Abstract
Data from animal models indicate that neonatal stress or pain can permanently alter subsequent behavioral and/or physiological reactivity to stressors. However, cumulative effects of pain related to acute procedures in the neonatal intensive care unit (NICU) on later stress and/or pain reactivity has received limited attention. The objective of this study is to examine relationships between prior neonatal pain exposure (number of skin breaking procedures), and subsequent stress and pain reactivity in preterm infants in the NICU. Eighty-seven preterm infants were studied at 32 (±1 weeks) postconceptional age (PCA). Infants who received analgesia or sedation in the 72 h prior to each study, or any postnatal dexamethasone, were excluded. Outcomes were infant responses to two different stressors studied on separate days in a repeated measures randomized crossover design: (1) plasma cortisol to stress of a fixed series of nursing procedures; (2) behavioral (Neonatal Facial Coding System; NFCS) and cardiac reactivity to pain of blood collection. Among infants born ≤ 28 weeks gestational age (GA), but not 29–32 weeks GA, higher cumulative neonatal procedural pain exposure was related to lower cortisol response to stress and to lower facial (but not autonomic) reactivity to pain, at 32 weeks PCA, independent of early illness severity and morphine exposure since birth. Repeated neonatal procedural pain exposure among neurodevelopmentally immature preterm infants was associated with down-regulation of the hypothalamic–pituitary–adrenal axis, which was not counteracted with morphine. Differential effects of early pain on development of behavioral, physiologic and hormonal systems warrant further investigation.
Keywords: Preterm infant, Pain, Morphine, Cortisol, Stress, Facial reactivity, Autonomic
1. Introduction
Repeated pain in immature neonates has long-term effects on the developing organism, including pain systems (Anand, 2000; Anand et al., 1999; Andrews and Fitzgerald, 1994; Grunau, 2002; Grunau et al., 1994; Ren et al., 2004). However, pain is one dimension in a broader context of stress reactivity, and the capacity of the individual infant to self-regulate (Grunau, 2003). Experimental animal studies have shown that exposure to neonatal stress of maternal separation, and environmental manipulations of handling, can permanently alter (increase or decrease respectively) development of both behavioral and physiological (hypothalamic–pituitary–adrenal axis; HPA) responsiveness to subsequent stressors (for reviews see Ladd et al., 2000; Pryce and Feldon, 2003). In contrast, while neonatal rats exposed to repeated physical pain exhibited long-term alterations in behavior as adults, no differences were found in HPA reactivity between pain-exposed and control animals (Anand et al., 1999; Walker et al., 2003).
In human preterm infants, higher procedural pain exposure is associated with altered behavior and cardiac reactivity to subsequent pain in the neonatal intensive care unit (NICU) (Grunau et al., 2001a; Johnston and Stevens, 1996). However, HPA function has not been examined in this context. After controlling for gestational age (GA) at birth, higher numbers of skin breaking procedures and greater exposure to postnatal corticosteroids, were associated with dampened behavioral and cardiac responses to pain in the NICU (Grunau et al., 2001a). Moreover, early exposure to morphine predicted more ‘normalized’ cardiac (but not behavioral) pain response (Grunau et al., 2001a). Since corticosteroids influence the activity of multiple physiological systems, exposure to postnatal dexamethasone may have influenced the earlier findings. Recently, medical practice in the NICU has changed with few infants currently receiving postnatal corticosteroids due to potential negative effects on neurodevelopment (Jobe, 2004; Yeh et al., 2004). Furthermore, few data specifically address infants born ≤ 28 weeks, who are exposed to the most procedural pain, and are the most vulnerable to altered developmental trajectories in childhood.
It is not known whether repeated neonatal pain in preterm infants affects only behavioral and cardiac pain reactivity, or also alters HPA reactivity, nor whether effects of prior pain differ between preterm infants born at varying physiologically maturity. Procedural handling (stress) and skin breaking procedures (pain) are considered to be on a continuum of stressors from mild to severe. Our goals were to evaluate whether, in preterm infants with no exposure to postnatal corticosteroids, cumulative prior neonatal procedural pain affects later: (1) cortisol response to stressors and (2) behavioral and cardiac reactivity to pain. We compared preterm infants born at extremely low gestational age (ELGA; 23–28 weeks) with more physiologically mature preterm infants born at very low gestational age (VLGA; 29–32 weeks). Further, we examined whether there is a relationship between cumulative neonatal pain and morphine exposure, in relation to later cortisol, behavioral, and cardiac responses to pain or stressors. To our knowledge this is the first study to examine relationships of early procedural pain and morphine exposure on the HPA axis in human infants.
