Abstract
In the subventricular zone of the adult mammalian forebrain, neural stem cells (NSCs) reside and proliferate to generate young neurons. We screened factors that promoted the proliferation of NSCs in vitro by a recently developed proteomics technique, the ProteinChip system. In this screen, we identified a soluble carbohydrate-binding protein, Galectin-1, as a candidate. We show herein that Galectin-1 is expressed in a subset of slowly dividing subventricular zone astrocytes, which includes the NSCs. Based on results from intraventricular infusion experiments and phenotypic analyses of knockout mice, we demonstrate that Galectin-1 is an endogenous factor that promotes the proliferation of NSCs in the adult brain.
Keywords: lectin, mobilization, stem cell niche
Recently, neural stem cells (NSCs) residing in the adult CNS have been studied to elucidate the mechanisms of ongoing tissue maintenance (1) and to develop strategies for regenerating the damaged CNS (2, 3).
Two neurogenic regions have been identified in the forebrain (FB): the subventricular zone (SVZ) of the lateral ventricle (LV) (4–6) and the subgranular layer of the hippocampal dentate gyrus (7–9). NSCs in these regions can generate functional neurons in the adult brain (10–12). Clinically, soluble factors that regulate these progenitor cells may be useful for regenerating the damaged CNS (13). The identification of additional factors that promote the proliferation of stem cells will contribute to NSC biology and to the development of innovative strategies for brain repair.
The proliferation and differentiation of various adult stem cells are regulated by common soluble factors (14). OP9 is a cell line that has been used to screen for factors that support hematopoietic stem cells (HSCs) (15, 16). In the present study, we found that OP9 conditioned medium (CM) promoted neurosphere formation, by which the proliferation of NSCs can be monitored in vitro (17). Using the ProteinChip system (18), we identified Galectin-1 as one of the molecules responsible for this activity.
Galectin-1 is a soluble carbohydrate-binding protein (19, 20) that has been implicated in a variety of biological events (21, 22). Carbohydrates on the cell surface may be involved in the intercellular interactions of various stem cells, including NSCs (23–25) and HSCs (26). A recent report suggested that Galectin-1 promotes the proliferation of HSCs in vitro (27). However, its functions in NSCs remain unknown. Here, we report on the expression and function of Galectin-1 in the adult mammalian brain.
Results and Discussion
Galectin-1 Was Found in OP9CM.
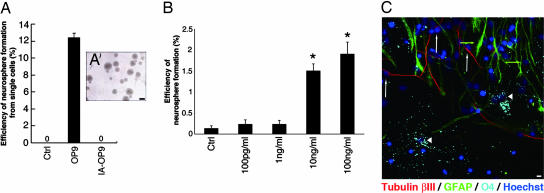
To examine how OP9 cells affect the proliferation of NSCs, we cultured neurosphere cells (17) with or without OP9CM. At a density of one cell per well, no neurospheres formed in cultures grown without OP9CM (Fig. 1A, Ctrl; n = 800 cells) (28). In contrast, at the same culture density, of the total cells grown with OP9CM (n = 400), 49 (12.4 ± 0.54%) initiated neurosphere formation (Fig. 1A, OP9). Interestingly, the CM from OP9 cells that were passaged repeatedly over 6 months (inactivated OP9, IA-OP9) did not support neurosphere formation (Fig. 1A, IA-OP9; n > 800).
Fig. 1.
Galectin-1 enhances neurosphere formation. (A) Effects of CM on neurosphere formation. Ctrl, control. (A′) The neurospheres initiated by OP9CM could be passaged more than five times. (B) Galectin-1 enhances neurosphere formation. Note that some neurospheres formed in the control cultures under this condition (culture density of 100 cells per well). ∗, P < 0.01. (C) The neurospheres initiated by Galectin-1 differentiated into neurons and glial cells. Representative images of differentiated cells from a neurosphere generated with recombinant Galectin-1 and immunostained with each neural lineage marker are shown. Tubulin βIII (neurons, red, white arrows), GFAP (astrocytes, green, green arrows), O4 (oligodendrocytes, light blue, arrowheads), and Hoechst (nucleus, dark blue) images were obtained by confocal laser microscopy. (Scale bars: A, 100 μm; C, 10 μm.)
