Abstract
Purpose
To demonstrate the efficacy of local corticosteroid therapy for the treatment of eyelid and orbital xanthogranuloma in adults.
Methods
We performed a retrospective chart review of six patients receiving local triamcinolone acetonide (40 mg/mL) injections for the treatment of eyelid and orbital xanthogranuloma at the University of Michigan. All patients underwent diagnostic biopsy prior to treatment. The effects of this therapy on symptoms and signs of the disease were assessed.
Results
All six patients complained of eyelid swelling or nodularity, and five had yellow discoloration of their eyelids. All lesions involved the eyelids and anterior orbit, and five were present bilaterally. Biopsy revealed necrobiotic xanthogranuloma in four patients and adult-onset xanthogranuloma in two patients. Triamcinolone acetonide was administered intralesionally as series of two to 25 injections. Local control was obtained in all six cases, with the reduction of symptoms and signs of the disease in five cases. Two patients with necrobiotic xanthogranuloma developed non-Hodgkin’s lymphoma. Average follow-up of patients whose treatment was not truncated by systemic chemotherapy was 52 months (range, 30 to 86 months). No complications occurred as a result of this treatment.
Conclusion
Intralesional injection of triamcinolone acetonide is an effective, safe treatment for orbital xanthogranuloma in adults. This modality avoids the side effects associated with systemic corticosteroid or cytotoxic agent therapy.
INTRODUCTION
Three types of idiopathic xanthogranuloma occur in the eyelids and orbits of adults: necrobiotic xanthogranuloma (NXG),1,2 adult-onset xanthogranuloma (AXG),3,4 and Erdheim-Chester disease.5,6 All three types of xanthogranuloma may be associated with systemic manifestations. NXG is commonly complicated by paraproteinemia and multiple myeloma or other lymphoproliferative disorders,1,2 whereas AXG may be associated with adult-onset asthma.3 Erdheim-Chester disease typically involves the posterior portion of the orbit and is associated with widespread systemic disease that results in death due to infiltration of vital organs.5,6 This disorder,7,8 like NXG9 and AXG,10 has been routinely treated with combinations of systemic corticosteroids, cytotoxic agents, and radiotherapy. However, NXG and AXG tend to affect the eyelids and anterior orbital tissue. The accessibility of NXG lesions to injection has prompted some to treat them with intralesional corticosteroids, yielding inconsistent results.11,12 We describe treatment of AXG and NXG with series of local injections of triamcinolone acetonide. This therapy controls the disease while avoiding the side effects of systemic or radiation treatment.
METHODS
A retrospective review was performed on the charts of six patients who presented to the Eye Plastic and Orbital Surgery Service of the University of Michigan Kellogg Eye Center. They sought treatment for relief of symptomatic eyelid swelling and discoloration. After computed tomographic (CT) scans were obtained in five of six cases, each patient underwent diagnostic biopsy, establishing the diagnosis (Figure 1). All six patients received intralesional triamcinolone acetonide (40 mg/mL) injections given into subcutaneous and anterior orbital tissue exhibiting palpable nodularity. The medication was delivered by means of a 25-gauge needle with 20 to 40 mg given into each site of nodularity up to a total dose of 120 mg. A series of repeated injections was given at 1- to 6-month intervals until nodularity was not palpable. Visual acuity was checked before and after each series of injections. The presence and severity of nodularity, skin discoloration, ptosis, and diplopia were documented before and after treatment. Also noted were patient age, sex, laterality of involvement, associated systemic disease, and complications. Informed consent for biopsy and treatment was obtained for all patients. This study was approved by the University of Michigan Institutional Review Board.
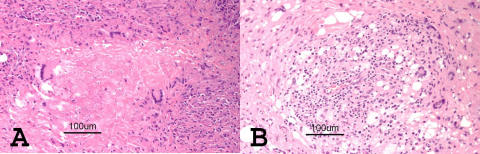
FIGURE 1.
Biopsy findings of necrobiotic xanthogranuloma (NXG) and adult-onset xanthogranuloma (AXG). A, Photomicrograph of patient 2 showing typical features of NXG, namely, granulomas with histiocytes containing lipid pallisading around areas of necrobiotic collagen. B, Photomicrograph of patient 4 showing AXG findings of lipogranulomatous inflammation with Touton-type giant cells and fibrosis.
RESULTS
A summary of the clinical data for all six patients is given in Table. Biopsy established the diagnosis of NXG in four patients and AXG in two patients. Equal numbers of men and women were affected. The average age was 53 years (range, 34 to 73 years). The average duration of symptoms prior to presentation was 29 months (range, 1.5 to 60 months).
TABLE.