2. Materials and methods
2.1. Study Participants
The study sample comprised 87 preterm infants (47 male and 40 female) born ≤ 32 completed weeks gestational age (GA), admitted to the level-III NICU in the Children's and Women's Health Centre of British Columbia, Vancouver, Canada. Infants who had received analgesics or sedatives within 72 h of the assessment, or who had significant intraventricular hemorrhage and/or parenchymal brain injury (IVH grade IV, or PVL), a major congenital anomaly, exposure to maternal illicit drug use during pregnancy, or any postnatal corticosteroids were excluded. All 87 infants had valid data for one or more outcome measures; missing data was due to face obscured on video, poor quality cardiac recording, or insufficient blood to assay cortisol (exact n provided in Table 3). The infants were 32 weeks postconceptional age (PCA) ±7 days at time of testing, with a mean of 3.5 (SD 2.8) days between test sessions. Infant characteristics are presented in Table 1. Because our aim was to examine multiple determinants of pain responses, sample size was determined based on requirements for multiple regression analysis of 10 participants per predictor variable (Licht, 1998).
Table 3.
Associations between prior pain exposure, prior morphine exposure and illness severity with facial and autonomic responses to heel lance and cortisol response to a series of nursing procedures
| Illness severity (snap II day I) | Pain exposure (number of skin breaking procedures) birth to 32 weeks | Morphine exposure (daily average mg/kg×days) birth to 32 weeks | Mechanical ventilation (days) | |
|---|---|---|---|---|
| Infants born ≤ 28 weeks GAa | ||||
| Facial (lance) n = 29 | —0.26 | —0.44* | —0.42* | —0.19 |
| HR (baseline to lance) n = 28 | —0.24 | —0.09 | —0.15 | 0.05 |
| Low frequency (baseline to lance) n = 28 | —0.02 | —0.28 | 0.05 | —0.31 |
| High frequency (baseline to lance) n = 28 | —0.25 | —0.19 | —0.34 | —0.27 |
| Cortisol (clustered care) n = 23 | —0.17 | —0.50* | —0.26 | —0.37 |
| Infants born 29—32 weeks GAb | ||||
| Facial (lance) n = 43 | 0.16 | 0.05 | 0.19 | —0.09 |
| HR (baseline to lance) n = 45 | —0.06 | 0.09 | 0.05 | 0.03 |
| Low frequency (baseline to lance) n = 45 | —0.36* | —0.10 | —0.11 | 0.07 |
| High frequency (baseline to lance) n = 45 | —0.13 | —0.11 | —0.15 | 0.15 |
| Cortisol (clustered care) n = 45 | 0.19 | 0.10 | —0.04 | 0.12 |
P < 0.05
n = 30 ELGA infants had one or more outcomes (facial, cardiac, cortisol).
n = 57 VLGA infants had one or more outcomes (facial, cardiac, cortisol).
Table 1.
Preterm infant characteristics (N=87: mean (SD))
| Preterm 22–28 weeks GA at birth, n=30 | Preterm 29–32 weeks GA at birth, n=57 | |
|---|---|---|
| Gestational age at birth (weeks) | 27.17 (1.31) | 30.93 (1.26) |
| Birth weight (g) | 960.83 (254.87) | 1532.19 (390.71) |
| Illness severity day 1 (SNAP-II) | 18.13 (11.22) | 8.14 (8.07) |
| Illness severity day 3 (SNAP-II) | 5.50 (5.52) | 0.67 (2.21) |
| Mechanical ventilation (days)a | 17.53 (14.67) | 1.40 (3.13) |
| Other respiratory support (days)a | 10.80 (8.71) | 5.0 (6.35) |
| Pain exposure (number of skin breaking procedures)a | 115.20 (64.71) | 40.47 (25.98) |
| IV Morphine exposure (daily average mg/kg × days)a | 1.90 (2.70) | 0.06 (0.17) |
| Sex (male/female) | 16/14 | 31/26 |
Recorded daily from birth to test day (32 weeks±6 days postconceptional age).
2.2. Cortisol
Blood samples were collected on ice and centrifuged at 4 °C. Plasma cortisol was measured by radioimmunoassay in the plasma extracted in 95% ethanol. Dextran-coated charcoal was used to absorb and precipitate free steroids after incubation. Samples were counted using ScintiSafe Econo 2 (Fisher Scientific, Ottawa, ON). The minimal detectable dose for cortisol was 0.02 μg/dl and the mid-range intra and interassay coefficients of variation were 1.55 and 4.26%, respectively.