To identify the OP9-derived molecules that enhanced neurosphere formation, we used an expression screen based on mass spectrometry (18) to detect molecules that were more abundant in OP9CM than in IA-OP9CM. A signal at ≈14.6 kDa showed a reproducible difference in peak height between the two CMs (Fig. 5, which is published as supporting information on the PNAS web site). This fraction was purified, concentrated, and separated by SDS/PAGE, and the band was cut from the gel and analyzed by tandem mass spectrometry (see Materials and Methods). We obtained two amino acid sequences (VRGEVASDAK and EDGTWGTEHR) that were identical to portions of the Galectin-1 protein (n = 3), suggesting that the 14.6-kDa peak was Galectin-1. Western blotting with a specific Ab showed that the OP9CM contained more Galectin-1 than did the IA-OP9CM (n = 3; P < 0.01).
Adding recombinant Galectin-1 protein (10 and 100 ng/ml) to the culture medium enhanced the formation of neurospheres cultured at 100 cells per well (Fig. 1B; P < 0.01, ANOVA). Galectin-1 did not mimic the full activity of the OP9CM, suggesting that there are other factors that enhance neurosphere formation in the OP9CM. The neurospheres grown with Galectin-1 (100 ng/ml) were larger (Fig. 6A, which is published as supporting information on the PNAS web site; P < 0.05), formed more secondary neurospheres (Fig. 6B; P < 0.05), and differentiated into neurons, astrocytes, and oligodendrocytes (Fig. 1C). Together, these results suggest that Galectin-1 is one of the factors in OP9CM that enhances neurosphere formation.
Expression of Galectin-1 in the Adult Mouse FB.
To investigate the in vivo function of Galectin-1, we examined its expression in the mouse brain by using a Galectin-1-specific Ab (29). To confirm the specificity and sensitivity of the staining procedures, brain sections from a galectin-1-null mutant mouse (30) were simultaneously incubated with the same anti-Galectin-1 Ab, and we observed no staining (Fig. 7, which is published as supporting information on the PNAS web site).
In the adult mouse FB, as reported previously (31), subsets of neurons in the cortex were Galectin-1+ (Fig. 7A′). In addition, we found Galectin-1 staining signals in the SVZ (Fig. 7A″) and dentate gyrus (Fig. 8A, which is published as supporting information on the PNAS web site), the two major adult neurogenic regions. RT-PCR analysis also showed that Galectin-1 is expressed in the SVZ (Fig. 8B). Almost all of the Galectin-1+ cells in the adult SVZ expressed glial fibrillary acidic protein (GFAP) (Fig. 2A; see Fig. 8C for the confocal image), Nestin (Fig. 8D), and S100β (Fig. 8E). Some of these Galectin-1+ cells were positive for Ki67 (Fig. 8F) (32), a nuclear marker of cellular replication. With regard to Galectin-1 expression in GFAP+ cells outside the SVZ, we found that subsets of GFAP+ cells in the subgranular layer and hilus were Galectin-1+ (Fig. 8A), whereas most of the GFAP+ cells in the cortex and striatum were Galectin-1− (Fig. 8 G and H). In the SVZ, the GFAP+ astrocytes (type B cells) have been shown to act as NSCs (33, 34), which generate Dlx+/Mash1+ type C cells (35, 36) (Toida Kazunori, personal communication) and subsequently differentiate into PSA-NCAM+/Dlx+/Mash1− type A cells (in which PSA-NCAM is the polysialylated neural cell adhesion molecule) (35, 36). Among striatal neurons (Fig. 8I), Mash1+ type C cells, and PSA-NCAM+ type A cells (Fig. 8J), none showed Galectin-1 immunoreactivity. Dissociated SVZ cells were stained with anti-Galectin-1 and anti-GFAP Abs to determine the percentages of each cell population in the SVZ. Of the total SVZ cells counted (n = 336 from three mice), 32.7 ± 6.38% (110 cells) were Galectin-1+, and 31.3 ± 6.99% (105 cells) were GFAP+. Of the GFAP+ cells, 71.4 ± 1.48% were also Galectin-1+. These results suggest that Galectin-1 is expressed in a subset of SVZ astrocytes.
