Abstract
Purpose
In colobomatous eyes, the risk of retinal detachment increases with age. This study elucidates the anatomic conditions and pathologic process of retinal detachment associated with colobomas.
Methods
The records, including histologic slides, of 14 children (1 day to 17 months old) and 7 adults (17 to 78 years old) with colobomas were examined.
Results
In children, colobomas were associated with lethal malformations. The extracolobomatous inner retinal layers extended centrally, forming the intercalary membrane. Duplication of the outer retinal layers and a horizontal shift of Müllerian glia created a triangle and a locus minoris resistentiae adjacent to the laterally displaced pigment epithelium. Part of the locus was an incomplete layer of photoreceptors excluding Müllerian glia.
In adults, atrophy of the intercalary membrane, manifested as central schisis, thinning of the neuroepithelium, and hole formation, was related to a paucity of blood vessels within and underneath the intercalary membrane and the size of colobomas. The margins featured blood vessels, pigment epithelial hypertrophy, and choroidal and scleral thickening in a compact, intertwined arrangement.
Conclusions
Glial atrophy, schisis, and hole formation in the intercalary membrane and separation of the locus minoris resistentiae from the pigment epithelium can disrupt barriers to fluid flow and set the stage for rhegmatogenous retinal detachment. That process is exacerbated by scleral ectasia, increasing vitreous traction at the margin, and retinovascular ischemia within the intercalary membrane. A vascularized, compact margin resembling a laser barrier, found predominantly in adults, may protect against retinal detachment.
INTRODUCTION
Even though coloboma of the choroid is a rare malformation,1 occurring in only 0.14% of the general population, 23% to 42% of those afflicted eventually develop retinal detachment.2,3 Many detailed histological descriptions of colobomas4–25 preceded any clinical description of associated retinal detachment.26–28 According to Jules Gonin,29 the father of retinal detachment surgery, pathoanatomy is critical “pour combattre utilement un processus pathologique il faut en connaître la nature et les conditions anatomiques.” Yet these early descriptions were forgotten long before scleral buckling (in the1960s) and vitreoretinal techniques (in the 1980s) provided some measure of therapeutic success.2,3,30–44 Clinically, however, surgeons specializing in retinal detachments were likely to see only colobomas of medium severity. Severity and age at onset are related in that severe colobomas are part of multiple malformations45,46 and were, therefore, more likely to be the subject of ophthalmic pathological study.47 Patients with less severe and isolated colobomas may reach adulthood, yet pass through a vulnerable period in the second and third decades of their lives, when retinal detachments are likely to occur (Table 1).
TABLE 1.
AGE AT FIRST PRESENTATION OF PATIENTS WITH COLOBOMA AND RETINAL DETACHMENT
| AUTHOR | DATE OF PUBLICATION | AGE/GENDER |
|---|---|---|
| Mannhardt | 1897 | 20-year-old female |
| Gilbert | 1906 | 20-year-old male |
| Brinton | 1922 | 18-year-old male |
| Wagener | 1925 | 37-year-old female |
| 40-year-old female | ||
| Komoto | 1926 | 25-year-old male |
| 18-year-old male | ||
| 22-year-old female | ||
| Jesberg | 1961 | 31-year-old male |
| 44-year-old male | ||
| 28-year-old male | ||
| 43-year-old male | ||
| 32-year-old female | ||
| 33-year-old male | ||
| 29-year-old male | ||
| Patnaik | 1981 | 9 males, 5 females, no ages given |
| Chams | 1984 | 8 males, 12 to 25 years old, average age 18 |
| Wang | 1985 | 19 males, 6 females, 11 to 29 years old |
| Steahly | 1990 | 35-year-old male |
| Gopal | 1991 | 12 males, 4 females, 6 to 37 years old, average age |
| Hanneken | 1991 | 42-year-old |
| 35-year-old | ||
| 24-year-old | ||
| 35-year-old, | ||
| 7-year-old | ||
| 7-year-old | ||
| 25-year-old | ||
| 11-year-old | ||
| eight eyes, five males and two females | ||
| McDonald | 1991 | 14-year-old female |
| 45-year-old female | ||
| 8-year-old female | ||
| 15-year-old female | ||
| 16-year-old female | ||
| 7-year-old male | ||
| 24-year-old male | ||
| Lee | 1992 | 77-year-old female |
| Jalali | 1994 | 21-year-old female |
| 19-year-old male | ||
| 25-year-old male | ||
| 28-year-old male | ||
| Daufenbach | 1998 | 5-month-old female |
| 29-month-old female | ||
| 10-year-old male | ||
| 15-year-old female | ||
| Unlu | 2002 | 19-year-old female |
| 37-year-old male | ||
| 69-year-old female | ||
| 10-year-old male | ||
| 45-year-old male | ||
| 12-year-old male | ||
| 42-year-old female |
Based on the large series of colobomas made available, this study was undertaken to evaluate the pathoanatomy of young and adult patients with lesions that might predispose to retinal detachment. Not surprisingly, the barriers represented by an intact intercalary membrane and a vascularized, compact, and cohesive margin emerged as potentially protective factors.
BACKGROUND: EMBRYOLOGY
Over the past two centuries, embryologic descriptions have gradually abandoned the vascular and inflammatory concept7,17 previously used to explain faulty closure of the embryonic fissure. According to that concept, the fissure closes at its anterior margin at the 10-mm embryonic stage.48 At this point, there is no iris, ciliary body, or choroid. The retina, which is at the end of its first period of differentiation, consists of only an inner marginal zone and a primitive neuroepithelium. During the same period in which the fissure closes, the retina progresses to the second stage of differentiation, developing an inner and an outer neuroblastic layer separated by the transient layer of Chievitz. The inner neuroblastic layer eventually forms ganglion, amacrine, and Müller cells; the outer neuroblastic layer, which develops at a slower rate, forms bipolar cells, horizontal cells, and photoreceptors.
The simultaneous closure of the fissure and retinal differentiation, together with subsequent retinal growth, are vital with respect to the pathoanatomy of the fissure in that only the inner neuroblastic layer of the retina, with its Müller cells, fuses across the fissure. The outer neuroblastic layer reverts and connects to the retinal pigment epithelium (RPE). The layer of Chievitz ends up parallel to the sclera, forming a triangle connecting the inner and outer retina. The margin of the cup becomes the peripheral retina. Thus, the retina does not close in the middle of the choroid (the “bridging” coloboma); rather, the anterior portion simply does not exist at the time of closure.
Moreover, vascularization of the retina occurs in the seventh month, 6 months after fissure closure. Not surprisingly, anatomic variations, including persistence of the layer of Chievitz, closure of the inner neuroblastic layer, reversal and late development of the outer neuroblastic layer, and faulty or deficient vascularization, are likely to occur. The perverse orientation of photoreceptors is possible only through late differentiation of the everted outer neuroblastic layer. The first cones appear at the 48-mm stage. The sclera matures at this time, forming from front to back.48–50
In summary, the structure resulting from faulty closure of the fissure is frozen in time at the margin of the coloboma. The tissue that actually bridges the defect is the inner neuroblastic layer, with its derivative ganglion, amacrine, and Müllerian cells. The tissue reverting is a mixture of bipolar, horizontal, and photoreceptor cells; that is, the laterally displaced RPE is likely to connect to the junctional system of photoreceptors and not to processes of Müllerian glia, the main structural backbone of the retina: hence, the locus minoris resistentiae. The severity and size of the malformation, together with ensuing cell death, differentiation, and ocular growth, will ultimately determine the structural composition and quality of the barriers, as well as the risk of hole formation and retinal detachment.
HISTOLOGICAL STUDIES, 1830–1931
History of Definitions, 1830–1908
By the beginning of the 20th century, definitions of the intercalary membrane, point of reversal, duplication of photoreceptors, locus minoris resistentiae, and scleral ridge had all been introduced and used descriptively. Von Ammon,1 in 1830, observed with the unaided eye the “mutilation” of colobomatous eyes, while Pause,6 in 1878, noted the absence of pigment epithelium in colobomas and provided the first description of the tissue bridging them, which he called “Intercalarplatte.”
