Abstract
Purpose
To evaluate the data on the safety, effectiveness, and stability of conductive keratoplasty (CK), a thermal, radiofrequency-based technique for treating 0.75 to 3.00 diopters (D) of spherical hyperopia.
Methods
A prospective, consecutive series, multicenter clinical trial involving 400 hyperopic eyes was conducted by 19 surgeons at 12 centers. The treatment goal was emmetropia. Cohort follow-up was up to 2 years.
Results
At 12 months postoperatively, data were available for 97.5% (354/363) of eyes for efficacy variables and 98% (391/400) of eyes for safety variables. A total of 54% of the CK eyes showed 20/20 or better uncorrected visual acuity, and 92% showed 20/40 or better at 12 months. The mean postoperative manifest refractive spherical equivalent was within 0.50 D in 61% and within 1.00 D in 88%. After CK, eyes were approximately 0.50 D myopic at month 1 and effectively emmetropic at 6 months. At 24 months, there was a 20% loss of initial effect. Safety results showed a 2-line loss of best-corrected visual acuity in 2% of the CK-treated eyes. The incidence of induced cylinder of 2.00 D or greater was below 1%.
Conclusion
The CK technique corrects low to moderate hyperopia effectively and safely and with acceptable stability. It spares the visual axis, does not require the creation of a large flap, and is not associated with postoperative dry eye. CK has value as an alternative to hyperopic LASIK for patients with hyperopia.
INTRODUCTION
Corneal curvature can be modified to correct ametropia and various pathological conditions by using lasers, incisions, or the application of heat. Heat as a means of shrinking stromal collagen fibers has been explored for more than 100 years, but investigators have failed to develop a technique with an ideal safety and efficacy profile. Unpredictability, excessive regression, and, to a lesser extent, complications such as scarring and irregular astigmatism have been major obstacles.
Conductive keratoplasty (CK) is a new nonablative, radiofrequency-based, collagen shrinking procedure to treat hyperopia by steepening the central cornea. The procedure is based on the delivery of precise amounts of radiofrequency energy through a fine tip inserted into the peripheral corneal stroma at 8 to 32 treatment points. As the current flows through the tissue immediately surrounding the tip, resistance to this energy creates heat. The tip itself remains cool. Collagen in the area surrounding the tip shrinks in controlled fashion and forms a column or cylinder. A full circle of CK spots applied to the peripheral cornea produces a “cinching” effect that increases the curvature of the central cornea.
Originally conceived by Antonio Mendez, MD, as an alternative to procedures that applied heat directly to the corneal surface,1 CK has evolved in its safety, efficacy, and predictability profile. In April 2002, the US Food and Drug Administration (FDA) granted approval of the ViewPoint CK System for the treatment of mild to moderate (0.75 diopter [D] to 3.00 D) previously untreated, spherical hyperopia. Other potential uses of the CK technique are the treatment of astigmatism, correction of hyperopia induced by previous refractive procedures, enhancing outcomes of cataract surgery, and the treatment of presbyopia through monovision surgery.
This thesis defends the hypothesis that CK is a safe and effective radiofrequency-based technique for treating low to moderate spherical hyperopia. To provide a foundation for understanding thermal techniques, the mechanism of action of current heat-induced collagen shrinkage procedures and the historical development of thermal techniques are reviewed. This is followed by a presentation of multicenter and single-center CK clinical data and a comparison of CK results with those of other modalities for treating hyperopia.
COLLAGEN SHRINKAGE: MECHANISM OF ACTION
Effect of Heat on Collagen Structure
Collagen, the principal component of corneal tissue, is present as chains wound in triple helices. Covalent bonds hold the polypeptide chains together in the spiral, triple-helix configuration, whereas hydrogen bonds give a particular shape to the polypeptide molecules. Although this conformation provides collagen with great tensile strength and thermal stability, exposure to raised temperatures for specific time periods breaks down its structure to various degrees.2
Slight heating breaks the hydrogen bonds so that the polypeptide segments are free to form different configurations. However, these realignments are temporary, and the molecules can revert back to normal configuration upon rehydration. Additional heating breaks the covalent bonds of the collagen backbone. These bonds can be restored upon cooling, but the final orientation is usually different from the original. Finally, when collagen is subjected to very high temperatures, it is reduced to gelatin, a mixture of polypeptides that solidifies into an amorphous structure upon cooling.
The change in collagen structure caused by heat is known as denaturation and has been described as a rate-process in which the native helical structure is transformed into a more random coiled structure.3 This configurational entropy is accompanied by contraction (shrinkage) of the collagen and changes in the mechanical behavior of the tissue.4 In hyperopia treatment procedures, the desired mechanical change is peripheral corneal flattening and the resulting central corneal steepening.
Denaturation of collagen can occur in various degrees: reversible, intermediate, and irreversible gelatin formation. Reversible changes follow minimal heating of collagen and do not contract tissue sufficiently to produce refractive change. Intermediate denaturation is more permanent because the brisk wound healing response5 may not completely replace the denatured collagen with new collagen. And finally, irreversible denaturation from heating to the gelatin state would induce replacement of denatured collagen with new collagen.6 The resulting structural changes in corneal collagen would not support a predictable refractive change, and scarring and damage to the endothelium would be possible. According to one study, some wound healing changes after thermal procedures are desirable, whereas others may contribute to postoperative regression and other unknown effects.5
Influence of Temperature and Time
Collagen denaturation depends not only on the temperature to which it is subjected, but also to the duration of exposure to the elevated temperature.3,7 Pearce and Thomsen3 demonstrated that thermal damage in laser-coagulated tissue is exponentially dependent on temperature and linearly dependent on time of exposure. The shrinkage temperature of corneal collagen has been extensively studied in in vitro models with corneal strips placed in heated water or oil baths for specific time periods (steady-state conditions).4,6–10 Sporl and associates8 reported that strips of porcine collagen held in an oil bath for approximately 1 minute began to shrink at 60° C and reached maximal shrinkage at 75° to 80° C. At higher temperatures, the shrinkage effect decreased because covalent intramolecular bonds were broken. The investigators concluded that temperatures of 70° to 85° C should be reached to coagulate tissue, but that higher temperatures were to be avoided because they destroyed collagen structure and decreased corneal tensile strength.