2.3. Facial activity (Neonatal Facial Coding System: NFCS)
The Neonatal Facial Coding System (NFCS; Grunau and Craig, 1987) is a reliable, well validated behavioral pain measure widely used in studies of preterm infants (Grunau et al., 2001a; Lindh et al., 1997; Stevens et al., 1994). Videotapes were coded in random order of events, and coders were blind to all clinical information about the infants and to events. In order to establish reliability, primary NFCS coder and the reliability coder were trained to achieve a reliability coefficient above 0.85 (Grunau and Craig, 1987). In addition, reliability coding was carried out on 20% of the infants, with a reliability coefficient of 0.88 on this sample.
2.4. Cardiac
Continuous electrocardiographic (ECG) activity was recorded from a single lead of surface ECG and was digitally sampled at 360 Hz off-line using a specially adapted computer acquisition system. Custom physiologic signal processing software (HRView Software, 1996) was used to acquire, process and analyze heart rate (HR) and heart rate variability (HRV). R waves were detected from the sampled ECG, and were used to form a smoothed instantaneous 4-Hz time series as described previously (Berger et al., 1989). Epochs of heart rate (HR; 2.2 min each) were selected for baseline and lance. The epoch selection criteria were based on quantitative signal stationarity, the presence of a stable behavioral state, and the absence of gross movement artifact. Power spectral estimates of HR were quantified (Saul et al., 1991) using the area (power) of the spectrum in low frequency (LF) HR variability (0.04–0.15 Hz), which reflects sources mediated by both sympathetic and parasympathetic influences, and high frequency (HF) variability (0.15–0.80 Hz), which reflects the influence of respiratory activity (respiratory sinus arrhythmia) mediated by parasympathetic influences alone. Residualized change scores were calculated to adjust response to lance for initial baseline values, to provide pain reactivity measures of autonomic activity.
2.5. Background data
A NICU-trained research nurse completed each clinical chart review while the infant was in the NICU, and obtained daily information from birth to day of testing including, but not limited to the following: birth weight, gestational age at birth, Apgar scores, illness severity using the Score for Neonatal Acute Physiology (SNAP-II; Lee et al., 1999), every dose of narcotic and other analgesic and sedative medications (adjusted for daily weight), number and type of each invasive skin breaking procedure, respiratory support. Invasive procedures were defined as those involving skin breaking such as heel lance, venipuncture, insertion of arterial and venous lines, lumbar puncture and chest-tube insertion.
2.6. Procedures
The infants were recruited by a NICU-trained research nurse, and written informed consent was obtained from a parent according to a protocol approved by the Clinical Research Ethics Board of the University of British Columbia, and the Children's and Women's Health Centre of BC Research Review Committee. Infant responses to two different stressors were studied on separate days in random order, in a repeated measures crossover design. On one day, a fixed series of nursing procedures (diaper change, measuring abdominal girth, temperature, mouth care) was timed to occur approximately 20 min prior to a scheduled clinical blood collection, which was used to obtain a small amount of extra plasma for cortisol assay. On the other day, behavioral and autonomic responses before, during and after blood collection were measured from continuous video recording and acquisition of cardiac signals. Infants were studied in the morning to control for time of day.
Each infant was undisturbed for a period of at least 30 min prior to recording. Heart rate data were collected by attaching the leads from the bedside monitor to a custom-designed computer data acquisition system. The video camera was positioned for close-up on the face, and attached to a custom made recording set-up on a moveable cart. The signals were fed directly to a VCR, and a time code was imprinted automatically. Each study phase was marked with an inaudible event cue signal recorded simultaneously on the videotape and physiologic acquisition systems. During recording, the incubator was partially covered with a blanket, and the infant's position was supported (nested) using a continuous roll around both sides and feet. For the blood collection procedure, the research nurse applied a foot warming pack 5 min before the lab technician drew the blood. The lab technician's standard protocol involved checking the infant's identification band on the incubator, removing the warming pack from the foot, swabbing the heel with a small gauze pad with disinfectant, lancing the heel, and then gently squeezing the heel intermittently until the amount of blood was collected which was required for clinical management. Unless the infant demonstrated physiological instability requiring intervention by the bedside nurse, the infant was handled only by the lab technician during the procedure. For the tactile nursing procedures, a single research nurse carried out clustered nursing procedures in a set order: changing the diaper, measuring abdominal girth, taking the axillary temperature, cleaning the mouth with gauze and sterile water. For both the pain and tactile procedures, a research technician set up the video camera and the VCR machines, operated the cardiac data acquisition computer and marked each phase during the procedure.