OVERVIEW OF FOUR CASES OF NECROBIOTIC XANTHOGRANULOMA AND TWO CASES OF ADULT-ONSET XANTHOGRANULOMA
| CASE NO. | AGE/SEX | DX | ASSOCIATED SYSTEMIC DISEASE | SYMPTOMS AND SIGNS | DURATION OF SYMPTOMS AND SIGNS (MONTHS) | INVOLVED SITE | ORBITAL INVOLVEMENT | TRIAMCINOLONE DOSE | NO. OF INJECTIONS | RESPONSE | COMPLICATION | FOLLOW-UP (MONTHS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60/M | NXG | None | Nodularity, discoloration, diplopia, swelling, ptosis | 32.0 | Bilateral | Bilateral superoanterior | 40 mg | 22 | Improved nodularity, discoloration, ptosis | None | 86 |
| 2 | 54/M | NXG | NHL | Nodularity, discoloration, diplopia | 1.5 | Bilateral | Unknown | 40 mg | 2 | Improved nodularity | None | 3 |
| 3 | 73/F | NXG | MGUS | Nodularity, discoloration, diplopia, proptosis, ptosis, pain | 6.0 | Bilateral | Left superoanterior | 30 to 120 mg | 9 | Improved nodularity, discoloration, diplopia | None | 37 |
| 4 | 41/M | NXG | CLL, MDS | Nodularity, discoloration, proptosis, swelling | 60.0 | Bilateral | Bilateral superoanterior and EOM | 30 mg | 1 | Stable | None | 96 (died of CLL) |
| 5 | 34/F | AXG | Eczema | Nodularity, discoloration, proptosis | 24.0 | Bilateral | Bilateral superoanterior | 32 to 70 mg | 3 | Improved all signs | None | 46 |
| 6 | 57/F | AXG | None | Nodularity, discoloration, diplopia, proptosis, ptosis | 48.0 | Right | Superoanterior | 40 to 90 mg | 2 | Improved all signs | None | 30 |
AXG = adult-onset xanthogranuloma; CLL = chronic lymphocytic leukemia; Dx = diagnosis; EOM = extraocular muscles; MDS = myelodysplastic syndrome; MGUS = monoclonal gammopathy of uncertain significance; NHL = non-Hodgkin’s lymphoma; NXG = necrobiotic xanthogranuloma.
Nodularity, swelling, or both were present in all cases. Also present were yellow discoloration (five patients), diplopia (four patients), proptosis (four patients), and ptosis (three patients). The disease process was bilateral in five of the six patients. Orbital involvement, localized primarily superiorly and anteriorly, was present in all five cases that underwent CT scanning.
Intralesional triamcinolone acetonide was delivered into regions of palpable tissue nodularity and into the anterior orbital tissues based on CT scans without adverse effects in all six patients. According to clinical findings, repeated series of injections were given in four patients, all of whom demonstrated dramatic improvements in their symptoms and signs (Figure 2), which were maintained in each patient during an average follow-up of 52 months (range, 30 to 86 months). Posttreatment CT scans of the two patients with AXG confirmed reduction of their periocular disease (Figure 3). Patients 2 and 4 (Table), both with NXG, developed systemic lymphoproliferative malignancies, which required systemic treatment with corticosteroids and cytotoxic agents. These patients underwent only two and one series of injections, respectively. Even with this short course, improvement was seen in one patient and stability in the other.
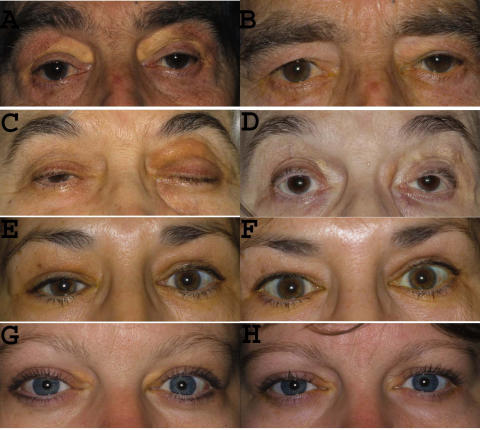
FIGURE 2.
Clinical response of necrobiotic xanthogranuloma (NXG) and adult-onset xanthogranuloma (AXG) to corticosteroid injections. A, Patient 1 with bilateral diffuse NXG lesions causing eyelid fullness, discoloration, and mechanical ptosis; numerous injections of intralesional triamcinolone resulted in resolution of lesions, maintained for 84 months (B). C, Patient 3 with NXG causing severe left ptosis, discoloration, and fullness; local corticosteroid treatment markedly decreased discoloration and fullness, and ptosis was corrected surgically after 37 months (D). E, Patient 5 with bilateral upper eyelid fullness, discoloration, and proptosis present at the time of diagnosis of AXG; all these parameters showed improvement following local triamcinolone injections that was maintained for 46 months (F). G, Patient 6 with AXG causing right upper eyelid fullness, discoloration, and mechanical ptosis; intralesional triamcinolone improved all of these findings as seen at 30 months follow-up (H).
