Summary
Despite recent advances in antibiotic therapy and intensive care, sepsis remains widespread problems in critically ill patients. The high mortality of sepsis is in part mediated by bacterial endotoxin, which stimulates macrophages/monocytes to sequentially release early (e.g., TNF, IL-1, and IFN-γ) and late (e.g., HMGB1) pro-inflammatory cytokines. In light of our recent discovery of HMGB1 as a late mediator of lethal systemic inflammation, and the observation that green tea (Camellia sinensis) dose-dependently attenuated bacterial endotoxin-induced HMGB1 release, we propose that regular tea intake might decrease the incidence of and mortality rates from lethal endotoxemia and sepsis.
Introduction
In 1347, a mysterious plague spread across the entire continent of Europe, taking the lives of 20 million people, wiping out approximately one third of the European population in the mere three years [1]. More than six hundred years have passed, yet our world continues to be plagued by infectious diseases. Being touted as modern day’s equivalent of the bubonic plague [1], sepsis refers to an overwhelming systemic inflammatory response to infection, and is defined by signs of organ dysfunction that include abnormalities in body temperature, heart rate, respiratory rate, and leukocyte counts. Despite recent advances in antibiotic therapy and intensive care, sepsis is still the most common cause of death in the intensive care units, claiming approximately 225,000 victims annually in the U.S. alone. Initiated by an infection, the pathogenesis of sepsis is attributable, at least in part, to dys-regulated systemic inflammatory responses characterized by excessive accumulation of various proinflammatory cytokines [2–5].
In response to bacterial toxins (e.g., lipopolysaccharide, LPS), macrophages/monocytes release reactive nitrogen intermediates (e.g., nitric oxide) [6], and various proinflammatory cytokines such as tumor necrosis factor (TNF) [7], interleukin (IL)-1 [8], interferon (IFN)-γ [9], and macrophage migration inhibitory factor (MIF) [10], which individually, or in combination, contribute to the pathogenesis of lethal endotoxemia or sepsis. For instance, neutralizing antibodies against TNF, the first cytokine elaborated in inflammatory cascade, reduces lethality in an animal model of endotoxemic/bacteremic shock [7]. However, the early release of TNF makes it difficult to target therapeutically in a clinical setting [7], prompting the search for late proinflammatory cytokines that may offer a wider therapeutic window for the treatment of lethal systemic inflammatory diseases.
HMGB1 as a late mediator of lethal endotoxemia and sepsis
We have discovered that a ubiquitous protein, high mobility group box 1 (HMGB1), is released by activated macrophages/monocytes [11–13], and functions as a late mediator of lethal endotoxemia and sepsis [11;14–16]. First, circulating HMGB1 levels are elevated in a delayed fashion (after 16–32 hours) in endotoxemic and septic animals [11;14], and in patients with sepsis [11]. Second, administration of recombinant HMGB1 to mice recapitulates many clinical signs of sepsis, including fever [17], allodynia [18], derangement of intestinal barrier function [19], lung injury [20], and lethal multiple organ failure [11]. Third, administration of anti-HMGB1 antibodies or inhibitors (e.g., ethyl pyruvate, nicotine, or stearoyl lysophosphatidylcholine) significantly protects mice against LPS-induced acute lung injury [20;21], and lethal endotoxemia [1;11;15;22;23]. Notably, these anti-HMGB1 reagents are also capable of rescuing animals from lethal experimental sepsis even when the first doses are given 24 hours after onset of sepsis [14;15;24], confirming a pathogenic role of HMGB1 in lethal experimental sepsis. Therefore, agents proven clinically safe, and yet still capable of attenuating HMGB1 release may hold potential in the prevention and treatment of inflammatory diseases.
Tea and human health
Tea has been brewed from the leaves of the Camellia sinensis plant for almost fifty centuries. In Maintaining Health by Drinking Tea, Eisai, the “Father of Tea” in Japan, called tea a “miraculous medicine for the maintenance of health.” Today, tea is cultivated in 30 different countries around the world, and its daily consumption ( 120 ml) is second only to water [25–28]. Although health benefits have been attributed to tea consumption since the beginning of its history, scientific investigations of this beverage and its constituents has been underway for less than three decades.
