Abstract
Purpose
To evaluate the validity of different methods of relative afferent pupillary defect (RAPD) detection and assess the usefulness of detecting an RAPD in glaucoma patients.
Methods
For this prospective observational study, we enrolled 70 of 153 consecutive glaucoma patients coming for examination. Exclusion criteria were cloudy corneas, recent surgery, fixed pupils, and other diseases producing RAPDs. Inclusion criteria were any type of glaucoma and absence of any exclusion criterion. Patients were examined for an RAPD by the swinging flashlight method (SFM), a magnifier-assisted swinging flashlight method (MAM), and automated pupillography. The SFM and MAM results were compared with those obtained by pupillography. Receiver operating characteristic curves and the area under the curve (AUC) were determined. Correlations of other optic disc and visual field indices of glaucoma damage with RAPD were determined.
Results
We confirmed RAPDs in 39 (56%) of the subjects by pupillography, finding a sensitivity of 41% and 84% and a specificity of 90% and 76% for SFM and MAM, respectively. The AUC was 0.86 for MAM and 0.61 for SFM. The correlations (r) between RAPD with intereye differences in the optic disc and visual field indices of glaucoma damage were moderate.
Conclusions
A modified swinging flashlight test with magnification increases the sensitivity of the test in detecting an RAPD. Because the detection of an RAPD may precede apparent optic disc and visual field damage, looking for an RAPD may be an important part of the assessment of patients with glaucoma.
INTRODUCTION
Glaucoma is the leading cause of preventable blindness in the world. In the United States, 3 to 6 million people are at risk for developing glaucoma over the next 5 to10 years.1 Such persons, however, cannot be identified reliably before they develop damage. The asymmetric nature of glaucoma suggests that diagnostic methods focused on this asymmetry may be helpful.
The pupillary light reflex is a relatively objective indicator of afferent input from the retina and optic nerve. The normal pupillary light reflex is symmetric when the right-eye stimulus is compared with the left-eye stimulus. An asymmetric pupillary light reflex or relative afferent pupillary defect (RAPD) is a useful way to detect asymmetric retinal or optic nerve diseases.2
Testing pupils for an RAPD was first described by Levatin in 1959,3 and since that time numerous modifications and refinements have been suggested. The alternating (swinging) flashlight test has become a routine part of many ophthalmic examinations.4 This test is not without problems, because some factors, such as end-point determination, unequal retinal illumination, and examiner bias, adversely affect reliability, and in people with dark irides or sluggish pupils, determination of the presence of an RAPD can be difficult.4,5
We hypothesized that use of magnification would allow better appreciation of the pupillary reactions and would increase the sensitivity of RAPD detection. We have developed a method of testing for an RAPD using a +20-diopter lens to produce magnification. The objectives of the present study were to compare this new method of testing for RAPD with other methods and to evaluate the validity of these methods. We also assessed the usefulness of detecting an RAPD in glaucoma patients by correlating the presence or absence of an RAPD with the extent of glaucoma damage.
METHODS
This prospective observational study was reviewed and approved by the Institutional Review Board of Wills Eye Hospital.
The medical records were screened of 153 consecutive glaucoma patients of a glaucoma referral practice physician (G.L.S.) on the Wills Eye Hospital Glaucoma Service; a wide range of optic nerve damage was represented. Exclusion criteria included cloudy corneas, any surgical or laser procedure within the past 90 days, any condition preventing accurate measurement or examination of the pupils, nonreactive pupils, and any other retinopathy, vasculopathy, or optic neuropathy with the potential for producing an RAPD. Inclusion criteria were the diagnosis of any type of glaucoma or ocular hypertension and the absence of any exclusion criterion. All eligible patients were examined.
Pupils were examined by two different methods to determine the presence or absence of an RAPD: swinging flashlight method (SFM)3 and magnifier-assisted swinging flashlight method (MAM).
