Abstract
Introduction
Because of the many ocular diseases that may benefit from genetic alteration, ophthalmology will be embroiled in the controversy over the ethics of stem cell research. In preparation for a major academic symposium at the Washington University School of Medicine, the direct and indirect effects of political efforts to determine whether this research should be funded were explored.
Methods
A symposium was held at Washington University School of Medicine sponsored by the Barnes-Jewish Hospital Medical Staff Association to discuss the scientific basis of stem cell research. The forces arrayed on either side of the argument are presented as well as the political context in which they are being played out.
Results
Two kinds of effects are expected from this controversy: direct effects on current and proposed research efforts from both within and outside the academic milieu and indirect effects on research funding from state and federal sources influenced by the political process.
Conclusion
This report serves to document the efforts of one scientific community to deal with a legislative initiative to criminalize stem cell research for both the scientist and the patient. The process and interim conclusions may be instructive for those involved in this endeavor to recognize the dynamics of the interaction between society and science when ethical issues influence how decisions are made.
INTRODUCTION
Stem cell research is at the heart of the genomic revolution that will transform our ability to affect the course of disease states in the next 100 years. The elucidation of the human genome has allowed us to begin to find the aberrations in gene function that result in phenotypic alterations in organ systems. Although we do not pretend to understand at this point how to reorganize base pair sequences to manufacture novel proteins, we know that at critical junctures substitutions, deletions, missense mutations, and early terminations of protein sequencing will impair the organism’s structure or function. What we are discovering is that we are able to “repair” these defects by adding or replacing the malfunctioning protein with a normal one or by introducing a cell population that has the potential to provide the correct substance and allow biochemical reactions to proceed as they would in a “normal” organism.
These alterations have enormous potential. They have been likened to the introduction of antibiotics 70 years ago. But the results from these investigations will come more slowly, their correct use will require trial and error, and their power also carries the opportunity for misuse. That this era is here we can have no doubt.
Two categories of stem cell production are at issue in the present debate: (1) those derived from fertilized eggs (embryonic stem cells or reproductive cloning) and (2) those derived from DNA taken from other sources (somatic nuclear cell transfer [SNCT]).
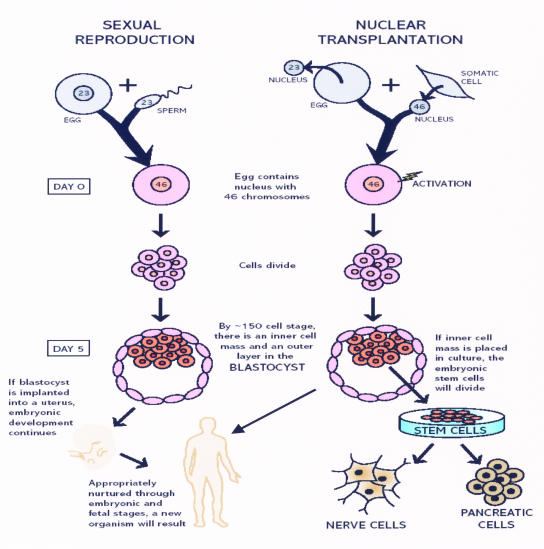
In the Figure, the differences in these techniques are illustrated. The source of the nuclear material differs according to the origin of the cell line. In embryonic stem cells, the source is sexual reproduction with one half of the chromosomal material from the ovum and half from the sperm. In SNCT, both halves of the genetic material come from the same donor, replacing the haploid material of the unfertilized ovum with diploid material from the nucleus of the somatic cell. Whereas, theoretically, both blastocysts, if implanted in a uterus, will form a fetus, the destination for the inner cell mass in the blastocyst in SNCT is a cell culture that will ultimately grow into an immortal cell line of skin cells, neurons, or other distinct cell types for use in replacing or supplementing missing proteins or enzymes in a variety of disease states.
Methods of creating stem cells: embryonic versus somatic nuclear cell transfer. (Reprinted with permission from Steven Teitelbaum, MD, Professor of Pathology, Washington University, St Louis, Missouri.)
METHODS
The first task is to separate out the scientific factors from the ethical and religious arguments for stem cell research. For the scientist, the goals are advancement of knowledge and treatment of disease. The pitfalls are perversion of efforts toward advancement of knowledge to self-aggrandizement, misrepresentation of the true effects of treatment, and use of these methods to affect attributes that are not disease states. Within the scientific community, the regulatory process includes peer review of proposals, institutional review by human studies committees, and a set of guidelines that specify what stem cells are being used, the sources from which they come, and how the resultant cell lines and cultures will be handled and guarded. Oversight, including input from lay personnel, remains a hallmark of the credentialing process and avoids the criticism that experiments are being conducted without transparency for all steps in the process. The explicit prohibition against cloning or use of the products of SNCT for reproduction has been incorporated into the legislation passed in California and New Jersey and in the United Kingdom.