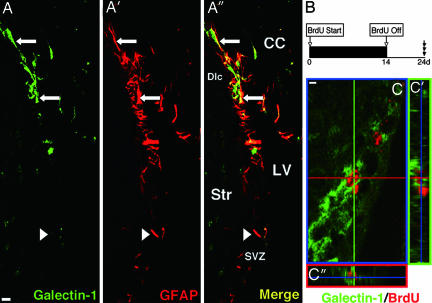
Fig. 2.
Galectin-1 is detected in SVZ astrocytes. (A) Low-magnification images of Galectin-1 (A) and GFAP (A′) double immunostaining in the coronal section through the LV. Galectin-1 and GFAP double-positive cells are seen in the SVZ (arrows). Note there are some Galectin-1-negative cells among the GFAP+ cells (e.g., arrowhead). (A″) Merged image. CC, corpus callosum; Str, striatum; Dlc, dorsolateral corner. (B) Experimental schema for marking slowly dividing cells. (C) Slowly dividing Galectin-1+ cell. High-magnification confocal image of a Galectin-1 (green in cell soma) and BrdU (red in nucleus) double-positive cell in the SVZ. Sections were made after 2 weeks of oral BrdU administration followed by 10 days of wash-out. (C′ and C″) 3D reconstruction images. (Scale bars: 5 μm.)
To detect the slowly dividing NSC population in the SVZ (37), we gave BrdU to mice in their drinking water for 2 weeks and killed the mice 10 days later (Fig. 2B); this delay allowed the BrdU in the rapidly dividing type C cell population to be washed out and the type A cell population to migrate to a more rostral region, out of the SVZ (37). A subpopulation of the long-term BrdU-retaining cells expressed Galectin-1 (Fig. 2C). Thus, we conclude that Galectin-1 is expressed in a subset of GFAP+ SVZ astrocytes that includes NSCs.
Galectin-1 Facilitates Proliferation of Neural Progenitor Cells in the Adult FB.
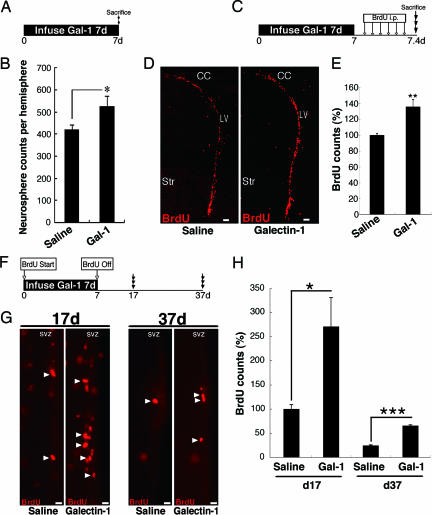
The in vitro functions and in vivo expression pattern of Galectin-1 led us to examine whether it promotes adult neural progenitor proliferation in vivo. Galectin-1 protein was infused into the mouse LV for 7 days, and the number of neurospheres derived from the SVZ was counted (Fig. 3A); this number should reflect the number of progenitor cell types in the SVZ, including type B and C cells (36). As expected, significantly more neurospheres were formed by SVZ cells from the Galectin-1-infused adult brains than by SVZ cells from the control brains (Fig. 3B). The neurospheres in these cultures retained the properties of stem cells in vitro, and the proportion of neurons produced from the spheres was not significantly different (Galectin-1, 7.61 ± 0.61%; control, 5.84 ± 0.47%).
Fig. 3.
Galectin-1 facilitates neural progenitor cell proliferation in the adult FB. (A) Experimental schema of the neurosphere formation assay after Galectin-1 infusion. (B) Galectin-1 significantly increased the number of neurosphere-initiating cells in the SVZ. ∗, P < 0.05. (C) Experimental schema of BrdU infusion after Galectin-1 infusion. (D) Low-magnification images of BrdU+ cells in the SVZ after Galectin-1 infusion. (E) Galectin-1 infusion significantly increased the number of BrdU+ cells in the SVZ. ∗∗, P < 0.01. (F) Experimental schema for labeling cells that retained BrdU long after Galectin-1 infusion. The mice were killed at the 17- or 37-day time point. (G) BrdU-labeled cells after infusion of saline or Galectin-1. (H) In the Galectin-1-infused brain, the number of BrdU+ cells was significantly greater than in the saline-treated brain at 17 and 37 days. ∗, P = 0.01; ∗∗∗, P = 0.0006. Str, striatum. (Scale bars: D, 50 μm; G, 15 μm.)