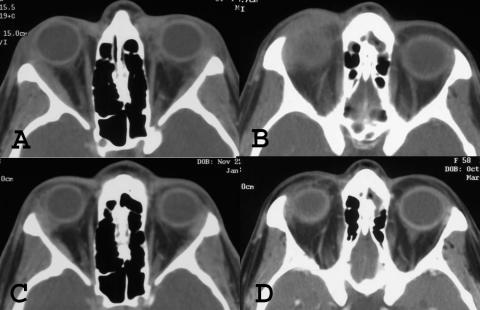
FIGURE 3.
Radiographic improvement of adult-onset xanthogranuloma (AXG) lesions to corticosteroid injections. Computed tomographic scans of both patients with AXG. Pretreatment involvement of eyelid and anterior orbital tissues by diffuse, infiltrating inflammatory masses involving both eyes of patient 5 (A) and the right eye of patient 6 (B); intralesional triamcinolone injections improved the radiographic findings in both cases (C and D), respectively.
DISCUSSION
Corticosteroid injection into the eyelids and orbit has been reported as a successful treatment for numerous periocular disease processes, such as capillary hemangioma,13 thyroid ophthalmopathy,14 chalazia,15 sarcoid,16 and vernal keratoconjunctivitis.17 More inconsistent results have been reported with sporadic use of intralesional corticosteroids for the treatment of periocular NXG.11,12 Assessment of the efficacy of intralesional corticosteroid treatment in NXG is hampered by the lack of documentation with respect to type, dose, and depth and site of injection. In addition, the need for repeated treatment, which we found to be necessary in our cases, is not mentioned in any of the reports. We feel the consistent results obtained in our series are likely due to the use of a long-acting corticosteroid, adequate depth of injection into the involved subcutaneous and anterior orbital tissue, and the delivery of repeated injections until adequate clinical response was achieved.
In our cases, the chief symptoms and signs of nodularity, swelling, and skin discoloration all responded to local corticosteroid treatment. Of the three cases with diplopia, one resolved following therapy. One of three cases of ptosis improved following treatment, whereas two required surgical correction. All cases achieved an improved and acceptable cosmetic result.
Periocular corticosteroid injections may be complicated by central retinal and ophthalmic artery occlusion18,19 as a result of embolization at the time of injection. Complications from local effects of the medication include eyelid necrosis,20 persistent glaucoma,21 and linear subcutaneous fat atrophy.22 Adrenal suppression has also been reported.23 In our series, none of these complications occurred.
We performed biopsies on all six patients. A tissue diagnosis is essential in these cases because each of these is associated with different, frequently life-threatening systemic diseases. The diagnosis serves as a basis for further directed systemic investigation and monitoring of the patient for these diseases so that prompt interventions may be implemented.
Intralesional corticosteroid injection is successful in controlling symptoms and signs of NXG and AXG eyelid and orbital involvement. This therapy avoids the use of systemic corticosteroids and cytotoxic agents, which are the currently accepted treatments for these disorders. In addition, this regimen avoids the complexity and expense of plasmapheresis and the significant local morbidity of radiation therapy, both of which are only partially effective.
