Abstract
Wild cotton species can contribute a valuable gene pool for agronomically desirable cultivated tetraploid cultivars. In order to exploit diploid cotton a regeneration system is required to achieve transformation based goals. The present studies aimed at optimizing the conditions for regeneration of local varieties as well as wild species of cotton. Different callus induction media were tested with varying concentrations of hormones in which sucrose was used as nutritional source. Different explants (hypocotyls, cotyledon, root) were used to check the regeneration of both local cotton plants and wild relatives using T & G medium, BAP medium, CIM medium, EMMS medium, and cell suspension medium. Different stages of embryogenicity such as early torpedo stage, late torpedo stage, heart stage, globular stage and cotyledonary stage were observed in wild relatives of cotton. The results of this study pave the way for establishing future transformation methods.
Keywords: Cotton, Callus, Somatic embryogenic, Wild species, Cell suspension culture
INTRODUCTION
Somatic embryogenesis resulting in regeneration of whole plant is an important step in any plant transformation method. Successful and stable transformation requires that a single cell gives rise to a plant. Ideal transformation scheme is that done via somatic embryogenesis, because from callus each transformed cell has the potential to produce a plant. Somatic embryogenesis and subsequent plant regeneration have been reported in most of the major crop species (Evans and Sharp, 1981). Soybean and cotton proved to be the most difficult to regenerate (Scowcroft, 1984). The regeneration of plants provides a unique stem cell system for investigating meristem formation in embryogenesis research and plant totipotency (Imin et al., 2005). Shoemaker et al.(1986) described somatic embryogenesis and plant regeneration from G. hirsutum var Coker 315 and 201. Trolinder and Xhixian (1989) obtained regenerated plants from cell suspensions of 38 genotypes and classified them into four classes as high, moderate, low and non embryogenic. Zhang et al.(1996) observed somatic embryogenesis and regenerated plants from Coker 201. Firoozabady and DeBoer (1993) demonstrated that somatic embryogenesis was rapid for Cokers (10~12 weeks), relatively slow for GSCs, and very slow for non-Coker cultivars. Further studies were also carried out on the effect of environmental conditions on culture induction, maintenance, somatic embryo development, somatic embryogenesis and plant regeneration. Zhang et al.(1996) reported the plant regeneration of Chinese cotton varieties, such as Simian 3, Zhongmian 12. Cell suspension culture of wild cotton G. klotzschianum and somatic embryo formation was shown by Price and Smith (1979). Finer and Smith (1984) also obtained somatic embryos of G. klotzschianum but most embryos either failed to mature or developed abnormal leaves and shoots. This study describes somatic embryogenesis and plant regeneration from embryogenic cultures of G. klotzschianum. Wild varieties are the naturally manipulating varieties. Tissue culture of wild varieties of cotton is an important step towards the regeneration of cotton.
MATERIALS AND METHODS
Delinting of seeds
This study was conducted at CEMB (Centre of Excellence in Molecular Biology), University of the Punjab, Lahore, Pakistan. Seeds used as source material were obtained from CCRI (Central Cotton Research Institute) Multan, Pakistan, and were delinted by using concentrated commercial H2SO4 (100 ml/kg of seeds). The seeds were continuously stirred in H2SO4 by spatula for 10~15 min until the shiny surface of seeds appeared. Some water was then added and stirred for a few seconds. The seeds were thoroughly washed five times with tap water to remove the acid completely, left in a beaker of water for few minutes, after which those floating on the water surface were discarded.
Sterilization of delinted cotton seeds was done in autoclaved majenta boxes. The seeds were washed in a solution containing a few drops of Tween 20 to which was added water and vigorously shaken and then thoroughly washed thrice by autoclaved water. Surface sterilization was done by using 0.1% HgCl2 to treat them for 20 min followed by 5 washings with autoclaved distilled water. The seeds were soaked in autoclaved distilled water for one hour. After that, the excess water was removed and the seeds were kept in the dark at 30 °C for germination. All sterilization work was performed in a laminar airflow cabinet.