Next, we tested the effects of Galectin-1 on cell proliferation in the SVZ by infusing it into the LV, followed by BrdU injections every 2 h for 10 h (Fig. 3C). Thirty minutes after the last BrdU injection, the mice were killed. We counted the number of BrdU+ cells in the SVZ of the LV and found a significant increase, on average, compared with the saline-infused control group (Fig. 3 D and E; P < 0.01; n > 3 mice each). There was no significant difference in the number of apoptotic cells in the SVZ between the two groups (Fig. 9 A and B, which is published as supporting information on the PNAS web site), suggesting that the Galectin-1-induced increase in BrdU+ cells was caused by increased proliferation rather than increased cell survival.
To determine what type of cells in the SVZ proliferated in response to the Galectin-1 infusion, we used cell-type markers (Fig. 10, which is published as supporting information on the PNAS web site) that allowed us to distinguish proliferating type B (BrdU+/Sox21+/Dlx−), type C (BrdU+/Mash1+), and type A (BrdU+/Dlx+/Mash1−) cells from others (BrdU+/Sox21−) (Table 1; see Materials and Methods for details). The Galectin-1 infusion significantly increased the number of proliferating type B and C cells (Table 1; P < 0.05, Mann–Whitney U test).
Table 1.
Galectin-1 increases the number of SVZ astrocytes in the adult brain
| Treatment | No. of cells |
||
|---|---|---|---|
| Type B | Type C | Type A | |
| Saline | 40 ± 14 | 200 ± 12 | 64 ± 6.6 |
| Galectin-1 | 66 ± 7.0* | 280 ± 2.0* | 64 ± 31 |
| Galectin-1/saline relative ratio | 1.7 | 1.4 | 1.0 |
Significantly increased numbers of SVZ astrocytes (type B cells) and type C cells were observed in the Galectin-1-infused brain. Data are the average counts from 50-μm sections. ∗, P < 0.05.
Infusion of Galectin-1 Protein Increases the Slowly Dividing SVZ Cells.
We next examined the effect of Galectin-1 on the number of slowly dividing cells. We gave BrdU to mice in their drinking water during the 1-week infusion of Galectin-1 protein and killed them 17 or 37 days after the beginning of the infusion (Fig. 3F). There were significantly more BrdU+ cells in the SVZ of the Galectin-1-infused brains than in that of the control brains at both time points (Fig. 3 G and H; day 17, P = 0.01, n > 3 each; day 37, P < 0.001, n > 3 each). These data suggest that Galectin-1 infusion increased the population of slowly dividing SVZ progenitor cells.
Galectin-1 exists in either a reduced or oxidized form, and only the reduced form possesses carbohydrate-binding activity (38). The infusion of CS-Galectin-1, a reduced form in which all of the cysteines responsible for its oxidation have been converted to serine (39), significantly increased the number of slowly dividing cells (Fig. 11, which is published as supporting information on the PNAS web site; day 17, P < 0.001, n > 5 mice each). In contrast, the oxidized Galectin-1 did not have a significant effect (Fig. 11C). These results suggest that the carbohydrate-binding capacity of the extracellularly administrated Galectin-1 is required for its effect on the number of slowly dividing cells. We also found that CS-Galectin-1 could bind to SVZ cells, including GFAP+ astrocytes (unpublished data), suggesting that these cells are responsive to Galectin-1.
Galectin-1-Null Mice Have Fewer Neural Progenitor Cells in the Adult Brain.