2.7. Data analysis
Pearson correlations were carried out to examine relationships among the neonatal variables, and between the neonatal and predictor variables. Hierarchical regression analysis was used to evaluate whether prior pain and morphine exposure were associated with stress and/or pain responses, after controlling for early illness severity. For a conservative estimate of associations, we used R2 adjusted for sample size.
3. Results
3.1. Preliminary analyses
3.1.1. Outliers
Cortisol and cardiac data were examined for outliers, defined as any value more than ±3 SD from the mean (Gunnar et al., 1989; Ramsay and Lewis, 2003). One infant had an outlier value for cortisol, and two infants for cardiac measures. These values were ‘winsorized’ following the method of Tukey (1977), which involves replacing the outlier value with the closest value within the 3 SD range, which is then used for data analyses.
3.1.2. Neonatal characteristics
The neonatal characteristics for the ELGA and VLGA infants are presented in Table 1. For the total sample of preterm infants the intercorrelations of the neonatal characteristics were examined prior to regression analysis to identify potential confounding factors (see Table 2). History of more painful procedures (higher number of skin breaking procedures since birth) was highly correlated with lower gestational age (r=–0.77, P=0.001) and with number of days of mechanical ventilation (r=0.80, P=0.001). In addition, a higher number of painful procedures was significantly correlated with lower birth weight (r=–0.64, P=0.001) and higher illness severity (SNAP-II) (r=0.58, P=0.001). Higher cumulative morphine exposure since birth was significantly associated with lower gestational age (r=–0.55, P=0.001), lower birth weight (r=–0.46, P=0.001), higher illness severity (r=0.39, P=0.001), and number of days on mechanical ventilation (r=0.69, P=0.001), and higher number of painful procedures (r=0.65, P=0.001).
Table 2.
Pearson intercorrelations of perinatal/neonatal characteristics for the ELGA and VLGA preterm infants combined (N=87)
| Gestational age at birth | Birth weight | Illness severity day 1 | Mechanical ventilation (days) | Oxygen (days) | Pain exposure (number of skin breaking procedures) | |
|---|---|---|---|---|---|---|
| Birth weight | 0.79** | |||||
| Illness severity day 1 (SNAP-II) | —0.52** | –0.39** | ||||
| Mechanical ventilation (days) | —0.76** | –0.61** | 0.43** | |||
| Other respiratory support (days) | —0.48** | –0.33** | 0.25* | 0.15 | ||
| Pain exposure (number of skin breaking procedures) | —0.77** | –0.64** | 0.58** | 0.80** | 0.38** | |
| Morphine exposure (daily average mg/kg X days) | —0.55** | –0.45** | 0.39** | 0.68** | 0.06 | 0.65** |
P < 0.01
P < 0.05
3.2. Neonatal experience and later pain/stress responses
Bivariate correlations were computed to examine the associations between the main predictor variables (prior procedural pain and morphine exposure since birth) and the outcome measures (cortisol, facial, and cardiac reactivity), for the ELGA and the VLGA infants separately (see Table 3). In the ELGA, but not VLGA group, higher neonatal pain exposure was significantly related to lower cortisol response to the stressor (r= −0.50, P=0.015) and to lower facial activity to pain (r=–0.44, P=0.02). Interestingly, higher morphine exposure was also associated with lower facial activity (r=–0.42, P=0.022), but not with cortisol response. In the VLGA group, higher illness severity was significantly associated with lower low frequency HRV (r=–0.36, P=0.017), i.e. greater cardiac arousal; no other significant associations were found.
To further examine relationships between prior pain (number of neonatal skin breaking procedures) and morphine exposure and later behavioral and cardiac responses to the pain of heel lance, and cortisol response to the stress of clustered nursing procedures, hierarchical multiple regression analyses were conducted separately for each outcome measure, controlling for early illness severity. In block 1, SNAP II scores at day 1 were entered to control for early illness severity; in block 2, pain exposure since birth (number of skin breaking procedures) was entered; and in block 3, intravenous morphine exposure since birth (total doses adjusted for daily weight) was entered.
3.2.1. Cortisol responses
In the ELGA group, after controlling for early illness severity, and independent of morphine exposure, higher neonatal procedural pain predicted lower plasma cortisol responses to stress (adjusted R2=0.23; standardized β=–0.59, t=–2.22, P=0.039) (see Fig. 2). No statistically significant relationships were found in the VLGA group.
Fig. 2.
