Abstract
Purpose
To characterize involution of retinopathy of prematurity (ROP) following treatment at threshold, to identify findings during involution that portend development of retinal detachment, and to assess the potential utility of preemptive vitrectomy for eyes with high-risk features.
Methods
The probability of ROP involution and of retinal detachment evolution over time was analyzed in 262 treated eyes of 138 infants in a retrospective observational non–case controlled series. Expected utility of preemptive reintervention in eyes with high-risk features was evaluated using decision analysis. Modifications were devised to enhance classification of advanced ROP.
Results
ROP fully involuted in approximately 80% of eyes within 28 days of treatment. Vitreous organization meeting the study’s clinically important definition was associated with a 31-fold (5.37 to 183.63; P < .0001) and a 13-fold (2.97 to 58.59; P < .0001) increase in the odds for retinal detachment for right and left eyes, respectively. Vitreous hemorrhage defined as clinically important was associated with a 38-fold (2.69 to 551.19; P = .007) and a 15-fold (1.65 to 144.12; P = .02) increase in the odds for retinal detachment for right and left eyes, respectively. As modeled, an expected utility of 0.85 was calculated for preemptive vitrectomy compared with 0.79 for deferred vitrectomy for eyes with clinically important vitreous organization.
Conclusions
Acute-phase ROP involuted quickly in most eyes. Vitreous organization and vitreous hemorrhage were predictive of eyes that developed a retinal detachment. Decision analysis suggests that preemptive vitrectomy for eyes with vitreous organization meeting specific criteria is not likely to be worse than deferred vitrectomy, and it could be advantageous in some scenarios.
HYPOTHESIS
Acute-phase retinopathy of prematurity involutes rapidly following diode laser photocoagulation of the peripheral avascular retina in eyes with threshold disease. Retinal detachments evolve slowly and are heralded by involutional features that are highly predictive of the condition. Preemptive vitreoretinal surgery has potential utility in preventing retinal detachment in eyes with detrimental features of involution.
INTRODUCTION AND BACKGROUND
Retinopathy of prematurity (ROP) is an important cause of preventable blindness in children. It is a vasoproliferative disorder of the retina primarily affecting severely premature infants. The World Health Organization considers the control of childhood blindness a top priority for several reasons. First, the number of “blind years” is extraordinarily high for a person blinded in infancy. Blind children have a lifetime of visual handicap ahead of them, and there are staggering associated emotional, social, and economic costs to the affected child, the child’s family, and society at large. Second, many causes of blindness in children, including ROP, are either preventable or treatable, rendering the staggering costs largely unnecessary. Third, childhood blindness from many causes is associated with increased child mortality in developing nations; thus control of blindness is linked to child survival.1
The first case reports of ROP were described by Theodore L. Terry2 in Boston in 1942. Within less than a year, he had personally seen seven cases, was aware of at least eight other cases seen by colleagues, and recognized that he was witnessing an epidemic of infant blindness from a previously unreported cause.3 Affected eyes exhibited a grayish-white, opaque membrane behind the crystalline lens. Unsure of the pathophysiology of the disease, he suggested that it might have resulted from “fibroblastic overgrowth of a persistent tunica vasculosa lentis.”4 Terry correctly suspected prematurity as a predisposing condition.
Silverman5 noted that premature infants achieved an important measure of prominence in the social and medical consciousness of the United States starting in 1949, when information about the duration of pregnancy and birth weight were added to standard birth certificates. This was soon followed by publication of special reports from the National Office of Vital Statistics indicating that birth weight of less than 2,500 gm accounted for a higher infant mortality rate than any other condition.
Neonatal care had begun to evolve in the 1930s and 1940s. Oxygen administration was recognized for its ability to improve the health of premature infants,6 and its use became common practice. Specialized centers to care for premature neonates began to proliferate in the 1940s, especially in the United States.
By 1951, most states provided some form of special care facility for premature infants. Many of these new facilities had oxygen outlets in the walls, reducing both the inconvenience and cost previously associated with oxygen administration.5 Unchecked, empiric use of oxygen became common in the mistaken belief that if a little was good, more was better. Soon, an epidemic of ROP, then called retrolental fibroplasia, followed in the United States and around the world. Silverman estimated that between 1940 and 1953, as many as 10,000 children (7,000 children in the United States) were blinded by the disease.5
The cause of ROP remained unclear until almost 10 years later. Following advice from colleagues about the possible role of oxygen, Kate Campbell reported the rate of ROP in three groups of infants under her care in Melbourne.7 Each group was managed similarly, except with regard to the amount of oxygen they received. ROP developed at a higher rate in the groups where higher levels of exogenous oxygen were administered. A randomized, prospective trial of oxygen therapy was carried out shortly thereafter by Patz and coworkers8 at Gallinger Municipal Hospital in Washington, DC. This study confirmed that higher levels of oxygen administration were indeed associated with a greater risk of developing ROP. Kinsey and coworkers9 subsequently confirmed the role of oxygen in the pathogenesis of the condition, but a “safe” level of inspired oxygen has never been established.
Animal experiments subsequently revealed that high levels of systemic oxygen resulted in permanent obliteration of blood vessels in the developing neonatal retina.10,11 Several theories regarding the role of oxygen in promoting the development of ROP have subsequently been proposed. Choroidal vessels cannot autoregulate under hyperoxic conditions, whereas retinal blood vessels can autoregulate. Investigators believe that this dichotomy in response to hyperoxia results in excess oxygen diffusion from the choroid to the retina, prompting constriction of retinal blood vessels to the point of irreversible obliteration.12,13 Oxygen free radicals have also been theorized to overwhelm antioxidant enzymes and other protective mechanisms in the neonate, resulting in damage to stem cells and thus interrupting the process of normal vessel migration and vasculogenesis.14
Vasoactive cytokines, such as vascular endothelial growth factor, are also believed to be important in the pathogenesis of ROP. High supplemental oxygen in animal models results in vascular endothelial growth factor suppression, which is thought to produce excessive pruning of retinal vessels.15 Studies have also proposed that later hypoxia results in excessive vascular endothelial growth factor expression, neovascularization, and the sequelae of ROP.15 Most recently, deficiency of insulin-like growth factor I has been proposed as an instigator of the disease.16
After exogenous oxygen administration was discovered to be a significant risk factor for ROP, its use was dramatically curtailed in the neonatal population.17 The rate of blindness in one survey dropped from 7.9 per 100,000 population to 1.2 per 100,000 population between 1950 and 1965.18 Unfortunately, concurrent with the decline in the rate of ROP, there was a substantial increase in the rate of neonatal mortality and serious morbidity,17,19 including cerebral palsy.20 Recognizing this dilemma, the use of oxygen was liberalized, but monitored carefully.21 Cross19 calculated the human cost of oxygen restriction to be the death of 16 infants for every case of blindness prevented, resulting in excess of 20,000 deaths in the United Kingdom and 150,000 deaths in the United States.22
Extremely premature low birth weight infants are known to be at the highest risk for developing ROP. A resurgence of ROP occurred in the 1970s and 1980s, concurrent with a marked increase in the survival rate of very low birth weight premature infants, especially those weighing less than 1,000 gm.23–25 Emsley and coworkers26 reported that survival of infants born in the 23rd to 25th weeks of gestation increased from 27% in the years 1984 to 1989 to 42% in the years 1990 to 1994.
During the 1970s and early 1980s, there were encouraging27–31 but sometimes conflicting32–34 reports on the effectiveness of laser photocoagulation and cryotherapy of the retina in preventing the progression of ROP to retinal detachment. A multicenter randomized, prospective trial (the Cryotherapy for Retinopathy of Prematurity [Cryo-ROP] study) conducted in the United States in the 1980s demonstrated a significant reduction in the rate of unfavorable structural outcomes in eyes treated with cryotherapy.35 However, despite the proven benefits of cryotherapy and laser photocoagulation, ROP remains an important cause of blindness and severe visual impairment in children born prematurely. There is conflicting evidence on the current incidence and severity of the disease, which has been reported to be decreasing in several single-center studies.36,37 A larger multicenter study on ROP, however, noted an unchanging incidence of ROP and suggested that single-center studies reporting a lower incidence of ROP are misleading because of a phenomenon known as regression toward the mean.38 This group noted that anytime one selects the extreme values from a distribution and then compares these with later results, there is a tendency to move closer to the mean for the group as a whole.38 Advances in the care of premature infants continue to produce improved infant survival, even for infants as young as 22 weeks in estimated gestational age, which is considered to be the official lowest age of viability in Japan.39 ROP in general and serious ROP in particular are known to occur more frequently in extremely premature infants.40 Coats and coworkers41 reported that ROP developed in all 97 eyes of 49 infants born at less than 25 weeks estimated gestational age, with 39 (40%) of those eyes developing threshold disease. Very low birth weight infants are also at risk for visual impairment from several other causes, though severe ROP is associated with the most devastating visual consequences.42,43
EPIDEMIOLOGY
Exact estimates of the prevalence of childhood blindness are difficult to obtain because the prevalence is low in relationship to the total population, whereas the sample size required to accurately estimate prevalence is prohibitively large and impractical. Most information about severe visual impairment and blindness in children is obtained through the study of children in schools for the blind and from blind children registries. Despite obvious methodological flaws in this approach, these studies provide the most accurate data presently available.
It is estimated that there are 1.5 million blind children in the world and that 72,000 of these children live in United States, Europe, and Japan. 44 In Europe, ROP is among the most common causes of severe visual impairment and blindness.44 Among causes of blindness in 17 surveys of schools for the blind and visually impaired in the Americas and the Caribbean, ROP was cited as the most frequent cause in seven surveys and ranked second in three other surveys, as summarized by Munoz and West.45 In a 1999 survey in the United States, ROP was found to be the second most common cause of childhood blindness and the leading cause of potentially preventable childhood blindness.46 The disease accounted for 320 of 2,553 cases of vision loss among students in schools for the blind and visually impaired in the United States (or approximately 13% of this population).46 Only cortical visual impairment was more common at 19%.
ROP is believed to account for 6% to 18% of childhood blindness in industrialized countries that have an infant mortality rate of less than 10 per 1,000 live births.47 In countries with an intermediate infant mortality rate of 10 to 60 per 1,000 live births, ROP is emerging as a major cause of childhood blindness as advances in neonatal care reach these areas. According to recent estimates, the number of cases of children with severe visual impairment and/or blindness due to ROP is thought to be around 9,000 in high-income countries and 29,000 in middle-income countries.1 Little ROP-related blindness occurs in countries where the infant mortality rate is greater than 60 per 1,000 live births, because at-risk premature infants generally do not survive.47 Because of the global relationship between economics and the effects or ROP, blindness from ROP is targeted by the Global Initiative for Elimination of Avoidable Blindness.48 The World Health Organization also identified ROP as an important cause of preventable blindness in its Vision 2020 program, especially in middle-income countries. 1
In a study from 17 Canadian neonatal units, extremely premature infants with an estimated gestational age of less than or equal to 25 weeks accounted for only 4% of neonatal intensive care unit admissions, but for 22% of deaths, 20% to 60% of major morbidities, 11% of days in the hospital, and 10% to 35% of major procedures.49 In addition, severe ROP (described as stage 3 or stage 4 disease) has been found to be a significant predictor of disability, which is defined as inability to perform self-care at age 4.26,50 Emsley and coworkers26 reported an increase in survival and associated deteriorating developmental outcomes in infants born at 23 to 25 weeks over an 11-year period. There was an increase in survival to discharge from 27% to 42%. This was accompanied by an increase in disability rates from 38% to 68%. The proportion of children with cerebral palsy as the cause of their disability did not change significantly and was 21% and 18%, respectively. Blindness due to ROP was a major contributor to increased disability, escalating from 4% to 18% and underscoring the full adverse impact that ROP may have on the premature infant.
The Cryo-ROP Cooperative Group51 reported that among children with “favorable” Teller acuity outcomes (defined as grating acuity ≥6.4 cycles per degree), 3.5% were classified as disabled compared with 67.4% disabled among those who did not achieve “favorable” Teller acuity outcomes. The Group noted that “…unquestionably, those children who retained vision in at least one eye functioned much more independently.” They cautioned that this does not necessarily mean that treatment to preserve vision might reduce disability rates, but rather that ROP unresponsive to cryotherapy might be indicative of children with severe brain injury. Regardless of the cause-and-effect relationship between poor ROP outcome and disability, retention of vision offers the potential for a disabled person to function at a higher level than otherwise possible.
ECONOMICS AND QUALITY OF LIFE
Blindness acquired in infancy is associated with a lifetime of direct and indirect costs. Direct costs include special education and training, loss of productivity, and disability cost. A significant amount of the socioeconomic burden, particularly disability and future medical care, is borne by federal and state programs. Successful treatment of ROP is associated with a substantial long-term economic gain.
Although economic factors are important, the quality of life of those affected with ROP is also essential in evaluating the benefits of treatment. To that end, Drummond52 estimated the health utility of a blind person. According to him, a well-adjusted blind person would have a health state utility of 0.48, whereas a poorly adjusted blind person would have a utility of 0.35, compared with a utility of 1.0 for complete well-being. A year of blindness, therefore, is equivalent to 0.48 quality-adjusted life-years (QALY) for a person who has been maximally rehabilitated. The potential gain in quality of life from preventing vision loss in a person with a sight-threatening disease, therefore, is estimated to be 0.52 per year. Although these estimates are useful, they do not take into account the severity of neurologic deficits that often complicate the rehabilitation of premature infants, and it is highly likely that a blind or severely visually impaired premature infant will have a lower QALY score than an otherwise healthy or even a poorly adjusted blind person. According to Drummond’s model, saving the sight of a single premature infant would yield a minimum utility of 40.14 QALY saved based on an average life expectancy of 77.2 years for a person born in 2001 (National Center for Health Statistics; http://www.cdc.gov/nchs/fastatsfasts/lifexpec.htm; accessed December 23, 2003).
COURSE OF ACUTE-PHASE ROP
The timing of events in the course of acute-phase ROP is better correlated with postconceptional age than with postnatal age.40 The median time of onset of stage 1 ROP was 34.3 weeks postconceptional age in the Cryo-ROP study.40 Acute-phase ROP progresses through several stages that have been carefully defined by the Committee for the Classification of Retinopathy of Prematurity.53 This classification system is known as the International Classification of Retinopathy of Prematurity (ICROP). The termination of blood vessels relative to the optic nerve is used to characterize the zone of ROP. Zone I is represented by a circle centered on the optic disc, the radius of which subtends an angle of 30° and extends from the disc to twice the distance from the disc to the center of the macula. Zone II includes retina in a ring defined by an area extending from zone I to the nasal ora serrata, and zone III includes the remaining peripheral retina, primarily located temporally. The extent of ROP is denoted by dividing the circumference of the vascular/avascular junction into 12 sectors, typically described as “clock-hours.” Progressive stages of acute-phase ROP include stage 1, visible to the clinician as a two-dimensional demarcation line at the junction of the vascular and avascular retina. Stage 2 is characterized as a ridge (a three-dimensional structure at the vascular/avascular junction), and stage 3 denotes the presence of neovascular tissue emanating from the posterior aspect of the ridge. Plus disease in ICROP is denoted when “the posterior veins are enlarged and the arterioles are tortuous” [sic]. Threshold ROP was later defined in the Cryo-ROP study as five contiguous or eight cumulative clock hours of stage 3 disease in zones I or II in the presence of plus disease.35 “Threshold” was felt to indicate an increased likelihood of developing a retinal detachment, a risk estimated to be approximately 50% by the study committee.54 This estimation proved to be accurate, with 47.4% of untreated threshold control eyes in the Cryo-ROP study developing an unfavorable outcome35 (defined as a retinal detachment involving zone I, macular fold, or retrolental tissue obscuring view of the posterior pole). The clinical findings that constitute plus disease have evolved over the past 20 years. Plus disease was more strictly defined in the recently concluded Early Treatment for Retinopathy of Prematurity (ETROP) Study as dilation and tortuosity of arterioles and venules in the posterior pole in at least two quadrants. (Early Treatment for Retinopathy of Prematurity Cooperative Group. Manual of Procedures. Springfield, Virginia: National Technical Information Service; 2004). The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity subsequently characterized the more advanced stages of the disease in 1987.55 Stage 4 ROP was used to denote a subtotal retinal detachment with stage 4A indicating that the macula was uninvolved and stage 4B indicating that the macula was involved. Stage 5 was used to denote a total retinal detachment.
PREVENTION
Many preventive and interdictive concepts have been studied in an attempt to reduce blindness from ROP. Even in the initial report on the disease, Terry2 described one infant who had received “x-ray therapy” and another who had undergone diathermy in an attempt to treat the disease, even though the pathophysiology of the disease had not yet been explored. The antioxidants vitamin E56–58 and D-penicillamine59 have been studied in randomized trials to test the theory that antioxidant use might reduce oxygen free radical damage to developing retinal tissues and prevent development or reduce the severity of ROP. Though each has shown some degree of promise, concerns over safety, controversy regarding efficacy, or both, have limited their use.
Because a relative hypoxic state is known to exist in the retina during late stages of acute-phase ROP, a multicenter randomized trial of supplemental oxygen administration was carried out in the 1990s.60 Infants with prethreshold ROP were randomly assigned to supplemental oxygen to maintain oxygen saturation between 96% and 99% (supplemental group) or to a control group in which the oxygen saturation was maintained between 89% and 94% (conventional group). Though the rate of disease progression in the supplemental group was lower than that in the conventional group, the differences were not statistically significant and the study failed to demonstrate that supplemental oxygen administered to infants with prethreshold ROP reduced the incidence of progression to threshold disease. Importantly, adverse pulmonary events were more common in the supplemental group, highlighting the need to consider the whole child, not just the eyes, when assessing potential treatments for ROP.60 Wide fluctuations in oxygen saturation have been noted as possibly having an adverse impact on development of ROP and on progression of the disease.61 Recent evidence has suggested that tighter control over oxygen administration to avoid fluctuation of oxygen saturation during the neonatal period may reduce the incidence and severity of ROP.62 Light stimulation of the retina was thought to potentiate the development of ROP by increasing the metabolic demands of the retina and formation of free radicals. A randomized multicenter study on light exposure in the nursery, however, reported lack of efficacy of light reduction in preventing ROP. 63
TREATMENT
Cryotherapy of the peripheral avascular retina was established in the late 1980s as an effective treatment for reducing, but not eliminating, the risk of an unfavorable outcome in eyes with threshold ROP.35 An unfavorable anatomical outcome was present 1 year after treatment in 25.7% of eyes treated with cryotherapy compared with 47.4% of untreated control eyes. Both argon and diode laser photocoagulation have been increasingly used in the treatment of advanced ROP over the last decade,64 though only small randomized trials comparing laser to cryotherapy have been published. Laser can be applied via a transscleral probe,65,66 but most trials have utilized a transpupillary approach through an indirect ophthalmoscope delivery system. Several studies have demonstrated that laser photocoagulation of the avascular retina is at least as effective as cryotherapy in the treatment of threshold disease,67–73 whereas others have suggested that laser treatment is associated with better structural and functional outcomes.74–76 Some investigators have also suggested that laser may be particularly beneficial for treatment of zone I disease.72,77,78 Although argon and diode laser photocoagulation have both been demonstrated to be effective, the complication of cataract development may be more common in eyes treated with argon laser79 than with diode laser.80
Recognizing that a significant number of eyes and infants still progressed to develop severe visual impairment or blindness when treated at threshold, clinicians began to consider treatment at an earlier stage in the disease. Fleming and coworkers81 used diode laser successfully to treat nine infants with prethreshold ROP in zone I or posterior zone II. Although their study was uncontrolled, they felt that the high rate of success relative to historical controls might represent an improvement in the timing of treatment for posterior ROP. In addition, the recently concluded ETROP study randomized infants to ablation of the avascular retina at high-risk prethreshold versus observation with treatment administered at threshold.82 An unfavorable structural outcome occurred in 9.1% of eyes treated at prethreshold, as compared to 15.6% of eyes treated at threshold. Functional outcomes were also superior for the early treatment group (14.5% unfavorable versus 19.5% for the threshold group).