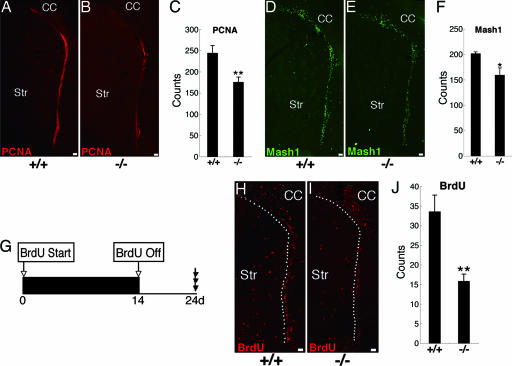
To study the function of endogenous Galectin-1 in adult neural progenitor cells, we analyzed the phenotype of adult galectin-1 mutant mice. First, we counted the number of cycling cells in the SVZ by using proliferating cell nuclear antigen (PCNA), a proliferation marker (40), and found significantly fewer in the galectin-1-null mice than in their wild-type littermates (Fig. 4A–C; P < 0.01; n > 3 each). We also examined the number of type C cells by counting the Mash1+ cells, of which there were also significantly fewer in the galectin-1-null mice than in wild-type littermates (Fig. 4 D–F; P < 0.05; n > 3 each). We then treated the animals with BrdU for 2 weeks, followed by a 10-day wash-out period (Fig. 4G), and found that there were significantly fewer slowly dividing cells in the SVZ of the galectin-1-null mice than in their wild-type littermates (Fig. 4 H–J; P < 0.01; n > 3 each). Furthermore, infusion of an anti-Galectin-1 neutralizing Ab (29) into the LV of adult wild-type mice significantly decreased the number of slowly dividing cells (Fig. 12, which is published as supporting information on the PNAS web site), a phenotype similar to that seen in galectin-1-null mutants (Fig. 4 G–J), suggesting that the endogenous Galectin-1 protein in the adult SVZ plays a role in the maintenance of neural progenitor cells. Moreover, the galectin-1-null mutation did not affect the number of apoptotic cells in the SVZ (Fig. 9 C and D). Together, these data suggest that Galectin-1 is required for the normal proliferation of type B and C cells in the adult brain.
Fig. 4.
Adult galectin-1-null mice show decreased neural progenitor cells. (A–C) Significantly fewer PCNA+ cells were seen in the SVZ of galectin-1-null mice than in wild-type littermates. ∗∗, P < 0.01; n > 3 each. (D–F) Significantly fewer Mash1+ type C cells were seen in the SVZ of galectin-1 null mice than in wild-type littermates. ∗, P < 0.05; n > 3 each. (G–J) Fewer slowly dividing cells were seen in galectin-1-null mice than in wild-type mice. Slowly dividing cells in the SVZ were visualized as described in the legend of Fig. 2B. Dotted lines are drawn around the SVZ in H and I. (I) Significantly fewer long-term BrdU-retaining cells were observed in the SVZ of galectin-1 mutant mice than in wild-type littermates. ∗∗, P < 0.01; n > 3 each. (Scale bars: 50 μm.)
The proliferation of adult and fetal NSCs is regulated by distinct mechanisms (41). Radial glial cells act as NSCs in the fetal and early postnatal brain and then may differentiate into astrocytes, expressing GFAP at approximately postnatal day (P) 7–15 (42, 43). Interestingly, Galectin-1 is not expressed before P9 in the FB (44). The brain of galectin-1-null mice at birth is indistinguishable from that of wild-type littermates (30, 45) except for an aberrant topography of olfactory axons (46). Therefore, Galectin-1 is most likely to play a role in adult NSCs rather than in embryonic NSCs. Taken together, our results demonstrate that Galectin-1 is expressed in slowly dividing SVZ astrocytes, which include the NSCs (34), and plays an important role in the proliferation of adult neural progenitor cells, including SVZ astrocytes. Stem cells reside in an area called a niche (47, 48), which has a characteristic cellular composition and signal mediators. In the niche, the state of each cell [i.e., cell cycle, apoptosis, and cell–cell or cell–extra cellular matrix (ECM) interactions] is strictly regulated to maintain stem cell homeostasis. Although ECM proteins are enriched in the SVZ (24, 49, 50), the niche for NSCs, the physiological significance of their carbohydrate structures has not been well characterized. In general, lectins exert their biological effects by binding to certain carbohydrate structures. Galectin-1’s carbohydrate-binding ability is required for some functions but not others (27, 39, 51, 52). The present study suggests that the carbohydrate-binding activity of Galectin-1 is required for its promotion of adult neural progenitor cell proliferation. The analysis of Galectin-1 function will help us understand the important roles of carbohydrate molecules in stem cell biology, which may lead to the development of innovative therapies for human diseases.
Materials and Methods
Evaluation of OP9CM Activity.