Callus initiation
The delinted sterilized cotton seeds were germinated on sterile blotting paper in petri dishes under 30 Einsteins/(m2·s) light and (28±2) °C. After three days of radical emergence, the hypocotyl was sectioned into 4 mm lengths. Epicotyl and leaf disc portions were isolated. Cotyledonary leaves were cut into 3~4 mm. The pH of the media was adjusted to 5.8 and growth regulators were added to the medium which was then autoclaved for 20 min at 121 °C and 103421.4387465 Pa pressure. Then explants were placed on the media in petri plates and incubated under a 16:8 h photoperiod under conditions of 60~90 micro-Einsteins/(m2·s) and (28±2) °C. Calli were visually evaluated twice, first after one month of initiation and then after two months of culture. Fast growing calli were dispersed in sterile water and examined under stereomicroscope for the characterization of different stages of embryogenesis and compared with those observed in vivo. Selection of a genotype for further studies was based on the level of embryogenicity, simplicity, convenience and the time period required.
Cell suspension, initiation and maintenance
Potential embryogenic calli were identified and transferred to liquid culture for 5~6 weeks. Weekly sub culturing was done after passing them through screens of various mesh sizes to supply nutrients for embryogenic cells. Suspension media were the same as those for callus initiation except that they contained no growth hormones and gelling agents. After six weeks, highly (differentiated into stages of embryogenicity) embryogenic cells were placed on various embryo maturation media which similar to suspension media but containing different concentrations of NAA (napthalene acetic acid)+kinetin, NAA+zeatin, NAA+BAP (6-benzylaminopurine), 2,4-D+BAP and BAP. Further development of globular to heart shaped emryos and torpedo stages was performed by subculturing after every two weeks on the same media for further proliferation. Afterwards, mature somatic embryos were isolated and cultured on half MS medium for germination.
RESULTS
Callus induction
1. 0.1+0.5 T & G medium
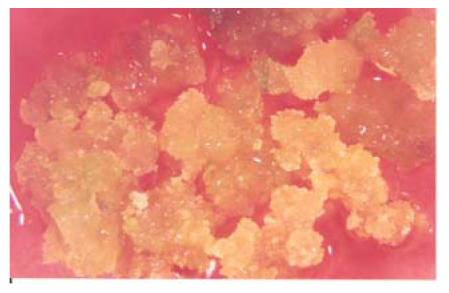
The MS (Murashige and Skoog) basal medium supplemented with 0.1 mg/L 2,4-D and 0.5 mg/L kinetin showed callus formation in almost all varieties but different varieties showed different types of callus formation on this medium. Furthermore callus initiation and production was maximized by increasing more contact between the cut surface of explants and media by longitunally dissecting explants (hypocotyls) placed on media. Hypocotyls produced more and more friable callus than other explants used. Root was found to be the lowest callus producing explant. Table 1 and Fig.1 show the characteristics of these calli. MS medium with 0.5 mg/L kinetin and 0.1 mg/L 2,4-D showed 75% calli formation/100 explants (i.e. cotyledon or hypocotyls or root), and callus easily proliferated.
Table 1.
Effect of different media on calli of different cotton varieties
| Variety of cotton | T & G 0.1+0.5 | T & G 0.1+0.1 | CIM medium | EMMS1 | EMMS2 | EMMS3 | EMMS4 | CSC medium | T & G Reg. medium | BAP1 medium | BAP2 medium | BAP3 medium | Embryogenicity |
| G. herbaceum | WRG | WWR | WWR | HWR | LAWR | LAWR | WWR | SOCOSCS | AWRBE | WWR | WWR | WWR | SSOE |
| G. bickii | HRGBE | HRG | HRG | HRG | HARG | HARGBE | LRG | SOCOSCS | FRGBE | HRG | HRG | HRGSE | SSE |
| G. inacanum | WGBE | WG | HLG | WLG | LTLG | ALG | LLG | SOCOSC | ALGBE | CLG | CLG | FLGSE | SSOE |
| G. anomalum | WGBE | WG | HGW | WGW | WGW | HGW | LGW | SOCNO | AGWSE | HGW | HGW | HGW | SSOE |
| G. davidsonii | WW | WW | WW | WW | WW | WW | LW | SOCNO | LW | LW | LW | LW | No |
| G. thurberri | WWBE | WW | WW | WW | WW | WW | WW | SOCNO | FWBE | WW | WW | WW | SSOE |
| G. herkesnessi | WW | WW | HG | WG | WW | WG | WG | – | WG | WG | WG | WG | No |
| G. australe | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CIM 497 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | CG | No |