INVOLUTION
ROP is a self-limiting condition. It has a clinically predictable time of onset and a definitive end stage when the disease process becomes inactive, regardless of maximum disease severity.83 Permanent retinal sequelae are directly related to maximum severity of acute-phase disease. Flynn and coworkers84 reported that ROP will last an average of 15 weeks from inception to resolution in eyes that regress. Whereas involution occurs in a harmless manner in most cases, it can be marked by the development of detrimental vitreoretinal abnormalities that result in permanent retinal damage, including retinal detachment and subsequent blindness in a significant number of eyes.
Though extensive study has been conducted regarding risk factors, development, progression, and treatment of ROP, as well as treatment of retinal detachments, little or no formal study on the process of involution following treatment of threshold ROP has been published. The only studies this author could identify that specifically addressed the process of ROP involution reported only on involution of nonthreshold disease.85,86 Preslan and Butler85 reported that acute-phase ROP often underwent a protracted course of resolution, not reaching zone III until 42 to 45 weeks. Repka and coworkers86 noted that acute-phase ROP typically began to involute at a mean of 38.6 weeks postmenstrual age and that 90% of eyes demonstrated onset of involution prior to 44 weeks postmenstrual age.
Important areas of potentially vision-saving research include not only prevention of ROP and interdictive treatments at or prior to threshold, but identification of those eyes with the highest risk for developing a retinal detachment following treatment and exploration of preemptive reintervention strategies designed to mitigate the effects of detrimental involution. Identification of factors likely to be associated with an unfavorable outcome could be of paramount importance
RETINAL DETACHMENT RISK FACTORS
Many patient-specific risks associated with development of severe ROP are beyond the influence of the treating ophthalmologist. The Cryo-ROP Cooperative Group87 demonstrated an increased risk of threshold ROP in infants with lower birth weight, lower estimated gestational age, white race, multiple births, and birth outside of a study center hospital. Local factors associated with an unfavorable outcome included zone I threshold disease, rapid progression to threshold disease, and extent of stage 3 disease.87 Zone I threshold disease was a particularly noteworthy prognostic indicator, with 75% of treated threshold zone I eyes developing an unfavorable outcome.35,87 Each sector of stage 3 neovascularization over 5 clock hours (the minimum prerequisite for threshold) was associated with a 26% increase in the odds for developing an unfavorable outcome; thus the circumferential extent of the disease was also found to be important.87 Systemic Candida infection has been shown in several studies to be associated with an increase in the incidence and severity of ROP88–90 and an increase in the risk of a retinal detachment.90 Other investigators have not been able to demonstrate an independent association between severity of ROP and systemic infection with Candida, but could not rule out such an association either.91
ROLE OF THE VITREOUS
The role of the vitreous in the development of severe cicatricial ROP has been previously recognized, although not subjected to detailed clinical study. The fact that ICROP does not take vitreous abnormalities into account in the classification of the disease may help to explain the lack of clinical study into the role vitreous pathology plays in the development of ROP retinal detachments. Though this author could find no detailed published clinical data on eye findings in the days or weeks preceding a retinal detachment in treated eyes, research on untreated eyes with cicatricial disease may be of value in better understanding the pathogenesis of detrimental involution that ultimately concludes with development of a retinal detachment. In a study on the natural history of ROP, Schulenberg and coworkers83 reported that progression from early ROP to development of neovascular tissue occurred rapidly, especially in eyes with posterior disease. In contrast, progression to retinal detachment occurred more slowly, requiring 4 to 5 weeks to develop. They noted that development of a retinal detachment was usually heralded by the sudden onset of vitreous haze 1 week before detachment occurred. Flynn and coworkers92 also characterized the vitreous as becoming “progressively hazy” as untreated acute-phase ROP progressed into a cicatrizing process.
Reporting on the natural history of ROP, Schulenberg and coworkers83 noted that when ROP remained confined to the retina, cicatricial ROP typically consisted of relatively minor preretinal fibrosis limited to surface tractional retinal changes only. Eyes that continued to progress after stage 3 often developed a vitreoretinopathy with “severe consequences.” They characterized cicatricial disease as being associated with extensive transvitreal retinal traction that invariably progressed to retinal detachment and blindness in zone I and zone II eyes.
Eyes with cicatricial ROP that have undergone cryotherapy or photocoagulation during acute-phase disease have several interesting and consistent characteristics. Hikichi and coworkers93 described the characteristics of the vitreous in previously treated children old enough to undergo slit-lamp biomicroscopy. They observed and photographed the retina and vitreous in 29 eyes of 15 children at a mean age of 9 years. Each had developed cicatricial ROP after undergoing cryotherapy, photocoagulation, or both, during acute-phase ROP. Marked vitreous liquefaction without a posterior vitreous detachment was observed, especially in the vitreous overlying areas that had undergone treatment.
Faris and coworkers94 described the vitreous changes in mild ROP in the cicatricial stage. They noted that vitreous organization was localized to the cortical layer and was composed of white and gray avascular fibrous tissue. Central portions of the vitreous were often liquefied into large lacunae. The vitreous between these lacunae was often condensed into bands and membranes that were connected to organized tissue at the vitreous base. The posterior cortical layer of the vitreous was always attached to retina folds. They stated that “…vitreous changes in the cicatricial stage of retrolental fibroplasia are contraction of the scar tissue located in the vitreous base, shrinkage of the cortical layer of the vitreous, formation of bands and membranes in the liquefied vitreous gel, and extensive syneresis in the central and posterior vitreous cavity. Posterior vitreous detachment was seldom observed …. ” Faris and coworkers believed that the posterior cortical layer of the vitreous was firmly adherent to the retina and that this firm adhesion was important in the pathogenesis of retinal disease in ROP. Fibrillar condensation of the vitreous takes the form of membrane-like fibers that traverse the vitreous cavity to the periphery of the retina.93 These fibers are most prevalent in areas where cryotherapy or photocoagulation has been applied. Optically empty spaces in the vitreous overlying chorioretinal treatment scars are typically larger in eyes treated with cryotherapy than in those treated with photocoagulation.93 These optically empty spaces are traversed by condensed vitreous strands.
Foos95 reported on the histopathologic features of eyes with advanced ROP retinal detachments. Extraretinal proliferation of nonvascular tissue was often seen in the area of the ridge. The vitreous overlying the ridge was noted to become opalescent, and linear vitreous condensations were often apparent. As this process continued, the retina was drawn anteriorly and a traction retinal detachment ensued.
Hirose and Sang96 described in some detail the vitreous findings in patients with acute and chronic cicatricial ROP. They noted that during the acute phase of ROP, central lacunae formed in the vitreous cavity associated with cell migration into the cortical vitreous. Extraretinal neovascularization extended into the vitreous cavity from the anterior edge of the vascularized retina in severe ROP, leading to the formation of fibrovascular tissue extending toward the lens. Contraction of these fibrous tissues produced pronounced traction on the retina, ultimately leading to development of a tractional retinal detachment.
deJuan and Machemer97 examined the histologic and ultrastructural characteristics of membranes removed during vitreoretinal surgery to treat ROP-related retinal detachments. They found that the internal limiting membrane was fragmented by retinal glial cells that had migrated into the vitreous along microvilli. These membranes were entirely avascular in inactive disease. These findings suggest that the proliferative membrane is firmly attached to the underlying retina. When vitreous membranes are severed at the time of open-sky vitrectomy, they have been noted to shrink dramatically, highlighting the contractile tension that is present.96 Soong and coworkers98 demonstrated the presence of linear bundles of actin in vitreous membranes excised during open-sky vitrectomy of ROP-related retinal detachments. Actin was also found to be concentrated in the cortical cytoplasm of cells, many of which appeared to be myofibroblast-like in appearance. Myofibroblasts are known to contract and have been suggested as causative factors in vitreoretinal disease states through contraction of transvitreal membranes. The proliferative vasculature in ROP has been shown to leak fluorescein,99 and an ROP animal model has demonstrated lack of a blood-retinal barrier early in the process of neovascularization.13 Chan-Ling and coworkers13 believe that proteins leaking into the retina and vitreous play an important a role in inducing vitreous pathology. Clinically obvious organization of vitreous membranes has been reported in a large percentage of eyes that developed a retinal detachment.100 Hartnett and McColm101 reported that progression to retinal detachment was predicted by absence of clear vitreous.
TREATMENT FAILURES
There is at present no reliable means for an ophthalmologist to predict with any degree of certainty which eyes with ROP will develop a retinal detachment. The criteria for choosing eyes for ROP treatment have undergone constant evolution as judgment and experience with the disease have advanced. Treatment of retinal detachments (or impending retinal detachments) should undergo the same constant evolution based upon experience as well as advances and knowledge reported in the literature. Little scientific data is available to guide reintervention strategies in eyes with detrimental involution following ablation therapy. Other than anecdotal information, this author could identify no published data on the timing, appropriateness, and effectiveness of reintervention after initial treatment, other than treatment of retinal detachments. Likewise, no published data on the typical process of ROP involution following treatment of threshold disease could be identified. Even routine follow-up recommendations for treated infants are sparse and anecdotal, leaving physicians to rely strictly on experience and judgment in the postoperative management of infants with ROP.
The management of stage 4A disease (extramacular, partial retinal detachment) is controversial, with no consensus as to the best treatment approach. Surgeons are often reluctant to intervene when a stage 4A retinal detachment is present, for a variety of reasons, including fear of causing harm, lack of evidenced-based medicine to clearly support intervention at this stage, and fear of medicolegal consequences. Treatment outcomes of the most advanced ROP, however, are not in doubt. Other than isolated reports of eyes with good vision following macula-off detachments,102 very few eyes have retained even form vision following repair of advanced ROP retinal detachments.103 In general, anatomical outcomes for treatment of stage 4A retinal detachments have been significantly better, and surgery is believed by some to reduce the risk of progression to more severe detachment profiles.104 Indeed, nonprogression of detachments beyond stage 4A has been reported to occur between 84% and 94% of eyes in several recent studies using lens-sparing vitrectomy with or without concurrent scleral buckling.104–107 Vitreoretinal surgery to treat less advanced detachments is technically easier and associated with fewer vision-threatening consequences, such as the requirement for lensectomy.104 Vision following lens-sparing vitrectomy for stage 4A detachments is generally good, according to M. T. Trese, MD (written communication, December 22, 2003), with an average visual acuity of 20/55 (20/20 to 20/200) for the 90% of retinas that were reattached in his recent analysis.
DECISION ANALYSIS
Determining optimal treatment pathways for infants with progressive ROP could yield further improvements in outcomes. Decision analysis may provide a means of exploring the potential utility of various treatment options. The decision sciences have been utilized in business for more than 30 years. They have been increasingly utilized in medicine over the past two decades to aid in medical decision making. Decision analysis is a mathematical tool used to emulate human decision making.108 It utilizes a systematic approach to consider multiple factors in a decision on a presumably rational basis to calculate the expected utility of a clinical scenario. Decision analysis involves devising simulated clinical scenarios and entry of the known probability of each event where available, and estimating probabilities of events that are not known. A decision tree is then populated with these values and calculations are made to yield a probability for each treatment arm. Expected utilities are then calculated at each major branch point. Though not a substitute for clinical judgment, it is a tool to facilitate clinical reasoning, and use of decision analysis can be of value in helping to analyze potential treatment options for ROP. Utility is an attribute that is felt to motivate individuals to choose among various options. In general, a utility of “1” is assigned to an optimal outcome, and “0” is assigned to the worst possible outcome. Intermediate outcomes are assigned a utility between the extremes. The plan of action with the highest expected utility is anticipated to be the preferred choice of treatment.109
Given the poor prognosis of advanced stages of ROP, exploration of the potential utility of surgical reintervention prior to development of a retinal detachment is a reasonable consideration. Data regarding the time course of retinal detachment and features heralding an impending retinal detachment are currently unavailable, so surgeons are left to rely on anecdotal experience and observation to make critical treatment recommendations. The purposes of this study, then, were to (1) systematically evaluate the process of ROP involution and retinal detachment evolution following diode laser photocoagulation to treat threshold disease, (2) analyze specific proposed features of the process of involution that portend development of a retinal detachment, and (3) analyze, using decision analysis, the theoretic utility of preemptive lens-sparing vitreoretinal surgery in eyes considered to be in a high-risk predetachment state. In the process of conducting this study, it became obvious that the present system for classification of late-stage ROP was insufficient, and an updated and expanded classification scheme was devised.
METHODS
The records of consecutive infants undergoing diode laser photocoagulation for threshold ROP from one large pediatric ophthalmology center were studied. Eyes requiring retreatment for skip areas were excluded because of the desire to study the process of involution in fully treated eyes. Institutional review board approval was obtained to conduct the study. Demographic data analyzed included estimated gestational age, birth weight, race, and gender. Pretreatment ophthalmologic factors analyzed included zone of threshold ROP according to ICROP53 and the number of sectors of stage 3 neovascularization present at the time of threshold diagnosis. All eyes underwent transpupillary diode laser photocoagulation within 72 hours of diagnosis, with most undergoing treatment within 48 hours of diagnosis. Photocoagulation was applied to the entire peripheral avascular retina from the vascular/avascular junction to the ora serrata for 360°. No treatment was applied on or posterior to the ridge. Treatment was applied in either an interrupted mode or a near-continuous mode. Up to one-half laser spot diameter was allowed between adjacent treatment spots to allow for postoperative spread of the laser burns. Laser power and duration were adjusted to create moderate intensity (gray-white) burns. These treatment patterns resulted in confluent treatment scars of the avascular retina, noted at the first examination 5 to 7 days after treatment.
All eligible infants had a birth weight of less than or equal to 1,500 gm and/or an estimated gestational age of less than or equal to 28 weeks, consistent with present screening recommendations.110 All infants were followed to a structural outcome examination at 9 ± 3 weeks after treatment, utilizing the longest follow-up examination during this interval to determine outcome. Eight infants (5.7%) initially lost to follow-up were seen after the outcome examination window. Data from these examinations were analyzed with the remainder of the outcome data. Although analysis of data acquired outside of the standard interval window has the potential to bias the results, this is less likely to occur for late data acquisition compared with early data acquisition. It was the intention at the outset of the study to evaluate all treated eyes. Therefore, analysis of these data was believed to represent less of a bias than their exclusion. Fundus examination was conducted at least every other week for the first 4 weeks after treatment. The study was limited to these time periods to minimize the number of patients lost to follow-up, because infants in our center are often followed up by other ophthalmologists after discharge from the hospital and are therefore lost to further follow-up at our center.
INVOLUTION AFTER TREATMENT
The key clinical findings on retinal examination were tabulated and analyzed for each treated eye during the first 4 weeks after diode laser treatment and again at 9 ± 3 weeks following treatment. If more than one examination occurred in the 9 ± 3–week interval, the examination corresponding to the longest follow-up was utilized. Involution parameters were established prior to initiating data collection. To minimize investigator bias, evaluation of the postoperative retinal status was strictly limited to those features of active ROP that could be clearly and unmistakably identified from clinical records.
Residual plus disease was considered to be present if any elements of plus disease remained, including any dilation or tortuosity of any posterior pole vessel. No attempt was made to characterize the severity of residual elements of plus disease, because this would have required subjective interruption of clinical data, and even existing clinical proposals for plus disease quantification are highly subjective, at best.111 Residual stage 3 neovascularization was characterized as present if any residual stage 3 disease remained. Examples of stage 3 disease have been published elsewhere.53 Vitreous organization (defined below) above the ridge with no clinically obvious vascular elements was not considered to be active neovascularization. Whereas limiting the classification of stage 3 disease and plus disease to a simple “present” or “absent” assessment does not reflect the full dynamics of the involution process, it captured the key clinically identifiable features of the process of involution and required no subjective interpretation of the clinical documentation, thus reducing investigator bias. ROP was considered completely involuted in eyes with no remaining active stage 3 disease and no remaining elements of plus disease, as defined above. Because no further useful information regarding involution was available once a retinal detachment was noted, eyes with retinal detachments were tabulated as fully involuted in this analysis, and retinal detachments were subsequently analyzed separately to investigate timing of retinal detachment diagnosis relative to treatment date.