To assay the CM activity (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site), neurospheres, prepared as described in ref. 53, were dissociated with trypsin and then FACS-sorted (Supporting Materials and Methods) at one cell per well directly into 96-well low-adhesion microtiter plates (Costar) containing each CM in a separate well. Human Galectin-1 was purchased from Genzyme Techne. To prepare the neurosphere CM (NSCM), neurospheres were cultured in the basal medium with 20 ng/ml human recombinant EGF (PeproTech, Rocky Hill, NJ) and FGF-2 (Genzyme Techne) for 48 h. To evaluate the activity of Galectin-1, the cells were sorted at 100 cells per well into NSCM-containing medium.
Molecular Identification of Galectin-1 in the CM.
The OP9CM and IA-OP9CM preparations were analyzed by using the ProteinChip system (Ciphergen Biosystems). To screen the differences in peak heights, chemical surface chips that were positively charged, negatively charged, hydrophobic, C4, and Zn were used in the range of 500–200,000 Da. The affinity of the protein for each chip was monitored by using wash buffers of several pH values. To purify the 14.6-kDa protein, 200 ml of the OP9CM was freeze-dried, and a condensed solution was run on a Q-100 column (General Electric). The fraction that was eluted with 200 mM NaCl was used for SDS/PAGE. The 14.6-kDa band was cut from the gel and used for amino acid sequencing by tandem mass spectrometry.
Infusion into the SVZ and Adult Neurosphere Culture.
Galectin-1 (2 or 14 μg), anti-Galectin-1 neutralizing Ab (rabbit IgG, 30 μg/ml, Kirin Brewery), or control rabbit IgG (30 μg/ml, Kirin Brewery) was dissolved in 0.9% saline with 1 mg/ml mouse serum albumin (Sigma) and infused into the LV as described in ref. 54 by using an osmotic pump (Alzet, Palo Alto, CA) at 0.5 μl/h for the given number of days. Adult neurosphere cultures from the infused brain samples were prepared as described in ref. 54.
Immunohistochemistry.
Brains were perfusion-fixed with 4% paraformaldehyde (PFA) and postfixed in the same fixative overnight, and 50-μm sections were cut on a vibratome. Differentiated neurospheres grown on coverslips were immersion-fixed in 4% PFA for 15 min at room temperature. After three rinses in PBS, the neurospheres or sections were incubated for 1 h in TNB blocking solution (Vector Laboratories), incubated with primary antibodies overnight, and incubated for 60 min at room temperature with biotinylated secondary antibodies (1:200) or Alexa Fluor-conjugated secondary antibodies (1:200; Molecular Probes), unless otherwise noted. Biotinylated antibodies were visualized by using the Vectastain Elite ABC kit and TSA (Vector Laboratories). Anti-Galectin-1-neutralizing Abs were prepared as described in ref. 39. Other primary antibodies used in this study are described in Supporting Materials and Methods.
BrdU Labeling.
For short-term labeling, after the intraventricular infusion of Galectin-1 for 7 days, mice received i.p. injections of BrdU (120 mg/kg dissolved in 0.007% NaOH in phosphate buffer; Sigma) every 2 h for 10 h and were killed 0.5 h after the last injection. For long-term labeling, 1 mg/ml BrdU was given to mice in their drinking water for 2 weeks (or 1 week in experiments involving the infusion of Galectin-1 or Abs). Mice were killed 10 or 30 days after the last day of infusion, and the brains were processed for immunohistochemistry.
Quantification of Histological Results.
To quantify each cell type, 20 coronal vibratome sections of the SVZ (50 μm thick) were obtained at the level of the caudate-putamen (1.0–0 mm rostral to the bregma) from each hemisphere. The sections were stained for three different markers with BrdU (Sox21/BrdU, Dlx/BrdU, or Mash1/BrdU). Single confocal images were taken as 1-μm optical sections (LSM-510; Zeiss) from each vibratome section. The BrdU+ nuclei that were positive for each marker (Sox21, Dlx, or Mash1) were counted, and the total number of BrdU+ cells was multiplied by the ratio of the cells of each type to BrdU+ cells, yielding the numbers for each cell type as follows: type B cells = total number of BrdU+ [(Sox21+/BrdU+) − (Dlx+/BrdU+)], type C cells = total number of BrdU+ (Mash1+/BrdU+), and type A cells = total number of BrdU+ [(Dlx+/BrdU+) − (Mash1+/BrdU+)]. The average number of each cell type per 50-μm section throughout the LV is indicated in each figure. Apoptotic cells were detected by using an ApoTag kit (Intergen, Purchase, NY). We quantified the cells in the LV contralateral to the infused side, because exposure to the increased concentration of reagent in the LV could have an artifactual effect.