| CIM 499 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
| CIM 473 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
| CIM 446 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
| CIM 482 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
| BH-118 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
| N-78 | WWG | WWG | HG | WG | WG | WG | WG | – | WG | WG | WG | WG | No |
Note: WRC: Watery reddish callus; WWR: Watery white reddish; HWR: Hard white reddish; LAWR: Low amorphous white reddish; SOCOSCS: Separation of cells occur show cell stages; AWRBE: Amorphous white reddish becomes embryogenic; SSOE: Showing stages of embryogenicity; HRGBE: Hard reddish green becomes embryogenic; HRG: Hard reddish green; HARG: Highly amorphous reddish green; HARGBE: Highly amorphous reddish green becomes embryogenic; LRG: Loose reddish green; FRGBE: Friable reddish green becomes embryogenic; HRGSE: Hard reddish green show embryogenicity; SSE: Show somatic embryos; WGBE: Watery greenish becomes embryogenic; WG: Watery greenish; HLG: Hard lush green; WLG: Watery lush green; LTLG: Loose texture lush green; ALG: Amorphous lush green; LLG: Loose lush green; ALGBE: Amorphous lush green becomes embryogenic; CLG: Compact lush green; FLGSE: Friable lush green show embryogenicity; HGW: Hard greenish white; WGW: Watery greenish white; LGW: Loose greenish white; SOCNO: Separation of cells not occur; AGWSE: Amorphous greenish white show embryogenicity; WW: Watery whitish; LW: Loose whitish; WWBE: Watery whitish becomes embryogenic; FWBE: Friable whitish becomes embryogenic; HG: Hard green; WG: Watery green; WWG: Watery whitish green; CG: Compact green
Fig. 1.
Effects of 0.1+0.5 T & G medium on callus
2. 0.1+0.1 T & G medium
MS basal medium with 0.1 mg/L 2,4-D and 0.1 mg/L kinetin showed some different results. On this medium the ratio of calli/100 explants was 40% lower than that of 0.1+0.5 T & G medium.
3. CIM medium
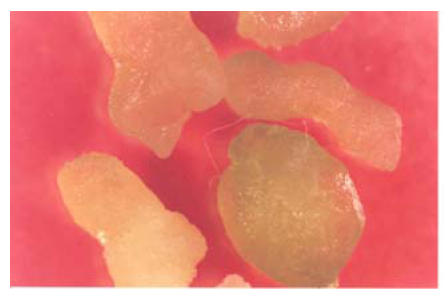
The application of 2.0 mg/L of NAA and 1.0 mg/L of kinetin resulted in the formation of compact callus. Large numbers of roots were found emerging from this compact callus but no shoot formation was found. Different varieties showed different types of response; G. anomalum showed good response and showed different stages of embryogenicity on this medium. The characteristics response of different varieties on this medium is shown in Table 1 and Fig.2.
Fig. 2.
Callus of G. inacanum showing stages of embryogenicity on 0.1+0.5 medium
Plant regeneration
1. EMMS medium
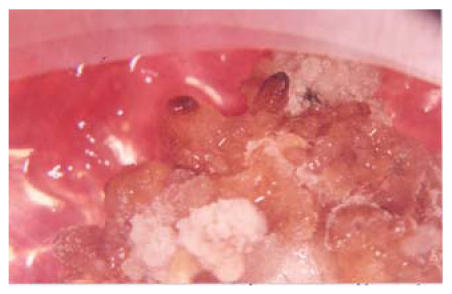
On EMMS medium with varying concentration of NAA and kinetin, different results in different varieties were observed. The medium containing 1 mg/L of NAA along with 0.1 mg/L kinetin called EMMS1 medium showed results tending towards callus differentiation. Most of the calli applied to this medium led to less amorphous callus formation, less further proliferation and no embryogenicity. This may be due to greater concentration of hormones i.e. NAA and kinetin, probably having inhibitory effects when used in greater concentration. Similarly the medium having 0.5 mg/L of NAA and 0.05 mg/L of kinetin also did not show satisfactory results. Callus applied to this medium also became less amorphous although somewhat better than that of EMMS1. It means that by lowering NAA concentration and 0.01 mg/L kinetin led to somewhat satisfactory results in the case of some varieties (especially G. bickii) tended towards maturation and became highly embryogenic and showed high amorphous state when this medium was used but could not achieve regeneration (Figs.3, 4 and 5). It means that 0.1 mg/L NAA and 0.01 mg/L kinetin is appropriate concentration for some varieties but not for all, which was why more variation in medium concentration was tested by applying 0.1 mg/L 2,4-D and 0.5 mg/L kinetin, but no change in callus differentiation was observed.