RETINAL DETACHMENT RISK FACTOR ASSESSMENT
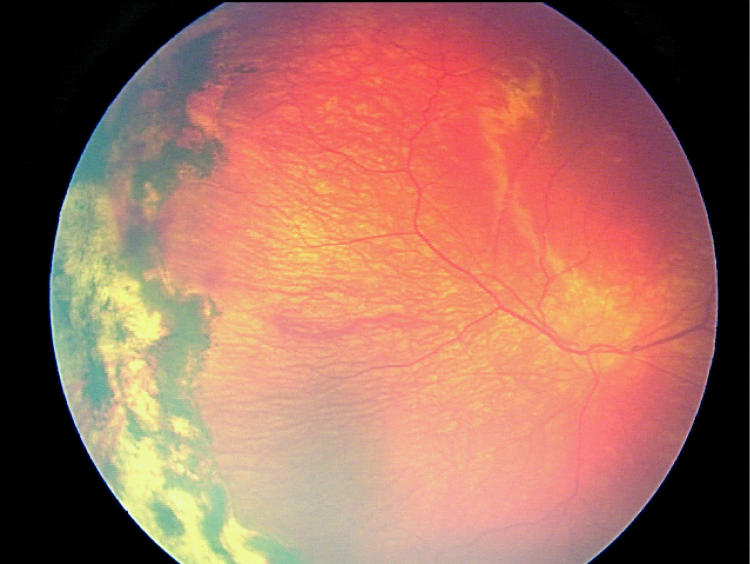
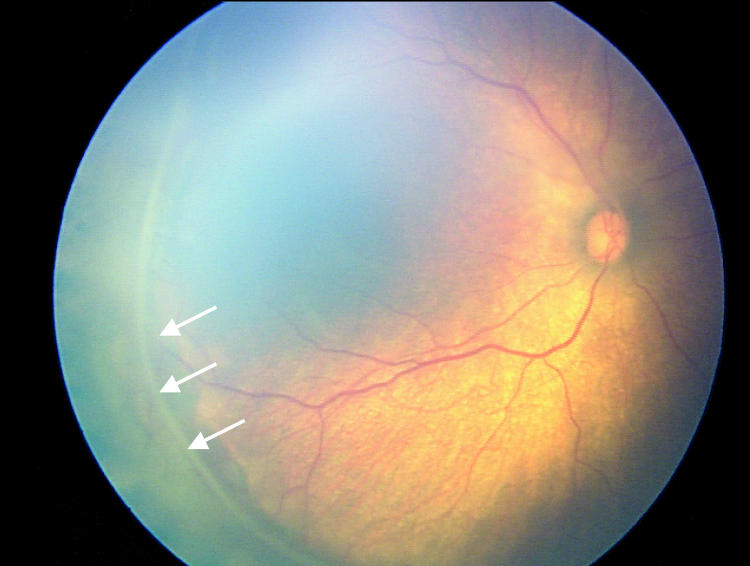
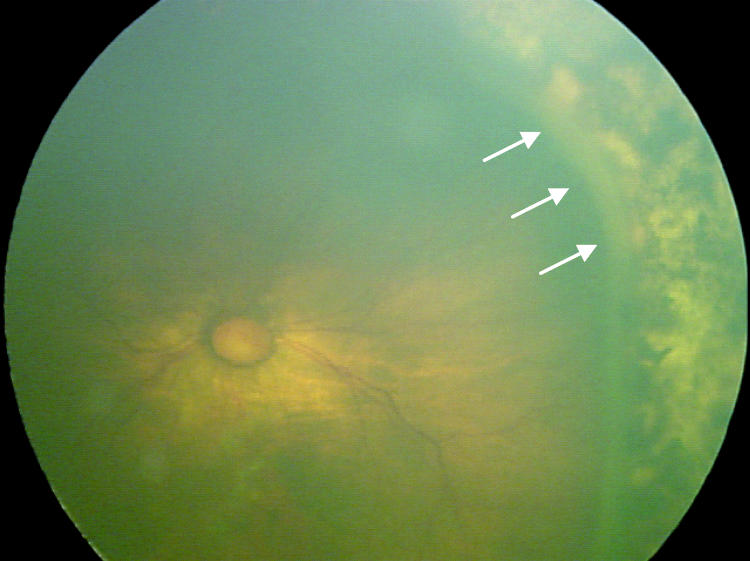
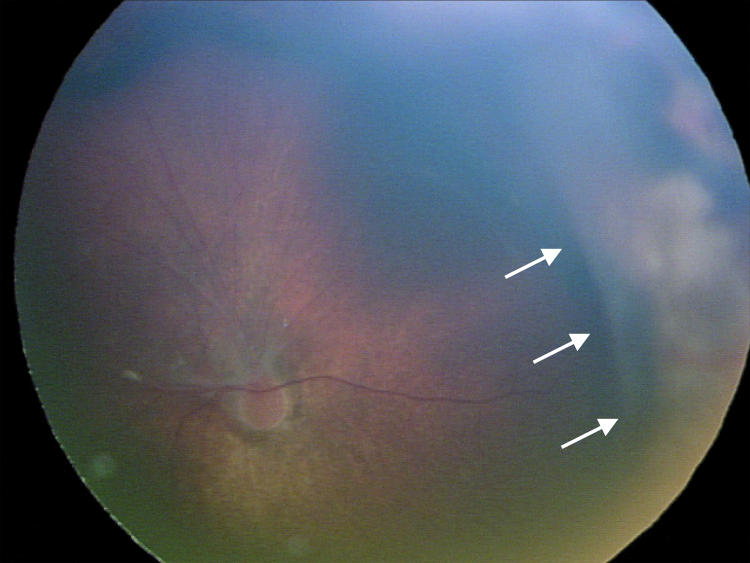
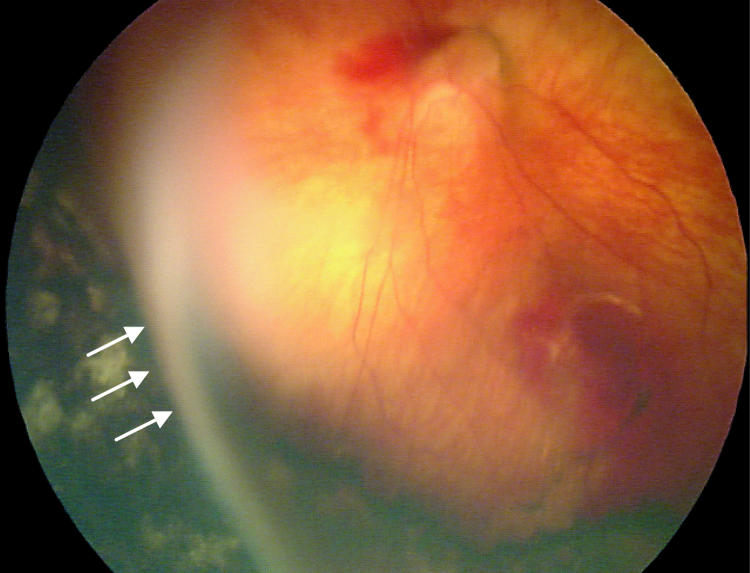
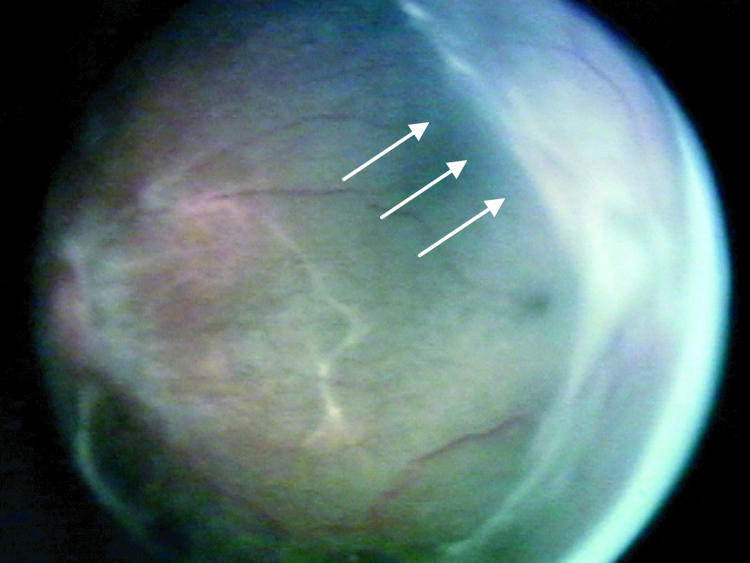
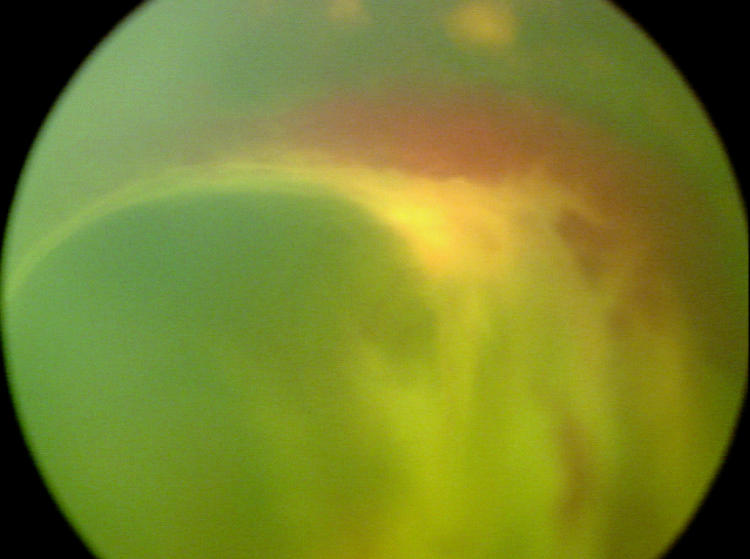
Factors suspected to be associated with an increased risk of retinal detachment were established prior to data collection based on clinical experience. These factors were (1) active stage 3 disease more than 21 days after treatment, (2) active elements of plus disease (dilation or tortuosity of any posterior pole vessel) more than 21 days after treatment, (3) clinically important vitreous organization (defined below), and (4) clinically important vitreous hemorrhage. Vitreous hemorrhage was considered clinically important if it was severe enough to completely obscure visualization of the underlying retina. Vitreous organization was considered to be present when white, fibrous-appearing opacification of the vitreous was seen above the vascular/avascular junction. Vitreous organization contained no clinical evidence of vascular elements, such as clinically visible blood vessels, hemorrhage, or pink/red coloration. Vitreous organization usually, but not always, had a visible connection to the underlying retina, but the bulk of the material was in the vitreous, not on the retina. Examples of vitreous organization are shown on page 291.
For vitreous organization to be considered clinically important, it had to span two or more contiguous clock hours and be dense enough to significantly reduce visualization of the underlining retina, but also be unassociated with a retinal detachment at the time of initial discovery. Tractional elevation of retinal blood vessels into the vitreous without detachment of the underlying retina was not considered a retinal detachment and, in isolation, was not considered vitreous organization. For the purposes of this study prior to data collection, this level of vitreous organization was classified as “clinically important” based on clinical experience, suggesting this degree of vitreous organization was frequently associated with development of a retinal detachment.
DATA ANALYSIS
A high correlation has been demonstrated between fellow eyes during progression of acute-phase ROP, strongly suggesting that systemic variables impact the progression of disease more than local eye factors.112 Though no such correlation has been reported during the involution phase of ROP in treated eyes, it was assumed that such a correlation might exist, so the data for involution of threshold disease were analyzed and reported for right and left eyes independently.
Study data were analyzed using Stata 8.0 (College Station, Texas). The probability of threshold ROP involution occurring over time was evaluated with the Kaplan-Meier estimator, using the weekly activity status of stage 3 disease and plus disease. Each factor was analyzed individually and in combination to generate a profile each for ROP, plus disease, and stage 3 involution. Because the post-28 interval is known to be very long and events are assumed to occur with equal probability in this interval, these data were interval censored, using the midpoint between day 28 and the final examination to more accurately reflect involution after 28 days. Events occurring prior to 28 days, where the interval was only 1 to 2 weeks, were treated as right censored (ie, assumed to have occurred at the end of the observation window). Censoring of data in this manner for analysis is standard practice in statistical analysis.113
Retinal detachments were characterized in the analysis as either present or absent, with no attempt made to distinguish severity of detachment. This was necessary because vitreoretinal surgical intervention was frequently performed in the early phases of detachment, limiting further analysis of the natural history of retinal detachment after laser treatment. The only exception to this was classification of retinal detachments at the time of initial discovery with regard to macular involvement. The probability of retinal detachment over time was studied using the Kaplan-Meier estimator.
Univariate and multivariate logistic regression models were constructed to explore the relationship between retinal detachment and several risk factors, including zone of threshold ROP, number of clock hours of stage 3 disease at threshold, plus disease activity after 21 days, stage 3 disease activity after 21 days, clinically important vitreous hemorrhage, and clinically important vitreous organization. The relative risk (computed as the odds ratio) of retinal detachment associated with each of the proposed risk factors was calculated.
TIME-EVENT ANALYSIS
Diagrams were constructed to visually represent the timing of key events during the process of involution in eyes that suffered a retinal detachment. These time-event diagrams were designed to depict the time between treatment and documentation of the following events: (1) full involution of stage 3 disease, (2) full involution of plus disease, (3) detection of clinically important vitreous organization, (4) detection of clinically important vitreous hemorrhage, and (5) detection of retinal detachment.
DECISION ANALYSIS
Decision analysis was performed using Data 4.0 (TreeAge Software, Inc, Williamstown, Massachusetts) in eyes having surgically manageable involutional risk factors detected prior to retinal detachment. Models were constructed to explore the expected utility of preemptive lens-sparing vitrectomy versus observation with vitrectomy performed after a retinal detachment developed. Utility is an attribute that is felt to motivate individuals to choose among various options and is usually quantified on a scale between “0” and “1” with “1” indicating the optimal outcome. Expected utility is calculated by multiplying an outcome utility by its probability of occurrence. Decision analysis rests upon the principle that the plan of action that results in the highest expected utility (ie, optimal for the individual) will be the preferred choice. 109
In the absence of optic nerve and central nervous system abnormalities, health of the macula is the most important factor in determining the visual prognosis of eyes with ROP. The status of the macula was used for possible outcomes in the model, and payoffs were assigned to the following four possible macular outcomes and associated factors: (1) macula remained attached following vitrectomy, (2) macula remained attached following vitrectomy but lensectomy required, (3) macula remained attached and vitrectomy not required, and (4) macula detached.
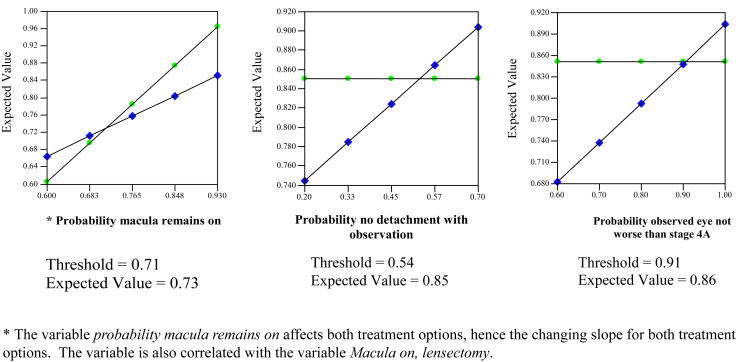
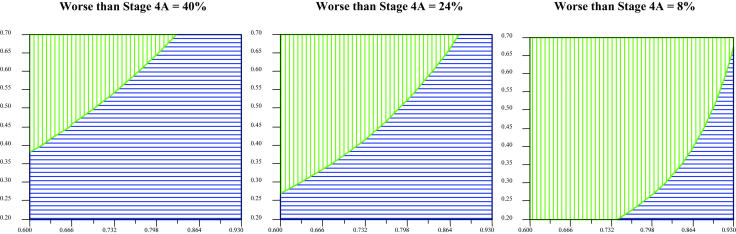
The purpose of decision analysis was to determine if preemptive vitrectomy had potential utility in preventing retinal detachments based on the findings of this study and findings reported in the published literature. It is important to recognize that decision analysis will not establish a treatment as beneficial but can provide initial evidence supporting a particular course of action. Decision analysis begins with construction of a decision tree. A decision tree is a diagram that outlines a clinical decision and plausible outcomes of that decision. A decision tree begins with a root decision that represents the active choice to be made by the patient and/or physician. Points of uncertainty are represented by a chance node (round), with branches of each chance node indicating possible health states. Each health state is associated with a probability of occurrence. Each branch ends on a terminal node (triangle), which represents a final outcome. One-way and three-way sensitivity analyses were performed over a range of plausible values to examine uncertainty in the model. A sensitivity analysis involves altering one or more of the variables in the decision tree to determine the threshold values for choosing one decision pathway over another. In other words, the calculations are repeated using multiple different variables.
The chance nodes used in this analysis represent the following points of uncertainty: (1) retinal detachment versus no retinal detachment with observation alone, (2) severity of retinal detachment developing in eyes undergoing observation, and (3) anatomical surgical outcome of lens-sparing vitrectomy for preemptive surgery and for surgery performed on stage 4A detachments. A utility related to macular status was assigned to each terminal node. Maintenance of macula and lens integrity was considered the optimal outcome, and a utility of “1” was assigned to eyes maintaining this status. A utility of “0.5” was assigned to eyes in which the macula remained attached but lensectomy was required or a cataract developed, and “0” was assigned to eyes in which the macula was detached, regardless of lens integrity. These utilities were chosen because maintenance of macular and lens integrity is generally associated with the best outcome, hence a score of 1. Detachment of the macula, on the other hand, is typically associated with light perception to count fingers vision at best, and this was scored as a payoff of “0” in the model. Macular integrity with loss of the lens is associated with useful but usually subnormal vision in the range of 20/200 or worse, a value considered 50% of normal according to the American Medical Association,114 hence a payoff score of 0.5. The model uses a conservative approach assuming that the anatomical outcomes and complications with early surgery will be the same as those associated with deferred surgery. In reality, intervention prior to development of more extensive vitreous membranes may be associated with better results.115 Alteration of the value of outcome possibilities in the model may alter the calculated expected utility but will not alter the relative difference between the calculated utilities.
Decision analysis often requires utilization of assumptions in the construction of a model. Assumptions were made based on best estimates and extrapolation from similar, but not identical, situations where published data were unavailable. Sensitivity analysis is used to challenge the assumptions used by recalculating the expected utilities using a variety of different parameters. The assumptions used were as follows:
Because the rate of retinal detachment was the same between right and left eyes, a single decision tree was constructed.
The success rate and complications profile of preemptive lens-sparing vitrectomy will be similar to that for lens-sparing vitrectomy of stage 4A retinal detachments, as reported in the literature during the past 3 years.104–106,107 More advanced disease is known to be associated with poorer outcomes. Thus, this assumption is considered to be conservative, and preemptive treatment may actually be associated with greater success and fewer complications.
Twenty percent of eyes with surgically manageable retinal detachment risk factors will progress beyond stage 4A during observation. This estimate is based on the number of eyes in this study that had macular-involving retinal detachments (nine of 38 eyes [23.6%]) upon initial detection of the retinal detachment. This may be a conservative estimate compared with published data on outcomes for partial retinal detachments.100
The rate of cataract development or need for lensectomy will not exceed 3%, on the basis of recent surgical complication estimates (M. T. Trese, MD, written communication, December 22, 2003).
Lens-sparing vitrectomy for stage 4A retinal detachments is beneficial and is preferable to surgery at more advanced stages of detachment.
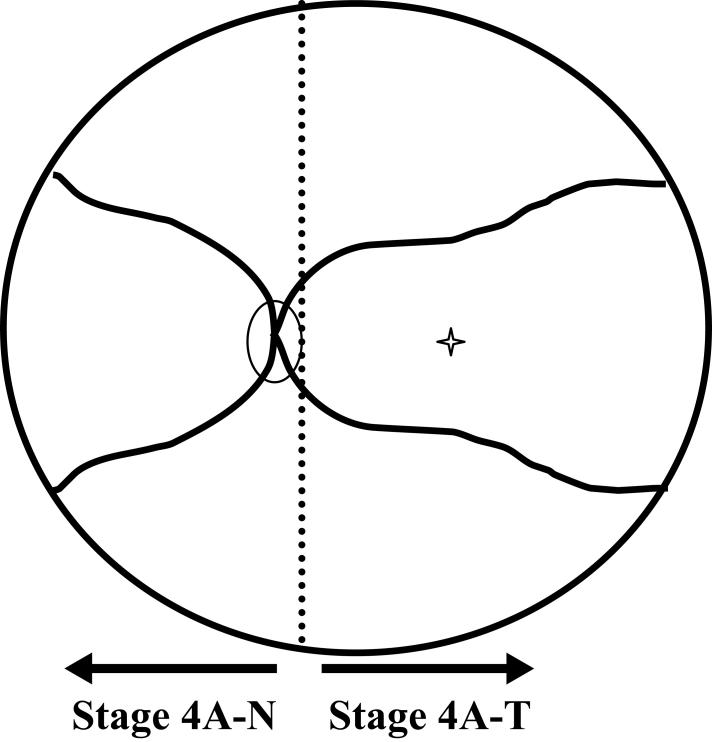
CLASSIFICATION OF LATE-STAGE ROP
In the process of conducting this study, it became apparent that the classification system for description of the late stages of ROP was insufficient for fully characterizing events during involution. No classification system has been accepted to address the vitreous component of ROP, and the nomenclature for ROP retinal detachments is too generalized to allow optimal comparison of data and patients from various studies. Thus, a system to account for the vitreous component of ROP was devised, and nomenclature to further stratify stage 4A retinal detachments was developed. This classification system was devised after data collection and analysis and was not used to evaluate outcomes in this study. It has been proposed to facilitate future study of ROP outcomes.