In 1881, Deutschman7 originated the inflammatory theory, according to which sclerochoroiditis caused colobomas. He defined the intercalary membrane, which he thought to be scar tissue, as duplication, or “Duplicatur der Membran,” and the locus minoris resistentiae, “durch Exsudat gesprengt.” Hannover,4 in 1888, thought that thickening of the sclera interfered with closure of the fissure. He was the first to describe the scleral ridge, which he called the “funiculus sclerae.”5 In 1891, Manz,11 a proponent of the fissure theory, described the point of reversal, which he thought to be a “peculiar anatomic landmark.” Van Duyse,13 in 1896, was more definite than the preceding authors about the retinal layers. He described the reversal of the outer layer of the retina and the continuation of the inner layer across the coloboma.
Mannhardt,14 in 1897, was the first to describe retinal detachment associated with coloboma, noting it in the eye of a 20-year-old girl. In this histological study, he noted that there were scleral ridges and that the intercalary membrane and the sclera were separated.
Von Hippel,17 in 1900, emphasized the importance of intraocular pressure for ocular growth. He wrote that the mere existence of the intercalary membrane proved that the fissure had been closed at some time. In 1906, Gilbert20 noted that the duplication of the retina at the margin was caused by an obstacle, either scleral or vascular. Seefelder,21 in 1908, using all of the previous definitions, concluded that the absence of pigment epithelium ultimately determines the extent of the defect.
The Center of Colobomas, 1878–1923
Although Pause6 and Deutschmann7 defined the intercalary membrane as forming the center of colobomas, Hess,9 in 1890, expanded this definition to encompass the inner retina, glial tissue, and thin connective tissue, including a scleral ridge. In 1893, Bock12 noted scleral ectasia bordered by scleral ridges, which were associated with detachment of the intercalary membrane. He also described atrophic membranes and rosettes. Van Duyse13 documented the internal limiting membrane and glial tissue that form the intercalary membrane and atrophic retina.
Mannhardt14 demonstrated that the detached retina overlies an outpouching of sclera. Von Hippel17 noted the presence of normal retina, as well as rosettes and gliosis, in the center of colobomas. In young patients, he found thick intercalary membranes; in adult patients, he found ectatic and atrophic membranes.
Levinsohn19 and Gilbert20 observed scleral thickening in the center of colobomas, where neural elements had been replaced by connective tissue. Seefelder21 found only glial tissue without structure or rosettes. He also observed scleral ridges at the margin of ectasia. Like von Hippel,17 he noted that in the center of colobomas, young patients had a well-differentiated retina, whereas elderly patients had a degenerated retina. In 1923, Baurman24 reported cases in which the retina firmly adhered to the sclera in the center of colobomas.
The Margins of Colobomas, 1881–1908
Following Deutschman’s definition of the duplication of the membrane and locus minoris resistentiae,7 both Hannover4 and Hess9 noted confluence of the inner and outer nuclear layers at the margins of colobomas. Bach, in 1901,15 described choroidal thickening and a wedge at the margin, which, together with the scleral ridge, were responsible for scleral choroidal adhesion. According to Bach, there was “melting” or fusion of the retina and choroid at the margin, which resulted in thickening so that the choroid seemed to hang over the coloboma. Van Duyse13 is credited with the first detailed description of the reversal of the outer nuclear layer and continuation of the inner layer across colobomas. He also noted choroidal thickening, or “augmentation de la masse du stroma choroidien.” Mannhardt14 saw vessels and scleral thickening at the margin (“protuberantia sclerae foetalis”).
Seefelder,21 in 1908, described the termination of the choriocapillaris, RPE, and Bruch’s membrane in the area of duplication of the retina. He noted that the RPE and retina were fused and that there was also a pocket-like fusion of retina and choroid, “taschenfoermige Einlagerung.”
Retinal Detachments Clinically Associated With Colobomas, 1897–1988
The early histologists, in their compulsive descriptions, documented the occurrence of both retinal detachments and colobomas, but made no connection between the two. In 1897, Mannhardt14 studied the colobomatous enucleated eye of a 20-year-old girl and found a retinal detachment. Similarly, in 1906, in the course of a histological study, Gilbert20 found a detachment in the eye of a 20-year-old boy. In 1922, Brinton26 presented the case of an 18-year-old boy who had retinal detachment associated with a coloboma. None of these investigators connected the occurrence of coloboma with that of retinal detachment. Indeed, ophthalmic authority Edward Jackson, who discussed Brinton’s paper at the Colorado Ophthalmological Society Meeting in 1922, commented that he had “never before seen a detachment of the retina in a case of choroidal coloboma.”26
Following Brinton’s lead, Wagener and Gipner,27 in 1925, reported a retinal detachment in a 37-year-old woman, stating that they had been unable to find any previous record of total detachment of the retina in a colobomatous eye. In 1926, Komoto,28 who had read Wagener and Gipner’s report, described a 25-year-old patient with a retinal detachment in a colobomatous eye. It is noteworthy that Brinton, Wagener and Gipner, and Komoto all emphasized that the detachment of the retina was almost total, occurring in all areas except that of the coloboma. Wagener and Gipner27 found that the margins of the coloboma were practically free from deposits of pigment or evidence of choroidal atrophy. They concluded that an abnormal adhesion was the factor that prevented extension of the detachment over the coloboma.
In 1985, Wang and Hilton33 surveyed eight general textbooks of ophthalmology and found that with respect to coloboma, “none of them mentioned the possibility of an associated retinal detachment.” Wang and Hilton also examined 15 textbooks exclusively devoted to retinal detachment and found that “only 4 of them mentioned the entity of coloboma detachment.”
Then, in 1988, Daufenbach and colleagues46 found six retinal and one choroidal detachment associated with coloboma, a prevalence of 8.1% in the 86 affected eyes they studied.
Although retinal detachments associated with choroidal coloboma had been histologically described since 1897 and clinically reported since 1922, no successful treatment for any retinal detachment existed before Jules Gonin’s operation, which became accepted in the 1930s. A typical statement was Komoto’s: “The patient remained under my treatment for some time, but with no therapeutic success.”28
CLINICAL DESCRIPTIONS
The Center of Colobomas, 1961–1995
In the 1940s and 1950s, Custodis51 and Schepens3 developed scleral buckling techniques that eventually were applied to colobomatous eyes. This advance changed the focus from the thorough description of colobomas to the identification and subsequent closure of retinal breaks. Like their anatomist counterparts, clinicians wrote papers that analyzed the center and margins of colobomas. Yet the structure of colobomas remained mysterious because modern clinicians had long ago forgotten their histological basis.
Jesberg and Schepens,2 in 1961, described the center of colobomas as an “island of unpigmented tissue, everted or ectopic sensory retina” that was subject to considerable variability in arrangement. There were cystic dilations, abnormal retinal tissue, and a “peculiar membrane” that stretched across the colobomatous area. Jesberg and Schepens also discussed the “diaphanous nature of the retinal membrane, flimsy membrane, abnormal preretinal tissue, maldeveloped retina, which lined the coloboma.” Twenty years later, in 1981, Patnaik and Kalsi30 described the center as a “thin, underdeveloped, diaphanous retina overlying the pigmentless, featureless area of choroidal coloboma.” Schepens,3 at the same time, described the center as “undeveloped retina, flimsy membrane which occasionally showed recent hemorrhages in it.” Chams and Chams,32 in 1984, described the retina as being reduced to a “thin dysplastic layer with fibroglial proliferations.” Wang and Hilton,33 in 1985, spoke of a “thin layer of rudimentary retina with a few blood vessels overlying the sclera which may be ectatic.” They also found “retinal breaks within the rudimentary retina of the coloboma.”
Gopal and colleagues,34 in 1991, described the central colobomatous area as consisting of a “thin layer of hypoplastic retinal tissue.” They noted that the choroidal and retinal pigment epithelium were not developed in this region and that the sclera underlying the colobomatous area was usually thin and ectatic, producing a staphyloma. These investigators observed not only that the diaphanous retinal tissue stretched over the coloboma, but also that coloboma-related detachments were associated with breaks in the retinal tissue covering the coloboma. In their experience, breaks sometimes occurred on the slope, but were more commonly located in the center of the colobomatous area. The floor of the coloboma was usually irregular and scalloped; the margins of the break could sometimes be seen to merge with the boundaries of the scalloped areas. The central breaks were usually oval and fairly large, more than one optic disc in diameter.