However, it must be emphasized that the temperatures mentioned above for specific collagen changes were derived under steady-state conditions. Thermokeratoplasty is a dynamic (not steady-state) heating process, and the state of collagen while undergoing thermokeratoplasty can be inferred, but not exactly defined, through steady-state temperature studies. The temperature effects of CK on corneal collagen are discussed later in this thesis.
REVIEW OF THERMOKERATOPLASTY DEVELOPMENTS
Thermokeratoplasty With Probes
The use of thermal techniques to shrink corneal collagen dates back to the rabbit studies of Lans11 in the 19th century (Table 1). Investigations continued into the next century and, in the 1970s, Gasset and Kaufman attempted to treat keratoconus by applying heated probes to flatten the central cornea.12 Although thermokeratoplasty was initially successful, corneal topography returned to preoperative status,13 and the complications were unacceptable.14,15 Results of thermokeratoplasty for the treatment of persistent corneal hydrops were more positive.16 All six cases cleared within 3 weeks, and transplantation was not needed in four cases. Persistent basement membrane defects, however, were reported after thermokeratoplasty procedures and raised concerns about the safety of these procedures.17
TABLE 1.
MILESTONES IN THERMOKERATOPLASTY
| DATE | INVESTIGATION | RESULT |
|---|---|---|
| 1889 | Lans, Rabbit studies | Radial burns steepened the cornea |
| 1970s | Gasset and Kaufman, Keratoconus treatment | Some success |
| Aquavella et al, persistent hydrops | Successful | |
| 1980s | Fyodorov, Hot needle keratoplasty for treatment of hyperopia | Unpredictable and unstable |
| Rowsey et al, Los Alamos Project, treatment of hyperopia | Clinically unsuccessful | |
| 1990 | Seiler et al, Holmium:YAG LTK for correction of hyperopia | Stable steepening in blind eyes |
| Horn et al, Co:MgF2 LTK for corneal curvature change | Stable corneal curvature changes in rabbits | |
| 1990s | Noncontact holmium laser thermal keratoplasty investigations (Sunrise, Inc.) | Some success in corneal steepening; FDA approval granted in 2000 with stipulation that correction may be temporary |
| 1990s | Mendez, conductive keratoplasty for hyperopia | Some success in denaturing collagen and corneal steepening. Procedure later refined by Refractec, Inc. |
| 2000 | Diode laser thermokeratoplasty (continuous wave) investigations (DTK, ProLaser Medical Systems, Inc, Dusseldorf, Germany) | Some success with hyperopia treatment. Tested outside of US only. Status uncertain. |
| 2002 | One-year US clinical trial results of safety and efficacy of conductive keratoplasty completed (Refractec, Inc) | Approved by the FDA as effective and safe for treatment of spherical hyperopia April 2002 |
| 2004 | One-year US clinical trial results of safety and efficacy of conductive keratoplasty for presbyopia (Refractec, Inc) | Approved by the FDA for the temporary induction of myopia in the nondominant eye of presbyopic hyperopes or emmetropes |
FDA = US Food and Drug Administration.
Thermokeratoplasty was first applied for purely refractive purposes by Fyodorov in Moscow in 1981, and the technique was refined in 1984. Using a retractable probe tip, Fyodorov and others applied controlled thermal burns in a radial pattern to treat spherical hyperopia and in a sectional pattern to treat hyperopic astigmatism. The tip was heated to 600° C, and penetration was reported to be 95% of corneal depth.18–20 Although corrections of 1.5 D to 8.0 D were achieved,18 later studies showed lack of predictability and substantial regression of effect.21–23 Evaluation of the procedure through well-designed clinical trials before adoption and dissemination was recommended.24
At approximately the same time as the Soviet investigations, Rowsey and colleagues attempted to minimize thermokeratoplasty complications by developing the Los Alamos thermokeratoplasty probe. This instrument heated stromal collagen by means of radiofrequency waves, rather than by direct thermal conduction from a heated instrument.25,26 The probe was designed to treat stroma 200 to 400 μm beneath the corneal epithelium, thereby minimizing damage to the endothelium. McDonnell and associates27 used the instrument on a cornea with keratoconus and found marked regression of effect within a few postoperative weeks. The Los Alamos thermokeratoplasty probe was withdrawn from experimentation because of the short-lived topographical effect (Rowsey JJ, ARVO Meeting, 1987, Abstract).
Thermokeratoplasty With Lasers
Not all attempts to permanently change corneal topography through the application of heat ended in failure. Horn and associates28 obtained stable curvature changes in rabbit eyes after treatment with a cobalt:magnesium fluoride laser, and Seiler and associates29 reported stable corneal steepening in blind eyes treated with a pulsed, 2.06-μm-wavelength holmium:YAG laser in the contact mode. These studies suggested that lasting topographic changes could be achieved if the stroma was heated to the necessary temperature and depth. Ideally, the temperature elevation would be maximal within the stroma and minimal at the epithelium and endothelium in order to achieve stromal shrinkage without ocular surface complications or loss of endothelial cells.