RESULTS
At total of 138 infants underwent transpupillary diode laser photocoagulation for threshold retinopathy in 130 right and 132 left eyes (Table 1). Threshold ROP was diagnosed at a mean postconceptional age of 35.8 (range, 31 to 44) weeks. Seventy-four (54%) of 138 infants were males and 58 (42%) of 138 were of a race other than Caucasian. The mean estimated gestational age was 25.5 weeks (range, 22 to 36) and the mean birth weight was 760 gm (range, 300 to 1,500). Fourteen infants (10%) had unilateral threshold disease. Ten (7.7%) of 130 right eyes and 10 (7.5%) of 132 left eyes had zone I threshold disease. Seventy-eight (60%) of 130 right eyes and 85 (64%) of 132 left eyes had more than five sectors (clock hours) of stage 3 neovascular disease at the time of threshold diagnosis. Diode laser photocoagulation was performed using an interrupted mode or a near-continuous mode (Table 2). Previous study has demonstrated no difference in outcomes or complications with these two treatment patterns (unpublished data).
TABLE 1.
DEMOGRAPHIC AND BASELINE CHARACTERISTICS OF INFANTS AND EYES TREATED FOR THRESHOLD ROP
| CHARACTERISTIC | VALUE |
|---|---|
| Male | 74 (54%) |
| Nonwhite race | 58 (42%) |
| Estimated gestational age, mean ± SD (range), weeks | 25.5 ± 1.9 (22 to 36) |
| Birth weight, mean ± SD (range), grams, | 760 ± 216 (300 to 1,500) |
| Zone I threshold | |
| Right eyes | 10 (7.7%) |
| Left eyes | 10 (7.5%) |
| >5 clock hours stage 3 disease | |
| Right eyes | 78 (60%) |
| Left eyes | 85 (64%) |
TABLE 2.
LASER POWER AND APPLICATION WITH TWO TREATMENT PATTERNS
| TREATMENT | RIGHT EYES, MEAN (RANGE) | LEFT EYES, MEAN (RANGE) |
|---|---|---|
| Interrupted | ||
| Power (mW) | 300 (185 to 800) | 294 (165 to 450) |
| Duration (msec) | 311 (300 to 500) | 309 (300 to 500) |
| Number of applications | 1,514 (531 to 2,600) | 1,464 (565 to 2,900) |
| Near continuous | ||
| Power (mW) | 253 (165 to 600) | 257 (130 to 600) |
| Duration (msec) | 9,000 | 9,000 |
| Number of applications | 107 (40 to 300) | 106 (45 to 519) |
INVOLUTION AFTER TREATMENT
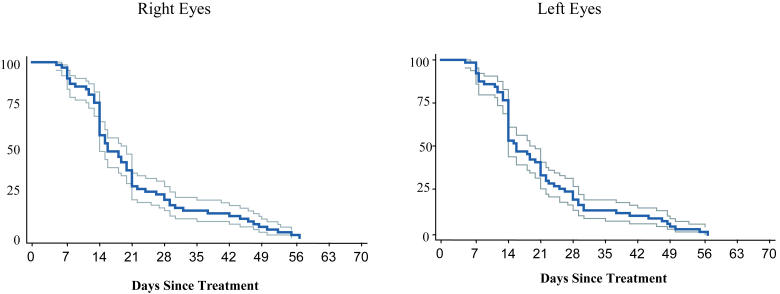
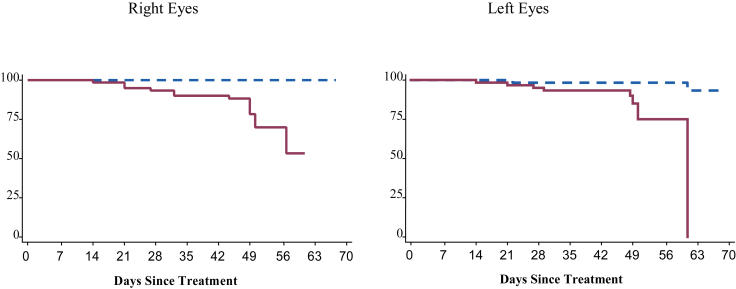
Information on involution after treatment has previously been published.116 Eyes were considered to still have active acute-phase ROP if they had any persistent stage 3 neovascularization and/or elements of plus disease. Development of a retinal detachment was tabulated as a fully involuted eye in this analysis, and retinal detachments were subsequently analyzed separately in a subgroup analysis. Figure 2 and Table 3 summarize the probability of complete ROP involution over time. The majority of eyes demonstrated rapid and complete involution of acute-phase ROP during the first 21 days after treatment. Within 14 days of treatment, only 58% of right eyes and 53% of left eyes had active acute-phase disease. By 21 days following treatment, only 29% of right eyes and 33% of left eyes had active disease, and by 28 days after treatment, only 22% of right eyes and 20% of left eyes continued to have active acute-phase ROP. All eyes had fully involuted or developed a retinal detachment by 9 ± 3 weeks after treatment. The Kaplan-Meier estimator method may have overestimated the proportion of eyes that continued to have active ROP, especially after 28 days, for two reasons: (1) eyes with vitreous hemorrhage obscuring visualization of the retina were considered to have active ROP until involution was confirmed upon clearing of the hemorrhage or a retinal detachment was diagnosed, and (2) only one examination was considered beyond 28 days after treatment. As mentioned earlier, the timing of events in this interval was assumed to occur midway between 28 days and the outcome examination.
FIGURE 2.
Full involution: Probability of full ROP involution over time with 95% confidence intervals (Kaplan-Meier estimator).
TABLE 3.
PROPORTION OF EYES WITH ACTIVE ACUTE-PHASE ROP (RESIDUAL ELEMENTS OF PLUS DISEASE AND/OR STAGE 3 DISEASE) AT WEEKLY INTERVALS FOLLOWING LASTER TREATMENT FOR THRESHOLD ROP
| DAYS SINCE TREATMENT | RIGHT EYES, % ACTIVE | LEFT EYES, % ACTIVE |
|---|---|---|
| 7 | 92 | 92 |
| 14 | 58 | 53 |
| 21 | 29 | 33 |
| 28 | 22 | 20 |
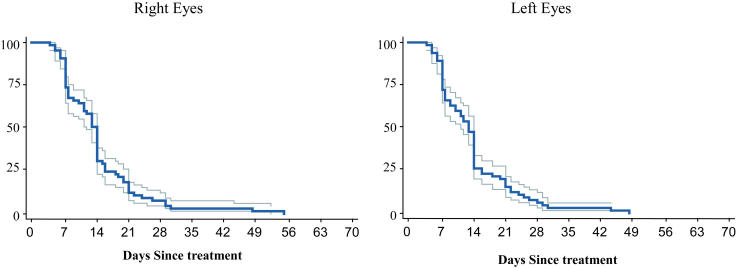
Plus disease is represented for right and left eyes in Figure 3 and Table 4. There was a sharp decline in the number of eyes with elements of persistent plus disease beginning in the first week and proceeding for the first 21 days following treatment. At 14 days following treatment, only 31% of right eyes and 26% of left eyes still had elements of persistent plus disease, and by 21 days only 12% of right eyes and 14% of left eyes demonstrated continued activity of plus disease. By 28 days after treatment, fewer than 10% of right and left eyes had persistent plus disease elements.
FIGURE 3.
Involution of plus disease: Probability of full involution of all elements of plus disease over time with 95% confidence intervals (Kaplan-Meyer estimator).
TABLE 4.
PROPORTION OF EYES WITH PERSISTENT ELEMENTS OF PLUS DISEASE AT WEEKLY INTERVALS FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| DAYS SINCE TREATMENT | RIGHT EYES, % ACTIVE | LEFT EYES, % ACTIVE |
|---|---|---|
| 7 | 73 | 72 |
| 14 | 31 | 26 |
| 21 | 12 | 14 |
| 28 | 7 | 6 |
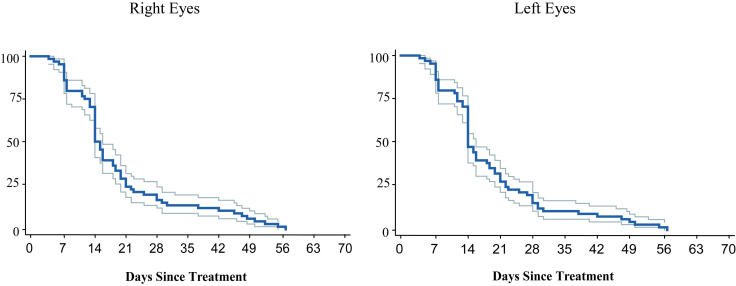
Resolution of stage 3 extraretinal fibrovascular proliferation is represented in Figure 4 and Table 5. There was a sharp decline in the number of eyes with stage 3 neovascular activity beginning in the first week after treatment. By 14 days after treatment, only 50% of right eyes and 47% of left eyes still had active stage 3 disease, and by 21 days only 28% of right eyes and 28% of left eyes demonstrated continued activity. By 28 days after treatment, fewer than 20% of right and left eyes continued to demonstrate active stage 3 disease. In general, stage 3 disease was slower to involute than plus disease.
FIGURE 4.
Involution of stage 3 disease: Probability of full stage 3 disease involution over time with 95% confidence intervals (Kaplan-Meier estimator).
TABLE 5.
PROPORTION OF EYES WITH ACTIVE STAGE 3 DISEASE AT WEEKLY INTERVALS FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| DAYS SINCE TREATMENT | RIGHT EYES, % ACTIVE | LEFT EYES, % ACTIVE |
|---|---|---|
| 7 | 85 | 85 |
| 14 | 50 | 47 |
| 21 | 28 | 28 |
| 28 | 17 | 15 |
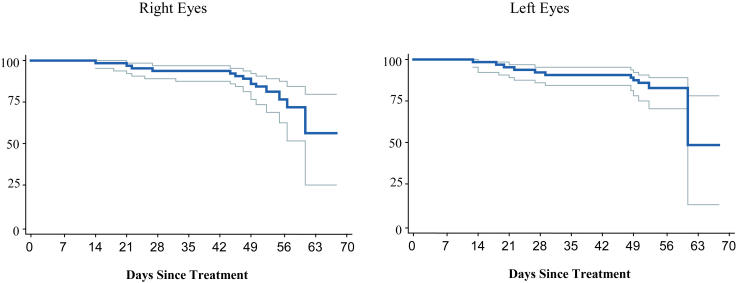
RETINAL DETACHMENT
Overall, 18 (13.8%) of 130 right eyes and 18 (13.6%) of 132 left eyes developed a retinal detachment. Fourteen (70%) of 20 eyes with zone I threshold disease and 32 (13%) of 242 eyes with zone II threshold disease developed a retinal detachment. Seven infants with zone I threshold disease developed retinal detachments, all of which were bilateral. Seventeen infants with zone II threshold disease developed retinal detachments. The detachment was bilateral in five (29%) of these 17 infants (Table 6). Figure 5 and Table 7 highlight the timing of retinal detachments. Notably, no retinal detachments were diagnosed in the first 7 days following treatment. By 14 days, only one (5%) of 18 right eye detachments and three (17%) of 18 left eye detachments had developed. During the third week after treatment, three additional right eyes (16%) and four additional left eyes (22%) developed a retinal detachment. A similar number occurred in the fourth week. Interestingly, a large proportion of retinal detachments did not develop until well after treatment: 11 (61%) of 18 right eyes and eight (44%) of 18 left eyes were not diagnosed with a retinal detachment until more than 28 days after treatment.
TABLE 6.
LATERALITY OF RETINAL DETACHMENTS FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
|
RETINAL DETACHMENT |
||
|---|---|---|
| ZONE | UNILATERAL | BILATERAL |
| I | 0 | 7 |
| II | 12 | 5 |
FIGURE 5.
Retinal detachment: Probability of retinal detachment over time with 95% confidence intervals (Kaplan-Meier estimator).
TABLE 7.
NUMBER OF RETINAL DETACHMENTS OCCURRING AT WEEKLY INTERVALS AFTER LASER TREATMENT FOR THRESHOLD ROP
| DAYS SINCE TREATMENT | RIGHT EYES | LEFT EYES |
|---|---|---|
| 0 to 7 | 0 | 0 |
| 8 to 14 | 1 | 3 |
| 15 to 21 | 3 | 4 |
| 22 to 28 | 3 | 3 |
| ≥29 days | 11 | 8 |
| TOTAL | 18 | 18 |
Univariate and multivariant logistic regression models were constructed to identify factors associated with development of a retinal detachment (Tables 8 and 9). In right eyes, two factors—active elements of plus disease and active stage 3 disease beyond 21 days after treatment—were not statistically associated with retinal detachment in either the univariate or multivariate model. The presence of more than 5 clock hours of stage 3 disease at threshold diagnosis was significantly associated with development of a retinal detachment in the univariate model but not in the multivariate model. Stratified analysis indicated that the relationship (association) between presence of stage 3 disease beyond 21 days after treatment and retinal detachment differed depending on the status of the zone I variable. To account for this, an interaction term for stage 3 disease and zone I was included in the multivariate logistic regression models. It is suspected that the interaction between these variables is due to a sampling anomaly because of the very small number of eyes with this covariate pattern. In left eyes, none of these factors were statistically associated with retinal detachment in either model.
TABLE 8.
UNIVARIATE LOGISTIC REGRESSION MODELS OF POTENTIAL RISK FACTORS ASSOCIATED WITH RETINAL DETACHMENT FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| RIGHT EYES | LEFT EYES | |||
|---|---|---|---|---|
| OR (95% CI) | PVALUE | OR (95% CI) | PVALUE | |
| RISK FACTOR | ||||
| Clinically important vitreous organization | 17.98 (5.60, 57.78) | <.0001 | 13.13 (4.22, 40.78) | <.0001 |
| Clinically important vitreous hemorrhage | 18.17 (4.02, 82.13) | <.0001 | 28.25 (5.12, 155.78) | <.0001 |
| Zone I threshold | 23.12 (5.22, 102.37) | <.0001 | 23.76 (5.37, 105.15) | <.0001 |
| Active stage 3 disease beyond 21 days | 3.38 (0.571, 19.95) | .180 | 2.27 (0.42, 12.23) | .34 |
| Active elements of plus disease beyond 21 days | 3.24 (0.278, 37.65) | .348 | 2.75 (0.49, 15.38) | .25 |
| >5 Clock hours stage 3 disease | 3.75 (1.03, 13.69) | .045 | 2.01 (0.62, 6.52) | .24 |
OR = odds ratio.
TABLE 9.
MULTIVARIATE LOGISTIC REGRESSION MODEL OF POTENTIAL RISK FACTORS ASSOCIATED WITH RETINAL DETACHMENT FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| RIGHT EYES | LEFT EYES | |||
|---|---|---|---|---|
| OR (95% CI) | PVALUE | OR (95% CI) | PVALUE | |
| RISK FACTOR | ||||
| Clinically important vitreous organization | 31.41 (5.37, 183.63) | <.0001 | 13.18 (2.97, 58.59) | <.0001 |
| Clinically important vitreous hemorrhage | 38.49 (2.69, 551.19) | .007 | 15.41 (1.65, 144.12) | .02 |
| Zone I threshold | 80.55 (7.82, 829.63) | <.0001 | 45.71 (5.45, 383.37) | <.0001 |
| Active stage 3 disease beyond 21 days | 1.41 (0.003, 622.55) | .91 | 0.54 (0.01, 27.15) | .76 |
| Active elements of plus disease beyond 21 days | 11.14 (0.02, 5337.68) | .45 | 7.58 (0.35, 165.80) | .20 |
| >5 Clock hours stage 3 disease | 7.39 (0.898, 60.91) | .06 | 1.55 (0.30, 7.94) | .60 |
| Zone I threshold and active stage 3 beyond 21 days* | 0.25 (.000011, 52.61) | .34 | 0.33 (0.001, 86.96) | .70 |
OR = odds ratio.
This interaction term was included to account for an interaction that was felt to be an anomaly based on the small number of eyes with this covariate pattern.
Clinically important vitreous organization was found to be an important factor associated with eventual development of a retinal detachment. In the multivariate logistic regression model, vitreous organization increased the odds of developing a retinal detachment by a factor of 31 (5.37 to 183.63; P < .0001) for right eyes and 13 (2.97 to 58.59; P < .0001) for left eyes. Figure 6 demonstrates the probability of retinal detachment over time in right and left eyes with and without clinically important vitreous organization. Overall, 90 (34%) of 262 eyes developed some degree of vitreous organization (Table 10). Sixty-four (24%) of 262 eyes had vitreous organization that was less severe than the definition of clinically important vitreous organization.
FIGURE 6.
Retinal detachment and vitreous organization: Probability of retinal detachment in eyes with and without clinically important vitreous organization over time (Kaplan-Meier estimator). Model adjusted for zone I disease, number of clock hours of stage 3 disease, and residual activity of elements of plus disease and stage 3 disease more than 21 days after treatment. Solid lines represent eyes with clinically important vitreous organization; dashed lines represent eyes without clinically important vitreous organization.
TABLE 10.
RATE OF RETINAL DETACHMENT FOR EYES WITH AND WITHOUT CLINICALLY IMPORTANT VITREOUS ORGANIZATION FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| STATUS OF VITREOUS ORGANIZATION | N | RETINAL DETACHMENT | NO RETINAL DETACHMENT |
|---|---|---|---|
| Clinically important | 26 | 17 (65%) | 9 (35%) |
| Nonclinically important | 64 | 2 (3%) | 62 (97%) |
| None | 172 | 17 (10%) | 155 (90%) |
Two (3%) of these 64 eyes with non–clinically important vitreous organization developed a retinal detachment, compared with 17 (65%) of 26 eyes with clinically important vitreous organization that developed a retinal detachment (odds ratio = 58.6 [10.4 to 559.3], P < .00001). Retinal detachment occurred in 17 (10%) of 172 eyes without any clinically obvious vitreous organization. Clinically important vitreous organization remained a significant risk factor when compared to the odds of detachment in the remaining 236 eyes without clinically important vitreous organization (odds ratio = 21.57 [7.71 to 61.62], P < .0001). The majority of eyes with clinically important vitreous organization were not diagnosed with a retinal detachment until 7 or more days after detection of clinically important vitreous organization, with many occurring several weeks later, indicating that retinal detachments occurring in association with vitreous organization often evolve slowly. This slow process of retinal detachment suggests existence of a potential window of opportunity for preemptive intervention.
Clinically important vitreous hemorrhage was also found to be associated with development of retinal detachment (Tables 8 and 9). Twelve (75%) of 16 eyes that experienced clinically important vitreous hemorrhage developed a retinal detachment. In the multivariate logistic regression model, clinically important vitreous hemorrhage increased the odds of developing a retinal detachment by a factor of 38 (2.69 to 551.19; P = .007) and 15 (1.65 to 144.12; P = .02) in right and left eyes, respectively. Because clinically important vitreous hemorrhage obscures visualization of the retina, it was not possible to determine if retinal detachment developed concurrent with, before, or after vitreous hemorrhage.