In the same year, Hanneken and coworkers35 characterized the center as “absence of normal retina, retinal pigment epithelium and choroid.” They observed that retinal breaks were usually small, narrow slits in regions of thin rudimentary retina within the colobomatous crater. The retina overlying the coloboma was extremely atrophic, thin, and transparent. Within the coloboma, however, the RPE frequently was absent and, when present, was abnormal.
McDonald and coworkers,36 also in 1991, stated that the center of colobomas consisted of thin hypoplastic retinal tissue overlying an area in which uveal tissue and the RPE were absent. Breaks within the coloboma were invariably round or oval. It was uncertain, they said, whether these breaks were related to vitreoretinal traction. The retinal detachments occurring within these breaks were, in their view, most likely related to vitreous liquefaction or progressive accumulation of subretinal fluid that had passed over the edge of the coloboma.
In 1995, Gopal and colleagues39 found that 63.8% of breaks inside colobomas were located within two disc diameters of the margin, whereas the rest of the breaks were located in the central portion, the distance of margin to center depending on the size of the coloboma. Where subretinal fluid was present within a coloboma (type II), the role of coloboma in causing retinal detachment needed to be determined. These authors noted that breaks of this second type were oval and atrophic, and occurred within the atrophic retinal tissue. At no point could a traction operculum be demonstrated in front of them.
The Margin of Colobomas, 1961–1995
Descriptions of the margin of colobomas have focused on pigmentation, demarcation, and barrier function. If a barrier was incomplete or missing, one could be enforced with a laser to limit either detachment or the risk thereof to one side of the margin.
Jesberg and Schepens,2 in 1961, expressed the view that the retina is not bound or attached to the margin of the defect, although an incomplete line of hyperpigmentation just peripheral to it suggested an attachment. Wang and Hilton33 also noted “a pigmented line along the edge of the coloboma, which suggested a demarcation line with some adhesion,” even though the pigment line seemed not to afford any barrier to the movement of subretinal fluid. Gopal and coworkers,34 in 1991, noted a demarcation line along part or all of the border of colobomas, which they felt might be confused with retinopathy scars in patients undergoing surgery.
Hanneken and associates35 remarked that endolaser, retinopexy, or endocryotherapy could be applied to the coloboma margin to create a border of chorioretinal adhesion and to separate normal from abnormal retina. In 1991, McDonald and coworkers,36 in a discussion of surgical technique, stated that in each case they attempted to drain either through the retinal break at the margin or within the choroidal coloboma. In two of the eyes included in this report, they administered intraoperative laser treatment to the entire margin of the RPE surrounding the coloboma. Despite retinal reattachment, visual acuity in these eyes failed to improve as a consequence of laser-induced nerve fiber damage.
In 1995, Gopal and associates,39 during fundus examination, paid particular attention to the margins of colobomas and thus identified a group of eyes in which retinal detachment did not extend at all inside the margin (type I). In the majority of these eyes, they saw a break at the edge of the detachment inside the coloboma; that is, if they traced a retinal detachment from the periphery beyond the colobomatous margins into the coloboma, they were able to note that the breaks occurred along the line where the detachment ended. In the literature, therefore, descriptions of the margin as a barrier remained inconclusive.
THE MARGIN AS A BARRIER
Early histological descriptions mentioned detachment of the intercalary membrane,12,14 whereas detachment of the extracolobomatous retina was observed only by later authors.26,28 Of the few investigators who noted both of these points, Komoto28 felt that the margin acted as a barrier: “The fundus was occupied by the detached retina on both sides, temporal and medial, with the exception of the place of coloboma, where the flow of fluid was prevented.”
Partial involvement of the margin with intercalary detachment was described in four of Jesberg and Schepens’ cases,2 three of Patnaik and Kalsi’s cases,30 seven of Chams and Chams’ series of eight cases,32 and 18 of the 26 cases studied by Wang and Hilton.33 Gopal and colleagues39 distinguished detachment of the extracolobomatous retina (type I) from detachment of the intercalary membrane (type II). In their type IIA, the detachment was limited to the intercalary membrane. They found types IIB through E, which included the extracolobomatous retina, indicating disruption of the barrier, in 88.9% (32) of 36 eyes with detachments in their series.
Two more recent reports have described spontaneous ruptures of the thin sclera forming the floor of chorioretinal colobomas.40,41 In both of the cases reported, a leaking fistula was complicated by hypotony and had to be repaired surgically. Even though these defects involved the sclera and intercalary membrane, there was no associated retinal detachment, a finding that is consistent with the suggestion that there is an intact barrier at the margin of colobomas.
AGE AT FIRST PRESENTATION OF PATIENTS WITH COLOBOMA AND RETINAL DETACHMENT
Table 1 shows the gender and age at presentation of all patients with coloboma and retinal detachment whose cases have been reported in the literature. This group includes 69 males and 33 females. The average age of those patients whose exact age was disclosed was 26 years. In addition, Chams and Chams32 reported on eight males ranging in age from 12 to 25 years, with an average age of 18 years; Wang and Hilton33 reported on 19 males and six females aged 11 to 29 years; and Gopal and coworkers34 reported on 12 males and four females aged 6 to 37 years, with an average age of 19.37 years. According to the cases reported in the literature, the typical patient with coloboma-associated retinal detachment is a male under the age of 26 years.
METHODS
This study, begun in the summer of 1999, involved examination of the 53 sets of slides and clinical records carrying a diagnosis of coloboma in the W. Richard Green Eye Pathology Laboratory of the Wilmer Eye Institute, Baltimore. The purpose was to determine the structure of choroidal colobomas and the associated risk and mechanism of retinal detachment in patients of different ages. Permission was obtained from the institutional review board for this study (IRB AAAB0415). In this report, the identity of patients has been masked by ranking the acquisition numbers and assigning patient numbers based on this list. Cases were excluded if the records were incomplete or if slides showing the colobomatous malformations were lacking. The following disorders were observed among the excluded cases of coloboma: myelinated nerve fibers (four cases); phthisis (three cases); total retinal detachment, autolysis, and lid colobomas (two cases each); and massive gliosis, atrophy of the RPE, myopia, and nanophthalmos (one case each). The remainder of the 32 excluded case records either included no slides or included none that showed evidence of coloboma.
Selected illustrations of five cases (patients 7, 8, 11, 12, and 20) had previously been published in book chapters on coloboma.47,52 Including these five, 21 cases remained. The patients whose records were used ranged in age from 1 day to 78 years. For the purpose of the study, they were divided into a pediatric group (14 patients, 1 day to 7 months of age) and an adult group (7 patients, 17 to 78 years of age). Tables 2 and 3 show patients’ ages and the associations of colobomas with other abnormalities in the pediatric and adult groups.
TABLE 2.
