reproductive growth of rice plant. Soil Sci Plant Nutr. 2002;48(3):341–345. [Google Scholar]
- 10.Kaufman PB, Petering LB, Smith JG. Ultrastructural development of cork-silica cell pairs in Avena internodal epidermis. Botanical Gazette. 1970;131(3):173–185. doi: 10.1086/336529. [DOI] [Google Scholar]
- 11.Kinrade SD, Del NJW, Schach AS, Stoan TA, Wilson KL, Knigh CTG. Stable five- and six-coordinated silicate anions in aqueous solution. Science. 1999;285(5433):1542–1545. doi: 10.1126/science.285.5433.1542. [DOI] [PubMed] [Google Scholar]
- 12.Kröger N, Deutzmann R, Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286(5442):1129–1132. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 13.Labaj J, Slameoova D, Lazarova M, Kosikova B. Lignin-simulated reduction of oxidative DNA lesions in testicular cells and lymphocytes of Sprague-Dawley rats in vitro and ex vivo. Nutrition and Cancer. 2004;50(2):198–205. doi: 10.1207/s15327914nc5002_10. [DOI] [PubMed] [Google Scholar]
- 14.Lawther JM, Sun R, Banks WB. Extraction and comparative characterization of ball-milled lignin (LM), enzyme lignin (LE) and alkali lignin (LA) from wheat straw. Cellulose Chemistry and Technology. 1996;30(5/6):395–410. [Google Scholar]
- 15.Lewis NG, Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41(1):455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y. Effect of silicon on enzyme activity, and sodium, potassium and calcium concentration in barley under salt stress. Plant and Soil. 1999;209(2):217–224. doi: 10.1023/A:1004526604913. [DOI] [Google Scholar]
- 17.Montgomery DJ, Parry WD. The ultrastructure and analytical microscopy of silicon deposition in the intercellular spaces of the roots of Molinia caerulea (L.) Moench. Ann Bot. 1979;44(1):79–84. [Google Scholar]
- 18.Parry DW, Smithson F. Types of opaline silica depositions in the leaves of British grasses. Ann Bot. 1964;28(1):169–185. [Google Scholar]
- 19.Parry DW, Kelso M. The distribution of silicon deposits in the roots of Molinia caerulea (L.) Moench. and Sorghum bicolor (L.) Moench. Ann Bot. 1975;39(5):995–1001. [Google Scholar]
- 20.Rodrigues FA, Vale FX, Datnoff LE, Prabhu AS. Effect of rice growth stages and silicon on sheath blight development. Phytopathology. 2003;93(5):256–261. doi: 10.1094/PHYTO.2003.93.3.256. [DOI] [PubMed] [Google Scholar]
- 21.Sun R, Rowolands P, Lawther JM. Rapid isolation and physico-chemical characterization of wheat straw lignins. Recent Research and Development of Agricultural and Food Chemistry. 2000;4:1–26. [Google Scholar]
- 22.Wang J, Naser N. Improved performance of carbon paste ampermeric biosensors through the incorporation of fumed silica. Electroanalysis. 1994;6(7):571–574. doi: 10.1002/elan.1140060707. [DOI] [Google Scholar]
- 23.Xing XR, Zhang L. Review of the studies on silicon nutrition of plants. Chinese Bulletin of Botany. 1998;15(2):33–40. (in Chinese) [Google Scholar]