Zone I threshold disease was confirmed to be an independent risk factor for development of a retinal detachment (Tables 8 and 9). The presence of threshold disease in zone I increased the odds of developing a retinal detachment by a factor of 81 (7.82 to 829.63; P < .0001) and 46 (5.45 to 383.37; P < .0001) for right and left eyes, respectively, in the multivariate logistic regression model.
TIME-EVENT ANALYSIS
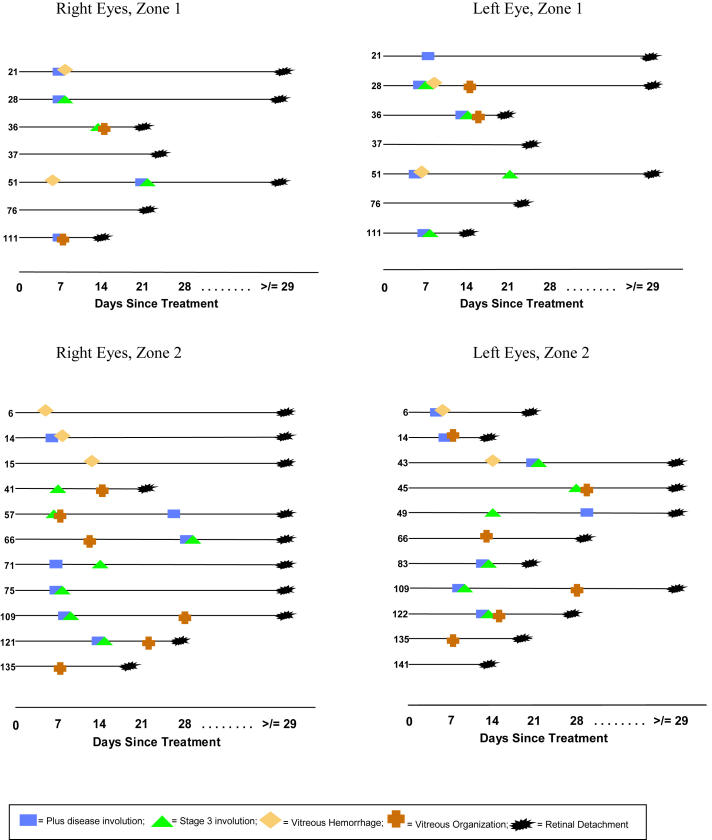
As mentioned, time-event diagrams were created to depict clinically identifiable key features that occurred or failed to occur during the process of involution. This evaluation was designed to demonstrate the time course of involution in eyes that ultimately developed a retinal detachment in a format that allowed analysis of the documentation of important events relative to each other. Events plotted included resolution of plus disease, resolution of stage 3 disease, detection of clinically important vitreous hemorrhage, detection of clinically important vitreous organization, and detection of retinal detachment.
Zone I and zone II eyes were plotted separately (Figure 7). Among the 14 zone I eyes (seven right eyes and seven left eyes) that developed a retinal detachment, four eyes (29%) developed clinically important vitreous organization prior to diagnosis of a retinal detachment. Four (29%) of 14 developed clinically important vitreous hemorrhage prior to diagnosis of retinal detachment. One eye (tabulated above) developed both vitreous organization and vitreous hemorrhage. Among eyes that did not develop severe vitreous hemorrhage or clinically important vitreous organization, acute-phase ROP had fully involuted in two (14%) of the remaining zone I threshold eyes prior to retinal detachment but had not fully involuted in the remaining five zone I eyes (36%) prior to retinal detachment.
FIGURE 7.
Time-event profile: Time-event diagrams for eyes developing a retinal detachment. Key events documented during involution denoted by unique symbols. Numbers on y-axis are patient numbers.
Among the 22 zone II eyes (11 right and 11 left eyes) that developed a retinal detachment, 12 (55%) of 22 developed clinically important vitreous organization prior to diagnosis of a retinal detachment (Figure 7). Five (23%) of 22 developed clinically important vitreous hemorrhage prior to retinal detachment diagnosis. Of the remaining zone II eyes that did not develop clinically important vitreous hemorrhage or clinically important vitreous organization, acute-phase ROP had fully involuted in four (18%) of 22 eyes, but had not fully involuted in one (5%) of 22 eyes prior to development of retinal detachment.
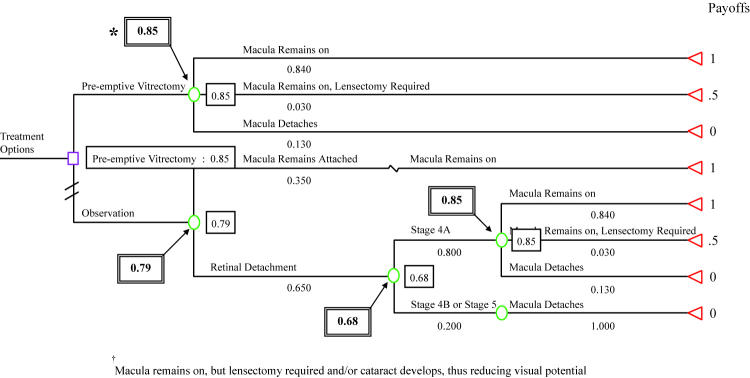
DECISION ANALYSIS
Decision analysis was performed to compare preemptive lens-sparing vitrectomy with vitrectomy deferred until retinal detachment developed in eyes with clinically important vitreous organization. Vitreous organization was known to occur prior to development of a retinal detachment in affected eyes and is a clinically distinct entity that can be surgically manipulated. The timing of vitreous hemorrhage relative to retinal detachment is not known, making assessment of the potential value of preemptive surgical intervention for clinically important vitreous hemorrhage with decision analysis problematic.
Decision analysis was performed using macular status as the final outcome state. The model revealed a slightly higher expected utility for preemptive lens-sparing vitrectomy compared with deferral of vitrectomy until retinal detachment occurred (Figure 8). The assumptions used in the model included a 35% probability of not developing a retinal detachment in eyes with clinically important vitreous organization (the nondetachment rate in eyes with clinically important vitreous organization in this study), an 80% chance that observed eyes would not progress beyond stage 4A while under observation (the proportion of eyes in this study with a retinal detachment less than stage 4B at initial detection), and an 84% chance of maintaining macular attachment and lens integrity following preemptive surgery and surgery for stage 4A detachments. This final variable represents the lowest reported success rate for stage 4A detachment repair in the past 3 years.107 Three percent of eyes were estimated to require a lensectomy, whereas 13% were estimated to progress despite intervention, as outlined under assumptions in the “Methods” section. Using this approach, the expected utility for preemptive vitrectomy was calculated to be 0.85, compared with 0.79 for deferred vitrectomy, with a utility of 1 representing the best possible outcome of maintaining macular and lens integrity. For example, in the “preemptive vitrectomy” branch, the value of 0.85 was calculated as follows: 1(0.84) + 0.5(0.03) + 0(0.013) = 0.85. For eyes that develop a retinal detachment while under observation, the expected utility with attempted repair was calculated as 0.68, compared with 0.85 for those in the preemptive surgery group.
FIGURE 8.
Decision analysis: What is the best treatment option for an eye with clinically important vitreous organization following treatment of threshold ROP? Star represents greatest expected utility; boxes represent expected utility at each decision node.
Sensitivity analysis was performed because decision analysis relies on probability and outcome values that could change depending upon the individual patient and physician and because assumptions must be made where data are not available. A sensitivity analysis involves altering one or more of the variables in the decision tree to determine the threshold values for choosing one decision pathway over another. One-way sensitivity analysis is presented in Figure 9. During one-way sensitivity analysis, only one variable was adjusted at a time to determine the threshold at which the expected value of the two treatment alternatives became equivalent. Preemptive lens-sparing vitrectomy continued to have a higher expected utility until the probability of the macula remaining on after surgery was reduced to 0.71 (expected utility = 0.73) from the original model of 0.84. This variable was adjusted concurrently for both preemptive vitrectomy and vitrectomy for stage 4A retinal detachments. Changes in this variable were correlated with the outcome macula remains on, lensectomy variable, to reflect changes in the number of eyes likely to require lensectomy or to develop a cataract following surgery as the success rate for vitrectomy was adjusted in the sensitivity analysis. One-way sensitivity analysis on probability of no retinal detachment occurring during observation demonstrated that preemptive lens-sparing vitrectomy continued to have a higher expected utility until the probability of not developing a detachment in an eye with clinically important vitreous organization was increased to 0.54 (expected utility = 0.85) from the original model of 0.35. Preemptive lens-sparing vitrectomy remained optimal in one-way sensitivity analysis on probability that an observed eye would not progress beyond stage 4A until the probability of not progressing increased to 0.91 (expected utility = 0.85) from the original model of 0.80.
FIGURE 9.
One-way sensitivity analysis: Comparison of expected utility of preemptive vitrectomy versus observation with vitrectomy only if retinal detachment occurs, over a wide range of plausible values for each variable: probability that macula remains on (left); probability that retinal detachment does not occur during observation (middle); probability that observed retinal detachment does not progress beyond stage 4A (right). x-axis = analyzed variable; y-axis = expected value; circles = preemptive vitrectomy; diamonds = observation. Intersection represents threshold at which surgery and observation have equal expected value.
Three-way sensitivity analysis is presented in Figure 10. In this analysis, all three variables were adjusted simultaneously. Threshold values cannot be determined in a three-way sensitivity model. Instead, results are presented as an animated region graph with one variable each on the x-axis and y-axis and the third variable modified within a designated range, with intervals presented as sequential graphs. The utility of one treatment option over another can be determined within the range of variables modeled by examination of the Figures. Only when the risk of progressing beyond stage 4A with observation was reduced to 8% or less did the greater expected value of preemptive surgery become critically challenged within the range of plausible values modeled for the other two variables. The probability that the macula remains on following surgery had the greatest impact on the model.
FIGURE 10.
Three-way sensitivity analysis: Probability macula remains on with preemptive vitrectomy (x-axis), probability that retinal detachment does not occur with observation (y-axis), and probability that observed detachment progresses beyond stage 4A (left 40%, center 24%, right 8%). Vertical lines favor observation; horizontal lines favor preemptive lens-sparing vitrectomy..
CLASSIFICATION OF LATE-STAGE ROP
ICROP53,55 does not reflect the important contribution of the vitreous to the pathogenesis of retinal detachments. Other than to note the presence of vitreous membranes, ICROP does not include vitreous features as part of the classification system for advanced disease. As such, formal means to describe and compare the vitreous features of late-stage ROP are unavailable.
Additionally, ICROP does not sufficiently stratify partial retinal detachments. For example, stage 4A nasal retinal detachments are considered by some surgeons to have a more favorable prognosis than stage 4A temporal retinal detachments, because of the latter’s proximity to the fovea. Therefore, modifications and additions to the current nomenclature were developed to remedy these deficiencies. This system, devised after data collection and analysis, was not used for analysis of risk in this study, but rather was devised in response to the findings of this study in an attempt to formalize enhanced classification of late-stage ROP.
Vitreous Status
In the proposed classification scheme, the vitreous component of the disease is classified on the basis of the location, extent, and severity of vitreous involvement. Location and extent are classified in exactly the same manner as recommended in ICROP for acute-phase disease.53 In this scheme, the zone of vitreous organization is classified by the location of the vascular/avascular junction, even if vitreous organization extends anteriorly or posteriorly, because the most tightly adherent vitreoretinal adhesions are thought to be present at or near the vascular/avascular junction.
The severity of vitreous organization appears to have prognostic significance and is divided into three grades as outlined in Table 11 and demonstrated in Figure 1. The term “grade” was chosen to avoid confusion with the stages of acute-phase disease. Grade 0 is used to indicate the absence of clinically obvious vitreous organization, recognizing the possibility that significant subclinical vitreous abnormalities might be present, as highlighted by the development of a retinal detachment in some eyes without preceding development of clinically important vitreous organization. Grade 1 is characterized as minimal to mild vitreous organization. Grade 2 is characterized as moderate to severe vitreous organization. Clinically, grade 2 vitreous organization is severe enough to significantly or completely obscure visualization of the underlying retina. The extent of vitreous organization is defined as the number of sectors (clock hours) of the highest grade of organization seen. Thus, an eye treated for zone I threshold disease noted to have moderate vitreous organization in three sectors would be classified Vitreous Status: Zone I, Grade 2, Extent 3, allowing better comparison between patients and studies.
TABLE 11.
CLASSIFICATION SYSTEM FOR CLINICAL SEVERITY OF VITREOUS ORGANIZATION FOLLOWING LASER TREATMENT FOR THRESHOLD ROP
| GRADE | SEVERITY OF VITREOUS ORGANIZATION |
|---|---|
| Grade 0 | None visible |
| Grade 1 | Minimal to mild (view of underlying retina not significantly reduced) |
| Grade 2 | Moderate to marked (view of underlying retina significantly reduced or obscured) |
FIGURE 1A.
Grade 0 (no visible organization).
FIGURE 1B.
Grade 1, mild vitreous organization
FIGURE 1C.
Grade 1, mild vitreous organization. Courtesy of Cynthia Toth, MD.
FIGURE 1D.
Grade 2, moderate vitreous organization.
FIGURE 1E.
Grade 2, severe vitreous organization. Courtesy of Cynthia Toth, MD.
FIGURE 1F.
Grade 2, severe vitreous organization.
FIGURE 1G.
Severe vitreous organization in association with retinal detachment. Courtesy of Cynthia Toth, MD.
Partial Retinal Detachments
Modifications were also developed to expand the nomenclature for stage 4A retinal detachments. These modifications recognize that threat to (and even unrecognized subclinical involvement of) the fovea is more likely to occur with a temporal retinal detachment than a nasal detachment and thus could influence timing and choice of intervention as well as final outcome. Nasal and temporal 4A retinal detachments are referred to simply as Stage 4A-N and Stage 4A-T disease, respectively. The optic disc is used as the dividing line for this designation, with detachments extending temporal to a vertical line extrapolated from the temporal edge of the optic disc classified as stage 4A-T (Figure 11). Detachments involving both the nasal and temporal aspects of the retina are classified as stage 4A-T to recognize the greater potential threat to vision of the temporal component of the detachment. The zone of the detachment is designated on the basis of the zone at treatment, and the number of detached segments is designated similarly to methods previously reported,100 except that only the area of vascularized retina is considered when tabulating the number of detached segments. Briefly, zone I and zone II contain one sector for each of 12 clock hours, and zone III contains one sector for each of 10 clock hours. A zone I eye with a nasal retinal detachment involving three sectors would be classified Retinal Detachment: Zone I, Stage 4A-N, 3 sectors.
FIGURE 11.
Classification of stage 4A retinal detachments: Designation of a stage 4A retinal detachment as stage 4A-N (nasal) or stage 4A-T (temporal) relative to the temporal border of the optic disc.
DISCUSSION
ROP remains a leading cause of severe visual impairment and blindness in industrialized nations, including the United States.46 It has been estimated that 9,000 surviving children in upper-income countries and more than 29,000 surviving children in middle-income countries are presently blind because of ROP.1 Already an important public health problem, the disease is gaining increasing importance as technology continues to push the lower limit of survival for premature infants. Infants as young as 22 weeks estimated gestational age may now survive.39
Since the original description of ROP,2 extensive basic and clinical research efforts have been directed toward better understanding of the pathogenesis of ROP. Many preventive and interdictive treatments have been attempted. Patz and coworkers8 and Kinsey and coworkers9 demonstrated that excessive supplemental oxygen did indeed result in a higher rate and severity of ROP, as suggested by Campbell,7 prompting exploration of oxygen manipulation to reduce the incidence of the disease. Studies on the antioxidant effects of vitamin E and D-penicillamine have demonstrated varying degrees of effectiveness56–59 but have failed to gain wide acceptance because of concerns about the potential for adverse side effects of these drugs. Supplemental oxygen at onset of prethreshold disease in an attempt to minimize hypoxia in the avascular retina failed to demonstrate a significant reduction in the rate of threshold development in a large multicenter randomized trial.60 Light is known to increase free radical formation in the retina, and thus the Light Reduction in Retinopathy of Prematurity study was conducted to determine the impact of nursery lighting on ROP.63 Unfortunately, it failed to show a difference in the rate of progression of ROP in infants shielded from nursery lighting.
Inositol supplementation has been shown to markedly reduce the rate of severe ROP development.117,118 Confirmation of this effect in a large randomized controlled trial, however, has not been done. Recently, noncontrolled studies have suggested a role for strict oxygen management in the prevention of ROP. A fourfold reduction in the rate of development of stage 3 and 4 ROP was noted in one center after strict practices to more precisely monitor oxygen administration were implemented in an effort to avoid repeated cycles of hypoxia-hyperoxia.118 Though the study was retrospective and was not conducted on concurrent infant populations, its results are nevertheless intriguing.
PRIMARY TREATMENT
There have been many attempts to mitigate damage caused by the cicatricial phase of ROP. The Cryo-ROP study solidly established the role of ablation of the peripheral avascular retina in the treatment of threshold disease, demonstrating a reduction in unfavorable structural outcomes from 47.4% in untreated control eyes to 25.7% in treated eyes.35 Despite this important advance, no treatment studied to mitigate damage has thus far achieved success for all eyes and all infants. In 1992, Tasman119 asked, “Is 25.7% an acceptable failure rate?” His negative answer was similar to that of most practicing ophthalmologists, and dissatisfaction with the “cure rate” from peripheral ablation resulted in numerous studies designed to improve outcomes. Although not definitively demonstrated in a large randomized study, the weight of evidence strongly suggests that laser photocoagulation is at least as effective as, and possibly superior to, cryotherapy in the treatment of threshold ROP in some settings.72,77,78 The ETROP study, which randomized high-risk eyes to treatment at prethreshold or at threshold, demonstrated a reduction in the rate of unfavorable structural and functional outcomes at 9 months of age for eyes treated at prethreshold. The majority of eyes in the ETROP study were treated with laser photocoagulation. Though the definitions for parameters that qualified an eye as having threshold disease were not identical in the Cryo-ROP and ETROP studies, the improved success rate for threshold treated eyes in the ETROP study compared with the Cryo-ROP study (15.6% versus 25.7%) strongly supports laser as a superior treatment option. Despite improvement with treatment at prethreshold, eyes treated at prethreshold still had a 9.1% unfavorable structural outcome and 14.5% unfavorable functional outcome.82 Clinicians should recognize, however, that each of these studies has only established that one form of treatment at a specific phase of the disease is effective. Little or no information is available on prophylactic treatment by reintervention for eyes in a high-risk predetachment state following laser treatment.
ADVANCED-STAGE ROP
Retinal Detachment Risk Factors
The Cryo-ROP Cooperative Group reported that for each sector of stage 3 neovascularization above five sectors, there was a 26% increase in the likelihood of developing an unfavorable outcome.87 Interestingly, this author did not find an independent association between the number of clock hours of stage 3 disease and retinal detachment. There are several potential explanations for this. First, retinal detachments are relatively uncommon, and the sample size may not have been adequate to demonstrate this association. Second, laser treatment could be associated with more complete ablation of the peripheral avascular retina when compared with cryotherapy. One of the purported advantages of laser treatment over cryotherapy is the ability of the surgeon to more clearly identify treated areas during the laser treatment.74 This could result in a greater number of skip areas for cryothreapy-treated eyes, resulting in progressive disease and less optimal outcomes by inadvertent failure to treat ischemic retina, which could continue to produce vascular growth factors. Even laser treatment has been shown to be associated with skip areas in zone I ROP.78,120 This may be due in part to difficulties associated with treatment of zone I eyes related to media clarity, poor laser uptake (particularly in the avascular retina just anterior to the vascular/avascular junction), and/or the fact that the neovascular shelf can extend over the avascular retina in zone I eyes.120 Finally, laser photocoagulation may simply be more effective than cryotherapy, thus reducing the adverse impact of increasing sectors of stage 3 disease.