OCULAR AND SYSTEMIC FINDINGS ASSOCIATED WITH PEDIATRIC COLOBOMAS
| RECORD NUMBER | AGE | RACE/GENDER | OCULAR FINDINGS | SYSTEMIC FINDINGS |
|---|---|---|---|---|
| Patient 1 | 17 mo | W/F | Microphthalmos; retinal aplasia; scleral ectasia; corneal scarring; optic atrophy; coloboma of iris, retina, and choroids | |
| Patient 2 | 3 mo | B/M | Cataract, posterior lens epithelial cells, persistent hyperplastic primary vitreous, retinal dysplasia, coloboma, sclera cartilage | Trisomy 13, intraventricular septal defect, patent ductus arteriosus, right ventricular hypertrophy, cystic disease of kidneys, hydrocele, polydactylism, umbilical hernia |
| Patient 6 | 2 days | W/M | Posterior embryotoxon cornea; incomplete cleavage of angle; coloboma of iris, choroid, opticus; tunica vasculosa lentis; microphthalmos | Trisomy 13 (Patau), tetralogy of Fallot, persistent left superior vena cava, low-set ears, cleft lip and palate, undescended testes, polydactyly (double 5th digit), varus deformity of feet |
| Patient 10 | 18 mo | W/F | Microphthalmos with cyst; cataract; incomplete angle cleavage; coloboma of optic nerve, choroid; glial proliferation in coloboma | |
| Patient 11 | 7 mo | W/F | Microphthalmos; tunica vasculosa lentis; coloboma of retina, disc, sclera; retinal dysplasia | Tetralogy of Fallot, Blalock-Taussig, aberrant right subclavian artery, malformation right ear, square cranium |
| Patient 12 | 2 days | W/M | Abnormal cleavage of angle; coloboma of iris, ciliary body, retina, sclera; cataract; tunica vasculosa lentis; persistent primary hyperplastic vitreous; hypoplasia of disc; retinal dysplasia; microphthalmos | Peculiar face; congenital heart disease; aortic atresia; skin tag, right ear; malrotation of bowel; hypoplasia of fingernails |
| Patient 16 | 8 mo | W/F | Microphthalmos, cataract, persistent primary hyperplastic vitreous, retinal detachment, aplasia optic nerve | |
| Patient 18 | 2 days | M | Synophthalmus; microphthalmos; retinal dysplasia; coloboma of iris, retina, choroids; hypoplasia of iris, ciliary body, meshwork; tunica vasculosa lentis | Holoprosencephaly, hypoplastic pituitary, hypoplastic adrenals |
| Patient 19 | 3 days | W/M | Microcornea; coloboma of iris, choroid, opticus; retinal dysplasia | Triploidy, hypoplasia of hypophysis |
| Patient 20 | 25 days | W/F | Coloboma of choroids, retinal dysplasia, optic nerve hypoplasia, overhang of choroid and sclera | Hydrocephalus, chromosomes normal, sepsis |
| Patient 21 | 1 day | W/M | Coloboma of iris, choroid, optic nerve; cataract; tunica vasculosa lentis; retinal dysplasia | Potter’s syndrome, trisomy 14, renal agenesis, omphalocele, patent foramen ovale, micrognathia, clubbed feet, cephalohematoma, ear displacement |
| Patient 23 | 8 days | W/F | Microphthalmos; coloboma of iris, ciliary body, choroid, sclera; persistent hyperplastic primary vitreous; retinal dysplasia; optic nerve hypoplasia | Trisomy 13 (Patau), low-set ears, polydactyly, abnormal pulmonary valve, intestinal malrotation, base of skull anomaly |
| Patient 24 | 8 days | B/F | Coloboma of iris, choroid, optic nerve; cataract; persistent primary hyperplastic vitreous; retinal dysplasia; cartilage | Trisomy 13 (Patau), patent ductus arteriosus, low-set malformed ears, 6 digits, hypertelorism, cardiomegaly, simian crease, septate vagina |
| Patient 25 | 3 mo | F | Coloboma of iris, ciliary body, choroid, macula; retinal dysplasia; ectopic retina in sclera | Charge syndrome, square face, malar flattening, anteverted nares, cardiac anomalies, large pinnae, renal malformation |
TABLE 3.
OCULAR AND SYSTEMIC FINDINGS ASSOCIATED WITH ADULT COLOBOMAS
| RECORD NUMBER | AGE | RACE/GENDER | OCULAR FINDINGS | SYSTEMIC FINDINGS |
|---|---|---|---|---|
| Patient 7 | 73 yr | W/M | Arteriolosclerosis of retina and choroids; microcornea; prominent Schwalbe’s ring; pars plana cyst, ciliary body hypoplasia; coloboma of iris, retina; anterior chamber angle, anomalous configuration; drusen; chronic choroiditis | Hypertension, atrial fibrillation, coronary artery disease, pulmonary emboli, degenerated discs L3-L4, smoked until 4 years previously |
| Patient 8 | 44 yr | W/M | Hyaloid remnants, coloboma of choroids, scleral thinning, retinal dysplasia | |
| Patient 9 | 73 yr | M | Hemorrhagic choroidal detachment, coloboma of choroids, arteriolosclerosis of retinal vessels, optic nerve atrophy, mild retinal gliosis and atrophy, endophthalmitis | |
| Patient 14 | 29 yr | B/M | Scleral staphyloma, chorioretinal atrophy, coloboma of disc and choroids | Mental retardation, subdural hematoma, dysmorphic cranium, diffuse brain atrophy, zygoma |
| Patient 15 | 17 yr | W/F | Blunt trauma, 5 years previously; postcontusion angle deformity; cataract; retinal atrophy; coloboma of choroids; optic nerve atrophy; buphthalmos and megalocornea | |
| Patient 22 | 78 yr | F | Embryotoxon of cornea; coloboma of iris, choroids; cataract; optic atrophy; scleral thinning | Congenital absence of left half of nose, inferiorly displaced orbit, absence frontal and maxillary sinus |
| Patient 26 | 36 yr | M | Nanophthalmos; postcontusion deformity angle; cataract; retinal atrophy; ?traumatic retinopathy; coloboma of choroid, optic nerve |
RESULTS
MATERNAL AND GESTATIONAL HISTORY
The three patients with coloboma whose records included their maternal history had colobomas in both eyes (patient 3), severe glaucoma (patient 4), rheumatic fever (patient 13), and the mother’s taking of LSD before pregnancy (patient 13). Gestational problems included maternal hypertension (patient 5), diabetes (patient 6 and patient 18), premature labor (patient 13), hyperemesis (patient 14), phlebitis (patient 17), and oligohydramnios (patient 21).
Sources of Specimens and Causes of Death
In the pediatric group, 11 eyes were obtained at autopspy (patients 2, 6, 11, 12, 18, 19, 20, 21, 23, 24, and 25) and three eyes were surgical specimens (patients 1, 10, and 16. The reasons for surgical removal were microphthalmos (patients 1 and 10) and leukocoria (patient 16). Eight patients in this group died of cardiac causes (patients 2, 6, 11, 12, 21, 23, 24, and 25). Patient 18 died as a consequence of multiple malformations, patient 19 of encephalomalacia, and patient 20 of hydrocephalus. The fates of the three surgical patients in this group are not known; at this writing, their ages would be 28, 37, and 79 years.
Three eyes in the adult group were obtained at autopsy (patients 7, 8, and 14), and four were surgical specimens (patients 9, 15, 22, and 26). The reasons for surgical removal of these eyes were keratitis (patient 9), glaucoma (patient 15), and blind eye (patients 22 and 26). Patient 7 died of cardiac arrest and patient 12 died of fever; the cause of death for patient 8 is unknown. The fates of the remaining four surgical patients are not known; at this writing, their ages would be 44, 44, 91, and 108 years.
Histologic Findings, Pediatric Group
In this group, colobomas were characterized by rosettes in the intercalary membrane, a central glial triangle, a point of reversal and duplication of the photoreceptor layer, a locus minoris resistentiae, and lateral displacement of the RPE (Table 4, Figure 1 left).
TABLE 4.
HISTOLOGIC FINDINGS ASSOCIATED WITH PEDIATRIC COLOBOMAS
| RECORD NUMBER | RETINAL INTERCALARY | TRIANGLE | POINT OF REVERSAL | PIGMENT EPITHELIUM | CHOROID INTERCALARY | CHOROID | SCLERA INTERCALARY | SCLERA | OTHER |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Schisis | (+) | + | − | − | Thin | Stretch | ||
| Patient 2 | (+) | + | + | − | − | Thin | Thin | Traction | |
| Patient 6 | Rosettes | + | + | + | − | − | Thin | Thin | |
| Patient 10 | − | (+) | + | ||||||
| Patient 11 | + | + | + | ++ | − | − | Thin | Ridge | |
| Patient 12 | Rosettes | − | + | − | + | Thin rosettes | Ridge | Rosettes | |
| Patient 16 | + | − | − | + | Retinal detachment | ||||
| Patient 18 | Rosettes | + | + | + | Vessels | Vessels | Thin | Thin | Rosettes |
| Patient 19 | − | − | − | ||||||
| Patient 20 | Rosettes | + | + | + | Vessels | Vessels | Thin | Ridge | |
| Patient 21 | Rosettes | (+) | + | + | + | Ridge | |||
| Patient 23 | Rosettes | ++ | + | + | − | Thin | |||
| Patient 24 | Rosettes | − | + | + | − | + | |||
| Patient 25 | Rosettes | − | + | Vessels | + | Cartilage |
Symbols: (+)= mild
+ = moderate
++ = severe
− = absent.