Epidemiological surveys have suggested a close association between green tea consumption and human longevity [29]. Indeed, tea contains abundant polyphenols that may be protective against chronic illness such as cardiovascular disease and cancer [25;26;28]. These beneficial effects have been well demonstrated in animal studies, but have subsequently confirmed only in a limited number of human studies. Confounding factors are multiple, and may include the insufficiency in the dosage of tea consumed by humans (as opposed to the dosage required to demonstrate the beneficial effects in animal models), as well as other lifestyle-related factors in different populations.
In addition, a number of pre-clinical animal studies have suggested some anti-inflammatory activities of green tea in models of collagen-induced arthritis [30] and pulmonary inflammation [31]. For example, polyphenolic fraction of green tea has been shown to markedly reduce the expression of pro-inflammatory cytokines such TNF and IFN-γ in joints of animals with collagen-induced arthritis [30]. However, it was previously unknown if green tea can attenuate bacterial endotoxin-induced release of late mediators of lethal endotoxemia and sepsis.
More tea for septic patients?
Accordingly, we examined the effects of natural Lipton (R) Green Tea (without artificial colors or additives) on bacterial endotoxin-induced HMGB1 release. As indicated in Figure 1, the Lipton green tea dose-dependently inhibited endotoxin-induced release of nitric oxide and HMGB1 (Fig. 1). At a dose as low as 10 μl/ml (equivalent of 75 mls /person, assuming a total body weight of 75 kg, and blood volume of 7,500 mls), green tea almost completely abolished endotoxin-induced HMGB1 release. Even at concentrations that almost completely abrogated HMGB1 release, green tea did not exhibit any cytotoxicity to macrophage cultures, because cell viability, as assessed by trypan blue exclusion, was not reduced [92%, for control; versus 91%, in the presence of LPS + tea (10 μl/ml)]. In light of the capacity of green tea to attenuate endotoxin-induced release of nitric oxide and HMGB1, as well as their pathogenic roles in lethal systemic inflammation [11;15;32–35], we propose that green tea might be beneficial for patients with systemic inflammation (such as endotoxemia and sepsis). Therefore, regular tea intake might provide an approach to decrease the incidence of and mortality from lethal endotoxemia and sepsis.
Figure 1. Green tea dose-dependently suppressed endotoxin-induced HMGB1 release.
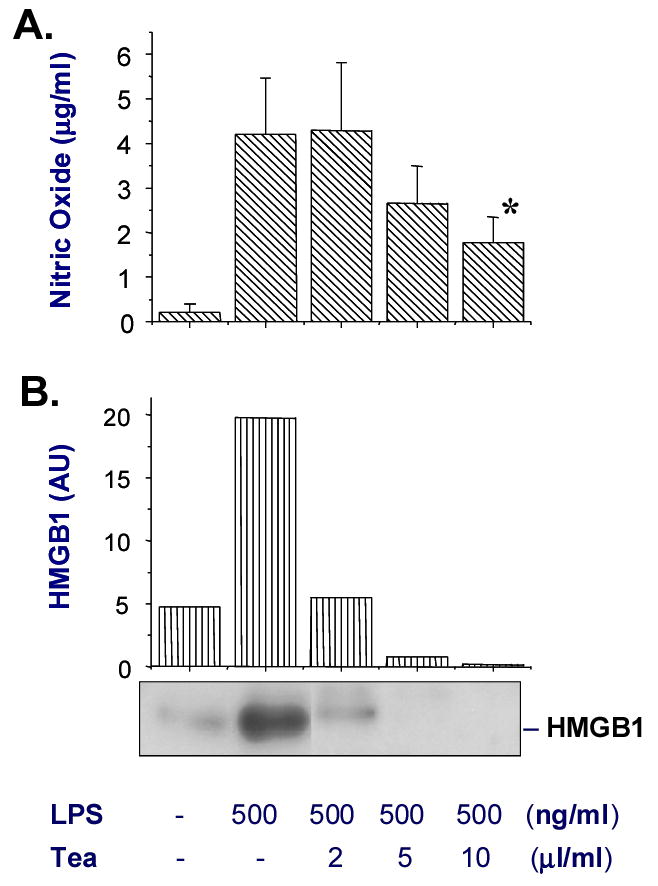
Murine macrophage-like RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in DMEM medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum and 2 mM glutamine. At 80–90% confluence, RAW 264.7 cells were washed twice with, and subsequently cultured in, serum-free DMEM medium before stimulation with bacterial endotoxin (lipopolysaccharide, LPS, E. coli 0111:B4, Sigma-Aldrich) alone, or in the presence of the green tea at indicated concentrations. Natural Lipton (R) Green Tea (3.2 gram/bag, Lipton, Englewood Cliffs, NJ, USA) was extracted in 100 ml hot water (100º C) for 10 minutes, and the aqueous extract was sterilized by filtration (through 0.22 μm filter) before adding to cell cultures. At 16 hours after stimulation, levels of nitric oxide and HMGB1 in the culture medium were determined by Griess reaction and Western blot, respectively [12]. Shown in the bar graph of panel A are levels of nitric oxide (expressed as mean ± SD of three experiments). Student’s t-test was performed and a P < 0.05 was considered significant (*). Shown in the bar graph of panel B are relative HMGB1 levels expressed as the optical band intensity (arbitrary units, AU). Shown in the lower portion of Panel B is a representative Western blot.