Only the holmium:YAG laser with a 2.1-μm (mid-infrared) wavelength was found to provide sufficient early evidence of safety and effectiveness for clinical evaluation. Based on the absorption characteristics of corneal tissue, holmium:YAG laser thermal energy was reported to penetrate to approximately 400 μm into the corneal stroma.30 In laser thermal keratoplasty (LTK) treatment, holmium:YAG laser energy can be delivered by a contact device29,31–34 (Holmium Laser Systems, Summit Technology, Waltham, Massachusetts, and the Holmium 25 Plus, Technomed, Baesweiler, Germany) or a noncontact device (Sunrise Technologies, Fremont, California).35–45 Studies with the Summit contact device that involved a hand-held fiberoptic probe were terminated by the FDA because of problems with regression.
However, two studies with the contact device by Technomed (Holmium 25 Plus, Baesweiler, Germany) were published fairly recently, one in 1999 regarding treatment of primary hyperopia46 and one in 2000 regarding treatment of secondary hyperopia.47 Hyperopia of up to 2.50 D was corrected, but predictability was low and astigmatism was induced. The investigators planned to refine the technique, but there have been no further publications, and the status of this laser’s development is unknown.
Noncontact Holmium:YAG Laser Thermal Keratoplasty
Early Parameter Studies
Early studies on the noncontact holmium:YAG laser in the technique that came to be known as noncontact LTK focused on design of the delivery system, application geometry on the cornea, and optimal pulse energy and repetition rates. Parel and associates35 designed a noncontact holmium:YAG laser (2.1-μm wavelength, 250-μsec pulse, 5-Hz repetition rate) delivery system coupled to a slit lamp. They also explored the laser treatment parameters in cadaver eyes and found that the central cornea was steepened when the diameter of the treatment zone was larger than 4 mm.36 Their third study of histology in animal eyes showed a cone-shaped zone of collagen shrinkage in the stroma after treatment.37
Another group that treated rabbit eyes with maximal energy levels demonstrated safety of endothelial cells and also showed efficacy (steepening of the central cornea) in eye-bank eyes treated with low energy levels in a ring pattern of 32 spots.38 Their histology specimens of rabbit eyes also showed a cone-shaped zone of stromal collagen coagulation.
A phase I study of the noncontact holmium:YAG laser (Sunrise Corneal Shaping System, Sunrise Technologies) in poorly sighted eyes examined the safety of various patterns and energy densities. Laser energy was delivered simultaneously to a single ring of eight spots with the slit-lamp delivery system. Energy densities sufficient to achieve mild changes in corneal curvature (mean, 1.10 D) did not produce any problems with epithelial healing or stromal necrosis or cause endothelial cell damage.39
Single Ring, 10 Pulses
The first trial of the Sunrise system in 17 sighted eyes was conducted internationally for the treatment of low hyperopia (up to +3.00 D).40 Eight holmium:YAG laser spots were delivered simultaneously in a symmetric octagonal array (single ring) at the 6-mm optical zone, 10 pulses at 5-Hz repetition frequency, and pulse energies of 159 to 199 mJ. At the 2-year follow-up, mean uncorrected Snellen visual acuity (UCVA) had improved from 20/125-1 to 20/50-2. The mean change in manifest refractive spherical equivalent (MRSE) was 0.79 D. No eye lost 2 or more lines of best spectacle-corrected visual acuity (BSCVA), and the mean induced astigmatism was 0.18 D.
Two Rings, 10 Pulses
In the Sunrise US phase IIA study of safety and effectiveness, 28 eyes were treated for low hyperopia (mean preoperative SE, +2.21 ± 0.89 D) with one or two rings of eight spots per ring at the 6-mm optical zone (one ring) or the 6- and 7-mm optical zones (two rings), 10 pulses at 5-Hz repetition rate, and pulse energies of 208 to 242 mJ.41–43 Two years after surgery, 73% gained 1 or 2 lines of UCVA, with a mean gain of 2.5 ± 2.2 lines in the one-ring group and a mean gain of 3.3 ± 2.7 lines in the two-ring group.42 The mean change in MRSE was −0.53 D ± 0.33 and −1.48 D ± 0.58 in the one- and two-ring groups, respectively. Because of the low corrections obtained with the one-ring group, the investigators recommended use of two rings in most cases.
In the two-ring treatment group, all eight eyes had reductions in hyperopia ranging from −0.38 D to −2.25 D. Regression between years 1 and 2 was a mean of 0.16 D. None of the eyes lost 2 or more lines of BSCVA or had sight-threatening complications. The investigators concluded that noncontact LTK treatment of low hyperopia was safe and produced modest but persistent corrections at 2-year follow-up.42
Two Rings, Five Pulses
In another study of 39 patients, the range of hyperopia treated was extended to up to +3.88 D, five pulses instead of 10 were applied, laser energy was fixed at 240 mJ, and the pattern of application was varied to the 5- and 6-mm optical zones (group A), the 6- and 7-mm optical zones (group B), and the 6.5- and 7.5-mm optical zones (group C). At 1 year, lines of visual acuity gained were 3.7 ± 0.5, 6.8 ± 2.7, and 5.3 ± 3.3 for groups A, B, and C, respectively. The mean changes in MRSE were −2.08 ± 1.13 D, −1.83 ± 0.88 D, and −1.22 ± 0.88 D, respectively. None of the eyes lost 2 or more lines of BSCVA. Longer follow-up and larger sample sizes were needed for stability determination.44
A SINGLE-CENTER STUDY
No other multicenter results of the Sunrise noncontact system for hyperopia correction have been published in the peer-reviewed literature. Alio and associates45 published their single-center study of 57 eyes treated with the Sunrise LTK system. Laser energy was applied according to the Sunrise energy/diopter algorithm in two or three rings of eight spots each. They found 58% with a mean MRSE within ±1.00 D of intended correction and 72% with UCVA of 20/40 at 15 months postoperatively. Complete regression of the effect was present in 31.5% of eyes at 15 months. The group recommended algorithms with an initial overcorrection and adjustments for patient age and corneal thickness.