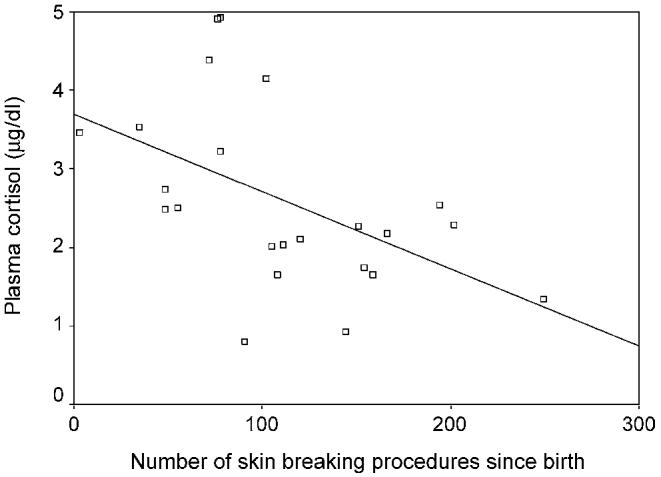
Cumulative procedural pain since birth in relation to cortisol stress response to a set of nursing interventions in infants born ≤ 28 weeks gestational age (n=23).
3.2.2. Behavior (NFCS)
In the ELGA group, after controlling for illness severity, higher numbers of skin breaking procedures since birth predicted dampened facial responses to lance (adjusted R2=0.14, standardized β=–0.41, t=–2.09, P=0.047) (see Fig. 1). However, at step 3 when cumulative morphine exposure was entered into the regression, no significant associations remained, indicating that effects of prior pain and morphine exposure (see correlations Table 3) were not independent. No significant associations were found for the VLGA group.
Fig. 1.
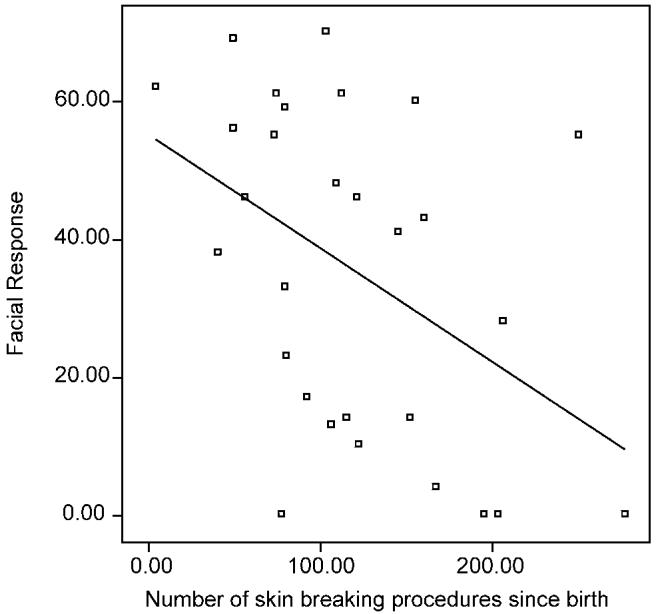
Cumulative procedural pain since birth in relation to facial reactivity to heel lance in infants born ≤ 28 weeks gestational age (n=29).
3.2.3. Cardiac reactivity
No significant associations were found between the neonatal predictors and heart rate or heart rate variability (low or high frequency power) during blood collection for either the ELGA or the VLGA group.
4. Discussion
This study is the first to examine relationships of cumulative early procedural pain and morphine exposure, with subsequent cortisol, behavioral, and cardiac responses in preterm infants in the NICU who were free from postnatal exogenous steroid exposure. Among infants born at extremely low gestational ages (≤ 28 weeks), greater cumulative exposure to painful (skin breaking) procedures since birth was associated with lower cortisol response to a stressor (clustered nursing procedures). This relationship was independent of illness severity on day 1 after birth, and of cumulative exposure to intravenous morphine since birth. Importantly, this down-regulation of cortisol response to the subsequent stressor was observed while the infants were still in the NICU. In contrast, in a previous study at 8 months of age corrected for prematurity, cortisol levels were elevated in infants exposed to higher numbers of skin breaking procedures since birth (Grunau et al., 2004), which is consistent with data from animal studies indicating sensitization following early exposure to stress (Anisman et al., 1998; Ladd et al., 2000; Pryce and Feldon, 2003). Thus, it appears that following higher levels of exposure to neonatal pain in physiologically immature infants, there may be a shift in HPA responsiveness over time, such that cortisol responses are dampened while infants are still in the hospital (and thereby exposed to ongoing environmental stress), but then increased later in infancy. Similarly, in a different study at age 8 months (adjusted for prematurity), cumulative neonatal pain was associated with elevated basal HR (Grunau et al., 2001b). Thus early pain may lead to altered developmental patterns in stress arousal systems.