Fig. 3.
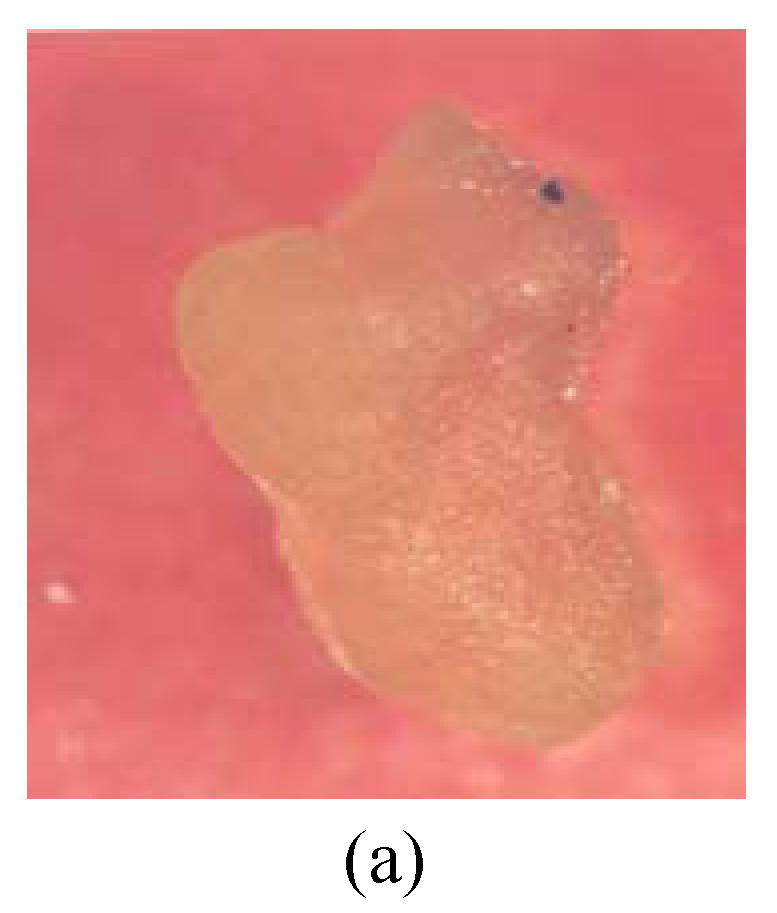
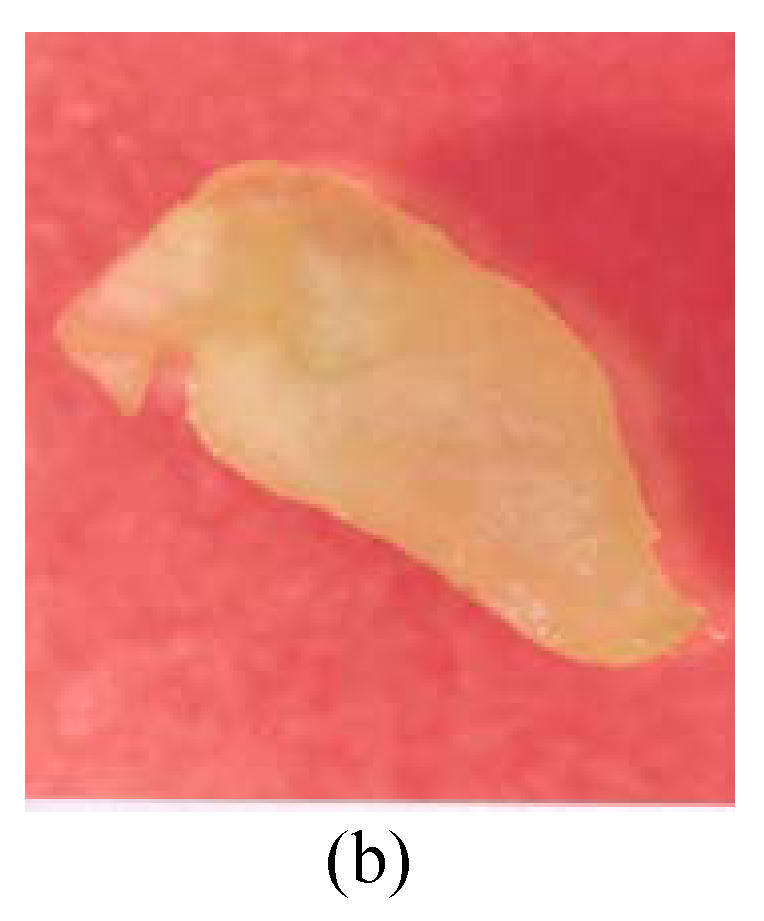
Callus of G. bickii and G. inacanum showing torpedo and early cotyledonary stage (a) Torpedo stage; (b) Early cotyledonary stage
Fig. 4.