It has long been recognized that the vitreous can play a central etiologic role in the development of ROP retinal events. Mintz-Hittner and Kretzer121 reported that remnant myofibroblasts in the vitreous contract 2 to 4 months following cryotherapy for ROP. They characterized central contraction of these elements as similar to tightening a purse string, which ultimately produces a traction retinal detachment.122 Their observations on the contraction of myofibroblasts are certainly consistent with the findings in the present study, in which half of the retinal detachments did not occur until more than 28 days after treatment of threshold disease. Ultrastructural evaluation of membranes removed at the time of vitreoretinal surgery demonstrated that these membranes are adherent to the retina.94,95 The presence of two contiguous clock hours of grade 2 vitreous organization was chosen as clinically important because of the general clinical impression prior to conducting this study that this degree of vitreous organization was commonly associated with severe retinal tractional forces, which were often associated with retinal detachment. This clinical impression was substantiated when 65% of eyes meeting the definition of clinically important vitreous organization were found to have developed a retinal detachment. Lower levels of vitreous organization, though, were also occasionally associated with development of a retinal detachment. Therefore, the degree of vitreous organization that is most likely to be associated with a retinal detachment warrants further study to verify the observations in this study and to more precisely determine which eyes are most at risk. The number of eyes developing a retinal detachment in this study is too small to make further statements in this regard.
Hutcheson and coworkers123 found that vitreous hemorrhage was associated with a high rate of progression to retinal detachment, with seven of eight eyes having vitreous hemorrhage (either before or after treatment) progressing to retinal detachment. In the present study, none of the eyes had significant vitreous hemorrhage prior to treatment. Retinal detachment was diagnosed in 75% of eyes with vitreous hemorrhage severe enough to completely preclude visualization of the retina.
When a vitreous hemorrhage that obscures visualization of the retina is diagnosed, a retinal detachment may then be present or may develop at any point in the future and go undiagnosed. In an experimental model, Ehrenberg and coworkers124 found that after injection of blood into the vitreous, almost all of the rabbit eyes in their testing developed glial membranes on the peripheral retina, resulting in local retinal traction. Thus it is reasonable to believe that vitreous hemorrhage may occur prior to retinal detachment in some, if not most, eyes. Ophthalmologic ultrasound has been shown to be useful for characterizing the configuration of retinal detachments due to ROP.125,126 If ultrasound technology was available and able to identify a low-grade retinal detachment or vitreous organization, interventional strategies might be altered. It can be said with certainty that the development of clinically important vitreous hemorrhage should prompt consultation of a vitreoretinal surgeon at a minimum, but more definitive statements regarding vitreous hemorrhage are not possible at this time. Further study of the timing of retinal detachment relative to onset of vitreous hemorrhage could identify a window of opportunity for preemptive reintervention in eyes with vitreous hemorrhage. This would have impacted 11 (31%) of 36 eyes that developed a retinal detachment in the present study. Overall, clinically important vitreous hemorrhage, clinically important vitreous organization, or both, were noted in 67% of eyes that developed a retinal detachment in the present study, highlighting the importance of addressing this question.
Beyond 21 days after laser treatment, prolonged activity of stage 3 disease and elements of plus disease were not demonstrated to be risk factors for development of a retinal detachment. The small number of eyes with residual activity after 21 days may have been insufficient to demonstrate such an association statistically. It seems intuitive that prolonged acute-phase disease could stimulate increased development of vitreoretinal interface abnormalities that could predispose to a retinal detachment, especially given experimental evidence of fluorescein leakage in human ROP99 and demonstration of an absent blood-retinal barrier in animal ROP models.13 Alternatively, reduction in activity of plus disease and stage 3 disease may be sufficient to reverse these abnormalities and reduce the risk of further disease progression, even if acute-phase disease has not fully involuted. Reduced activity was not evaluated in the present study. Certainly, eyes with prolonged activity of stage 3 disease and elements of plus disease should be observed closely until more data are available.
Retinal Detachment
Although scleral buckling and/or vitrectomy for stage 4B and stage 5 ROP may provide a superior anatomical outcome compared with the natural history of the disease, this approach is associated with dismal visual results.127 Electroretinograms performed after partial or complete surgical retinal reattachment for stage 5 ROP have been uniformly nonrecordable,128 confirming that severe retinal dysfunction persists despite successful anatomic reattachment.
All treatments that have been demonstrated in randomized prospective trials to reduce the risk of unfavorable sequelae suffer from the same problem in the final analysis. No treatment has yet been shown to eliminate the risk of an unfavorable outcome in all infants and all eyes, without causing potentially unwanted side effects. Even the ETROP study demonstrated a structural failure rate of 9.1% with early treatment.82 The question that Tasman119 asked in 1992 should be asked again today: Is a failure rate of 9.1% acceptable? Most would argue that it is not and that further progress is needed. It is unlikely that further attempts to mitigate damage through the isolated use of ablative procedures prior to or at threshold will render additional cures while maintaining a reasonable risk-to-benefit ratio. Given the poor visual outcome associated with repair of advanced retinal detachment, the next series of cures is likely to result from efforts that occur during the process of involution following treatment. This is likely to remain the case until agents to prevent development of severe ROP are discovered or better methods to delay premature birth are identified.
Topilow and coworkers129 noted early on that even if cryotherapy was successful, subsequent vitreous traction to “fibrotic remnants” could lead to development of a traction retinal detachment with cicatricial damage to the macula and severe vision loss. Machemer130 described retinal detachments in ROP as occurring as a result of several factors, including contraction of proliferative tissue at the ridge and proliferation of tissues into the vitreous from the shunt. He noted that detachments typically start in the periphery, later progressing posteriorly.131 Subretinal organization was found to be the cause of surgical failure in up to 10.3% of eyes in which retinal detachment repair was attempted in one study.132
In a study of the natural history of untreated ROP, Flynn and coworkers84 reported that definite signs of cicatricial disease and tractional detachments developed an average of 25 weeks after diagnosis of ROP. These investigators also reported that there were no obvious signs that set these infants apart from infants who did not develop cicatricial disease. In the present study, data on the slow evolution of retinal detachments in treated eyes are comparable in that the majority of eyes did not develop a retinal detachment until 3 or more weeks after laser treatment. In contrast, however, the predisposing feature of clinically important vitreous organization was present in 26 (10%) of 262 eyes in the present study, with 17 (65%) of these 26 eyes developing a retinal detachment, clearly identifying a subgroup of ultra-high-risk eyes. These findings parallel a report by Uemura,27 who described a classification system for the Joint Committee for the Study of Retrolental Fibroplasia in Japan in 1977. He reported two distinctive clinical courses during the natural process of acute-phase ROP in untreated eyes. Group 1 passed through defined stages, many typical of those later adopted by ICROP.53 Leakage and proliferation into the vitreous was the final step toward retinal detachment. Group 2 developed retinal detachment more rapidly and did not pass through these characteristic stages. A similar dichotomy appears to have occurred in the evolution of retinal detachments after treatment in patients in the present study, with almost half of detached eyes showing evolution of clinically important vitreous organization as a precursor to retinal detachment, whereas the remaining detachments occurred without what was believed to be important preceding visible vitreoretinal interface abnormalities.
Partial tractional retinal detachments in ROP are not stable. Gilbert and coworkers100 reported that in the setting of a 4A detachment, the best chance of interrupting the processes that lead to more advanced detachment occurs when a 4A detachment has not progressed beyond three retinal segments. They divided the retina into 34 segments: one segment for each of the 12 clock hours in zone I and II and one segment for each of the 10 clock hours in zone III. Eyes that were randomized in the Cryo-ROP study having a partial retinal detachment at 3 months were followed until 4.5 years of age. An unfavorable outcome was noted in 92% of eyes with 13 or more detached segments, none of which achieved final visual acuity better than 20/200. The number of eyes with only one to three detached segments was small (eight), but four of eight such eyes had vision of light perception or worse at 4.5 years. The message from this study was that most eyes with a partial retinal detachment had poor visual acuity or were blind 4.5 years after treatment, regardless of initial foveal involvement.
Quinn and coworkers103 reported on the prognosis of eyes in the Cryo-ROP study that had developed a total retinal detachment by 3 months after treatment. Approximately half of the eyes had undergone lensectomy and vitrectomy and half had not. Partial or complete retinal reattachment was present at 5.5 years in only 21% of eyes that had undergone lensectomy and vitrectomy. Vision was poor regardless of whether or not vitreoretinal surgery had been performed, with all but one eye having light perception or no light perception vision. The admonition by the Cryo-ROP Cooperative Group that retinal detachment should be prevented remains unchallenged.103
The literature offers no clear scientific guidance as to when reintervention should be considered if high-risk features are seen during the process of involution. There is no substitute for the clinical gestalt that an experienced ophthalmologist develops in understanding the general severity of a particular infant’s ROP for planning future observation and estimating a prognosis. Detailed examination of the individual components that generate this gestalt can improve the clinician’s ability to manage individual patients by clarifying the role of specific findings. Because there is at present no reliable clinical means to predict which eyes will progress to development of a tractional retinal detachment following treatment, infants at risk should be examined frequently after treatment and strategies to prevent impending retinal detachment by reintervention devised and carefully studied. Although causation cannot actually be observed, it can be inferred from observed patterns of disease and event sequences. In the present study, it was observed that an eye that ultimately develops a retinal detachment following treatment of ROP frequently exhibits features that are highly predictive of an adverse outcome, thus presenting a window of opportunity to intervene in many eyes.
Involution
The first step in developing reintervention strategies involves more clearly understanding the process of involution following treatment. The present study has revealed several important features regarding the process of ROP involution. Like acute-phase ROP, involution of ROP after treatment of threshold disease proceeds similarly in fellow eyes. The clinically obvious features of plus disease and stage 3 disease involute rapidly following laser treatment in most eyes. Almost half of treated eyes had fully involuted within 14 days after treatment, and almost 80% had fully involuted by 28 days.
The ability to predict which eyes are most prone to develop a retinal detachment has several important implications. Education of families and attending physicians to prepare these individuals for potentially adverse outcomes is of the utmost value. It is difficult enough for a family to accept that a child will be irreversibly blind due to ROP, but to be surprised by this revelation, rather than to be aware that it is a real and significant potential risk, makes this eventuality more difficult to accept. Surprise over a bad outcome may be more likely to be associated with a decision to pursue medicolegal action than is the bad outcome itself.133
Detailed study of the process of ROP involution after treatment and study of retinal detachment risk factors can also help to clarify follow-up needs during the initial period after treatment. There are at present no recommended published guidelines on how frequently an infant should be examined after treatment. None of the major US multicenter studies on ROP followed children for protocol visits at frequent intervals after treatment and thus offered no advice in this regard. This author recommends examination at least biweekly during the first 6 weeks after treatment, with more frequent examination of infants whose eyes begin to develop vitreous organization or continue to have active stage 3 disease, elements of plus disease, or both. Consultation with a vitreoretinal surgeon is recommended any time a high-risk predetachment state is believed to exist. Ophthalmologic ultrasound, vitreoretinal consultation, or both, are also recommended as soon as is practical when severe vitreous hemorrhage is noted.
PREVENTIVE REINTERVENTION
Supplemental and Posterior Photocoagulation
From the standpoint of reintervention with laser, two approaches have been reported. These include supplemental photocoagulation for skip areas when active acute-phase ROP persists67,78,134 and preemptive photocoagulation posterior to the ridge to reduce the risk of progression of low-grade detachments.135 Skip areas, which represent untreated areas of avascular retina and may range from small to large in size, are generally inadvertent and often occur in areas where visualization was suboptimal during treatment. Untreated areas may continue to produce vascular growth factors that could exacerbate acute-phase disease and impede involution after treatment. McNamara and coworkers67 discussed reintervention strategies used during a randomized trial of 18 children who received cryotherapy to one eye and argon laser photocoagulation to the fellow eye for treatment of threshold disease. Retreatment was performed within 17 days of initial treatment if untreated “skip areas” were present in association with persistent plus disease and when either of the following features adjacent to the skipped area were seen: (1) segmental, shallow retinal detachment or (2) progressive extraretinal fibrovascular proliferation contiguous with a skip area. The occasional need to repeat treatment on a patient following initial ablation therapy is not an unanticipated event. This may be especially likely to occur in zone I eyes,78,120 and skip areas could, in part, help to explain the high rate of treatment failure for zone I eyes. Capone and coworkers78 reported the need for retreatment of skip areas in two (6.7%) of 30 eyes with zone I disease, both of which had persistent plus disease.
Sternberg and coworkers134 reported that most eyes responded rapidly to cryotherapy within 7 to 10 days after treatment, although they did not provide any data to support this claim. They recommended that if no response to treatment had been noted within this time frame, the periphery should be inspected carefully to assess for skip areas. Supplemental cryotherapy was recommended for untreated areas, though clear guidelines on timing and approach to treatment were not provided. Tsitsis and coworkers136 stated that they considered supplemental laser treatment when neovascularization had not regressed within 2 to 3 weeks of treatment. Hartnett115 recommended careful monitoring of eyes after laser treatment for threshold ROP and made empiric reintervention recommendations. Without clarification of the timing of reintervention relative to the initial treatment, she recommended that additional laser be applied if involution of plus disease and neovascularization were not noted. In the absence of skip areas, the data in the present study do not support reintervention with additional ablative therapy to treat persistently active acute-phase ROP.
O’Keefe and coworkers135 reported the use of laser reintervention posterior to the fibrovascular ridge in eyes with a 4A retinal detachment or significant retinal traction in an attempt to halt progression of the detachment. They treated seven eyes that had previously undergone photocoagulation to the avascular retina and one eye with advanced ROP and a stage 4A detachment at the time of initial treatment. A large amount of treatment was applied posterior to the fibrovascular ridge, extending to “about one disc diameter” nasal to the optic disc and three disc diameters temporal to the fovea. The detachments had not progressed at last follow-up, though the duration of follow-up was not clearly stated for all of the treated infants.
Davis and coworkers65 noted that they were among the few investigators to provide details on the process of involution after treatment. They reported on the results of transscleral diode laser treatment of 14 eyes of eight patients with threshold or near threshold ROP. Though nonspecific regarding details, they indicated that all 14 eyes showed “regression” of plus disease at 1 week after treatment. They also noted that 12 eyes “began” to show regression of abnormal neovascularization at 2 weeks. Both eyes of one infant rapidly progressed to development of a stage 4 detachment at 2 weeks, despite initial regression of plus disease. Another infant subsequently developed a preretinal fibrotic band in one eye and a late traction retinal detachment. Their findings, though not presented in great detail, parallel our experience in that the clinically active features of plus disease and stage 3 disease involuted quickly after treatment in most eyes and that retinal detachment can occur even after complete involution of acute-phase disease, sometimes many weeks after treatment. However, these and other anecdotal recommendations available in the literature do not provide sufficient reintervention guidelines to clinicians caring for infants with ROP.
Insufficient data are available to make definitive recommendations on reintervention with repeated ablative therapy or laser posterior to the ridge to reduce the risk of retinal detachment progression. With regard to skip areas, logic dictates that reintervention by supplemental laser to skip areas offers high potential value with little additional risk. The stakes are high in eyes that develop advanced ROP. The goal of treatment is to prevent the retina from detaching. Skip areas very clearly provide opportunity for untreated ischemic retina to continue producing vascular growth factors, which have the potential to exacerbate acute-phase ROP. It would be difficult, if not impossible, to conduct a study on the effectiveness of reintervention for skip areas for a variety of reasons, including recruitment difficulties and ethical concerns. Thus, common sense should prevail. This author recommends supplemental laser treatment to skip areas when detected any time following treatment in eyes where full involution of all elements of plus disease and stage 3 disease has not occurred. Lower-intensity laser burns may not destroy the entire retina, and even treated areas may have viable remaining retina.137,138 Such areas could be a source of vascular growth factor production, and even retreatment of previously treated areas could potentially be of value in some situations and may justify consideration. No comments on the utility of this potential mode of reintervention can be made based on data from the present study.
Vitrectomy for Eyes in a High-Risk Predetachment State
This author now characterizes eyes with clinically important vitreous organization as being in a high-risk predetachment state. Eyes in the Cryo-ROP study that developed a retinal detachment were also more likely to have organized vitreous membranes.100 Among 61 eyes with a partial retinal detachment noted during the 3-month examination in the Cryo-ROP study, organized vitreous membranes were present in 57% as compared with 36% for the entire randomized group.100 Only five (8%) of 61 of these partially detached eyes had not developed disease involving the macula at the 4.5-year examination, and 38% of the eyes were considered “blind.”
The question is, then, whether preemptive surgery should be considered for eyes in a high-risk predetachment state or whether surgery should be deferred until a detachment ensues. In 1990, Mintz-Hittner and Kretzer121 conceptualized ROP as involving angiogenic stimulation emanating from spindle cells and tractional forces resulting from myofibroblasts. They reported an elaborate scenario to disrupt the angiogenic stimulation via sequential cryotherapy. They further believed that a prophylactic scleral buckle should be applied concomitant with cryotherapy to prevent development of macular distortion and ectopia. Although this concept of staged surgery never gained wide acceptance, their aggressive stance with early intervention to reduce tractional forces may be quite valid in high-risk eyes.
The poor visual prognosis of eyes that have developed high-grade retinal detachments pleads for exploration of alternative treatment strategies. One potential option is preemptive surgical reintervention for eyes deemed to be in a high-risk predetachment state. Lens-sparing vitrectomy of stage 4A disease has been suggested as offering hope for interrupting the cycle of events that ultimately leads to higher-grade detachments.104 Vitreous organization was among those factors associated with a poor surgical outcome in the repair of stage 4A retinal detachments in one study.115
We must wonder, then, if earlier intervention could reduce the failure rate of vitrectomy. As vitreous organization develops, growth of fibrovascular tissue from the ridge toward the anterior aspect of the eye makes it more difficult to successfully remove the offending membranes without the need to remove the lens and without the complication of creating an iatrogenic retinal break.115 Thus, intervention prior to the development of a stage 4A detachment when vitreous membranes are less well developed could, in theory, offer potential advantages. Additionally, despite slow evolution of many retinal detachments, once a retinal detachment develops, progression can be rapid. This author has observed two eyes of two infants that progressed from a stage 4A retinal detachment to a total detachment in less than 24 hours. Whereas most retinal detachments probably initially start out as stage 4A detachments, it is unlikely that follow-up can be reasonably rendered at a frequency sufficient to detect all detachments at this early stage. Observation with deferral of vitrectomy until a detachment develops is thus destined to result in missed opportunities for optimal intervention, assuming surgery for stage 4A detachments is superior to surgery at more advanced stages of detachment. For such eyes, the visual prognosis is poor even with successful reattachment surgery. Finally, it is postulated that subclinical foveal involvement may be more likely in the presence of partial detachment when compared to eyes in a predetachment state. Determining the optimal window for surgical reintervention is therefore of the utmost importance.