Noncontact Holmium:YAG Laser Histology and Wound Healing
Studies of tissue responses to 10-pulse noncontact Ho:YAG LTK were performed in three human corneas before undergoing penetrating keratoplasty and in six rabbits.5 The human and rabbit corneas showed a broad spectrum of histologic changes in the epithelium and stroma, and the rabbit eyes showed a brisk wound healing response. Some of the changes were noted as desirable to produce refractive change, whereas others may have contributed to postoperative regression. Among the questions that remained after study completion was the extent of postoperative regression induced by new collagen formation during stromal wound healing.
Current Sunrise Hyperion LTK System Parameters
The noncontact system currently used includes the holmium:YAG laser with a 2.10-μm wavelength and seven pulses simultaneously delivered to eight spots in a circular pattern at the 6- and 7-mm optical zones. The energy range is 226 to 258 mJ. The spot size is 0.6 mm in diameter. Three algorithms are provided that indicate the pulse energy that is to be delivered versus the age of the patient. Less laser energy is indicated for older patients to achieve the same effect (0.10 to 0.20 D for each decade of life). The first algorithm (“Most Effect—24 Month Endpoint”) provides the most refractive effect at 2 years postoperatively but is associated with the most overcorrection initially. The second and third algorithms have less long-term effect but also less initial overcorrection (Sunrise Hyperion LTK Physician Training Manual, Sunrise Technologies, Fremont, California, December 2001). Current physician labeling information for the approved Sunrise Hyperion LTK system limits the use of the device for treatment of patients 40 years of age or older, with an MRSE of 0.75 D to 2.50 D at the spectacle plane and cylinder ≤ 0.75 D.
FDA approval of the Hyperion LTK device in June 2000 was based on a study cohort of 612 eyes, with 493 of 612 available for analysis at 12 months postoperatively and 72 of 612 available at 24 months.48 Key safety and efficacy outcomes are presented later in this report in the section comparing CK, LTK, and excimer laser results for the treatment of hyperopia.
DIODE LASER THERMAL KERATOPLASTY STUDIES
Early Parameter Studies
Brinkmann and associates49 investigated threshold temperatures and exposure time for various lasers to coagulate corneal collagen. They reported that the pulsed holmium:YAG laser did not expose tissues to the necessary temperature long enough to produce a stable refractive change, and they recommended use of a continuously emitting laser source, such as the continuous-wave diode laser in the 2-μm (mid-infrared) wavelength range. Subsequently, the diode laser in continuous (not pulsed) mode, emitting at 1.9 μm, was described as a new instrument by Bende and associates50 in 1999 and tested in cadaver eyes. Laser energy was applied in a contact mode through an optical fiber with a diameter of 600 μm and output of up to 250 mW on the target site. The application was in a concentric ring pattern of eight coagulation spots on cadaver corneas. The investigators found that continuous-wave diode laser treatment produced more steady collagen temperature increases, avoided the temperature variations of the pulsed holmium:YAG laser, and optimized laser-collagen interaction for inducing refractive changes.50
Further basic studies of laser coagulation showed that the wavelength window useful for LTK depended on the absorption coefficient of water, with too much absorption leading to superficial coagulations and too little absorption damaging underlying tissues.51 Using that information, application parameters, geometries, and dosing of the tunable continuous-wave diode laser were studied in pig eyes with the goal of increasing corneal curvature.52 The researchers applied equidistant coagulation spots in a ring concentric about the corneal apex while varying laser power through the tunability feature of the laser. Refractive changes were measured by corneal topography.
The results showed that continuous-wave diode laser power of 125 to 200 mW could produce refractive changes of up to 10.0 D. Histologic analyses showed that overcoagulated zones were to be avoided because completely disintegrated collagen would not maintain the necessary stresses in the midperiphery to steepen the central cornea and would also bring out a pronounced healing response.52
Blind Eyes
A study in eight blind human eyes attempted to optimize laser parameters to achieve safe and effective clinical treatment.53 Eight coagulation spots were placed concentrically, whereas the laser energy and number of rings were varied. Two rings of coagulation resulted in up to 5.7 D of correction, and the effect was reported to be stable by 6 months postoperatively. A wavelength of 1.87 μm caused minimal endothelial cell damage. Further studies of corneal endothelial effects after diode laser treatment showed the potential for endothelial cell damage as well as possible limitation of damage through manipulation of laser power and wavelength.54
Clinical Study
Results of continuous-wave diode laser thermokeratoplasty (DTK, ProLaser Medical Systems Inc, Dusseldorf, Germany) in living, human eyes have not yet been reported in the literature. However, results of a European multicenter study have been presented (G. Waring, “Diode Thermal Keratoplasty,” International Society of Refractive Surgery meeting, November 2000, New Orleans, Louisiana). The laser energy was applied manually in a circular pattern at the 6- or 7-mm optical zones of 305 eyes. Eyes were initially overcorrected but stabilized in 6 to 9 months. Up to 4.7 D of correction was obtained. An age-related effect was observed, with younger eyes showing more regression. Compensation for this effect was possible by changing the laser parameters (tuning the laser). This laser was reported to be in evolution, with refinement of age effects and depth in progress.