Compact callus of G. anomalum on CIM medium
Fig. 5.
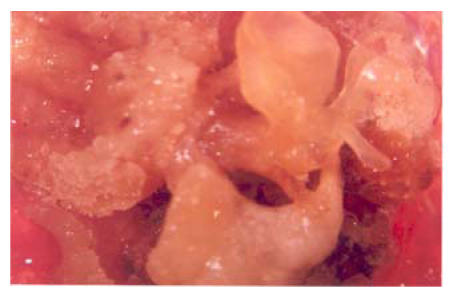
G. bickii showing morphogenic structure formation by T & G regeneration medium
2. BAP medium
Different concentrations of BAP and 2,4-D were applied to the calli of different varieties of cotton. Medium containing 125 μl/L of BAP and 100 μl/L 2,4-D (Stock 4 mg/ml) was BAP1 medium. The response of varieties varied accordingly on this medium. The callus of G. bickii when applied to this medium became red while the callus of G. thurberri showed rapid proliferation of cells on this medium, no change was observed in the other calli. Medium with 250 μl/L of BAP and 100 μl/L of 2,4-D, called BAP2 medium, had the same results as that of BAP1, except that the callus of G. bickii and G. thurberri became dry and hard. While the callus applied to BAP3 medium with 375 μl/L of BAP and 100 μl/L of 2,4-D showed prominent results from the embryogenic point of view. The callus of G. bickii when observed under microscope showed various stages of embryogenicity (Fig.3). Similarly the callus of G. inacanum became lush green and friable and showed different embryogenic stages.
Somatic embryogenesis
1. T & G regeneration medium
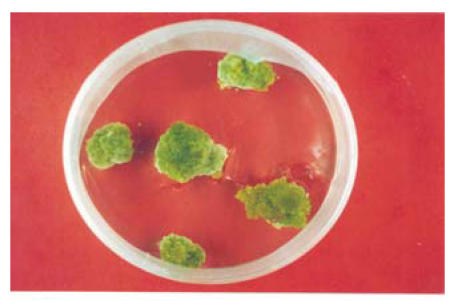
All the fifteen varieties of callus when applied to this medium showed different response. The callus of four varieties showed prominent results on application of the callus on this medium from the embryogenic point of view. G. bickii, G. inacanum, G. thurberri and G. anomalum showed all the stages of embryogenicity on application of this medium and G. bickii showed somatic embryo formation (Fig.5) when it was applied to this medium. While calli of the remaining wild varieties became compact first and latter became brown (Table 1. Figs.5 and 6).
Fig. 6.
Morphogenic stages of G. bickii
2. Cell suspension initiation and maintenance
Cell suspension culture was started with embryogenic calli. An embryogenic callus is generally friable, granular and yellowish green. The capability of a plant genotype to produce huge amounts of callus is not indicative of its regenerability. It very often happened that only a very small portion of a massive callus, formed from either hypocotyls or cotyledon explants, showed embryogenic potential. So, it is very crucial to identify and select potential embryogenic calli for getting highly regenerable cultures. After initiation, the embryogenic callus was selected, proliferated and transferred to cell suspension media for 5~6 weeks. Similar to solid media, callus morphology and callus colour in liquid culture varied drastically. Callus colors were yellowish green, yellow, whitish and black. The liquid medium consistently initiated and produced more somatic embryos than the solid media used for this purpose. About six varieties were applied to this medium out of which three showed all the stages of embryogenicity when seen under the microscope. The results are shown in Table 2, stages of embryogenicity in Figs.2 and 3.
Table 2.