SWINGING FLASHLIGHT METHOD
With no dilating drop in the eyes, the patient was placed in a dimly lit examination room for 5 minutes and instructed to look in the distance. A Finoff halogen transilluminator (Welch Allyn, Skaneateles Falls, New York) with maximum light intensity (halogen HPX lamp with 3250 degrees K color temperature) was held 15° below the visual axis to illuminate the retina. The light was shined first into the right eye for approximately 3 seconds and then moved rapidly to the left eye for approximately 3 seconds. This same cycle was repeated several times until the nature of the pupillary response in each eye was clear by virtue of noting the same type of response with each swing of the flashlight, which usually occurred after four to six swings. While swinging the flashlight, care was taken to move the light quickly from one eye to the other. The presence of an RAPD in the illuminated eye was indicated by any of three findings: a small initial momentary constriction followed by greater dilation, initially no apparent change followed by dilation, or initial immediate dilation.6 In cases where only one pupil was reactive, if the eye with the reactive pupil showed a marked, maintained contraction when the light was shined in it, but dilated when the light was shined in the eye with the fixed pupil, this was considered to indicate an RAPD in the eye with the fixed pupil.
MAGNIFIER-ASSISTED SWINGING FLASHLIGHT METHOD
The second method was exactly the same as the first except that a high plus magnifying lens (+20 diopter, Nikon) was held close to, and in front of, the right eye and, after observing the response in that eye, over the left eye.
BRIGHTNESS DISCRIMINATION TEST
Patients also were asked to compare the brightness of a light shined first into the right eye and then into the left eye. The eye that saw the light more brightly was graded as 100+, and the patient was asked to give the same or a lower figure to describe the brightness experienced by the other eye.
AUTOMATED BINOCULAR INFRARED PUPILLOGRAPHY
The pupillary movements were also independently recorded by automated dynamic binocular infrared pupillography (P2000 SA, Procyon Instruments Ltd, United Kingdom). This pupillograph has a spatial resolution of greater than 0.1 mm and temporal resolution of up to 25 Hz. The instrument was set to illuminate the eyes alternatively. The stimulation sequence used for this study was 3 seconds “light on” followed by 1 second “light off,” which was repeated for six cycles. The illumination level was set to low-mesopic illumination (0.4 lux). We chose this setting in order to simulate the SFM of testing for an RAPD. The system captured 25 pupillary images per second, providing a measurement period of about 30 seconds. The images were processed and objectively analyzed using Pupilfit software. The software recognized the size of the circle that matched the pupil’s border and recorded the horizontal pupil diameter. Time-dependent responses of pupillary reflex, such as the rested and constricted horizontal diameters of the pupils, the amplitude of pupillary constriction, and the latency of pupillary constriction, were analyzed using the pupillography software.
With reference to the previous studies done by Kawasaki and others,4,7–10 we defined an RAPD in terms of the difference in average pupillary constriction between the two eyes. The pupillography software (Pupilfit) determined the pupil contraction amplitude for each pupil and each stimulus cycle. The pupil response curves were generated, and the differences in pupil contraction amplitudes were calculated by averaging the right and left pupil contractions resulting from right-eye stimulation minus the average of right and left pupil contraction resulting from left-eye stimulation.
Variability exists in all biologic systems, including pupils. The variability in pupillary constriction may produce errors in clinical estimations of RAPD. To verify the variation in pupillary constriction between normal eyes, we studied 20 age-matched individuals (mean age, 66.5 ± 10.8 years) with no history of ocular problems or no abnormalities on eye examination. Pupillography using the same paradigms was done. The average amplitude of pupillary constriction of pupils was recorded for each stimulated eye separately. The mean difference in average amplitude of pupillary constriction between two eyes was 0.18 ± 0.06 mm (95% confidence interval). A mean difference in average amplitude of pupillary constriction between two eyes greater than 0.25 mm was considered indicative of an RAPD.
EXAMINATIONS BY THREE MASKED EXAMINERS
After giving their informed consent, each of the 70 enrolled patients underwent a fixed sequence of examinations by three masked examiners. The sequence of examination was as follows: The first examiner checked the pupils using SFM, looking for the presence or absence of an RAPD as described above. He then checked the pupils using MAM as described above. The second and third examiners followed the same sequence of examinations. All examiners were masked to each other.