Animals.
For the adult mouse study, 8-week-old male mice were killed by anesthetic overdose. galectin-1 knockout mice (129SJ background) are described in ref. 30. The animals were maintained on a 12-h light/12-h dark cycle with unlimited access to food and water. All experiments on live animals were performed in accordance with Keio University guidelines and regulations.
Statistical Analysis.
Values are expressed as the mean ± SE. An unpaired t test (for two groups) or ANOVA with the Bonferroni correction (for more than three groups) was used to evaluate the differences of the averages, unless otherwise noted.
Supplementary Material
Acknowledgments
We thank K. Sakurada, S. Kaneko, Masatake Osawa, H. Miyoshi, K. Nakashima, T. Imai, and S. Yamanaka for critical advice; T. Nakano (Osaka University, Japan), T. Kitamura (University of Tokyo), T. Seki (Juntendo University, Tokyo), H. Ohba (Keio University), K. Adachi (Keio University), O. Yamada (Keio University), and G. Panganiban (University of Wisconsin, Madison) for experimental materials; R. Wakatabe, Y. Fukase, Mitsujiro Osawa, Y. Morita, T. Yamashita, K. Sango, S. Kuno, Y. Hayakawa, and Y. Fujita for technical assistance; M. Ito, K. Inoue, and A. Hirayama for secretarial assistance; and S. Sakaguchi for critical advice and continuous support. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); Core Research for Evolutional Science and Technology of the Japan Society and Technology Agency (to H. Okano); the 21st Century Centers of Excellence Program of MEXT (to Keio University and Tokyo Medical and Dental University); Association pour la Recherche sur le Cancer, Lingue Nationale Française Contre le Cancer, and Association Française Contre les Myopathies (to F.P.); and the National Institutes of Health and National Institute of Neurological Disorders and Stroke (to M. Sakaguchi).
Abbreviations
- SVZ
subventricular zone
- LV
lateral ventricle
- FB
forebrain
- GFAP
glial fibrillary acidic protein
- PCNA
proliferating cell nuclear antigen
- NSC
neural stem cell
- CM
conditioned medium
- IA-OP9
inactivated OP9.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Alvarez-Buylla A., Lim D. A. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Okano H. J. Neurosci. Res. 2002;69:698–707. doi: 10.1002/jnr.10343. [DOI] [PubMed] [Google Scholar]
- 3.Lie D. C., Song H., Colamarino S. A., Ming G. L., Gage F. H. Annu. Rev. Pharmacol. Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 4.Lois C., Alvarez-Buylla A. Proc. Natl. Acad. Sci. USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levison S. W., Goldman J. E. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 6.Luskin M. B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 7.Altman J., Das G. D. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 8.Bayer S. A., Yackel J. W., Puri P. S. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson P. S., Perfilieva E., Bjork-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carleton A., Petreanu L. T., Lansford R., Alvarez-Buylla A., Lledo P. M. Nat. Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 12.Gheusi G., Cremer H., McLean H., Chazal G., Vincent J. D., Lledo P. M. Proc. Natl. Acad. Sci. USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., Nakafuku M. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 14.Reya T., Morrison S. J., Clarke M. F., Weissman I. L. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Takakura N., Kodama H., Nishikawa S. J. Exp. Med. 1996;184:2301–2309. doi: 10.1084/jem.184.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno H., Sakita-Ishikawa M., Morikawa Y., Nakano T., Kitamura T., Saito M. Nat. Immunol. 2003;4:457–463. doi: 10.1038/ni916. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds B. A., Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 18.Fung E. T., Thulasiraman V., Weinberger S. R., Dalmasso E. A. Curr. Opin. Biotechnol. 2001;12:65–69. doi: 10.1016/s0958-1669(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 19.Teichberg V. I., Silman I., Beitsch D. D., Resheff G. Proc. Natl. Acad. Sci. USA. 1975;72:1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K., et al. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 21.Perillo N. L., Marcus M. E., Baum L. G. J. Mol. Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 22.Leffler H. Results Probl. Cell Differ. 2001;33:57–83. doi: 10.1007/978-3-540-46410-5_4. [DOI] [PubMed] [Google Scholar]