FIGURE 1.
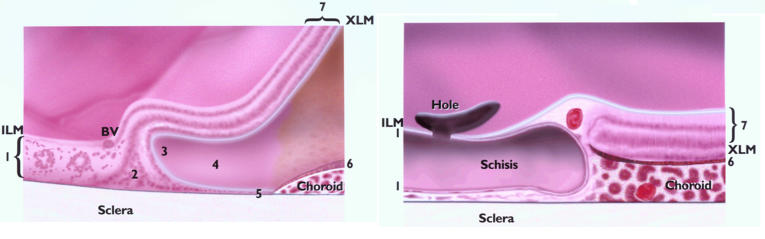
Schematic comparison of the margin of pediatric (left) and adult (right) colobomas. 1 = intercalary membrane, 2 = glial triangle, 3 = point of reversal, 4 = duplication, 5 = locus minoris resistentiae, 6 = pigmented epithelium, 7 = extracolobomatous retina. Young colobomas are characterized by features 1 through 7, adult by 1, 6, and 7. The absence of 2 through 5 possibly results in a more resilient barrier.
The Center
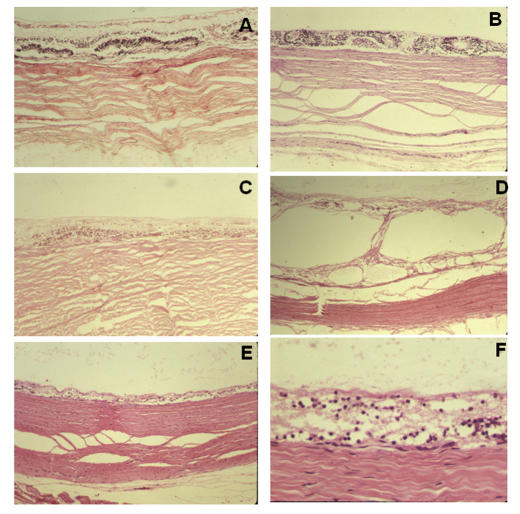
In all 14 cases, the intercalary membrane was in direct contact with the sclera; the choroid and RPE were absent centrally (Figure 2, A through F). Neuronal elements were variably preserved. Rosette formation was found in eight cases (Figure 2, A and B); schisis was present in one case (Figure 2, D).
FIGURE 2.
Histologic findings at the center of colobomas in pediatric patients. In general, the retinal pigment epithelium and choroid are lacking and the retina (intercalary membrane) is directly apposed to the sclera. Special features are rosette formation (A, B), schisis (D), and variable neuronal atrophy (E, F). A, Patient 6, hematoxylin-eosin, ×10. B, Patient 20, hematoxylin-eosin, ×10. C, Patient 11, hematoxylin-eosin, ×10. D, Patient 1, hematoxylin-eosin, ×4. E, Patient 20, hematoxylin-eosin, ×4. F, Patient 20, hematoxylin-eosin, ×40.
The Margin
Intercalary Membrane.
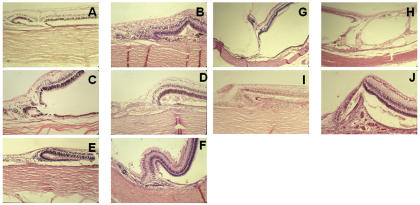
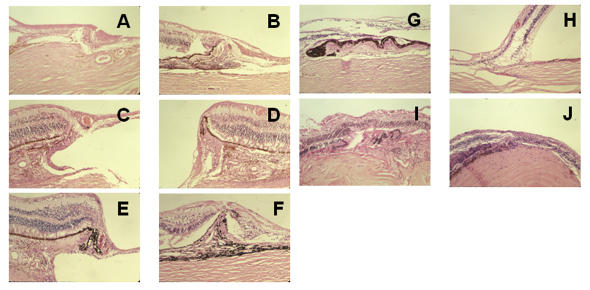
The inner layer of the retina continued centrally as intercalary membrane in all cases (Figure 3, A through J). In one case, a prominent attachment of the vitreous resembled a cystic retinal tuft (Figure 3, G).
FIGURE 3.
Histologic examination of the margins of colobomas in pediatric patients. In general, the inner retinal layer extends centrally, and the outer layer reverts to meet the laterally displaced retinal pigment epithelium (RPE). The perverse orientation of the photoreceptors is shown (A, B, C, E), as are the radial glia oriented parallel to scleral fibers forming a triangle between the inner and outer retina (D, E, F, I). There is exceptional vascular support of the margin (D), vitreous traction (G), and schisis of the transition to intercalary membrane (H). There is choroidal thickening and RPE hyperplasia (I, J), as well as subchoroidal invasion by the retina (I), which resembles a pocket. Rosettes are part of the margin (A, C). A, Patient 6, hematoxylin-eosin, ×10. B, Patient 21, hematoxylin-eosin, ×10,. C, Patient 18, hematoxylin-eosin, ×10. D, Patient 20, hematoxylin-eosin, ×10. E, Patient 18, hematoxylin-eosin, ×4. F, Patient 23, hematoxylin-eosin, ×10. G, Patient 2, hematoxylin-eosin, ×4. H, Patient 1, hematoxylin-eosin, ×4. I, patient 11, hematoxylin-eosin, ×10. J, Patient 20, hematoxylin-eosin, ×10.
The Glial (Bipolar) Triangle.
The bipolar cells of the retina were rotated so that their axes were parallel to the scleral fibers, a finding that is related to the central continuation of the inner retinal (ganglion cell) layer and the reversal of the photoreceptor layer (Figure 3, A through I). Aligned with the bipolar cells, the Müllerian cells spanned the internal limiting membrane to the external limiting membrane. The inner and outer layers continued in opposite directions, forming a triangle, the base of which rested on the sclera (Figure 1 left). Such triangles, having variable degrees of vascular support, were found in nine of the 14 pediatric cases (Table 4).
Point of Reversal of Photoreceptor Layer.
Central to the termination of the RPE and choroid, a clear point of reversal of the photoreceptor layer was found in 12 of the 14 cases. In four cases, the reversal was a complete duplication in that outer segments faced outer segments (Figure 3, A, B, C, and E).
RPE and Locus Minoris Resistentiae.
The RPE was laterally displaced in all cases and supported by the choroid in six of the 14 cases. The locus minoris resistentiae, consisting of dispersed noncohesive neuronal cells, extended from the point of reversal to the RPE. Variable degrees of vascularization characterized the junction between the external limiting membrane and the RPE. In most cases, there were no vessels, but in a few, vascularization was abundant (Figure 3, D and J).
Choroid.
The choroid ended together with the RPE. In two cases, choroidal thickening (Schwiele) was noted, with formation of a pocket (Tasche) (Figure 3, I and J)
Sclera.
Scleral thinning was the rule. A scleral ridge (Skleralleiste) at the margin was noted in four of 14 cases. Intrascleral rosettes were present in two of the 14 cases.
Histologic Findings, Adult Group
Colobomas in adult eyes were characterized by marginal retinal vessels, marginal choroidal thickening, and hyperplasia of the RPE (Table 5). Thus, the transition between the intercalary membrane and extracolobomatous retina was closer to the border of the choroid and RPE, more compact, and more distinct than in the pediatric sample (Table 5, Figure 1 right). The glial triangle, point of reversal, duplication, and locus minoris resistentiae were largely absent, and with them the structural weakness they caused.
TABLE 5.