Next, automated binocular infrared pupillography (with the above-mentioned setting) was done by a masked technician. After the clinical and pupillographic examinations of all of the patients, one of the examiners (D.L.) retrieved the required information from the medical charts. He graded the most recent Humphrey 24-2 threshold visual field test (HVF 24-2 within previous 6 months) based on the field damage likelihood scale (FDLS)11 and Hodapp-Parrish-Anderson (HPA)12 visual field staging system. The FDLS is a method of estimating the amount of field loss using any perimetric system. It is based on the HPA system but has seven stages, rather than the four stages used in the HPA visual field staging system. The intereye differences for each of the visual field staging systems (Δ FDLS and Δ HPA) were recorded. The mean deviation (MD) of each eye and the intereye differences in MD (Δ MD) for all subjects were calculated. The optic discs were graded according to the disc damage likelihood scale system (DDLS),13 a 10-stage scale, with each successive score representing a greater level of optic disc damage. The mean amounts of intereye asymmetry of the DDLS scores (Δ DDLS) were determined.
Data were collected and analyzed by using SPSS for Windows version 10.1 (SPSS Inc, Chicago, Illinois). Descriptive statistics were performed for the frequency of detected RAPD by both SFM and MAM. Pearson’s correlation was used to compare the changes in RAPD and amounts of mean deviation. Spearman’s correlation was used to compare the presence or absence of an RAPD with intereye differences in DDLS score (Δ DDLS), FDLS score (Δ FDLS), and HPA score (Δ HPA).
The sensitivity and specificity of both methods were determined. The receiver operating characteristic (ROC) curves for SFM and MAM were calculated, and the area under the curve (AUC) was determined for each method separately.
RESULTS
Seventy of the 153 screened consecutive glaucoma patients were eligible for the study, and all were enrolled. Patient characteristics are shown in Table 1. The mean and range of the horizontal diameters of the pupil before and after light stimulus are shown in Table 1.
TABLE 1.
CHARACTERISTICS OF 70 GLAUCOMA PATIENTS EXAMINED FOR PRESENCE OF RELATIVE AFFERENT PUPILLARY DEFECT
| CHARACTERISTIC | NUMBER |
|---|---|
| Gender | |
| Male | 22 (31.4%) |
| Female | 48 (68.5%) |
| Age (years) | |
| Mean | 70.8 ± 11.55 |
| Range | 27 – 94 |
| Type of glaucoma | |
| Primary open-angle | 46 (66%) |
| Chronic closed-angle | 7 (10%) |
| Average pressure | 4 (5.7%) |
| Ocular hypertension | 3 (4.3%) |
| Exfoliation syndrome | 2 (2.9%) |
| Pigment dispersion syndrome | 2 (2.9%) |
| Glaucoma suspect | 2 (2.9%) |
| Other | 4 (5.7%) |
| Right pupil, at rest (mm) | |
| Mean | 4.55 ± 0.87 |
| Range | 2.40 – 6.32 |
| Left pupil, at rest (mm) | |
| Mean | 4.31 ± 0.84 |
| Range | 2.63 – 6.30 |
| Right pupil , contracted (mm) | |
| Mean | 3.01 ± 0.82 |
| Range | 1.42 – 4.94 |
| Left pupil, contracted (mm) | |
| Mean | 2.80 ± 0.83 |
| Range | 1.58 – 5.08 |
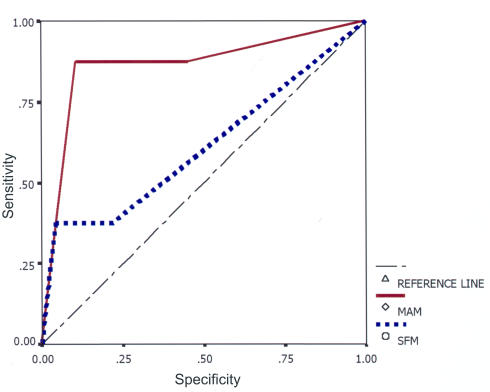
The sensitivity and specificity of both methods were calculated by using automated pupillography as the “gold standard.” For MAM, the sensitivity was 84% and the specificity 70% as compared with pupillography. For SFM, the sensitivity was 41% and the specificity 90% (Table 2). The AUC (Figure 1) was 0.86 for MAM and 0.61 for SFM. An AUC of 0.75 to 0.92 was classified as good for MAM and an AUC of 0.50 to 0.75 was classified as fair for SFM.
TABLE 2.