The decision to recommend preemptive vitreoretinal surgery must be weighed against the potential iatrogenic risks of retinal breaks, surgical aphakia, cataract formation, and endophthalmitis. Very few data have been reported on complications of vitrectomy for ROP, especially for stage 4A detachments. Among 35 eyes (30 with stage 4B or 5) treated with vitreoretinal surgery or scleral buckle, Hartnett115 reported that one eye developed glaucoma, one developed cataract, and 17 had persistent retinal detachment. The report does not make clear if the two eyes that developed glaucoma and cataract were also eyes that remained detached. Ferrone and coworkers139 reported on the incidence of cataract formation following pediatric lens-sparing vitrectomy. Twenty-six percent (12 of 45) of eyes that underwent lens-sparing vitrectomy for ROP detachments developed a cataract. Though all but three of the cataracts developed in eyes with stage 4B or 5 ROP, a statement regarding the risk of cataract formation following vitrectomy at or prior to stage 4A retinal detachment cannot be made on the basis of their data. Advances in surgical treatment and increased experience of vitreoretinal surgeons have resulted in fewer vision-threatening complications during vitrectomy for stage 4A disease. M. T. Trese, MD (written communication, December 22, 2003) believes that the rate of cataract development or need for lensectomy is probably 3% by 27 months after surgery. Enzymatic-assisted vitrectomy has also been suggested as an adjunct in the treatment of vitreous pathology140 and could potentially play a role in future treatment of ROP.
DECISION ANALYSIS
The decision sciences have evolved primarily from techniques that were applied to business and later adapted to medicine. Decision analysis is a mathematical tool used to emulate human decision making.108 The advantages of decision analysis are its systematic approach and its ability to consider multiple factors in a decision on a presumably rational basis. It is not a substitute for clinical judgment but is merely a tool to facilitate clinical reasoning. Expected utility is calculated by multiplying an outcome utility by its probability of occurrence. Decision analysis rests upon the principle that the plan of action that results in the highest expected utility (ie, optimal for the individual) will be the preferred choice.109
Some potential problems associated with decision analysis involve data accuracy. Schwartz141 pointed out the difficulties of populating the arms of decision trees with data that are accurate and relevant. Outcome data from systematic studies are commonly derived from tightly controlled patient populations and may not accurately reflect an individual patient’s situation. Additionally, a decision analysis attempts to simplify the elements of the decision process and may not consider all ramifications for a given patient. In addition, Kucey has pointed out that physicians often have difficulty with the concept of thinking probabilistically.108 Many physicians prefer generally applicable clinical maxims and clear-cut choices, rather than dealing with chance or probability.
The utilities calculated are valid given the range of assumptions tested. An important issue with regard to decision analysis is that of accuracy, which is dependent on attributes input into the equation. When hard data are not available, estimates based on anecdotal experience and/or related clinical data must be used. This is a known limitation of decision analysis, and results of decision analysis must be viewed with an understanding of these issues. Despite these limitations, decision analysis is felt to be an excellent means of analyzing the potential impact of possible treatment pathways. To help mitigate this problem, sensitivity analysis is performed to test the model under a wider range of assumptions.
Kucey108 described the process of decision analysis modeling for the surgeon. He identified four basic steps. The first step includes identification, definition, and bounding of the decision problem. The second step is to structure the decision problem by creating a decision tree. A decision tree displays a logical sequence of events in the clinical decision problem. Each decision tree has several basic structural elements, including (a) the clinical problem or starting point, (b) available alternative actions, which form the major branches of the tree, and (c) events that follow from each of these actions, including potential consequences of each action and potential outcomes. The third step is to assign a value and a probability to each possible outcome. The fourth and final step is to roll up the decision tree, a process that involves calculation of the expected utility at each branch point and identification of the action with the highest expected utility.
Vitreous organization is a tangible entity with a known pathogenesis and known propensity to contract and produce retinal traction. A potential treatment option is available, and as such, intervention in the form of lens-sparing vitrectomy has potential utility to prevent progression to retinal detachment. Decision analysis can thus assist in evaluating the theoretical utility of this proposed treatment. It cannot, however, prove that preemptive reintervention will be beneficial, but merely provides a preliminary analysis of expectations based on the parameters used in the model. This author modeled vitrectomy very conservatively, in order not to inflate results and believes it is likely that lens-sparing vitrectomy may have an even greater utility than the 0.85 that was calculated using a macular integrity model. For example, a surgical success rate of 84% was assumed, the lowest among surgical success rates reported for stage 4A detachments in the last 4 years.104–107 Using the highest reported success rate of 94% over the last 4 years,106 the expected utility of preemptive lens-sparing vitrectomy was 0.93 compared with 0.83 for vitrectomy deferred until a detachment developed. Decision analysis suggests that preemptive vitrectomy is not likely to be associated with worse outcomes compared with deferred vitrectomy and may well be superior if success rates are significantly higher when surgery is performed prior to development of a retinal detachment.
It was also assumed that only a small number of eyes treated initially with observation would progress beyond the 4A stage. Although this author believes that most retinal detachments start out as partial peripheral detachments, invariably many will progress to more advanced profiles during observation. It is not likely that any reasonable follow-up schedule will be sufficient to detect all retinal detachments at the earliest stages, when intervention is most likely to be associated with a favorable outcome.
While the assumptions utilized in the decision analysis may not be applicable to all infants and all eyes, a higher expected utility was found for preemptive vitrectomy in eyes with clinically important vitreous organization over a wide range of plausible values. It is not the purpose of the present study to recommend carte blanche the use of preemptive vitreoretinal surgery for eyes with high-risk predetachment features; rather, it is hoped that this study will stimulate evaluation of current treatment paradigms and stimulate further study on the potential utility of discernible changes to current approaches in the management of ROP retinal detachments and impending retinal detachments. Given the rate of progression to retinal detachment in eyes with vitreous organization, such studies appear justified.
CLASSIFICATION OF LATE-STAGE ROP
Much as the data and outcomes between physicians and studies were difficult to compare prior to adoption of ICROP,53,55 the classification system for late-stage ROP no longer appears sufficient to adequately describe and compare patients during ROP involution, particularly when clinically obvious vitreous pathology ensues. Vitreoretinal surgery for ROP-related retinal detachments was in its infancy when the current classification system was devised, making standardized nomenclature for vitreous pathology less important than it is today. Advances in the treatment of late-stage ROP have shaped a need for an updated nomenclature. The proposed classification scheme, then, includes classification of vitreous pathology which is known to substantially contribute to the pathogenesis of retinal detachment but is essentially ignored by the present classification system of late-stage ROP.55
The need for better classification of subtotal retinal detachments is also recognized. The proposed modifications better stratify stage 4A detachments, acknowledging that a temporal retinal detachment may be a more substantial threat to vision than a nasal detachment. In this scheme, retinal detachments are further classified based on the zone and on the number of sectors of vascularized retina involved in the detachment. This author believes that this system will allow better comparison of partial retinal detachment outcomes and may facilitate future studies involving surgical repair. The proposed modifications were designed to parallel the current ICROP53,55 as closely as possible and, like ICROP, remain simple to understand and easy to apply and compare between eyes. Adoption of this scheme or a similar classification system is encouraged.
SUMMARY
Since the initial description of retrolental fibroplasia (now referred to as ROP) in 1942,2 much progress has been made in defining the cause and pathogenesis of ROP. Significant advances have been made in reducing the incidence of ROP, especially threshold ROP, and in reducing the occurrence of severe visual impairment and blindness resulting from the disease. The best results from a randomized controlled study of an interdictive treatment (treatment at prethreshold) still resulted in a poor structural outcome in almost one in 10 treated eyes.82 Despite significant treatment advances, ROP will likely remain an important cause of severe childhood visual impairment and blindness.
It is the responsibility of clinicians to offer effective, proven therapies to their patients. It is also the obligation of clinicians to remain dissatisfied with the status quo when significant room for improvement exists. The advancement of new ideas and new treatments in a responsible and safe manner remains of paramount importance. In this study, the probability of acute-phase ROP involution over time following diode laser photocoagulation for threshold disease has been characterized in a systematic manner for the first time. Clinically important vitreous organization was identified as a key detrimental factor during involution and was associated with a substantial increase in the risk of developing a retinal detachment. Clinically important vitreous hemorrhage was also strongly associated with retinal detachment, though the timing of vitreous hemorrhage relative to retinal detachments was not clear. If it can be demonstrated that clinically important vitreous hemorrhage occurs prior to development of retinal detachment, preemptive reintervention strategies may be reasonable to consider when the condition is present.
ROP should not be thought of as merely a retinopathy, but as a vitreoretinopathy. Though retinal detachment could not be predicted in advance in all of the patients in this study, it was heralded in almost half of eyes by onset of clinically important vitreous organization. It follows, then, that a window of opportunity for preemptive vitreoretinal rescue is theoretically available for this subgroup of eyes. Conservatively modeled decision analysis suggested that preemptive surgery is not likely to result in worse outcomes, compared with surgery deferred until after a retinal detachment has occurred, and potentially could offer advantages in some situations. Future detailed study of the process of involution and standardized classification of vitreous abnormalities offers the hope of identification of additional eyes that may be salvaged.
Until a means to prevent premature birth and/or to prevent development of advanced ROP is discovered, the further salvaging of eyes from ROP-related vision loss is likely to require interdictive treatment after ablative treatment to the avascular retina and before a retinal detachment ensues and/or progresses. Careful study of preemptive vitreoretinal surgery appears warranted.
ACKNOWLEDGMENTS
I want to acknowledge Kirk R. Wilhemus, MD, PhD, MPH, Baylor College of Medicine, and Chuck Huber, MA, MS, University of Texas School of Public Health, for their assistance with the statistical evaluation of study data and Mohamed A.W. Hussein, MD, Baylor College of Medicine, and Aaron Miller, MD, Baylor College of Medicine, for their assistance with review and tabulation of study data.
Footnotes
Supported in part by Knight’s Templar Eye Foundation, Inc, Chicago, Illinois, and by an unrestricted grant from Research to Prevent Blindness, Inc, New York, New York.
REFERENCES
- 1.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020—the right to sight. Bull World Health Organ. 2001;79:227–232. [PMC free article] [PubMed] [Google Scholar]
- 2.Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. I. Preliminary report. Am J Ophthalmol. 1942;25:203–204. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. II. Report of case—clinical aspects. Arch Ophthalmol. 1943;29:36–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. III. Report of cases—etiologic factors. Arch Ophthalmol. 1943;29:54–68. [Google Scholar]
- 5.Silverman WA. Retrolental Fibroplasia. A Modern Parable New York: Grune & Stratton; 1980.
- 6.Hess JH. Oxygen unit for premature and very young infants. Am J Dis Child. 1934;47:916–917. [Google Scholar]
- 7.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med J Aust. 1951;2:48–50. [PubMed] [Google Scholar]
- 8.Patz A, Hoech LE, De La Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol. 1952;35:1248–1253. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 9.Kinsey VE, Jacobus JT, Hemphill FM. Retrolental fibroplasia. Cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol. 1956;56:481–547. [PubMed] [Google Scholar]
- 10.Patz A, Eastham A, Higginbotham DH, et al. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522. [PubMed] [Google Scholar]
- 11.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dollery CT, Bulpitt CJ, Kohner EM. Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Invest Ophthalmol. 1969;8:588–594. [PubMed] [Google Scholar]
- 13.Chan-Ling T, Tout S, Hollander H, et al. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992;33:2128–2147. [PubMed] [Google Scholar]
- 14.Katz ML, Robison WG., Jr Autoxidative damage to the retina: potential role in retinopathy of prematurity. Birth Defects Orig Artic Ser. 1988;24:237–248. [PubMed] [Google Scholar]
- 15.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 17.Avery ME, Oppenheimer EH. Recent increase in mortality from hyaline membrane disease. J Pediatr. 1960;57:553–559. doi: 10.1016/s0022-3476(60)80083-2. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield EM. Blindness in infants and young children. Sight Sav Rev. 1972;42:69–89. [PubMed] [Google Scholar]
- 19.Cross KW. Cost of preventing retrolental fibroplasia? Lancet. 1973;2:954–956. doi: 10.1016/s0140-6736(73)92610-x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487–491. doi: 10.1097/00008480-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Patz A. The need for further research in retrolental fibroplasia. Sight Sav Rev. 1970;40:7–11. [PubMed] [Google Scholar]
- 22.Bolton DP, Cross KW. Further observations on cost of preventing retrolental fibroplasia. Lancet. 1974;1:445–448. doi: 10.1016/s0140-6736(74)92395-2. [DOI] [PubMed] [Google Scholar]
- 23.Bauer CR. The occurence of retrolental fibroplasia in infants of birth weight 1000 grams and less (abstract) Clin Res. 1978;26:824A. [Google Scholar]
- 24.Gunn TR, Aranda JV, Little J. Incidence of retrolental fibroplasia. Lancet. 1978;1:216–217. doi: 10.1016/s0140-6736(78)90660-8. [DOI] [PubMed] [Google Scholar]
- 25.McCormick AQ. Retinopathy of prematurity. Curr Probl Pediatr. 1977;7:1–28. [PubMed] [Google Scholar]
- 26.Emsley HC, Wardle SP, Sims DG, et al. Increased survival and deteriorating developmental outcome in 23 to 25 week old gestation infants, 1990–4 compared with 1984–9. Arch Dis Child Fetal Neonatal Ed. 1998;78:F99–104. doi: 10.1136/fn.78.2.f99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uemura Y. Current status of retrolental fibroplasia. Report of the Joint Committee for the Study of Retrolental Fibroplasia in Japan. Jpn J Ophthalmol. 1977;21:366–378. [Google Scholar]
- 28.Sasaki K, Yamashita Y, Maekawa T, et al. Treatment of retinopathy of prematurity in active stage by cryocautery. Jpn J Ophthalmol. 1976;20:384–395. [Google Scholar]
- 29.Hindle NW. Cryotherapy for retinopathy of prematurity to prevent retrolental fibroplasia. Can J Ophthalmol. 1982;17:207–212. [PubMed] [Google Scholar]
- 30.Mousel DK, Hoyt CS. Cryotherapy for retinopathy of prematurity. Ophthalmology. 1980;87:1121–1127. doi: 10.1016/s0161-6420(80)35121-x. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Sira I, Nissenkorn I, Grunwald E, et al. Treatment of acute retrolental fibroplasia by cryopexy. Br J Ophthalmol. 1980;64:758–762. doi: 10.1136/bjo.64.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keith CG. Visual outcome and effect of treatment in stage III developing retrolental fibroplasia. Br J Ophthalmol. 1982;66:446–449. doi: 10.1136/bjo.66.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingham JD. Acute retrolental fibroplasia. II. Treatment by cryosurgery. Arch Ophthalmol. 1978;96:2049–2053. doi: 10.1001/archopht.1978.03910060437008. [DOI] [PubMed] [Google Scholar]
- 34.Harris GS, McCormick AQ. The prophylactic treatment of retrolental fibroplasia. Mod Probl Ophthalmol. 1977;18:364–367. [PubMed] [Google Scholar]
- 35.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome—structure and function. Arch Ophthalmol. 1990;108:1408–1416. [PubMed] [Google Scholar]
- 36.Rowlands E, Ionides AC, Chinn S, et al. Reduced incidence of retinopathy of prematurity. Br J Ophthalmol. 2001;85:933–935. doi: 10.1136/bjo.85.8.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullard SR, Donahue SP, Feman SS, et al. The decreasing incidence and severity of retinopathy of prematurity. J AAPOS. 1999;3:46–52. doi: 10.1016/s1091-8531(99)70094-7. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds JD, Hardy RJ, Palmer EA. Incidence and severity of retinopathy of prematurity [letter] J Aapos. 1999;3:321–322. doi: 10.1016/s1091-8531(99)70032-7. [DOI] [PubMed] [Google Scholar]
- 39.Oishi M, Nishida H, Sasaki T. Japanese experience with micropremies weighing less than 600 grams born between 1984 to 1993. Pediatrics. 1997;99:E7. doi: 10.1542/peds.99.6.e7. [DOI] [PubMed] [Google Scholar]
- 40.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 41.Coats DK, Paysse EA, Steinkuller PG. Threshold retinopathy of prematurity in neonates less than 25 weeks’ estimated gestational age. J AAPOS. 2000;4:183–185. [PubMed] [Google Scholar]
- 42.O’Connor AR, Stephenson T, Johnson A, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Rudanko SL, Fellman V, Laatikainen L. Visual impairment in children born prematurely from 1972 through 1989. Ophthalmology. 2003;110:1639–1645. doi: 10.1016/S0161-6420(03)00498-6. [DOI] [PubMed] [Google Scholar]
- 44.Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86:716–722. doi: 10.1136/bjo.86.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz B, West SK. Blindness and visual impairment in the Americas and the Caribbean. Br J Ophthalmol. 2002;86:498–504. doi: 10.1136/bjo.86.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinkuller PG, Du L, Gilbert C, et al. Childhood blindness. J AAPOS. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert C, Rahi J, Eckstein M, et al. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350:12–14. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 48.Global Initiative for the Elimination of Avoidable Blindness. Vol WHO/PBL/97.61. Geneva: World Health Organization; 1998. [PMC free article] [PubMed]
- 49.Chan K, Ohlsson A, Synnes A, et al. Survival, morbidity, and resource use of infants of 25 weeks’ gestational age or less. Am J Obstet Gynecol. 2001;185:220–226. doi: 10.1067/mob.2001.115280. [DOI] [PubMed] [Google Scholar]
- 50.Msall ME, Buck GM, Rogers BT, et al. Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks’ gestation. Clin Pediatr (Phila) 1993;32:521–527. doi: 10.1177/000992289303200903. [DOI] [PubMed] [Google Scholar]
- 51.Msall ME, Phelps DL, DiGaudio KM, et al. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Behalf of the Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics. 2000;106:998–1005. doi: 10.1542/peds.106.5.998. [DOI] [PubMed] [Google Scholar]
- 52.Drummond MF. Economic aspects of cataract. Ophthalmology. 1988;95:1147–1153. doi: 10.1016/s0161-6420(88)33063-0. [DOI] [PubMed] [Google Scholar]
- 53.The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 54.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106:471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 55.The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. Arch Ophthalmol. 1987;105:906–912. [PubMed] [Google Scholar]
- 56.Hittner HM, Godio LB, Rudolph AJ, et al. Retrolental fibroplasia: efficacy of vitamin E in a double-blind clinical study of preterm infants. N Engl J Med. 1981;305:1365–1371. doi: 10.1056/NEJM198112033052301. [DOI] [PubMed] [Google Scholar]
- 57.Finer NN, Schindler RF, Grant G, et al. Effect of intramuscular vitamin E on frequency and severity of retrolental fibroplasia. A controlled trial. Lancet. 1982;1:1087–1091. doi: 10.1016/s0140-6736(82)92276-0. [DOI] [PubMed] [Google Scholar]
- 58.Johnson L, Schaffer D, Boggs TR., Jr The premature infant, vitamin E deficiency and retrolental fibroplasia. Am J Clin Nutr. 1974;27:1158–1173. doi: 10.1093/ajcn/27.8.1158. [DOI] [PubMed] [Google Scholar]
- 59.Lakatos L, Lakatos Z, Hatvani I, et al. Controlled trial of use of D-Penicillamine to prevent retinopathy of prematurity in very low-birth-weight infants. In: Stern L, Oh W, Friis-Hansen B, eds. Physiologic Foundations of Perinatal Care. Vol 2 New York: Elsevier; 1987:9–23.