A bioptics procedure using laser in situ keratomileusis (LASIK) followed by DTK 2 months later for high hyperopia has been reported. The combination of methods was safe and effective. Regression was a mean of 1.50 D at a mean 10.5 months of follow-up.55 Nonablative systems for hyperopia correction are shown in Table 2.
TABLE 2.
NONABLATIVE SYSTEMS FOR HYPEROPIA CORRECTION
| DESCRIPTION | CONDUCTIVE KERATOPLASTY | SUNRISE HYPERION NONCONTACT LASER THERMAL KERATOPLASTY (LTK) | CONTINUOUS-WAVE DIODE LASER THERMOKERATOPLASTY (DTK) |
|---|---|---|---|
| Manufacturer | Refractec, Inc, Irvine, California | Sunrise Technologies, Fremont, California | Rodenstock; ProLaser Medical Systems, Inc, Dusseldorf, Germany |
| Approval status | FDA approved for 0.75 D to 3.00 D of spherical hyperopia | FDA approved for 0.75 D to 2.50 D of spherical hyperopia | Open Investigational Device Exemption |
| Type of device/energy delivery | Radiofrequency energy, 350 kHz, contact probe | Ho:YAG laser, pulsed 226 to 258 mJ, 5 Hz, noncontact | Continuous-wave diode laser, contact device |
| Wavelength | Not applicable | 2.1 μm | 1.87 μm |
| Temperature during treatment | Highest at center of cylinder | Highest on corneal surface | Radial and axial temperature gradient |
| Radial (side-to-side) temperature gradient | Radial and axial temperature gradient | ||
| No axial (surface to bottom) temperature gradient | |||
| Consistent thermal effect along the probe | |||
| Treatment depth | 80% of corneal depth, based on porcine histology | Wider on surface than in deep stroma | Not available |
| Footprint | Cylindrical | Conical | Conical |
| Number of spots | 8, 16, 24, or 32 | 16 to 32 | In development |
| Nomogram adjustment to increase effect | Increase number of treatment spots | Increase laser energy/temperature | Increase number of treatment spots |
EXCIMER LASER PROCEDURES FOR HYPEROPIA
Hyperopic Photorefractive Keratectomy
Results with hyperopic photorefractive keratectomy (PRK) in eyes with and without astigmatism have varied, but effectiveness and predictability have generally decreased with increasing level of attempted correction.56–61 Hyperopic PRK procedures have also shown a greater tendency toward regression than myopic procedures.
Photorefractive keratectomy studies of higher levels of hyperopia (>+4.00 D) have generally shown low efficacy and predictability. In a study of 34 eyes, Pietila and associates59 found PRK to be safe but only moderately effective (6.3% with UCVA of 20/20) or predictable (40% within ±1.00 D of emmetropia) for hyperopia ranging from +1.00 to +6.50 D. Although most eyes were said to be relatively stable at 3 months, the investigators concluded that regression is a constant finding after PRK for hyperopia.
O’Brart and associates60 reported 63% of eyes with UCVA of 20/40 or better at 6 months and, in the subgroup of eyes with less than +2.00 D of hyperopia, 71% within ±1.00 D. Dausch and associates61 reported 97% of eyes with UCVA of 20/40 or better, 40% with 20/20 or better UCVA, and 81% within ±1.00 D of intended refraction in eyes with a high range of hyperopia (+2.00 to +8.25 D). Regression was most apparent in eyes with higher hyperopia corrections.
Hyperopic LASIK
Hyperopic PRK was, for the most part, abandoned in favor of hyperopic LASIK procedures. LASIK is thought to produce better results than PRK for the treatment of hyperopia because Bowman’s layer is not damaged, postoperative pain is markedly less, and the presence of a flap reduces haze, epithelial healing, and subsequent regression.62 One study reported on predictability and safety of LASIK corrections using different ablation zone diameters in eyes with low to moderate spherical hyperopia.63 The investigators found a tendency toward overcorrection in the large ablation zone group, but the procedure was safe, with no eyes experiencing a loss of 2 or more lines of BSCVA at last examination. The investigators concluded that predictability would be expected to improve following nomogram adjustments. More recent hyperopic LASIK studies have shown successful outcomes,64–75 which can be compared with CK outcomes.
CONDUCTIVE KERATOPLASTY MULTICENTER CLINICAL STUDY
Background
Conductive keratoplasty is a “laserless” collagen shrinking procedure for correcting mild to moderate hyperopia that works through the conduction of radiofrequency energy. The procedure is performed by inserting a thin probe that emits low-energy, high -requency radio waves (350 kHz) into the stroma in delineated spots encircling the cornea outside of the visual axis. The number and location of spots determine the amount of refractive change. As the current flows through the tissue immediately surrounding the probe tip, resistance to the current creates heat. Collagen in the area surrounding the tip shrinks uniformly and forms a column or cylinder. Following a full circle of treatment spots, the peripheral cornea flattens and the central corneal steepens. This section presents the 1- and 2-year, single-treatment results of CK used to treat 400 eyes enrolled in a US phase III FDA clinical trial. Some of the 1-year results were previously published.76.77
METHODS
Study Design
The study was a prospective, consecutive series, multicenter clinical trial (FDA phase III) evaluating the safety, efficacy, and stability of the CK procedure for the treatment of 0.75 to 3.00 D of hyperopia and ≥0.75 D of cylinder. The 12 participating centers were a mix of university and private practice settings.
All treatments were based on the patients’ cycloplegic refractive spherical equivalent (CRSE) with the treatment goal of a full correction of spherical hyperopia (emmetropia). No cylinder corrections or retreatments were performed. Data on the complete 12-month cohort and partial 24-month cohort are reported here.