SENSITIVITY, SPECIFICITY, POSITIVE PREDICTIVE VALUE (PPV), AND NEGATIVE PREDICTIVE VALUE (NPV) OF TWO DIFFERENT METHODS OF RAPD DETECTION IN GLAUCOMA PATIENTS
| METHOD | SENSITIVITY | SPECIFICITY | PPV | NPV |
|---|---|---|---|---|
| Swinging flashlight | 41% | 90% | 84% | 55% |
| Magnifier-assisted | 84% | 70% | 76% | 79% |
RAPD = relative afferent pupillary defect.
FIGURE.
Receiver operating characteristic curve for magnifier-assisted method (MAM) and swinging flashlight method (SFM).
The correlation coefficient (r) was 0.51 for MAM and 0.43 for SFM. Both correlations were significant at the .01% level. The overall correlation coefficient for SFM, MAM, and pupillography was moderate (0.30 to 0.38, P ≤ .01). The correlation was highest for the magnifier-assisted method (0.38, P = .001) and lowest for SFM (0.30, P = .01). The correlation between intereye difference in indices of MD, DDLS and visual field defect (graded by HPA and FDLS) was moderate (P ≤ .03). The correlation between FDLS and HPA was better than that between the other indices (r = 0.63, P = 0). Table 3 shows Spearman’s correlation between different indices of glaucoma damage.
TABLE 3.
CORRELATION COEFFICIENTS BETWEEN INTEREYE DIFFERENCES FOR VARIOUS PARAMETERS AND INDICES OF GLAUCOMA DAMAGE
| ΔHPA | ΔFDLS | ΔDDLS | ΔBRIGHTNESS | |
|---|---|---|---|---|
| Δ MD | 0.28 | 0.40 | 0.22 | 0.32 |
| Δ HPA | 0.63 | 0.35 | 0.14* | |
| Δ FDLS | 0.44 | 0.27 | ||
| Δ DDLS | 0.34 |
DDLS = difference in intereye disc damage likelihood scale; Δ FDLS = difference in intereye field damage likelihood scale; Δ HPA = difference in intereye Hodapp-Parrish-Anderson staging system; Δ MD = difference in intereye mean deviation.
Not significant (P = .24). All other values were significant (P ≤.03).
Other indicators of glaucoma damage, including the patients’ subjective sense of change in brightness, optic disc changes graded by DDLS staging system, and visual field defects quantified by MD, HPA, and FDLS, were evaluated. The mean intereye differences for these indices were calculated for both RAPD-positive and RAPD-negative groups detected by each method. Then they were compared by an independent samples t test (Table 4). The mean intereye difference between RAPD-positive and RAPD-negative cases within each tested method were statistically significant for DDLS, FDLS, and pupillary amplitude (Table 4).
TABLE 4.
MEAN INTEREYE DIFFERENCE FOR VARIOUS INDICES OF GLAUCOMA DAMAGE FOR RAPD-POSITIVE AND RAPD-NEGATIVE GROUPS TESTED BY THE SWINGING FLASHLIGHT METHOD AND MAGNIFIER-ASSISTED METHOD (MEAN ± SD)
| MAGNIFIER-ASSISTED METHOD | |||
|---|---|---|---|
| NO RAPD (N = 29) | RAPD (N = 40) | P | |
| Δ DDLS | 0.79 ± 0.77 | 2.40 ± 2.10 | .000 |
| Δ FDLS | 1.31 ± 1.47 | 2.10 ± 1.72 | .050 |
| Δ HPA | 0.97 ± 1.09 | 1.21 ± 1.40 | .420 |
| Δ Amplitude | 0.18 ± 0.18 | 0.60 ± 0.45 | .000 |
| Δ MD | 1.17 ± 4.73 | 4.41 ± 10.49 | .100 |
| SWINGING FLASHLIGHT METHOD | |||
| NO RAPD (N = 49) | RAPD (N = 20) | P | |
| Δ DDLS | 1.06 ± 0.94 | 3.40 ± 2.43 | .000 |
| Δ FDLS | 1.27 ± 1.27 | 3.00 ± 1.89 | .001 |
| Δ HPA | 0.13 ± 1.39 | 0.21 ± 2.30 | .830 |
| Δ Amplitude | 0.31 ± 0.28 | 0.70 ± 0.54 | .005 |
| Δ MD | 0.80 ± 4.38 | 9.0 ± 13.40 | .025 |
Amplitude = mean intereye difference in amplitude of pupillary constriction (mm); Δ DDLS = mean intereye difference in disc damage likelihood scale units (0 – 10); Δ FDLS = mean intereye difference in field damage likelihood scale units (0 – 7); Δ HPA = mean intereye difference in Hodapp-Parrish-Anderson score (0 – 4); Δ MD = mean intereye difference in mean deviation (db); RAPD = relative afferent pupillary defect.