HISTOLOGIC FINDINGS ASSOCIATED WITH ADULT COLOBOMAS
| RECORD NUMBER | RETINAL INTERCALARY | TRIANGLE | POINT OF REVERSAL | PIGMENT EPITHELIUM | CHOROID INTERCALARY | CHOROID | SCLERA INTERCALARY | SCLERA | OTHER |
|---|---|---|---|---|---|---|---|---|---|
| Patient 7 | Schisis, sclerosis | (Wedge) | − | Drusen | − | + | Thin | Ridge | Old vessels |
| Patient 8 | Atrophy, schisis | Wedge | (+) | ++ | − | Thick | Retinal schisis | ||
| Patient 9 | Rosettes | − | + | ++ | |||||
| Patient 14 | Schisis, atrophy | Wedge | (+) | ++ | − | Thick | |||
| Patient 15 | Schisis, atrophy | + | + | + | |||||
| Patient 22 | Schisis, atrophy Sclerosis | − | − | Drusen | − | Thin | Ridge | ||
| Patient 26 | Sclerosis |
Symbols: (+)= mild
+ = moderate
++ = severe
− = absent.
The Center
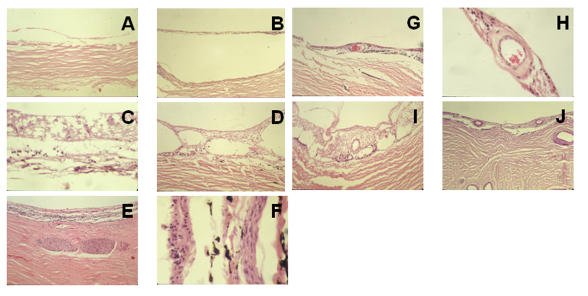
The intercalary membrane was atrophic and hypocellular and had defects in three of the seven eyes (Figure 4, A through C). In five eyes, the intercalary membrane was split (Table 5 and Figure 4, D). Vessels in the membrane showed varying degrees of intimal hyperplasia (Figure 4, G through J). Centrally, the choroid was absent in all cases. The sclera was thinned and showed intrascleral nerves in two cases (Figure 4, E and F).
FIGURE 4.
Histologic examination of the center of colobomas in adult patients. In general, the variably atrophic intercalary membrane is directly apposed to the sclera. Neuronal atrophy (A through J), schisis (D, I), and arteriosclerosis of intercalary vessels (G through J) are consistent with aging. The thinned sclera contains nerves (E, F). A, Patient 7, hematoxylin-eosin, ×10. B, Patient 8, hematoxylin-eosin, ×10. C, Patient 8, hematoxylin-eosin, ×40. D, Patient 14, hematoxylin-eosin, ×10. E, Patient 26, hematoxylin-eosin, ×10. F, Patient 14, hematoxylin-eosin, ×40. G, Patient 8, hematoxylin-eosin, ×10. H, Patient 8, hematoxylin-eosin, ×40. I, Patient 14, hematoxylin-eosin, ×10. J, Patient 22, periodic acid–Schiff stain, ×10.
The Margin
Intercalary Membrane.
The inner retinal layer continued across the coloboma. In five of six cases of schisis, the outer layer emerged from the RPE and lined the concavity of the choroid (Tasche) and sclera (Figure 5, C and E).
FIGURE 5.
Histologic examination of the margin in adult patients. In general, there is an abrupt termination of the retinal pigment epithelium and choroid, which are often hyperplastic and thickened. Both are centrally displaced, eliminating the point of reversal and locus minoris resistentiae and thus adding to structural stability. Moreover, abundant vascular supply of the margin is seen (A, C, D, E). Choroidal thickening and pockets anchor the intercalary membrane and form a seal (B through F), and intercalary schisis and hole formation reduce traction on the margin (C, E). Drusen-like deposits are consistent with aging (G). In smaller colobomas, well-vascularized intercalary membranes show preservation of inner layers (I, J). A, Patient 7, hematoxylin-eosin, ×10. B, Patient 8, hematoxylin-eosin, ×4. C, Patient 8, hematoxylin-eosin, ×10. D, Patient 8, hematoxylin-eosin, ×10. E, Patient 8, hematoxylin-eosin, ×10. F, Patient 14, hematoxylin-eosin, ×4. G, Patient 22, hematoxylin-eosin, ×10. H, Patient 15, hematoxylin-eosin, ×10. I, Patient 9, hematoxylin-eosin, ×4. J, Patient 26, hematoxylin-eosin, ×10.
Glial (Bipolar) Triangle.
This feature was largely absent in this group. The inner nuclear layer connected to the margin of RPE hyperplasia (Figure 5, A through G, I). Central to this, the inner layer continued as the intercalary membrane, which showed a vessel at the margin in four eyes (Figure 5, C through F).
Point of Reversal.
This point was less distinct in older eyes than in the pediatric sample. With concentration, it could be identified in four eyes. Whereas the point of reversal was central to the RPE in young eyes, in the older group the reversal of the photoreceptor layer was peripheral to the RPE hyperplasia (Figure 5, B through E).
Retinal Pigment Epithelium.
In four cases, the RPE was hyperplastic (Figure 5, D through G) and was supported by a wedge of thickened choroid that resulted in central elevation and overhung the pigmented margin (Figure 5, B and F). Drusen were noted in two of the seven eyes (Figure 5, G).
Choroid.
There was central absence of choroid in all cases. The marginal choroid was thickened, creating a peripheral ridge that supported the hyperplastic RPE.
Sclera.
A scleral ridge was noted in two of the seven eyes and was a prominent feature in most of these four eyes.
DISCUSSION
Colobomas encompass a range of malformations related to faulty closure of the embryonic fissure (Figures 2 through 5). In this study, the grouping of cases as pediatric versus adult meant, implicitly, severe versus mild. Severe colobomas are the ones most likely to be associated with other malformations.53,54 Also, patients with severe malformations, in addition to being more likely to undergo histologic examination at a young age, have a low likelihood of survival (Table 2). Indeed, patients with colobomas who make it to adulthood are relatively rare (Table 3). Therefore, most eyes from pediatric patients are obtained at autopsy, although a few are surgically removed to rule out a tumor or for cosmetic reasons. Fewer adult eyes are obtained at autopsy; the reasons for surgical removal are blindness, keratitis, and glaucoma. The survivors’ colobomas tend to be less severe and to occur as isolated malformations. Therefore, colobomas appear to represent two distinct phenotypes and populations with respect to association and survival. It is not clear whether the pediatric phenotype can transform into the adult phenotype with the passing of years; that is, whether, as opposed to being born with protective features, patients could develop them.
As Table 1 indicates, in patients who survive, and especially in males, colobomas are structurally challenged beginning in the second decade of life, long after the completion of ocular growth. The main challenge may be mechanical stress along the entire extent of the malformed tissue barriers. Stretching of the thin ocular walls and collapse of the vitreous generate tractional forces that possibly are related to minor trauma.55 Alternatively, these forces may be generated chronically through elevated intraocular pressure (patient 15) or by everyday activities. Apart from nonrhegmatogenous pathomechanisms, retinas stay attached because of intact neuroepithelial barriers and lack of traction on them. Two such neuroepithelial barriers to fluid conduit are present in colobomas, one in the center and the other at the margin.56 Structural changes in the barriers related to aging may either result in or protect against retinal detachment.
THE INTERCALARY MEMBRANE AS A FIRST BARRIER
The center of the coloboma, derived from the inner neuroblastic layer, is the structural equivalent of the inner retina, with glial cells contributing to its mechanical strength. Many clinicians have observed the formation of holes, most often in the center of colobomas or just central to their margins.34-36,39,42,57 Although the formation of holes is related to aging and traction, the main cause may be malnutrition of this tissue. Because of the absence of the RPE and choroid, the intercalary membrane must rely entirely on its hypoplastic retinal vasculature.
Choroidal neovascularization favors the margin, with vessels tending to grow toward the intercalary membrane, possibly in response to an ischemic stimulus.58-60 The endarterial retinal vessels may occlude, further compromising the integrity of this thin layer. Jesberg and Schepens2 and Schepens3 found hemorrhages in the intercalary membrane associated with retinal detachments. These resembled hemorrhagic infarcts of the central nervous system associated with tissue necrosis and defects.