Varietial comparision for embryogenicity
| Variety | Embryogenicity | Stages of embryogenicity |
||||
| Globular shape | Heart shape | Early torpedo | Late torpedo | Cotyledonary stage | ||
| G. herbaceum | Yes | √ | √ | × | × | × |
| G. bickii | Yes | √ | √ | √ | √ | √ |
| G. inacanum | Yes | √ | √ | √ | √ | √ |
| G. anomalum | Yes | √ | √ | √ | √ | √ |
| G. davidsonii | No | × | × | × | × | × |
| G. thurberri | Yes | √ | √ | √ | √ | √ |
| G. herkessnessi | No | × | × | × | × | × |
| G. australe | No | × | × | × | × | × |
| CIM497 | No | × | × | × | × | × |
| CIM499 | No | × | × | × | × | × |
| CIM473 | No | × | × | × | × | × |
| CIM446 | No | × | × | × | × | × |
| CIM482 | No | × | × | × | × | × |
| BH-118 | No | × | × | × | × | × |
| N-78 | No | × | × | × | × | × |
DISCUSSION
Somatic embryogenesis is an important step in any successful plant transformation scheme. Stable transformation required that a single cell gives rise to a plant. The ideal transformation scheme is that via somatic embryogenesis, because from callus each transformed cell has the potential to produce a plant. Somatic embryogenesis and subsequent plant regeneration has been reported in most of the major crop species (Evans and Sharp, 1981). Soybean and cotton proved to be the most difficult to regenerate (Scowcroft, 1984). In this study different callus induction media with varying concentrations of hormones and sucrose as nutritional source were tested by using different explants (hypocotyls, cotyledon, root) and different cotton varieties.
The results showed that callus induction with 0.1+0.5 T & G medium achieved 75% callus formation with almost all three types of explants. However, only four varieties achieved somatic embryogenesis. These results are similar to those in (Zhang et al., 2001; Trolinder and Xhixian, 1989) who classified cotton varieties on the basis of somatic embryogenesis and plant regeneration into four categories. First were the varieties with high ability for somatic embryogenesis and plant regeneration, such as Coker 201, Coker 312 which have become the model varieties in cotton tissue culture and genetic transformation. Second were those with moderate ability for somatic embryogenesis and plant regeneration that could produce some embryos and plantlets after many subcultures. Many varieties are included in this class e.g. Coker 310, Siokra 1-4, Coker 315, etc. Third class had poor ability, although somatic embryogenesis could be found but show no regeneration. Fourth class included some genotypes from embryo formation had not been observed. The varieties used during any study belonged to third and fourth class. Four varieties which led towards somatic embryogenesis belong to third class while all cultivated varieties and few wild varieties belong to fourth class. Similarly if we compare 0.1+0.5 T & G medium with 0.1+0.1 T & G medium, it is observed that only by varying the concentration of phytohormones, they play some roles in somatic embryogenesis. In 0.1+0.1 T & G medium, only by decreasing the concentration of kinetin, we got entirely different results. In the case of 0.1+0.5 T & G medium, four wild varieties showed somatic embryogenesis but in the case of 0.1+0.1 T & G medium no variety achieved somatic embryogenesis. These results are similar to those in (Zhang et al., 1996) which showed that suitable medium for callus induction was found to be. According to them the suitable medium for callus induction was found to be MS+B5 VIT+0.1 mg/L 2,4-D+0.5 mg/L kinetin. It is clear from this discussion that suitable concentration of kinetin plays some roles in this case. It was also found that explants hypocotyls produce more friable callus than other explants used in experiments. These results are similar to those in (Chee et al., 1990).
CIM medium showed formation of compact callus with application of NAA and kinetin. It was also seen that from this compact callus, roots came out in all directions and only one wild variety showed different stages of embryogenicity. It is clear from the above results that calli of different varieties show different response to different concentration of NAA. G. anomalum forms compact callus and shows all the stages i.e. globular shape embryo, heart shaped embryo, early torpedo, late torpedo embryo but no other variety achieved embryogenesis on this concentration of NAA. It means that by decreasing and increasing the concentration of NAA in MS medium we can get different results. These results are similar to those in (Chee et al., 1990; Stamp and Henshaw, 1982; Pérez et al., 1983; Lazzeri et al., 1987). According to them the response of somatic embryos to phytohormones varies with species.