Long-term effects of early stress in animal models appear to differ depending on the nature of the stressor. Our findings of elevated cortisol responses to stressors in ELGA preterm infants at 8 months who had the most exposure to painful procedures since birth are consistent with those found in animal models of maternal separation stress, which reported sensitization of the HPA axis to subsequent stressors (e.g. Plotsky and Meaney, 1993). In contrast, in animal studies of neonatal pain exposure, alterations in HPA reactivity in adulthood are typically not observed (Anand et al., 1999; Walker et al., 2003). However Walker et al. (2003) found that maternal care is increased when rat pups have been exposed to pain. They propose that the lack of potentiation of the HPA axis in neonatal rats exposed to early repeated physical pain may be due to moderating effects of this increase in maternal care. Maternal behavior is precluded for long periods of time in the maternal separation paradigm, which may explain these differential results.
The dampened cortisol response in the ELGA infants in the NICU may have occurred, at least in part, because preterm infants have varying degrees of immaturity in HPA axis function during the neonatal period (Bolt et al., 2000, 2002a,b; Korte et al., 1996). In particular, infants born at lower gestational ages who are sickest commonly show low basal cortisol and adrenal insufficiency (e.g. Hanna et al., 1997; Kari et al., 1996; Ng et al., 2001; Scott and Watterberg, 1995). In addition, preterm infants recovering from chronic lung disease may show decreased cortisol in response to ACTH 3 weeks after birth (Watterberg et al., 2001). In our study it is unlikely that the diminished cortisol response was caused by relative insensitivity of the adrenal cortex, as we examined stress reactivity at 32 weeks PCA; after approximately 30–32 weeks, adrenal sensitivity appears to be comparable to that in term infants (Winter, 1998).
Another major focus of this study was the relationships between prior cumulative procedural pain and subsequent facial and cardiac reactivity to pain during blood collection. Previously we found that higher exposure to neonatal dexamethasone contributed to dampened biobehavioral response to subsequent pain in the NICU at 32 weeks PCA (Grunau et al., 2001a). In the present study, in infants without exposure to postnatal dexamethasone, we replicated our earlier finding that dampened facial reactivity to pain during blood collection at 32 weeks in the NICU was significantly related to higher number of pain procedures since birth. However, it must be noted that the magnitude and direction of effects of both cumulative prior pain (r= −0.44) and cumulative morphine exposure (r=–0.42) in relation to facial pain response was the same (i.e. both dampened facial pain response). Thus morphine did not appear to ameliorate long-term effects of prior pain on subsequent behavioral reactivity to pain. It is noteworthy that the association between cumulative prior pain and both facial reactivity and cortisol response showed a downward direction. This suggests that both behavioral and cortisol reactivity were reflecting alterations to underlying stress response systems. However, unlike facial response where separate effects of prior pain and morphine were not distinguishable, cortisol was specifically associated with prior pain. In contrast, we found no relationships between pain or morphine exposure and HR or HRV in these infants who were free of exposure to postnatal corticosteroids. In our earlier NICU study (Grunau et al., 2001a), higher dexamethasone exposure since birth was associated with dampened biobehavioral response, and morphine appeared to ameliorate this association using a measure which primarily tapped sympathetic reactivity. However, given the relatively high doses of dexamethasone used at that time in medical care of extremely preterm infants, and the actions of corticosteroids on multiple physiologic systems, it appears likely that the differences between the present and prior studies was the exclusion of infants exposed to postnatal steroids in the present study.
Another important question is whether a ‘cutoff’ can be identified in the number of procedures at which point the relationship between prior pain and subsequent dampened reactivity ‘kicks-in’. In our earlier paper, preterm infants who received less that 20 procedures showed vigorous responses to blood collection, whereas more than 20 procedures was associated with dampened response (Grunau et al., 2001a,b). In the present sample we were unable to identify a ‘cutoff’ as shown in Figs. 1 and 2, where considerable variability was evident.
Advances in knowledge of the developmental neurobiology of pain and stress systems (Fitzgerald, 1993; Fitzgerald and Beggs, 2001) have increased attention on the long-term effects of pain on preterm infants in the NICU (Fitzgerald, 2004; Walker et al., 2001). However, recently there is evidence that early morphine in mechanically ventilated infants may not be particularly efficacious in ameliorating pain in these infants (Simons et al., 2003), and furthermore, may not decrease major adverse sequelae (Anand et al., 2004). In the present study we did not find evidence that cumulative morphine ameliorated relationships between early pain and later dampened facial or cortisol responses.