In Missouri, the two major institutions that stand poised to implement stem cell techniques in their research efforts are Washington University and the Stowers Institute in Kansas City. Washington University is the site of the federally funded Human Genome Project that resulted in the elucidation of the human genome.
Efforts at biomedical engineering have been encouraged from both private and public sources. The Stowers Institute for Medical Research was founded by James and Virginia Stowers to advance knowledge in the field of genomics.
Simultaneous bills were introduced in the Missouri Senate and House in early 2005 to criminalize stem cell research. The biomedical and research community was slow to respond because of a lack of understanding of the legislative process, a “cottage industry” approach to the global effect of the restrictions advocated, a leadership vacuum, and a reluctance to confront the forces arrayed against stem cell research. The bills have been tabled, as will be discussed below, but the potential issues and forces arrayed on each side may be assessed. Although the parameters of a research presentation do not conform exactly to the mechanics of a social or political process, I will attempt to approach this subject in an epidemiologic fashion.
The second task is to look at the efforts to regulate stem cell transplantation. The two sources of regulation are political and ethical/moral/religious. For legislative regulation to be successful, it must be explicit and clearly defined. Sources of stem cells that are permitted and forbidden need to be delineated and the uses to which they are put outlined to avoid ambiguity, suspicion, and legal challenge. More difficult is the distinction about egality of moral belief systems. As Ambassador John Danforth commented, “The proposal to criminalize cell regeneration research calls for a choice between two understandings of human life. On the one hand, we have the millions of people who suffer from ALS, Alzheimer’s, juvenile diabetes, Parkinson’s, spinal cord injuries, and cancer—and the loved ones who care for them and suffer by their sides. On the other hand, we have tiny bundles of unfertilized cells existing in Petri dishes. Supporters of the legislation (to criminalize stem cell research) should explain to the afflicted and their loved ones why they care more about those cell bundles than they do about the people.”1
The bills presented to the Missouri Legislature (identical in the House and Senate) sought to levy a fine and impose imprisonment on both the physician and the patient involved in stem cell research. The legislation is so far-reaching that it actually may be invoked if a patient receives treatment that involves stem cell research in another state and returns to Missouri. Governor Matt Blunt stated that he would veto the bill in its present form, but he has risked alienating part of his constitutency with his stance.
RESULTS
Stem cell research holds the prospect of relief from suffering for groups of people who have acute and chronic conditions that may become less onerous and life-threatening if the missing substrates and enzymes are replaced by cells that replace the abnormal DNA. Those who oppose stem cell research argue that the mechanism for relieving that suffering represents an illegal transfer of human cells and cite that the relief provided to those in need makes the deliverers and recipients equally criminal. They justify their belief by stating that the stem cells are a by-product of human organisms. These same arguments were utilized in the world-wide debate on organ transplant; however, these procedures have become an accepted practice for which no penalty is exacted in almost all cultures.
Unfortunately, scientific research will continue to extend into spheres that are considered “sacred.” If the safeguards of morality and legislative penalty for acts beyond the scope of respected medical practice are enforced, the risk that the privilege of using unfertilized ova for somatic nuclear cell transfer will be abused is small.
A symposium entitled “Stem Cell Research: Current Status and Future Challenges” was hosted by the Barnes-Jewish Hospital Medical Staff Association, the Washington University School of Medicine, and the Institute for the Study of Ethics and Human Values at Washington University and was held on February 15, 2005. The speakers limited their discussions to the science of stem cell research. Four hundred members of the university community attended, and the symposium was recorded on DVD and disseminated to those who could not attend. Challenges to the format of the meeting were raised both from within the medical community and from the St Louis community, objecting to the absence of a discussion of the ethics and religious views. An additional meeting was organized on April 14, 2005, to address these topics. The organizers of the symposium felt that understanding the nature of SNCT and its potential to relieve suffering and save lives should be emphasized in order to counteract the criminalization legislation.