When SFM was used for RAPD detection, the mean intereye differences in MD were significant between the RAPD-positive and RAPD-negative groups (P = .025), but when it was tested by MAM, it was not significant
The interobserver variations between the different observers are shown in Table 5.
TABLE 5.
INTEROBSERVER AGREEMENT BETWEEN TWO METHODS OF RAPD DETECTION
| EXAMINER 1 MAM | EXAMINER 2 SFM | EXAMINER 2 MAM | EXAMINER 3 SFM | EXAMINER 3 MAM | |
|---|---|---|---|---|---|
| Examiner 1 SFM | 70% | 91% | 67% | 90% | 71% |
| Examiner1 MAM | 71% | 87% | 67% | 94% | |
| Examiner 2 SFM | 71% | 90% | 73% | ||
| Examiner 2 MAM | 69% | 93% | |||
| Examiner 3 SFM | 69% | ||||
| Mean interobserver agreement for SFM: 90.3% | |||||
| Mean interobserver agreement for MAM: 91.3% | |||||
| Mean interobserver agreement between two methods: 70.3% | |||||
MAM = magnifier-assisted method; RAPD = relative afferent pupillary defect; SFM = swinging flashlight method.
DISCUSSION
The main objectives of the present study were to determine the accuracy of RAPD detection by our magnifier-assisted method in general and to evaluate its potential usefulness in glaucoma patients in particular.
We believe that the potential value and usefulness of detecting RAPDs to evaluate asymmetric glaucoma damage are underestimated. Few studies mention the occurrence of RAPDs in glaucoma. Using quantitative infrared pupillometry, Jonas and coworkers10 found an RAPD in 7 (41%) of 17 glaucoma patients. They concluded that quantitative pupillometry can be helpful in diagnosing glaucoma. Using infrared pupillography, we found an RAPD in 39 (56%) of 70 glaucoma subjects. Our study found an RAPD in 42 (60%) of 70 glaucoma subjects by using MAM and in 20 (29%) of 70 glaucoma subjects by using the standard swinging flashlight method. It is often difficult to notice small pupillary excursions, dilation, or contraction, because they are too small for the naked eye to appreciate. Recognizing this problem, Glazer-Hockstein and colleagues14 suggested using the magnification provided by a slit-lamp biomicroscope for the purpose of better detecting small changes. However, we found their method to be inconvenient and excessively awkward. Their idea of using magnification to improve the ability to detect a response, however, is original and sound. Our study, we believe, confirms the value of their contribution. In our population, an RAPD was more frequent than reported previously.10
One of the authors (G.L.S.) has been using MAM for RAPD detection in glaucoma patients for many years, and in the current study, pupillography was used as a “gold standard” to evaluate the validity of his method of RAPD detection (Table 2). We found a higher sensitivity and negative predictive value for MAM than for SFM (Table 2). The ROC curves (Figure 1) show a larger area under the curve for MAM compared with SFM.
In common clinical practice, the examiner first uses SFM to determine whether an RAPD is present. If an RAPD is indicated, then the next step may be to quantify the detection using, among other methods, neutral density filters,15 a Sbisa bar,16 a cross-polarized filter,17 pupillometry,10 or pupillography.4,7 These methods, however, are used to quantify the extent of an abnormal response, not to find an abnormal response. In our experience, if SFM is used to detect an RAPD, almost half of the cases will be missed.
We also wished to evaluate any correlations between the presence of an RAPD and other indicators of asymmetric damage in glaucoma patients. These indicators included amount of optic disc damage, amount of visual field change, and the patient’s subjective sense of difference in brightness.
We found a moderate correlation between intereye DDLS difference and presence or absence of an RAPD as detected by the two methods. However, in 12% of the eyes in which an RAPD was found by pupillography, there were no apparent differences between the two eyes as determined by optic disc appearance as graded by using the DDLS. This could be a sign that looking for an RAPD may be a helpful method of detecting glaucoma, even in those cases in which no disc asymmetry is apparent.