The first 54 eyes were treated with the original nomogram,76 and the rest were treated with the current (modified) nomogram.77 The modification included reduction of the maximum CK treatment of spherical hyperopia from 4.00 D to 3.25 D.65 Treatment with CK was bilateral and performed at the same session if the fellow eye met the same treatment criteria as the first treated eye (ie, 0.75 to 3.00 D of hyperopia and ≤0.75 D of cylinder).
Study Device
The ViewPoint CK System (Figure 1) used to perform the CK procedure consists of a portable, radiofrequency energy-generating console, a hand-held, reusable, pen-shaped handpiece attached by a removable cable and connector, a foot pedal that controls release of radio frequency energy (350 kHz), and a speculum that provides a large surface for an electrical return path. The energy level default is 60% of 1 W, and the exposure time default is 0.6 seconds for each application spot. Attached to the probe is a single-use, disposable, stainless-steel Keratoplast tip, 90 μm in diameter and 450 μm long, that delivers the current directly to the corneal stroma (Figure 2). The tip has a proximal bend of 45° and a distal bend of 90° to allow access to the cornea over the patient’s brow and nasal regions. At the very distal portion of the tip is an insulated Teflon-coated stop (cuff) that ensures correct depth of penetration. The impedance in corneal tissue of the current results in an optimal thermal profile for shrinking collagen along the entire length of the probe. This provides a homogenous and uniform cylinder of constricted collagen to approximately 80% of the peripheral corneal thickness.
FIGURE 1.
The ViewPoint CK System consists of a 14-pound portable console, a handpiece that holds the 450-μm-long and 90-μm-wide Keratoplast Tip, a corneal marker, a choice of three specula, and a foot pedal. The speculum is a return path for the radiofrequency energy. Reprinted with permission from Ophthalmology 2002;109:1979.
FIGURE 2.
The single-use Keratoplast tip, 450 μm long and 90 μm wide, with a cuff that ensures correct depth of penetration. Reprinted with permission from Ophthalmology 2002;109:1979.
Patients
Institutional review board approval was obtained at each clinical center that participated in the multicenter trial. A total of 401 patients at 12 centers in the United States who met eligibility requirements were enrolled consecutively into the study from February 10, 1999, to December 1, 2000, and signed informed consent forms. Four hundred patients had eight to 32 treatment spots applied to the cornea during the CK procedure (one patient, one eye did not have energy applied during the treatment).
Enrolled patients had no existing ocular or chronic systemic disease, previous ocular surgery, history of herpes infection, steroid-responsive increase in intraocular pressure (IOP), intractable keratoconjunctivitis sicca, history of keloid formation, or unstable, progressive hyperopia. Contact lens wearers were to discontinue hard contact lens wear 3 weeks before the final measurements and the procedure, and soft contact lens wear 2 weeks before the final measurements and the procedure. They also had to have clear, undistorted mires on the central keratometry examination. Eyes with pachymetry readings of less than 560 μm at the 6-mm optical zone and those with distance UCVA better than 20/32 were to be excluded from study participation.
Examination Methods
The preoperative and postoperative examinations for all eyes included manifest and cycloplegic refractions; uncorrected and best spectacle-corrected visual acuity using Early Treatment Diabetic Retinopathy Study visual acuity charts or Bailey-Lovie charts (distance) and Jaeger visual acuity charts (near); slit-lamp and funduscopic examination; and computerized corneal topography. Cycloplegic refraction was measured 30 minutes after two applications of one or two drops of 1% cyclopentolate 5 minutes apart. Intraocular pressure was measured with the surgeon’s choice of standard applanation instruments, including Goldmann, Perkins, or Draeger tonometry.
The preoperative examinations were performed by the surgeons or their assistants 1 to 60 days before the CK procedure. Postoperative examinations were performed on days 1 and 7 and months 1, 3, 6, 9, 12, and 24, and results were recorded on standardized data forms. Patients were also asked to subjectively evaluate the quality of their postoperative vision and indicate their level of satisfaction on standardized forms.
Mesopic contrast sensitivity testing was performed with the Optec 1600X, both with and without a glare source, in a subset of 158 study patients preoperatively and at 6, 12, and 24 months postoperatively. Testing without glare was performed with target illumination set to 5 cd/m2 and the glare source turned off. A trial frame with the patient’s best correction and test slide number 1 was inserted. The patient was asked to make a forced response to the direction (left or right) of the point of the line in boxes A1 through A8. The box number (ie, A6) of the last correct response was recorded. This was repeated with test slide number 2. The procedure was repeated with lines B1 through B8, C1 through C8, D1 through D8, and E1 through E8. The procedure for testing with glare was the same except that glare illumination was set to 2 lux.
Specular microscopy studies were carried out to study the effect of the treatment on the corneal endothelium on 162 eyes at five of the 13 investigational sites. Measurements were taken with the Konan NonCon Robopachy at the central cornea, the 3-mm optical zone, and the 6-mm optical zone. Changes from preoperative levels at these sites were calculated using paired-differences statistical testing.
Corneal haze was evaluated by slit lamp on a five-level scale of clear, minimal, trace, mild, moderate, and marked. The protocol failed to specify that haze only in the central cornea was to be reported and that haze at the treatment site is expected.
Confocal microscopy, a noninvasive technique for revealing histology, was performed on a subset of patients 12 months after the procedure using the Nidek Confoscan II. This technique optically sections living tissues to allow in vivo examination of individual layers.