The effect of EMMS1, EMMS2, EMMS3 and EMMS4 media showed that varying concentration of different hormones leads to different results from the embryogenic point of view. EMMS medium at various combinations of NAA and kinetin applied to different varieties shows different results. EMMS1 medium with 1 mg/L of NAA and 0.1 mg/L of kinetin results in slow proliferation of callus, less amorphous callus and less embryogenicity. This probably may be due to inhibitory effect of NAA and kinetin when used in greater concentration; similarly medium containing 0.5 mg/L of NAA and 0.05 mg/L of kinetin also does not show good results, although results were somewhat better than EMMS1 from the differentiation point of view. It means low concentration of NAA plays some roles in this respect. EMMS3 medium containing 0.1 mg/L of NAA and 0.01 mg/L kinetin shows good results in some varieties, especially callus of G. bickii leads towards embryogenesis, and becomes friable and amorphous if applied to this medium but cannot lead towards regeneration. It means 0.1 mg/L of NAA and 0.01 mg/L kinetin is appropriate concentration for some varieties but not for all which is why there is need to test these calli on media with more variations in hormonal concentration. EMMS4 medium with 0.1 mg/L of 2,4-D and 0.5 mg/L of kinetin shows no change in callus differentiation. These results are similar to those in (Sakhanokho et al., 2001; Chee et al., 1990; Stamp and Henshaw, 1982; Pérez et al., 1983; Lazzeri et al., 1987).
Similarly BAP1, BAP2 and BAP3 medium showed three different combinations of BAP with 2,4-D. BAP1 and BAP2 medium with low concentration of BAP but constant concentration of 2,4-D have almost similar results with the varying response of different varieties to these media. But when calli of different varieties were applied to BAP medium containing 375 μl/L of BAP and 100 μl/L of 2,4-D, they yielded somewhat different result. While the callus of G. bickii becomes embryogenic and when seen under microscope shows different stages i.e. globular shaped, embryo shaped, heart shaped embryo, early torpedo, late torpedo and cotyledonary stage, the callus of G. inacanum becomes lush green, hard, friable, and shows different stages of embryogenicity. No effect was observed on other calli. Perhaps greater concentration of BAP has some effects on the calli of different varieties.
T & G regeneration medium showed different effects on the calli of different varieties. Four wild varieties G. inacanum, G. bickii, G. thurberri and G. anomalum showed embryogenicity on application of this medium. Somatic embryogenesis was also observed in G. bickii on this medium containing KNO3 and other salts but no hormone. It means that after a month or two if we transfer the calli of different varieties to hormone free medium, then a change is observed in the nature of the callus. Although phytohormones can enhance callus growth, after two months phytohormones are not necessary for callus growth and the embryogenicity of calli can be obtained on simple MS medium without phytohormones. Gayle and Hamilton (1983) had the same results. Moreover it is also clear from the result that NO3 of KNO3 also plays some roles in the development of embryonic stages (Zhang et al., 2000).
The results of cell suspension media showed that when six wild varieties showing mature callus were shifted to suspension culture, three varieties showed the complete separation of cells, and when observed under microscope showed all stages of embryogenesis but no effect was observed on the other three varieties. When their cells were shifted to solid regeneration medium, no further process was observed in the calli properties. Many reseachers (Finer, 1988; Fredrick et al., 1990; Rajasekaran et al., 1996; Sakhanokho et al., 1999) used different cotton varieties in the suspension and got nearly the same results.
Table 2 shows the expression of different varieties from the regeneration point of view. From this table it is clear that five cotton genotypes have the ability to show different embryogenic stages on different media but that these varieties also could not have the ability of regeneration, local varieties showed very negative response toward regeneration.