THE MARGIN AS A SECOND BARRIER
As holes form in the intercalary membrane, detaching it, the margin has to seal the extracolobomatous subretinal space and prevent extension of the rhegmatogenous detachment of the intercalary membrane. In colobomas, the marginal barrier can be traced to the outer neuroblastic layer.48 Because of the reversal of that layer, the separation between the extensions of Müllerian glia and the RPE, which results in the locus minoris resistentiae, can be seen most clearly in the pediatric age group. The “locus” deserves this time-honored designation because glia neither bridge the gap nor contribute to its junctional system, the external limiting membrane. Instead, noncohesive neuronal cells are found between the point of reversal and the RPE in the pediatric population. The compromised barrier has variable degrees of choroidal vascular support; lack of adequate support further limits its survival. In the absence of a mechanical barrier, a break in the intercalary membrane is likely to result in extracolobomatous retinal detachment. In contrast, in adult colobomas, a compact cohesive margin is found. Müllerian glia resembling scars connect to the hyperplastic RPE. The triangle formed by bipolar cells and radial glia, the point of reversal, the duplication of retinal layers, and the locus minoris resistentiae are largely absent, reinforcing the resilience of the margin. Additional features suggesting the structural stability of this barrier are marginal vessels and a thickened, overhanging choroid that supports the hyperplastic RPE.
Overall, it appeared in these samples that with advancing age, the margin had moved peripherally toward the RPE and choroid, bringing the junctional complexes of glia and the RPE closer together (Figure 1). Moreover, in adult patients, the intercalary membrane was either split or detached from the sclera. Gluing holes in a split or atrophied intercalary membrane may not be effective because only the internal layer of the schisis will be sealed, so that progressive atrophy may enlarge the break.35 Contraction of the intercalary membrane may be stimulated by vitreoretinal surgery and tamponade. Probably for this reason, Wang and Hilton33 supported the margin with a buckle, while Gopal and colleagues34 incised and weakened the intercalary membrane and meticulously removed the vitreous. One might therefore surmise that attachment of the extracolobomatous retina depends on a firm seal at the margin of the coloboma and a lack of traction mediated by both the intercalary membrane and vitreous.
EFFECT OF AGE ON THE BARRIERS
Although the distinction between young and elderly patients is somewhat arbitrary, retinologists are most likely to be confronted with those who have survived into their second decade. Based on observation of the histological material used in the present study, one cannot tell which of the young colobomas, given enough time, would have developed retinal detachment. However, it was possible to observe the features of colobomatous eyes in which, over a long period, detachment had not occurred. On this basis, the conditions that are necessary for structural survival of the retina appear to be a vascular supply by marginal vessels, a thickened choroid, RPE hyperplasia, and an atrophic split in the intercalary membrane; that is, a strong marginal seal and a weakened center. Few of the young colobomas had these features. Colobomas had in common the malnourished glial center, either with or without holes, but differed in the quality of their margins.
SUMMARY
Jules Gonin29 said, “To effectively combat a pathological process one must know the nature and the anatomic conditions.” Following this precept, the present study was based on the thesis that colobomatous malformations age, if allowed to do so. During that process, the specific pathoanatomy of barriers to fluid flow (the intercalary membrane, glial triangle, point of reversal, duplication, locus minoris resistentiae, RPE, and extracolobomatous retina) may, in a subset of moderate colobomas, either protect against or result in retinal detachment.
Because retinal detachments associated with colobomas frequently occur during the second decade of life, pediatric and adult structures were compared and served as the basis for a speculation on the pathomechanism involving two barriers to fluid conduit. The adult features presented are likely to be present in colobomas seen in retinal practice. In the future, their morphology may be corroborated by imaging techniques. If the speculation offered here regarding the two barriers has merit, then detachments currently can best be treated by vitreoretinal surgical techniques to reduce traction on the margin exerted by the vitreous and intercalary membrane and to reinforce the margin.
ACKNOWLEDGMENTS
I thank D. Richard Green for the example he set, and for his scholarship and generosity. The study presented both lived and was limited by the sections of cases and their history that he made available and allowed to be shared.
Footnotes
Supported by an unrestricted grant from Mr Walter Klein.
REFERENCES
- 1.Von Ammon. Anatomische Untersuchung von Coloboma bulbi. Z Ophthalmol 1830: I.
- 2.Jesberg D, Schepens C. Retinal detachment associated with coloboma of the choroid. Arch Ophthalmol. 1961;65:163–173. doi: 10.1001/archopht.1961.01840020165003. [DOI] [PubMed] [Google Scholar]
- 3.Schepens CL. Retinal Detachment and Allied Diseases Vol 2. Philadelphia: WB Saunders; 1983:615–617.
- 4.Hannover A. Cited by Hess C. Zur Pathogenese des Microphthalmus. Archiv Ophthalmol. 1888;34:162. [Google Scholar]
- 5.Hannover A. Funiculus sclerae. Séance de L’academie des sciences danoise. 8. XII. 1876.
- 6.Pause H. Anatomischer Befund bei einem Colobom der Iris und Choriodea. Arch Ophthalmol. 1878;24:84–92. [Google Scholar]
- 7.Deutschmann R. Zur pathologischen Anatomie des Iris und Aderhautcoloboms, als Grundlage eines Erklaerungsversuches der sogenannten Hemmungsbildungen ueberhaupt. Klin Monatsbl Augenheilkd. 1881;19:101–113. [Google Scholar]
- 8.Hess C. Zur Pathogenese des Microphthalmus. Arch Ophthalmol. 1888;34:147–193. [Google Scholar]
- 9.Hess C. Weitere Untersuchungen ueber angeborene Missbildungen des Auges. Arch Ophthalmol. 1890;36:135–153. [Google Scholar]
- 10.Hess C. Pathologisch-anatomische Studien ueber einige seltene angeborene Missbildungen des Auges. I. Ueber die Pathogenese der Orbitalcysten. Arch Ophthalmol. 1896;42:214–224. [Google Scholar]
- 11.Manz W. Ueber das angeborene Colobom des Schnerven. Arch Augenheilkd. 1891;23:1. [Google Scholar]
- 12.Bock E. Die angeborenen Kolobome des Augapfels. Eine anatomische und klinische Studie Wien: Verlag von Josef Safar; 1893:93–121.
- 13.Van Duyse. Contribution a l’etude des colobomes de l’oeil. Arch D’Ophtalmol. 1896;16:432–449. [Google Scholar]
- 14.Mannhardt F. Das Colobom der Aderhaut und seine Folgen. Arch Ophthalmol. 1897;43:127–145. [Google Scholar]
- 15.Bach L. Weitere Beitraege zur Kenntnis der angeborenen Anomalien des Auges mit besonderer Beruecksichtigung der Genese der Korektopie. Z Augenheilkd. 1901;6:359–370. [Google Scholar]
- 16.Bach L. Pathologisch-anatomische Studien ueber verschiedene Missbildungen des Auges. Arch Ophthalmol. 1898;45:1–74. [Google Scholar]
- 17.Von Hippel E. Die Missbildungen und angeborenen Fehler des Auges. In: Graefe-Saemisch, ed. Handbuch der Augenheilkunde 2 Aufl. II Bd. Berlin: Verlag von Julius Springer;1900:18–38.
- 18.Von Hippel E. Embryologische Untersuchungen ueber die Entstehungsweise der typischen angeborenen Spaltbildungen (Colobome) des Augapfels. Graefes Arch Ophthalmol. 1903;55:507–548. [Google Scholar]
- 19.Levinsohn G. Kurzer Beitrag zur Histologie angeborener Augenanomalien. Arch Ophthalmol. 1903–04;57:266–276. [Google Scholar]
- 20.Gilbert W. Beitraege zur Kenntnis der pathologischen Anatomie der angeborenen Colobome des Augapfels mit besonderer Beruecksichtigung des Sehnerven. Arch Ophthalmol. 1906–07;65:185–218. [Google Scholar]
- 21.Seefelder R. Pathologisch anatomische Beitraege zur Kenntnis der angeborenen Colobome des Auges. Graefes Arch Ophthalmol. 1908;68:275–353. [Google Scholar]
- 22.Seefelder R. Ein pathologisch-anatomischer Beitrag zur Frage der Kolobome und Grubenbildungen am Sehnerveneintritt. Graefes Arch Ophthalmol. 1915;90:127–137. [Google Scholar]
- 23.Meisner W. Ein Colobom der Aderhaut und Netzhaut mit Aplasie des Sehnerven. Arch Ophthalmol. 1911;79:308–328. [Google Scholar]
- 24.Baurmann M. Ueber die Entstehung von Scleralausbuchtungen unter dem Sehnerveneintritt an Kolobomaugen. Arch Ophthalmol. 1923;112:495–505. [Google Scholar]
- 25.Henke F, Lubarsch O. Handbuch der speziellen pathologischen Anatomie und Histology Vol 11. Auge Teil 2. Berlin: Springer; 1931;7–36.