Surgical Procedure
Topical anesthesia was induced with one drop of 0.5% tetracaine, administered three times at 5-minute intervals. Pilocarpine was not administered. A lid speculum was placed in the eye to be treated to obtain maximal exposure and to provide the electrical return path; the fellow eye was taped closed. Illumination was provided by the operating microscope. While the patient fixated on the microscope’s light, the cornea was marked with a gentian-violet-dampened, eight-intersection CK marker that marks the 7-mm optical zone and makes radial marks that extend from the 6-mm to the 8-mm optical zones. The surface of the cornea was dried with a fiber-free sponge to avoid dissipation of applied energy through a damp surface.
The surgeons placed the Keratoplast tip on the cornea at the treatment markings, attempting to place it perpendicular to the corneal surface. The cuff around the probe, which settles perpendicular to the cornea, helped to achieve perpendicular placement. Light pressure was applied until the tip penetrated the stroma to its insulator stop. Energy was applied by depressing the foot pedal. All eyes were treated at the default setting of 350 kHz, 60% power, for 0.6 seconds.
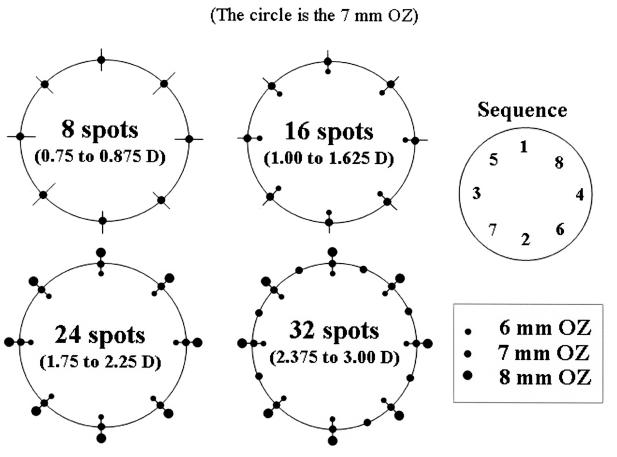
All eyes after the first 54 were treated with the number of spots indicated by the current nomogram (Figure 3). Larger-diopter corrections require application of a larger number of rings instead of an increase in power (power is kept constant at the 60% default setting). For example, eight spots (one ring) are placed for correcting up to +0.875 D, but 16 spots (two rings) are placed to correct +1.00 to +1.625 D. The sequence of spot placement is also shown in Figure 3. The probe tip was cleaned of tissue debris with a fiber-free sponge after each treatment spot. All eyes received a single CK treatment (ie, no retreatments were performed).
FIGURE 3.
Conductive keratoplasty (CK) treatment application. An increasing number of spots are placed for increasing levels of hyperopia. For example, eight spots (one ring) are placed for correcting up to +0.875 D, but 16 spots (two rings) are placed to correct +1.00 to +1.625 D. The sequence of spot placement is also shown at the top right. Reprinted with permission from Ophthalmology 2002;109:1980.
Postoperative Care
After treatment, one drop of topical antibiotic solution and one drop of diclofenac sodium 0.1% (Voltaren, Ciba Vision Ophthalmics) were administered every 4 hours during waking hours and continued for 2 days. Unpreserved artificial tear solution was the only ocular medication permitted in the study. The treated eye was not patched.
RESULTS
Accountability and Demographics
The total number of enrolled and treated patients was 233, and the number of treated eyes was 400 of the 401 enrolled. One eye was enrolled but not treated, and the patient was later determined to be ineligible on account of angle closure and was discontinued from the study. The original nomogram was used for the first 54 eyes, and the modified (current) nomogram for the rest of the treated eye. Of the 54 eyes treated with the original nomogram, 25 were in the correct treatment range and received the appropriate number of treatment spots specified by the current nomogram. The remaining 29 eyes fell outside of the treatment ranges defined in the current nomogram, and their refractive outcomes did not reflect the effectiveness of the current nomogram. On this basis, these 29 eyes were excluded from effectiveness outcomes (ie, UCVA, accuracy of intended [target] to achieved refraction, and mean postoperative refraction). An additional eight eyes were treated outside of the current nomogram as a result of protocol deviations or surgeon error and have also been excluded from analyses of effectiveness. All eyes (400) have been included in analyses of safety outcomes. The full population of eyes was also used for analyses of refractive stability, because stability shows changes over time irrespective of which nomogram was used. Accountability of patients through 24 months is shown in Table 3.
TABLE 3.
MULTICENTER CONDUCTIVE KERATOPLASTY STUDY PATIENT ACCOUNTABILITY: ALL ENROLLED EYES
| AVAILABILITY | MONTH 1 | MONTH 6 | MONTH 12 | MONTH 24 | ||||
|---|---|---|---|---|---|---|---|---|
| For safety analysis | 390/400 | 97.5% | 389/400 | 97.0% | 391/400 | 98.0% | 322/400 | 80.0% |
| For efficacy analysis | 354/363 | 97.5% | 352/363 | 97.0% | 354/363 | 97.5% | 285/363 | 78.5% |
At 12 months, follow-up data were available for 97.5% (354 of 363) of eyes for efficacy variables and 98% (391 of 400) of eyes for safety variables. The mean age of enrolled patients was 55.3 ± 6.4 years (range, 40 to 74). Female patients numbered 136 and male, 97. Most (81%) of the patients were white and the others were black, Asian, or “other,” or the race was not recorded. Demographic information is shown in Table 4.
TABLE 4.
DEMOGRAPHIC AND BASELINE INFORMATION: MULTICENTER CONDUCTIVE KERATOPLASTY STUDY
| CATEGORY | NUMBER |
|---|---|
| No. of patients/eyes | 233/401 |
| Mean age (SD) | 55.3 years (6.4) |
| Range | 40 to 74 |
| Median | 55.6 |
| Range of treatment CRSE | |
| Mean (SD) | +1.86 D (0.63) |
| Range | +0.75 D to +4.00 D |
| Median | +1.75 D |
| Range of treatment MRSE | |
| Mean (SD) | +1.80 D (0.64) |
| Range | −0.38 D* to +3.75 D |
| Median | +1.75 D |
CRSE = cycloplegic refractive spherical equivalent; MRSE = manifest refractive spherical equivalent.