References
- 1.Chee RP, Schultheis JR, Cantliffe DJ. Plant recovery from sweet potato somatic embryos. Hortscience. 1990;25:795–797. [Google Scholar]
- 2.Evans DA, Sharp WR. Growth and Behavior of Cell Cultures: Embryogenesis and Organogenesis. In: Trevor AT, editor. Plant Tissue Cultures. New York: Academic Press; 1981. pp. 45–113. [Google Scholar]
- 3.Finer JJ. Plant regeneration from somatic embryogenesis suspension culture of cotton (Gossypium hirsutum L.) Plant Cell Rep. 1988;7:399–402. doi: 10.1007/BF00269522. [DOI] [PubMed] [Google Scholar]
- 4.Finer JJ, Smith RH. Initiation of callus and somatic embryos from explants of mature cotton (Gossypium Klotzschianum Andress L.) Plant Cell Rep. 1984;3(1):41–43. doi: 10.1007/BF00270228. [DOI] [PubMed] [Google Scholar]
- 5.Firoozabady E, DeBoer DL. Plant regeneration via somatic embryogenesis in many cultivars of cotton (Gossypium hirstum L.) In vitro Cell Dev Biol Plant. 1993;29:166–173. [Google Scholar]
- 6.Fredrick JR, Randy WD, Tony AA, Roy LF, Stevin RS, Johnson T, GandDavis AF. Insect resistant cotton plant. Biotechnology. 1990;11:1151–1156. [Google Scholar]
- 7.Gayle HD, Hamilton HR. Plant regeneration from callus tissue of (Gossypium hirsutum L.) Plant Science Letters. 1983;3:89–93. [Google Scholar]
- 8.Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG. Proteomic analysis of somatic embryogenesis in Medicago truncatula. Explant cultures grown under 6-benzylaminopurine and 1-naphthalene-acetic acid treatments. Plant Physiol. 2005;137(4):1250–1260. doi: 10.1104/pp.104.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzeri PA, Hildebrand DF, Collins GB. Soybean somatic embryogenesis: effect of nutritional, physical and chemical factors. Plant Cell, Tissue and Organ Culture. 1987;10(3):209–220. doi: 10.1007/BF00037305. [DOI] [Google Scholar]
- 10.Pérez C, Fernández B, Rodríguez R. In vitro plantlet regeneration through asexual embryogenesis in cotyledonary segments of Corylus avellana L. Plant Cell Rep. 1983;2(5):226–228. doi: 10.1007/BF00269146. [DOI] [PubMed] [Google Scholar]
- 11.Price HJ, Smith RH. Somatic embryogenesis in suspension cultures Gossypium klotzschienum Andress. Planta. 1979;145(3):305–307. doi: 10.1007/BF00454456. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran K, Grula JW, Hudspeth RL, Pofelis S, Anderson DM. Herbicide resistant Acala and Coker transformed with a native gene encoding mutant forms of acetohydroxy acid synthase. Molecular Breeding. 1996;2(4):307–319. doi: 10.1007/BF00437909. [DOI] [Google Scholar]
- 13.Sakhanokho H, Sharma GC, Zipf A, et al. Tissue Culture Potential of Diverse Diploid and Tetraploid Cotton Genotypes. Proc. Beltwide Cotton Conf.; San Diego, CA. 1999. pp. 590–592. [Google Scholar]
- 14.Sakhanokho HF, Zip FA, Rajasekaran K, Saha S, Sharma GC. Induction of highly embryogenic calli and plant regeneration in upland (Gossypium hirsutum L.) and Pima (Gossypium barbadense L.) cottons. Crop Science. 2001;41:1235–1240. [Google Scholar]
- 15.Scowcroft WR. Tech. Rep. Internative Board for Plant Gen. Res. (AGPG:IBPGR) 1984. p. 41.
- 16.Shoemaker RC, Couche LJ, Galbraith DW. Characterization of somatic embryogenesis and plant regeneration in cotton (Gossypium hirsutum L.) Plant Cell Rep. 1986;5(3):178–181. doi: 10.1007/BF00269112. [DOI] [PubMed] [Google Scholar]
- 17.Stamp JA, Henshaw GG. Somatic embryogenesis in cassava. Z Pflanzenphysiol. 1982;105:183–187. [Google Scholar]
- 18.Trolinder NL, Xhixian C. Genotype specificity of the somatic embryogenesis response in cotton. Plant Cell Rep. 1989;8(3):133–136. doi: 10.1007/BF00716824. [DOI] [PubMed] [Google Scholar]
- 19.Zhang BH, Feng R, Li FL, Li XL. Anther culture and plant regeneration of cotton (Gossypium klotzchianum Anderss) Chinese Science Bulletin. 1996;41:145–148. [Google Scholar]
- 20.Zhang BH, Liu F, Yao CB, Wang KB. Plant regeneration via somatic embryogensis in cotton. Plant Cell, Tissue and Organ Culture. 2000;60(2):89–94. doi: 10.1023/A:1006488119200. [DOI] [Google Scholar]
- 21.Zhang BH, Wang LQ, Liu F. Phenotypic variation in cotton (Gossypium hirsutum L.) regenerated plants. Current Science. 2001;81:1112–1115. [Google Scholar]