- 60.The STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I. Primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 61.Cunningham S, Fleck BW, Elton RA, et al. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 62.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds JD, Hardy RJ, Kennedy KA, et al. Lack of efficacy of light reduction in preventing retinopathy of prematurity. Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) Cooperative Group. N Engl J Med. 1998;338:1572–1576. doi: 10.1056/NEJM199805283382202. [DOI] [PubMed] [Google Scholar]
- 64.Wallace DK, Coats DK, Paysse EA. Retinopathy of prematurity survey results [letter] J AAPOS. 1998;2:65–66. doi: 10.1016/s1091-8531(98)90065-9. [DOI] [PubMed] [Google Scholar]
- 65.Davis AR, Jackson H, Trew D, et al. Transscleral diode laser in the treatment of retinopathy of prematurity. Eye. 1999;13(Pt 4):571–576. doi: 10.1038/eye.1999.141. [DOI] [PubMed] [Google Scholar]
- 66.Seiberth V, Linderkamp O, Vardarli I. Transscleral vs transpupillary diode laser photocoagulation for the treatment of threshold retinopathy of prematurity. Arch Ophthalmol. 1997;115:1270–1275. doi: 10.1001/archopht.1997.01100160440008. [DOI] [PubMed] [Google Scholar]
- 67.McNamara JA, Tasman W, Brown GC, et al. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98:576–580. doi: 10.1016/s0161-6420(91)32247-4. [DOI] [PubMed] [Google Scholar]
- 68.McNamara JA, Tasman W, Vander JF, et al. Diode laser photocoagulation for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1992;110:1714–1716. doi: 10.1001/archopht.1992.01080240054029. [DOI] [PubMed] [Google Scholar]
- 69.O’Keefe M, O’Reilly J, Lanigan B. Longer-term visual outcome of eyes with retinopathy of prematurity treated with cryotherapy or diode laser. Br J Ophthalmol. 1998;82:1246–1248. doi: 10.1136/bjo.82.11.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeJonge MH, Ferrone PJ, Trese MT. Diode laser ablation for threshold retinopathy of prematurity: short-term structural outcome. Arch Ophthalmol. 2000;118:365–367. doi: 10.1001/archopht.118.3.365. [DOI] [PubMed] [Google Scholar]
- 71.White JE, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy for threshold retinopathy of prematurity: 3-year outcome. J Pediatr Ophthalmol Strabismus. 1997;34:83–87. doi: 10.3928/0191-3913-19970301-06. quiz 121–122. [DOI] [PubMed] [Google Scholar]
- 72.Hammer ME, Pusateri TJ, Hess JB, et al. Threshold retinopathy of prematurity. Transition from cryopexy to laser treatment. Retina. 1995;15:486–489. [PubMed] [Google Scholar]
- 73.Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993;100:238–244. doi: 10.1016/s0161-6420(93)31664-7. [DOI] [PubMed] [Google Scholar]
- 74.Paysse EA, Lindsey JL, Coats DK, et al. Therapeutic outcomes of cryotherapy versus transpupillary diode laser photocoagulation for threshold retinopathy of prematurity. J Aapos. 1999;3:234–240. doi: 10.1016/s1091-8531(99)70008-x. [DOI] [PubMed] [Google Scholar]
- 75.Connolly BP, Ng EY, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2. Refractive outcome. Ophthalmology. 2002;109:936–941. doi: 10.1016/s0161-6420(01)01015-6. [DOI] [PubMed] [Google Scholar]
- 76.Ng EY, Connolly BP, McNamara JA, et al. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 1. Visual function and structural outcome. Ophthalmology. 2002;109:928–934. doi: 10.1016/s0161-6420(01)01017-x. discussion 935. [DOI] [PubMed] [Google Scholar]
- 77.Noonan CP, Clark DI. Trends in the management of stage 3 retinopathy of prematurity. Br J Ophthalmol. 1996;80:278–281. doi: 10.1136/bjo.80.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Capone A, Jr, Diaz-Rohena R, Sternberg P, Jr, et al. Diode-laser photocoagulation for zone 1 threshold retinopathy of prematurity. Am J Ophthalmol. 1993;116:444–450. doi: 10.1016/s0002-9394(14)71402-3. [DOI] [PubMed] [Google Scholar]
- 79.O’Neil JW, Hutchinson AK, Saunders RA, et al. Acquired cataracts after argon laser photocoagulation for retinopathy of prematurity. J Aapos. 1998;2:48–51. doi: 10.1016/s1091-8531(98)90110-0. [DOI] [PubMed] [Google Scholar]
- 80.Paysse EA, Miller A, Brady McCreery KM, et al. Acquired cataracts after diode laser photocoagulation for threshold retinopathy of prematurity. Ophthalmology. 2002;109:1662–1665. doi: 10.1016/s0161-6420(02)01169-7. [DOI] [PubMed] [Google Scholar]
- 81.Fleming TN, Runge PE, Charles ST. Diode laser photocoagulation for prethreshold, posterior retinopathy of prematurity. Am J Ophthalmol. 1992;114:589–592. doi: 10.1016/s0002-9394(14)74488-5. [DOI] [PubMed] [Google Scholar]
- 82.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 83.Schulenburg WE, Prendiville A, Ohri R. Natural history of retinopathy of prematurity. Br J Ophthalmol. 1987;71:837–843. doi: 10.1136/bjo.71.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flynn JT, Bancalari E, Bachynski BN, et al. Retinopathy of prematurity. Diagnosis, severity, and natural history. Ophthalmology. 1987;94:620–629. [PubMed] [Google Scholar]
- 85.Preslan MW, Butler J. Regression pattern in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 1994;31:172–176. doi: 10.3928/0191-3913-19940501-09. [DOI] [PubMed] [Google Scholar]
- 86.Repka MX, Palmer EA, Tung B. Involution of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 2000;118:645–649. doi: 10.1001/archopht.118.5.645. [DOI] [PubMed] [Google Scholar]
- 87.Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993;100:230–237. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 88.Friedman S, Richardson SE, Jacobs SE, et al. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. Pediatr Infect Dis J. 2000;19:499–504. doi: 10.1097/00006454-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101:654–657. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 90.Noyola DE, Bohra L, Paysse EA, et al. Association of candidemia and retinopathy of prematurity in very low birthweight infants. Ophthalmology. 2002;109:80–84. doi: 10.1016/s0161-6420(01)00841-7. [DOI] [PubMed] [Google Scholar]
- 91.Karlowicz MG, Giannone PJ, Pestian J, et al. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</=1000 g) neonates? Pediatrics. 2000;105:1036–1040. doi: 10.1542/peds.105.5.1036. [DOI] [PubMed] [Google Scholar]
- 92.Flynn JT, Cassady J, Essner D, et al. Fluorescein angiography in retrolental fibroplasia: experience from 1969–1977. Ophthalmology. 1979;86:1700–1723. doi: 10.1016/s0161-6420(79)35329-5. [DOI] [PubMed] [Google Scholar]
- 93.Hikichi T, Nomiyama G, Ikeda H, et al. Vitreous changes after treatment of retinopathy of prematurity. Jpn J Ophthalmol. 1999;43:543–545. doi: 10.1016/s0021-5155(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 94.Faris B, Tolentino FI, Freeman HM, et al. Retrolental fibroplasia in the cicatricial stage. Fundus and vitreous findings. Arch Ophthalmol. 1971;85:661–668. doi: 10.1001/archopht.1971.00990050663003. [DOI] [PubMed] [Google Scholar]
- 95.Foos RY. Chronic retinopathy of prematurity. Ophthalmology. 1985;92:563–574. doi: 10.1016/s0161-6420(85)34007-1. [DOI] [PubMed] [Google Scholar]
- 96.Hirose T, Sang DN. Vitreous changes in retinopathy of prematurity. Bull Soc Belge Ophtalmol. 1987;223 Pt 1:165–177. doi: 10.1007/978-1-4757-1901-7_11. [DOI] [PubMed] [Google Scholar]
- 97.deJuan E, Jr, Machemer R. Retinopathy of prematurity. Surgical technique. Retina. 1987;7:63–69. [PubMed] [Google Scholar]
- 98.Soong HK, Eller AW, Hirose T, et al. In situ actin distribution in excised retrolental membranes in retinopathy of prematurity. Arch Ophthalmol. 1985;103:1553–1556. doi: 10.1001/archopht.1985.01050100129033. [DOI] [PubMed] [Google Scholar]
- 99.Flynn JT, O’Grady GE, Herrera J, et al. Retrolental fibroplasia: I. Clinical observations. Arch Ophthalmol. 1977;95:217–223. doi: 10.1001/archopht.1977.04450020019002. [DOI] [PubMed] [Google Scholar]
- 100.Gilbert WS, Quinn GE, Dobson V, et al. Partial retinal detachment at 3 months after threshold retinopathy of prematurity. Long-term structural and functional outcome. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1996;114:1085–1091. doi: 10.1001/archopht.1996.01100140287005. [DOI] [PubMed] [Google Scholar]
- 101.Hartnett ME, McColm JR. Retinal features predictive of progressive stage 4 retinopathy of prematurity. Retina. 2004;24:237–241. doi: 10.1097/00006982-200404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuchino Y, Hayashi H, Kono T, et al. Long-term follow up of visual acuity in eyes with stage 5 retinopathy of prematurity after closed vitrectomy. Am J Ophthalmol. 1995;120:308–316. doi: 10.1016/s0002-9394(14)72160-9. [DOI] [PubMed] [Google Scholar]
- 103.Quinn GE, Dobson V, Barr CC, et al. Visual acuity of eyes after vitrectomy for retinopathy of prematurity: follow-up at 5 1/2 years. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1996;103:595–600. doi: 10.1016/s0161-6420(96)30647-7. [DOI] [PubMed] [Google Scholar]
- 104.Capone A, Jr, Trese MT. Lens-sparing vitreous surgery for tractional stage 4A retinopathy of prematurity retinal detachments. Ophthalmology. 2001;108:2068–2070. doi: 10.1016/s0161-6420(01)00809-0. [DOI] [PubMed] [Google Scholar]
- 105.Chandran R, Worrall MA, Sun R, et al. Combined lens-sparing vitrectomy and sclreal buckling in stage 4 retinopathy of prematurity with significant vitreoretinal traction (abstract) Invest Ophthalmol Vis Sci. 2002;43:E-Abstract 4016. [Google Scholar]
- 106.Moshfeghi AA, Banach MJ, Salam GA, et al. Lens-sparing vitrectomy for tractional stage 4A retinopathy of prematurity retinal detachments (abstract) Invest Ophthalmol Vis Sci. 2003;44:E-Abstract 604. [Google Scholar]
- 107.Cherwek DH, O’Keefe JS, Burian G, et al. Lens-sparing vitrectomy for tractional retinal detachment due to retinopathy of prematurity (ROP) (abstract) Invest Ophthalmol Vis Sci. 2003;44:E-Abstract 605. [Google Scholar]
- 108.Kucey DS. Decision analysis for the surgeon. World J Surg. 1999;23:1227–1231. doi: 10.1007/s002689900653. [DOI] [PubMed] [Google Scholar]
- 109.Elwyn G, Edwards A, Eccles M, et al. Decision analysis in patient care. Lancet. 2001;358:571–574. doi: 10.1016/S0140-6736(01)05709-9. [DOI] [PubMed] [Google Scholar]
- 110.American Academy of Pediarics, Section on Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001;108:809–811. doi: 10.1542/peds.108.3.809. [DOI] [PubMed] [Google Scholar]
- 111.Wallace DK, Kylstra JA, Chesnutt DA. Prognostic significance of vascular dilation and tortuosity insufficient for plus disease in retinopathy of prematurity. J Aapos. 2000;4:224–229. doi: 10.1067/mpa.2000.105273. [DOI] [PubMed] [Google Scholar]
- 112.Quinn GE, Dobson V, Biglan A, et al. Correlation of retinopathy of prematurity in fellow eyes in the cryotherapy for retinopathy of prematurity study. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1995;113:469–473. doi: 10.1001/archopht.1995.01100040089032. [DOI] [PubMed] [Google Scholar]
- 113.Cleves MA, Gould MW, Gutierrez RG. An Introduction to Survival Analysis Using Stata, Revised Edition College Station, Texas: Stata Press; 2004.
- 114.Cocchiarella L, Andersson GGJ. Guides to the Evaluation of Permanent Impairment 5th ed. USA: AMA Press; 2001.
- 115.Hartnett ME. Features associated with surgical outcome in patients with stages 4 and 5 retinopathy of prematurity. Retina. 2003;23:322–329. doi: 10.1097/00006982-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Coats DK, Miller AM, Brady McCreery KM, et al. Involution of threshold retinopathy of prematurity after diode laser photocoagulation. Ophthalmology. 2004;111:1894–1898. doi: 10.1016/j.ophtha.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 117.Hallman M, Bry K, Hoppu K, et al. Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med. 1992;326:1233–1239. doi: 10.1056/NEJM199205073261901. [DOI] [PubMed] [Google Scholar]
- 118.Friedman CA, McVey J, Borne MJ, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. J Pediatr Ophthalmol Strabismus. 2000;37:79–86. doi: 10.3928/0191-3913-20000301-06. [DOI] [PubMed] [Google Scholar]
- 119.Tasman W. Threshold retinopathy of prematurity revisited. Arch Ophthalmol. 1992;110:623–624. doi: 10.1001/archopht.1992.01080170045021. [DOI] [PubMed] [Google Scholar]
- 120.Shaikh S, Capone A, Jr, Schwartz SD, et al. Inadvertent skip areas in treatment of zone 1 retinopathy of prematurity. Retina. 2003;23:128–131. doi: 10.1097/00006982-200302000-00033. [DOI] [PubMed] [Google Scholar]
- 121.Mintz-Hittner HA, Kretzer FL. The rationale for cryotherapy with a prophylactic scleral buckle for Zone I threshold retinopathy of prematurity. Doc Ophthalmol. 1990;74:263–268. doi: 10.1007/BF02482617. [DOI] [PubMed] [Google Scholar]
- 122.Kretzer FL, Hittner HM. Pathogenesis and antioxidant suppression of retinopathy of prematurity. In: Miquel J, Quintanilha AT, Weber H, eds. Handbook of Free Radicals and Antioxidants in Biomedicine. Vol II. Boca Raton, Florida: CRC Press; 2000:325.
- 123.Hutcheson KA, Nguyen AT, Preslan MW, et al. Vitreous hemorrhage in patients with high-risk retinopathy of prematurity. Am J Ophthalmol. 2003;136:258–263. doi: 10.1016/s0002-9394(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 124.Ehrenberg M, Thresher RJ, Machemer R. Vitreous hemorrhage nontoxic to retina as a stimulator of glial and fibrous proliferation. Am J Ophthalmol. 1984;97:611–626. doi: 10.1016/0002-9394(84)90382-9. [DOI] [PubMed] [Google Scholar]
- 125.de Juan E, Jr, Shields S, Machemer R. The role of ultrasound in the management of retinopathy of prematurity. Ophthalmology. 1988;95:884–888. doi: 10.1016/s0161-6420(88)33090-3. [DOI] [PubMed] [Google Scholar]
- 126.Pulido JS, Byrne SF, Clarkson JG, et al. Evaluation of eyes with advanced stages of retinopathy of prematurity using standardized echography. Ophthalmology. 1991;98:1099–1104. doi: 10.1016/s0161-6420(91)32171-7. [DOI] [PubMed] [Google Scholar]
- 127.Seaber JH, Machemer R, Eliott D, et al. Long-term visual results of children after initially successful vitrectomy for stage V retinopathy of prematurity. Ophthalmology. 1995;102:199–204. doi: 10.1016/s0161-6420(95)31035-4. [DOI] [PubMed] [Google Scholar]
- 128.Cherry TA, Lambert SR, Capone A., Jr Electroretinographic findings in stage 5 retinopathy of prematurity after retinal reattachment. Retina. 1995;15:21–24. doi: 10.1097/00006982-199515010-00004. [DOI] [PubMed] [Google Scholar]
- 129.Topilow HW, Ackerman AL, Wang FM. The treatment of advanced retinopathy of prematurity by cryotherapy and scleral buckling surgery. Ophthalmology. 1985;92:379–387. doi: 10.1016/s0161-6420(85)34038-1. [DOI] [PubMed] [Google Scholar]
- 130.Machemer R. Description and pathogenesis of late stages of retinopathy of prematurity. Ophthalmology. 1985;92:1000–1004. doi: 10.1016/s0161-6420(85)33925-8. [DOI] [PubMed] [Google Scholar]
- 131.Machemer R, deJuan E. Retinopathy of prematurity: approaches to surgical therapy. Aust N Z J Ophthalmol. 1990;18:47–56. doi: 10.1111/j.1442-9071.1990.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 132.Steidl SM, Hirose T. Subretinal organization in stage 5 retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2003;241:263–268. doi: 10.1007/s00417-003-0632-x. [DOI] [PubMed] [Google Scholar]
- 133.Bettman JW. Seven hundred medicolegal cases in ophthalmology. Ophthalmology. 1990;97:1379–1384. doi: 10.1016/s0161-6420(90)32406-5. [DOI] [PubMed] [Google Scholar]
- 134.Sternberg P, Jr, Lopez PF, Lambert HM, et al. Controversies in the management of retinopathy of prematurity. Am J Ophthalmol. 1992;113:198–202. doi: 10.1016/s0002-9394(14)71535-1. [DOI] [PubMed] [Google Scholar]
- 135.O’Keefe M, Burke J, Algawi K, et al. Diode laser photocoagulation to the vascular retina for progressively advancing retinopathy of prematurity. Br J Ophthalmol. 1995;79:1012–1014. doi: 10.1136/bjo.79.11.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsitsis T, Tasman W, McNamara JA, et al. Diode laser photocoagulation for retinopathy of prematurity. Trans Am Ophthalmol Soc. 1997;95:231–236. discussion 237–245. [PMC free article] [PubMed] [Google Scholar]
- 137.Smiddy WE, Hernandez E. Histopathologic characteristics of diode laser-induced chorioretinal adhesions for experimental retinal detachment in rabbit eyes. Arch Ophthalmol. 1992;110:1630–1633. doi: 10.1001/archopht.1992.01080230130037. [DOI] [PubMed] [Google Scholar]
- 138.Young TL, Anthony DC, Pierce E, et al. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997;1:105–110. doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 139.Ferrone PJ, Harrison C, Trese MT. Lens clarity after lens-sparing vitrectomy in a pediatric population. Ophthalmology. 1997;104:273–278. doi: 10.1016/s0161-6420(97)30325-x. [DOI] [PubMed] [Google Scholar]
- 140.Trese MT. Enzymatic-assisted vitrectomy. Eye. 2002;16:365–368. doi: 10.1038/sj.eye.6700193. [DOI] [PubMed] [Google Scholar]
- 141.Schwartz WB. Sounding board. Decision analysis: a look at the chief complaints. N Engl J Med. 1979;300:556–559. doi: 10.1056/NEJM197903083001011. [DOI] [PubMed] [Google Scholar]