In clinical studies it is difficult to ‘unpack’ specific effects of cumulative pain exposure versus biological immaturity, which are highly intercorrelated. We attempted to address this by examining associations of cumulative procedural pain separately for infants born extremely low (≤ 28 weeks) and very low (29–32 weeks) gestational age. Time in the NICU and postconceptional age are identical, and inversely related to gestational age, all indicating degree of biological immaturity. Repeated pain exposure likely acts synergistically together with neonatal physiological immaturity to drive altered pain and stress responses, both in the NICU and later after hospital discharge.
While experimental randomized studies are the only way to address causality, there are limits to these as well. With human infants, the ethical issues presented by needing to provide pain relief in clinical care lead to a high crossover rate in randomized pharmacologic trials (e.g. Anand et al., 2004). In studies using animal models, the intervention itself can induce alterations in maternal care (Walker et al., 2003). Our results are consistent with recent findings that reduction of environmental sources of stress in the NICU promotes improved outcomes (Als et al., 2004).
There are a number of limitations in this study. First, total skin breaking procedures reflect not only pain, but also cumulative exposure to stress since birth. Secondly, we did not evaluate effects of different types of procedures which vary in intensity. Lastly, we could not separate biological immaturity from prior pain/stress exposure, however, this limitation affects this field in general.
5. Conclusions
Cumulative stress exposure in the NICU, in conjunction with extreme physiologically immaturity, appears to alter HPA and behavioral reactivity in human infants. The most specific association was with a reduction in cortisol response, which is the primary stress hormone in humans. Further investigation is warranted concerning the dissociations found in outcomes of HPA, autonomic and behavioral systems, as well as whether effects persist later in childhood. Overall our findings support the perspective that prolonged and repeated neonatal stress (Als, 1995) and pain exposure in vulnerable preterm infants may alter self-regulation in multiple systems (Grunau, 2003). Of greatest concern is that these biobehavioral changes may underlie long-term learning and behavior difficulties in this population.
Acknowledgements
We thank the staff and families of the Special Care Nursery at BC Children's Hospital for participating in this study, and the research staff in the Early Experience Unit, Centre for Community Child Health Research, BC Research Institute for Children's and Women's Health. Funding was provided by the National Institute of Child Health and Human Development grant HD39783, Canadian Institutes of Health Research grant MOP42469, Human Early Learning Partnership (HELP) and the British Columbia Ministry of Children and Family Development grant 02-2410, HELP Post-doctoral Fellowship (DH), Senior Scholar Award Michael Smith Foundation for Health Research (REG), Canadian Institutes of Health Research/Canadian Occupational Therapy Foundation Post-Doctoral Fellowship (LH).
References
- Als H. The preterm infant: a model for the study of fetal brain expectation. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: a psychobiological perspective. Erlbaum; Hillsdale, NJ: 1995. pp. 439–71. [Google Scholar]
- Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, Warfield SK, Huppi PS, Butler SC, Conneman N, Fischer C, Eichenwald EC. Early experience alters brain function and structure. Pediatrics. 2004;113:846–57. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Effects of perinatal pain and stress. Prog Brain Res. 2000;122:117–29. doi: 10.1016/s0079-6123(08)62134-2. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–64. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Berger RD, Saul JP, Cohen RJ. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am J Physiol. 1989;256:H142–H52. doi: 10.1152/ajpheart.1989.256.1.H142. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, Weissenbruch MM, Sweep CGJ, Popp-Snijders C, Lefeber HN, Delemarre-van de Waal H. The cortisol–cortisone shuttle in preterm infants during the first week of life: its relation with gestational age and body size. Horm Res. 2000;53:s23. [Google Scholar]
- Bolt RJ, Van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Development of the hypothalamic–pituitary–adrenal axis in the fetus and preterm infant. J Pediatr Endocrinol Metab. 2002a;15:759–69. doi: 10.1515/jpem.2002.15.6.759. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA. Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002b;52:405–10. doi: 10.1203/00006450-200209000-00017. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Development of pain pathways and mechanisms. In: Anand KJS, McGrath PJ, editors. Pain in neonates. Elsevier; Amsterdam: 1993. pp. 19–37. [Google Scholar]