- 26.Brinton WT. Coloboma of iris and choroid: retinal detachment. Am J Ophthalmol. 1922;5:806. [Google Scholar]
- 27.Wagener H, Gipner J. Coloboma of iris, choroid and optic disc with detachment of the retina. Am J Ophthalmol. 1925;8:694–697. [Google Scholar]
- 28.Komoto J. Detachment of retina with coloboma of iris and choroid. Am J Ophthalmol. 1926;9:414–415. [Google Scholar]
- 29.Gonin J. Le decollement de la retine Lausanne, Switzerland: Librairie Payot; 1934:13.
- 30.Patnaik B, Kalsi R. Retinal detachment with coloboma of the choroid. Indian J Ophthalmol. 1981;29:345–349. [PubMed] [Google Scholar]
- 31.Gonvers M. Temporary use of silicone oil in the treatment of special cases of retinal detachment. Ophthalmologica. 1983;187:202–209. doi: 10.1159/000309327. [DOI] [PubMed] [Google Scholar]
- 32.Chams H, Chams G. Colobome chorio-retinien et decollement de la retine. A propos de 8 cas. Bull Mem Soc F Ophthalmol. 1984;95:333–335. [PubMed] [Google Scholar]
- 33.Wang K, Hilton G. Retinal detachment associated with coloboma of the choroid. Trans Am Ophthalmol Soc. 1985;83:49–62. [PMC free article] [PubMed] [Google Scholar]
- 34.Gopal L, Kini M, Badrinath S, et al. Management of retinal detachment with choroidal coloboma. Ophthalmology. 1991;98:1622–1627. doi: 10.1016/s0161-6420(91)32075-x. [DOI] [PubMed] [Google Scholar]
- 35.Hanneken A, deJuan E, McCuen B. The management of retinal detachments associated with choroidal colobomas by vitreous surgery. Am J Ophthalmol. 1991;111:271–275. doi: 10.1016/s0002-9394(14)72309-8. [DOI] [PubMed] [Google Scholar]
- 36.McDonald HR, Lewis H, Brown G, et al. Vitreous surgery for retinal detachment associated with choroidal coloboma. Arch Ophthalmol. 1991;109:1399–1402. doi: 10.1001/archopht.1991.01080100079046. [DOI] [PubMed] [Google Scholar]
- 37.Corcostegui B, Guell J, Garcia-Arumi J. Surgical treatment of retinal detachment in the choroidal colobomas. Retina. 1992;12:237–241. doi: 10.1097/00006982-199212030-00006. [DOI] [PubMed] [Google Scholar]
- 38.Jalali S, Das T. Selection of surgical technique for retinal detachment with coloboma of the choroid. Indian J Ophthalmol. 1994;42:27–30. [PubMed] [Google Scholar]
- 39.Gopal L, Badrinath SS, Sharma T, et al. Pattern of retinal breaks and retinal detachments in eyes with choroidal coloboma. Ophthalmology. 1995;102:1212–1217. doi: 10.1016/s0161-6420(95)30888-3. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, Narang S, Gupta V, et al. Successful closure of spontaneous scleral fistula in retinochoroidal coloboma. Arch Ophthalmol. 2001;119:1220–1221. doi: 10.1001/archopht.119.8.1220. [DOI] [PubMed] [Google Scholar]
- 41.Viola F, Morescalchi F, Gandolfo E, et al. Ocular hypotony secondary to spontaneously ruptured sclera in choroidal coloboma. Arch Ophthalmol. 2004;122:1549–1551. doi: 10.1001/archopht.122.10.1549. [DOI] [PubMed] [Google Scholar]
- 42.Gopal L, Badrinath S, Sharma T, et al. Surgical management of retinal detachments related to coloboma of the choroid. Ophthalmology. 1998;105:804–809. doi: 10.1016/S0161-6420(98)95018-7. [DOI] [PubMed] [Google Scholar]
- 43.Lee KJ, Peyman GA, Paris CL, et al. Management of retinal detachment associated with choroidal coloboma using perfluoroperhydrophenanthrene (Vitreon) Ophthalmic Surg. 1992;23:553–554. [PubMed] [Google Scholar]
- 44.Unlu N, Kocaoglan MA, Acar BS, et al. Surgical management of retinal detachment with choroidal coloboma. Eur J Ophthalmol. 2002;12:299–303. doi: 10.1177/112067210201200408. [DOI] [PubMed] [Google Scholar]
- 45.Chestler R, France T. Ocular findings in CHARGE syndrome. Ophthalmology. 1988;95:1613–1619. doi: 10.1016/s0161-6420(88)32968-4. [DOI] [PubMed] [Google Scholar]
- 46.Daufenbach D, Ruttum M, Pulido J, et al. Chorioretinal colobomas in a pediatric population. Ophthalmology. 1998;105:1455–1458. doi: 10.1016/S0161-6420(98)98028-9. [DOI] [PubMed] [Google Scholar]
- 47.Green WR. Uveal tract. In: Spencer WA, ed. Ophthalmic Pathology: An Atlas and Textbook Vol. 3. 6th ed. Philadelphia: WB Saunders; 1986:1415–1429.
- 48.Mann IC. The Development of the Human Eye London: Cambridge University Press; 1928;68–115.
- 49.Duke-Elder S. Normal and abnormal development. Congenital deformities Vol III, part 2. St Louis: CV Mosby; 1963;456–481.
- 50.Klien B. The pathogenesis of some atypical colobomas of the choroid. Am J Ophthalmol. 1959;48:509–607. doi: 10.1016/0002-9394(59)90450-7. [DOI] [PubMed] [Google Scholar]
- 51.Custodis E. Bedeutet die Plombenaufnaehung auf die Sklera einen Fortschritt in der operativen Behandlung der Netzhautabloesung? Deutsche Ophthalmol Gesell. 1953:102–105. [Google Scholar]
- 52.Michels R, Wilkinson C, Rice T. Retinal Detachment St Louis: CV Mosby; 1990:156.
- 53.James P, Karseras A, Wybar K. Systemic associations of uveal coloboma. Br J Ophthalmol. 1974;58:917–921. doi: 10.1136/bjo.58.11.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagon R. Ocular coloboma. Surv Ophthalmol. 1981;25:223–236. doi: 10.1016/0039-6257(81)90092-8. [DOI] [PubMed] [Google Scholar]
- 55.Mori S, Komatsu H, Watari H. Spontaneous posterior bulbar perforation of congenital scleral coloboma and its surgical treatment: a case report. Ophthalmic Surg. 1985;16:433–436. [PubMed] [Google Scholar]
- 56.Schubert HD. Schisis-like rhegmatogenous retinal detachment associated with choroidal colobomas. Graefes Arch Clin Exp Ophthalmol. 1995;233:74–79. doi: 10.1007/BF00241475. [DOI] [PubMed] [Google Scholar]
- 57.Gopal L, Badrinath S, Kumar K, et al. Optic disc in fundus coloboma. Ophthalmology. 1996;103:2120–2127. doi: 10.1016/s0161-6420(96)30380-1. [DOI] [PubMed] [Google Scholar]
- 58.Leff SR, Britton WA, Brown GC, et al. Retinochoroidal coloboma associated with subretinal neovascularization. Retina. 1985;5:154–156. doi: 10.1097/00006982-198500530-00004. [DOI] [PubMed] [Google Scholar]
- 59.Maberley A, Gottner M, Antworth M. Subretinal neovascularization associated with retinochoroidal colobomas. Can J Ophthalmol. 1989;24:172–174. [PubMed] [Google Scholar]
- 60.Steahly L. Laser treatment of a subretinal neovascular membrane associated with retinochoroidal coloboma. Retina. 1986;6:154–156. doi: 10.1097/00006982-198600630-00004. [DOI] [PubMed] [Google Scholar]