Since the symposium at Washington University School of Medicine, 2,300 individuals have joined the Missouri Coalition for Lifesaving Cures. A poll taken by the St Louis Post-Dispatch has revealed that 66% of Missourians are in favor of stem cell research. Several religious organizations have threatened elected officials that a vote against the bills would engender a concerted opposition to their next candidacy.2 Preachers in many pulpits have made their views known to their congregations both for and against the legislation. In some Christian denominations, threats of denial of access to the Sacraments were made, although they have not caused widespread concern. A midterm election for a State Senate seat occasioned by the resignation of the present Senator turned into a referendum on the stem cell research legislation with groups outside of the district and even outside of Missouri contributing large sums to each candidate.3 The candidate supported by those opposed to stem cell research won by a narrow margin.
In Missouri, the debate in the Senate was terminated, and the bill was withdrawn by its sponsor. The Republican majority became divided when faced with the political dilemma of choosing between the business considerations of driving potentially remunerative scientific endeavor from Missouri and the putative political damage that voting on the bill would have caused among the electorate.4
DISCUSSION
The use of nuclear material from somatic cells implanted in unfertilized ova permits the creation of stem cells that are close to the omnipotent cells released by the blastocyst after fertilization but before implantation in the uterine wall. These cells grown in culture are capable of becoming immortal and of being used to release proteins that may influence the course of genetically modifiable diseases whether incipient or established in the new host.
Those who argue against stem cell research state that, no matter how beneficial the results from this form of scientific inquiry, the fact that life has been destroyed in order to create stem cells is sufficient evidence that the research is unethical. For this argument, the responses that SNCT involves an unfertilized egg, that the female creates and discards several unfertilized eggs per month, or that these eggs if harvested would not in the process of SNCT create life, because they would not produce a differentiated organism, nor destroy life, because the unfertilized egg with haploid genetic material would not be able to reproduce itself, fall on deaf ears.
Those who argue in favor of stem cell research fall into two categories. In the first are those who feel that they must refute the arguments of those who oppose stem cell research. They fail to take into account that their opponents’ argument depends on the definition of when life begins, a sacred text that yields no truth because it is potentially “unknowable.” The second group chooses to reframe the question in terms of benefit. The criticism of this argument is the moral relativism of choice between helping many at the expense of a few. With SNCT, we are directing life to promote life, not exchanging one life for another. In much the same way, the conception of a child to provide bone marrow to a sibling with a reticuloendothelial or hematologic malignancy is countenanced because the younger child will be loved not only for the donation but for itself.
The successful support for ethical stem cell research must come from reframing the argument. Some have chosen to discuss the justice of helping others. Others have invoked the exploration of the unknown as a primary obligation of science. Still others have used the teleologic argument for survival. But ethical justification supersedes scientific intellectual reasoning when the consequences of misused genetic material may be devastating. Each step in the scientific investigation must hold the reasonable promise of a beneficial result. Pain or morbidity may be acceptable but only with the expectation of gain. Thus, we may cut the skin and integument to reveal the diseased organ whose removal or repair will make the body whole again. Similarly, we may lyse the cell membrane of an unfertilized egg as long as we allow other eggs to be fertilized to grow into new individuals and as long as we reconstitute the cell membrane of the unfertilized egg into a new cell that we intend to use to make the organism whole or to relieve suffering.
Our task is difficult. First, the scientific community must present its view of the processes and consequences of stem cell research in a balanced fashion: who will benefit, who will be harmed, from what sources stem cells may come, and to what uses they will be put. Second, the distinction between stem cell research and cloning of individuals must be clarified. Third, the scientific community must address the opposing arguments and must emphasize that the goals of stem cell research are the preservation and improvement of life.
Both the economics and the ethics of stem cell research are under attack. Effective education and discussion of risks and benefits are the weapons of mass dissemination that will allow this research to proceed. Criminalization and subsequent migration of researchers from unfavorable to favorable environments have already begun. The battle is being fought on a state-by-state basis. Our efforts to understand and educate ourselves and our legislators will affect the course of stem cell research in the United States for many years.
We stand at a crossroads, looking toward freedom from the ravages of chronic diseases. As we have done so many times in the past, we face the challenge of responsible leadership and making the individual and collective effort to inform and influence the path of knowledge and understanding. The stakes are high, but the promise of a cellular approach to the repair of genetic dysfunction should impel us to use our best efforts to aid our patients.
REFERENCES
- 1.Danforth J. In the name of politics. New York Times. March 30, 2005:17.
- 2.Franck M. Missouri Senate shelves proposal for ban. St Louis Post-Dispatch (Jefferson City Bureau). April 6, 2005. Available at: http://www.PostNet.com
- 3.Voters pick 3 new state legislators. Associated Press. April 5, 2005.
- 4.Zagier AS. In Missouri, GOP is riven over embryonic stem-cell research. Boston Globe. April 10, 2005.