A moderate correlation was found between the presence or absence of an RAPD detected by MAM and visual field defects graded by the FDLS. Loss of ganglion cells may occur prior to reproducible visual field defects in glaucoma patients.18 We suggest that our magnifier-assisted method might detect an RAPD before an asymmetric visual field defect becomes apparent.19 Kardon and associates19 showed that there is a significant correlation between the severity of an RAPD and the degree of asymmetric visual loss. In the present study, we were primarily interested in determining whether an RAPD was present, not in determining the intensity of any RAPD that was detected. Consequently, we cannot confirm or negate Kardon’s findings.
There was a statistically significant correlation between the intereye difference in the MD and the presence or absence of an RAPD as detected by SFM, but no correlation between the intereye difference in the MD and the presence or absence of an RAPD as detected by MAM. This may reflect the “noisiness” of the visual field data: real differences between the two eyes may have been present, but differences in the visual field scores of the two eyes could not be detected because the field tests were not accurate representations of the actual fields. The correlation between the presence or absence of an RAPD and the asymmetry of visual field loss was better when the severity of field loss was estimated using the FDLS as opposed to the MD or HPA score. The MD does not consider the location of an abnormality of the field, but the FDLS does. Defects in central field are counted more heavily in the FDLS, because such spots are more heavily represented in the brain. As such, the FDLS would be expected to represent the actual number of lost neurons more accurately than MD. The HPA method has so few stages that it probably is not able to differentiate mild differences in sensitivity of damage. In a similar way, Folk and colleagues20 found that a detached retina in the macular area (roughly comparable to the four central points in a Humphrey visual field) is more likely to be associated with an RAPD than when the macula is not involved.20
The perception that a light is seen more brightly in one eye than the other can indicate asymmetry of optic nerve function.21 In our study, we found only a low-moderate correlation between the asymmetric brightness test and the presence of an RAPD and concluded that the “brightness test” yields results that are too variable to be clinically useful. Peter and coworkers21 showed that there was poor association between decreased brightness scores and asymmetric field defects as determined by the Humphrey Field Analyzer.
The agreement among the examiners in detecting an RAPD using each method was high (90% to 91%) but not absolute. Bell and associates6 reported 78% agreement among examiners using SFM.
We used pupillography as the “gold standard” against which testers compare SFM and MAM. However, the validity of pupillography has not been established. In fact, according to pupillography, one patient considered completely normal clinically was considered to have an RAPD; neither SFM nor MAM found an RAPD in this patient. It seems likely that the pupillographic diagnosis was wrong. It is possible that in our study, when there was an RAPD found by MAM that was not found by pupillography, the MAM was correct and the pupillography wrong.
There are significant limitations to our study. First, if an RAPD was found by SFM, it was natural to expect to find that defect by MAM, Thus, the sensitivity of MAM may have been erroneously interpreted as greater than that of SFM. Second, the study patients, recruited from our large referral practice, may not be representative of the general population of patients with glaucoma. Third, although the parameters that we used for pupillography are the most widely accepted ones, they are not necessarily the correct ones. Fourth, the number of cases studied is relatively small. Finally, the field and disc data were acquired from charts retrospectively, and there may have been systematic errors in how they were recorded.