- Fitzgerald M. Painful beginnings. Pain. 2004;110:508–9. doi: 10.1016/j.pain.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–57. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Grunau R. Early pain in preterm infants. Clin Perinatol. 2002;29:373–94. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Grunau RE. Self-regulation and behavior in preterm children: effects of early pain. In: McGrath PJ, Finley GA, editors. Pediatric pain: biological and social context. Progress in pain research and management. Vol. 26. IASP Press; Seattle, WA: 2003. [Google Scholar]
- Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely low-birth-weight premature toddlers and preterm and full-term controls. Pain. 1994;58:341–6. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks' postconceptional age. Pediatrics. 2001a;107:105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Morison SJ, Saul JP. Pain reactivity in former extremely low birth weight infants at corrected age 8 months compared with term born controls. Infant Behav Dev. 2001b;24:41–55. [Google Scholar]
- Grunau RE, Whitfield MF, Weinberg J. Neonatal procedural pain exposure and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Connors J, Isensee J. Lack of stability in neonatal adrenocortical reactivity because of rapid habituation of the adrenocortical response. Dev Psychobiol. 1989;22:221–33. doi: 10.1002/dev.420220304. [DOI] [PubMed] [Google Scholar]
- Hanna CE, Jett PL, Laird MR, Mandel SH, Lafranchi SH, Reynolds JW. Corticosteroid binding globulin, total serum cortisol, and stress in extremely low-birth-weight infants. Am J Perinatol. 1997;14:201–4. doi: 10.1055/s-2007-994127. [DOI] [PubMed] [Google Scholar]
- HRView Software. Boston Medical Technologies; Boston: 1996. [Google Scholar]
- Jobe AH. Postnatal corticosteroids for preterm infants—do what we say, not what we do. N Engl J Med. 2004;350:1349–51. doi: 10.1056/NEJMe048031. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–30. [PubMed] [Google Scholar]
- Kari MA, Raivio KO, Stenman UH, Voutilainen R. Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestational age and dexamethasone therapy. Pediatr Res. 1996;40:319–24. doi: 10.1203/00006450-199608000-00021. [DOI] [PubMed] [Google Scholar]
- Korte C, Styne D, Merritt TA, Mayes D, Wertz A, Helbock HJ. Adrenocortical function in the very low birth weight infant: improved testing sensitivity and association with neonatal outcome. J Pediatr. 1996;128:257–63. doi: 10.1016/s0022-3476(96)70404-3. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lee SK, Ohlsson A, Synnes AR, Peliowski A, Sankaran KBR, Chien LY. The Canadian NICU Network, Mortality variations and illness severity (SNAP-II) in Canadian NICUs. Pediatr Res. 1999:248A. [Google Scholar]
- Licht MH. Reading and understanding multivariate statistics. American Psychological Association; Washington, DC: 1998. Multiple regression and correlation; pp. 19–64. [Google Scholar]
- Lindh V, Wiklund U, Sandman PO, Hakansson S. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Hum Dev. 1997;48:131–42. doi: 10.1016/s0378-3782(96)01851-8. [DOI] [PubMed] [Google Scholar]
- Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, Chan IH, Wong E. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed. 2001;84:F122–F4. doi: 10.1136/fn.84.2.F122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Dev. 2003;74:456–64. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and reinflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–96. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261:H1231–H45. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Scott SM, Watterberg KL. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res. 1995;37:112–6. doi: 10.1203/00006450-199501000-00021. [DOI] [PubMed] [Google Scholar]
- Simons SH, Van Dijk M, Van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, Van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. J Am Med Assoc. 2003;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- Stevens BJ, Johnston CC, Horton L. Factors that influence the behavioral pain responses of premature infants. Pain. 1994;59:101–9. doi: 10.1016/0304-3959(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Addison-Wesley; Don Mills, ON: 1977. [Google Scholar]
- Walker C, Anand KJS, Plotsky PM. Development of the hypothalamic–pituitary–adrenal axis and the stress response. In: McEwen B, Goodman H, editors. Coping with the environment: neural and endocrine mechanisms. Vol. 4. Oxford University Press; Oxford: 2001. pp. 237–70. [Google Scholar]
- Walker CD, Kudreikis K, Sherrard A, Johnston CC. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res Dev Brain Res. 2003;140:253–61. doi: 10.1016/s0165-3806(02)00611-9. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res. 2001;50:190–5. doi: 10.1203/00006450-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Winter JSD. Fetal and neonatal adrenocortical physiology. In: Polin R, Fox W, editors. Fetal and neonatal physiology. W.B. Saunders; Philadelphia, PA: 1998. pp. 2447–59. [Google Scholar]
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]