This hypothesis can be tested in pre-clinical animal, and clinical human studies. For instance, if green tea protects animals against lethal endotoxemia and experimental sepsis, it will support the above hypothesis. Similarly, if ingestion of green tea reduces mortality rate of patients with lethal sepsis, it will further support tea’s protective effects against lethal endotoxemia and sepsis. However, caution should be excised while using high doses of tea for disease prevention, because ingestion of large amount of caffeine by patients with intestinal or stomach ailments (e.g., ulcer) or kidney problems may cause dehydration, which may worsen the existing problems. Therefore, further studies are needed to elucidate the biologic activities of tea and to determine the optimal amount of tea consumption for possible health-beneficial effects.
Acknowledgments
This research was supported in part by the National Institutes of Health Grants (R01GM063075, R01GM070817, to HW), and the Institute for Medical Research at North Shore-LIJ (Faculty Research Award, to HW).
References
- 1.Wang H, Czura CJ, Tracey KJ. Lipid unites disparate syndromes of sepsis. Nat Med. 2004;10:124–5. doi: 10.1038/nm0204-124. [DOI] [PubMed] [Google Scholar]
- 2.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6 (Suppl 1):S27–S38. [PubMed] [Google Scholar]
- 3.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling--regulation of the immune response in normal and critically ill states. Crit Care Med. 2000 Apr;28(4 Suppl):N3–12. doi: 10.1097/00003246-200004001-00002. 28: N3–12. [DOI] [PubMed] [Google Scholar]
- 6.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–9. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–10. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–4. [PubMed] [Google Scholar]
- 10.Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 12.Rendon-Mitchell B, Ochani M, Li J, et al. IFN-gamma Induces High Mobility Group Box 1 Protein Release Partly Through a TNF-Dependent Mechanism. J Immunol. 2003;170:3890–7. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Li J, Ochani M, et al. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol. 2004;76:994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–31. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor KA, Hansen MK, Rachal PC, et al. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–65. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Chacur M, Milligan ED, Gazda LS, et al. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 19.Sappington PL, Yang R, Yang H, et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and causes derangements in intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 20.Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 21.Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–6. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 22.Yan JJ, Jung JS, Lee JE, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–7. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Li J, Qiang X, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–7. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Ulloa L, Ochani M, Yang H, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–6. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 26.McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 27.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130:2409–12. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- 28.Yang CS. Tea and health. Nutrition. 1999;15:946–9. doi: 10.1016/s0899-9007(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 29.Sueoka N, Suganuma M, Sueoka E, et al. A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. 2001;928:274–80. doi: 10.1111/j.1749-6632.2001.tb05656.x. [DOI] [PubMed] [Google Scholar]
- 30.Haqqi TM, Anthony DD, Gupta S, et al. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci U S A. 1999;96:4524–9. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dona M, Dell'Aica I, Calabrese F, et al. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–41. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 32.Vincent JL, Zhang H, Szabo C, et al. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. 2000;161:1781–5. doi: 10.1164/ajrccm.161.6.9812004. [DOI] [PubMed] [Google Scholar]
- 33.Fink MP, Payen D. The role of nitric oxide in sepsis and ARDS: synopsis of a roundtable conference held in Brussels on 18–20 March 1995. Intensive Care Med. 1996;22:158–65. doi: 10.1007/BF01720723. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Yang H, Czura CJ, et al. HMGB1 as a Late Mediator of Lethal Systemic Inflammation. Am J Respir Crit Care Med. 2001;164:1768–73. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Chen DZ, Li J, et al. Pathogenic role of HMGB1 in SARS? Med Hypotheses. 2004;63:691–5. doi: 10.1016/j.mehy.2004.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