REFERENCES
- 1.Kossars S, Winkelmann RK. Necrobiotic xanthogranuloma with paraproteinemia. Am Acad Dermatol. 1980;3:257–270. doi: 10.1016/s0190-9622(80)80189-7. [DOI] [PubMed] [Google Scholar]
- 2.Brazzo BG, Saffra N. Dermatofibrosarcoma protuberans of the brow and eyelid. Ophthal Plast Reconstr Surg. 2004;20:332–334. doi: 10.1097/01.iop.0000132178.23770.e1. [DOI] [PubMed] [Google Scholar]
- 3.Jakobiec FA, Mills MD, Hidayat AA, et al. Periocular xanthogranulomas associated with severe adult-onset asthma. Trans Am Ophthalmol Soc. 1993;91:99–125. [PMC free article] [PubMed] [Google Scholar]
- 4.Rose GE, Patel BC, Garner A, et al. Orbital xanthogranuloma in adults. Br J Ophthalmol. 1991;75:680–684. doi: 10.1136/bjo.75.11.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields JA, Shields CL. Clinical spectrum of histiocytic tumors of the orbit. Trans Pa Acad Ophthalmol Otolaryngol. 1990;42:931–937. [PubMed] [Google Scholar]
- 6.Hammond MD, Neimi EW, Ward TP, et al. Adult orbital xanthogranuloma with associated adult-onset asthma. Ophthal Plast Reconstr Surg. 2004;20:329–332. doi: 10.1097/01.iop.0000132177.20945.e9. [DOI] [PubMed] [Google Scholar]
- 7.Sheidow TG, Nicolle DA, Heathcote JG. Erdheim-Chester disease: two cases of orbital involvement. Eye. 2000;14:606–612. doi: 10.1038/eye.2000.151. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann EM, Muller-Forell W, Pitz S, et al. Erdheim-Chester disease: a case report. Graefes Arch Clin Exp Ophthalmol. 2004;242:803–807. doi: 10.1007/s00417-004-0928-5. [DOI] [PubMed] [Google Scholar]
- 9.Ugurlu S, Bartley GB, Gibson LE. Necrobiotic xanthogranuloma: long-term outcome of ocular and systemic involvement. Am J Ophthalmol. 2000;129:651–657. doi: 10.1016/s0002-9394(99)00469-9. [DOI] [PubMed] [Google Scholar]
- 10.Shah KC, Poonnoose SI, George R, et al. Necrobiotic xanthogranuloma with cutaneous and cerebral manifestations. J Neuro-surg. 2004;100:1111–1114. doi: 10.3171/jns.2004.100.6.1111. [DOI] [PubMed] [Google Scholar]
- 11.Codere F, Lee RD, Anderson RL. Necrobiotic xanthogranuloma of the eyelid. Arch Ophthalmol. 1983;101:60–63. doi: 10.1001/archopht.1983.01040010062009. [DOI] [PubMed] [Google Scholar]
- 12.Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma. Arch Dermatol. 1992;128:94–100. [PubMed] [Google Scholar]
- 13.Kushner BJ. Intralesional corticosteroid injection for infantile adnexal hemangioma. Am J Ophthalmol. 1982;93:496–506. doi: 10.1016/0002-9394(82)90140-4. [DOI] [PubMed] [Google Scholar]
- 14.Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol. 2004;88:1380–1386. doi: 10.1136/bjo.2004.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzarello LD, Jakobiec FA, Hofeldt AJ, et al. Intralesional corticosteroid therapy of chalazia. Am J Ophthalmol. 1978;85:818–821. doi: 10.1016/s0002-9394(14)78111-5. [DOI] [PubMed] [Google Scholar]
- 16.Bersani TA, Nichols CW. Intralesional triamcinolone for cutaneous palpebral sarcoidosis. Am J Ophthalmol. 1985;99:561–562. doi: 10.1016/s0002-9394(14)77958-9. [DOI] [PubMed] [Google Scholar]
- 17.Holsclaw DS, Whitcher JP, Wong IG, et al. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am J Ophthalmol. 1996;121:243–249. doi: 10.1016/s0002-9394(14)70271-5. [DOI] [PubMed] [Google Scholar]
- 18.Wesley RE, Johnston DT, Gutow GS. Central retinal artery occlusion. Ophthalmic Surg. 1987;18:123–125. [PubMed] [Google Scholar]
- 19.Egbert JE, Schwartz GS, Walsh AW. Diagnosis and treatment of an ophthalmic artery occlusion during an intralesional injection of corticosteroid into an eyelid capillary hemangioma. Am J Ophthalmol. 1996;121:638–642. doi: 10.1016/s0002-9394(14)70629-4. [DOI] [PubMed] [Google Scholar]
- 20.Sutula FC, Glover AT. Eyelid necrosis following intralesional corticosteroid injection for capillary hemangioma. Ophthalmic Surg. 1987;18:103–105. [PubMed] [Google Scholar]
- 21.Akduman L, Kolker AE, Black DL, et al. Treatment of persistent glaucoma secondary to periocular corticosteroids. Am J Ophthalmol. 1996;122:275–277. doi: 10.1016/s0002-9394(14)72027-6. [DOI] [PubMed] [Google Scholar]
- 22.Droste PJ, Ellis FD, Sondhi N, et al. Linear subcutaneous fat atrophy after corticosteroid injection of periocular hemangiomas. Am J Ophthalmol. 1988;105:65–69. doi: 10.1016/0002-9394(88)90122-5. [DOI] [PubMed] [Google Scholar]
- 23.Weiss AH. Adrenal suppression after corticosteroid injection of periocular hemangiomas. Am J Ophthalmol. 1989;107:518–522. doi: 10.1016/0002-9394(89)90497-2. [DOI] [PubMed] [Google Scholar]