- 23.Capela A., Temple S. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 24.Mercier F., Kitasako J. T., Hatton G. I. J. Comp. Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa M., Liour S. S., Yu R. K. J. Neurochem. 2004;91:804–812. doi: 10.1111/j.1471-4159.2004.02750.x. [DOI] [PubMed] [Google Scholar]
- 26.Pipia G. G., Long M. W. Nat. Biotechnol. 1997;15:1007–1011. doi: 10.1038/nbt1097-1007. [DOI] [PubMed] [Google Scholar]
- 27.Vas V., Fajka-Boja R., Ion G., Dudics V., Monostori E., Uher F. Stem Cells. 2005;23:279–287. doi: 10.1634/stemcells.2004-0084. [DOI] [PubMed] [Google Scholar]
- 28.Tropepe V., Sibilia M., Ciruna B. G., Rossant J., Wagner E. F., van der Kooy D. Dev. Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 29.Horie H., Inagaki Y., Sohma Y., Nozawa R., Okawa K., Hasegawa M., Muramatsu N., Kawano H., Horie M., Koyama H., et al. J. Neurosci. 1999;19:9964–9974. doi: 10.1523/JNEUROSCI.19-22-09964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirier F., Robertson E. J. Development (Cambridge, U.K.) 1993;119:1229–1236. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]
- 31.Joubert R., Kuchler S., Zanetta J. P., Bladier D., Avellana-Adalid V., Caron M., Doinel C., Vincendon G. Dev. Neurosci. 1989;11:397–413. doi: 10.1159/000111916. [DOI] [PubMed] [Google Scholar]
- 32.Brown D. C., Gatter K. C. Histopathology. 1990;17:489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 33.Seri B., Garcia-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M., Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 35.Parras C. M., Galli R., Britz O., Soares S., Galichet C., Battiste J., Johnson J. E., Nakafuku M., Vescovi A., Guillemot F. Embo. J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doetsch F., Petreanu L., Caille I., Garcia-Verdugo J. M., Alvarez-Buylla A. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 37.Morshead C. M., Reynolds B. A., Craig C. G., McBurney M. W., Staines W. A., Morassutti D., Weiss S., van der Kooy D. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 38.Whitney P. L., Powell J. T., Sanford G. L. Biochem. J. 1986;238:683–689. doi: 10.1042/bj2380683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inagaki Y., Sohma Y., Horie H., Nozawa R., Kadoya T. Eur. J. Biochem. 2000;267:2955–2964. doi: 10.1046/j.1432-1033.2000.01311.x. [DOI] [PubMed] [Google Scholar]
- 40.Yu C. C., Filipe M. I. Histochem. J. 1993;25:843–853. [PubMed] [Google Scholar]
- 41.Shi Y., Chichung Lie D., Taupin P., Nakashima K., Ray J., Yu R. T., Gage F. H., Evans R. M. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 42.Tramontin A. D., Garcia-Verdugo J. M., Lim D. A., Alvarez-Buylla A. Cereb. Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Buylla A., Garcia-Verdugo J. M., Tramontin A. D. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 44.Poirier F., Timmons P. M., Chan C. T., Guenet J. L., Rigby P. W. Development (Cambridge, U.K.) 1992;115:143–155. doi: 10.1242/dev.115.1.143. [DOI] [PubMed] [Google Scholar]
- 45.Colnot C., Fowlis D., Ripoche M. A., Bouchaert I., Poirier F. Dev. Dyn. 1998;211:306–313. doi: 10.1002/(SICI)1097-0177(199804)211:4<306::AID-AJA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Puche A. C., Poirier F., Hair M., Bartlett P. F., Key B. Dev. Biol. 1996;179:274–287. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- 47.Schofield R. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 48.Spradling A., Drummond-Barbosa D., Kai T. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 49.Campos L. S., Leone D. P., Relvas J. B., Brakebusch C., Fassler R., Suter U., Ffrench-Constant C. Development (Cambridge, U.K.) 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 50.Emsley J. G., Hagg T. Exp. Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 51.Liu F. T., Rabinovich G. A. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 52.Wells V., Mallucci L. Cell. 1991;64:91–97. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- 53.Shimazaki T., Shingo T., Weiss S. J. Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J. C., Weiss S. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.