An RAPD may precede apparent optic disc and visual field damage. Our swinging flashlight test modified with magnification can provide a simple, inexpensive, and highly sensitive method of detecting an RAPD. Therefore, it may be a valuable help in diagnosing ocular hypertension and glaucoma. In addition, finding an RAPD in a patient in whom one was not previously present or noting the disappearance of an existing RAPD is a strong sign of optic nerve deterioration in the previously better eye, or improvement in the worse eye.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergamin O, Zimmerman MB, Kardon RH. Pupil light reflex in normal and diseased eyes: diagnosis of visual dysfunction using waveform partitioning. Ophthalmology. 2003;110:106–114. doi: 10.1016/s0161-6420(02)01445-8. [DOI] [PubMed] [Google Scholar]
- 3.Levatin P. Pupillary escape in disease of the retina or optic nerve. Arch Ophthalmol. 1959;62:768–779. doi: 10.1001/archopht.1959.04220050030005. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki A, Moore P, Kardon RH. Variability of the relative afferent pupillary defect. Am J Ophthalmol. 1995;120:622–633. doi: 10.1016/s0002-9394(14)72209-3. [DOI] [PubMed] [Google Scholar]
- 5.Lagreze WD, Kardon RH. Correlation of relative afferent pupillary defect and estimated retinal ganglion cell loss. Graefes Arch Clin Exp Ophthalmol. 1998;236:401–404. doi: 10.1007/s004170050096. [DOI] [PubMed] [Google Scholar]
- 6.Bell RA, Waggoner PM, Boyd WM, et al. Clinical grading of relative afferent pupillary defects. Arch Ophthalmol. 1993;111:938–942. doi: 10.1001/archopht.1993.01090070056019. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki A, Moore P, Kardon RH. Long-term fluctuation of relative afferent pupillary defect in subjects with normal visual function. Am J Ophthalmol. 1996;122:875–882. doi: 10.1016/s0002-9394(14)70385-x. [DOI] [PubMed] [Google Scholar]
- 8.Cox TA. Pupillography of a relative afferent pupillary defect. Am J Ophthalmol. 1986;101:320–324. doi: 10.1016/0002-9394(86)90827-5. [DOI] [PubMed] [Google Scholar]
- 9.Cox TA. Initial pupillary constriction in the alternating light test. Am J Ophthalmol. 1986;101:120–121. doi: 10.1016/0002-9394(86)90480-0. [DOI] [PubMed] [Google Scholar]
- 10.Jonas JB, Zach FM, Naumann GO. Quantitative pupillometry of relative afferent defects in glaucoma. Arch Ophthalmol. 1990;108:479–480. doi: 10.1001/archopht.1990.01070060025009. [DOI] [PubMed] [Google Scholar]
- 11.Polansky JR, Juster RP, Spaeth GL. Association of the myocilin mt.1 promoter variant with the worsening of glaucomatous disease over time. Clin Genet. 2003;64:18–27. doi: 10.1034/j.1399-0004.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 12.Bayer A, Harasymowycz P, Henderer JD, et al. Validity of a new disk grading scale for estimating glaucomatous damage: correlation with visual field damage. Am J Ophthalmol. 2002;133:758–763. doi: 10.1016/s0002-9394(02)01422-8. [DOI] [PubMed] [Google Scholar]
- 13.Henderer JD, Liu C, Kesen M, et al. Reliability of the disk damage likelihood scale. Am J Ophthalmol. 2003;135:44–48. doi: 10.1016/s0002-9394(02)01833-0. [DOI] [PubMed] [Google Scholar]
- 14.Glazer-Hockstein C, Brucker AJ. The detection of a relative afferent pupillary defect. Am J Ophthalmol. 2002;134:142–143. doi: 10.1016/s0002-9394(02)01503-9. [DOI] [PubMed] [Google Scholar]
- 15.Thompson HS, Corbett JJ, Cox TA. How to measure the relative afferent pupillary defect. Surv Ophthalmol. 1981;26:39–42. doi: 10.1016/0039-6257(81)90124-7. [DOI] [PubMed] [Google Scholar]
- 16.McCormick A, Bhola R, Brown L, et al. Quantifying relative afferent pupillary defects using a Sbisa bar. Br J Ophthalmol. 2002;86:985–987. doi: 10.1136/bjo.86.9.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg ML, Oliva A. The use of crossed polarized filters in the measurement of the relative afferent pupillary defect. Am J Ophthalmol. 1990;110:62–65. doi: 10.1016/s0002-9394(14)76939-9. [DOI] [PubMed] [Google Scholar]
- 18.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 19.Kardon RH, Haupert CL, Thompson HS. The relationship between static perimetry and the relative afferent pupillary defect. Am J Ophthalmol. 1993;115:351–356. doi: 10.1016/s0002-9394(14)73587-1. [DOI] [PubMed] [Google Scholar]
- 20.Folk JC, Thompson HS, Farmer SG, et al. Relative afferent pupillary defect in eyes with retinal detachment. Ophthalmic Surg. 1987;18:757–759. [PubMed] [Google Scholar]
- 21.Peter E, Thomas R, Muliyil J. Brightness discrimination test is not useful in screening for open angle glaucoma. J Glaucoma. 1996;5:182–186. [PubMed] [Google Scholar]