Includes two ineligible eyes with minus MRSE values.
Uncorrected Visual Acuity
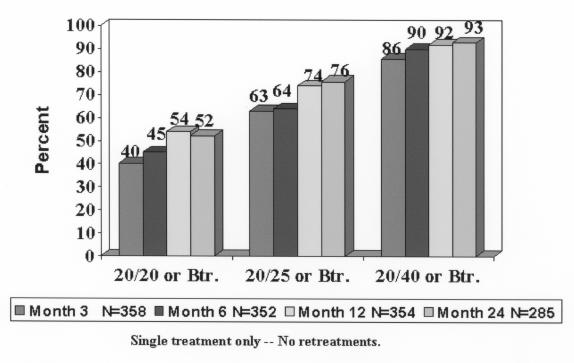
Before treatment, 5 of 363 eyes (1%) had 20/20 or better UCVA (these were enrolled in violation of study criteria), 96 of 363 (26%) had UCVA of 20/40 or better, and 358 of 363 (99%) had 20/200 or better. Postoperatively, at 1 year, 192 of 354 eyes (54%) had UCVA of 20/20 or better, 262 of 354 (74%) had 20/25 or better, and 325 of 354 (92%) had 20/40 or better. Results were similar at 24 months. Figure 4 shows postoperative UCVA over time, and Figure 5 shows UCVA changes over time for each acuity level. Uncorrected VA progressively improved with time for acuity levels of 20/20 or better and 20/25 or better and showed no leveling off. However, the 20/40 acuity level stabilized at 6 months and 20/80 leveled off at approximately 1 month.
FIGURE 4.
Postoperative uncorrected visual acuity over time. A total of 54% and 52% of eyes had 20/20 or better uncorrected visual acuity at 12 and 24 months, respectively. Reprinted with permission from Ophthalmology 2002;109:1981.
FIGURE 5.
Uncorrected visual acuity; change over time for various acuity levels. Acuity levels of 20/20, 20/25, and 20/40 showed a similar pattern over time. Reprinted with permission from Ophthalmology 2002;109:1982.
Accuracy and Stability
Accuracy.
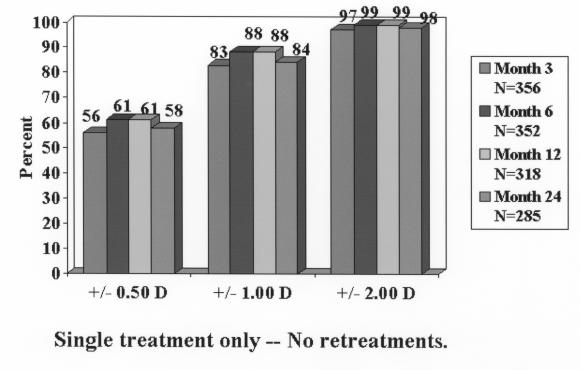
At 1 year, 217 of 344 eyes (63%) were within ±0.50 D of emmetropia, 312 of 354 (88%) were within ±1.00 D, and 351 of 354 (99%) were within ±2.00 D (Figure 6). At 24 months, accuracy percentages dropped off slightly. Table 5 shows a summary of efficacy variables (UCVA and accuracy) over time up to 24 months.
FIGURE 6.
Accuracy of achieved refraction. At 12 and 24 months, respectively, 61% and 58% of eyes were within 0.50 D of the intended correction (emmetropia). Reprinted with permission from Ophthalmology 2002;109:1982.
TABLE 5.
SUMMARY EFFICACY VARIABLES: MULTICENTER CONDUCTIVE KERATOPLASTY STUDY
| EFFICACY VARIABLE | 3 MONTHS n = 354 | 6 MONTHS n = 352 | 12 MONTHS n = 354 | MONTH 24 n = 285 |
|---|---|---|---|---|
| UCVA ≤ 20/20 | 40% | 45% | 54% | 52% |
| UCVA ≤ 20/25 | 63% | 64% | 74% | 76% |
| UCVA ≤ 20/40 | 86% | 90% | 92% | 93% |
| MRSE ± 0.50 D | 56% | 61% | 61% | 58% |
| MRSE ± 1.00 D | 83% | 88% | 88% | 84% |
| MRSE ± 2.00 D | 97% | 99% | 99% | 98% |
MRSE = manifest refractive spherical equivalent; UCVA = uncorrected visual acuity.
Table 6 shows the efficacy variables at 9 months postoperatively stratified by diopter group. Eyes treated for the lower amounts of correction (8 and 16 spots) showed mostly better visual and accuracy results than eyes treated for higher corrections (24 and 32 spots).
TABLE 6.
EFFICACY OF TREATMENT AT NINE MONTHS, STRATIFIED BY DIOPTRIC GROUP: MULTICENTER CONDUCTIVE KERATOPLASTY (CK ) STUDY
| 8 SPOTS | 16 SPOTS | 24 SPOTS | 32 SPOTS | |
|---|---|---|---|---|
| CRSE RANGE FOR NUMBER OF SPOTS | ||||
| GROUP | 0.75 – 0.875 D | 1.00 – 1.625 D | 1.75 – 2.25 D | 2.375 – 3.00 D |
| UCVA ≤ 20/20 | 7/14 (50%) | 80/140 (57%) | 53/122 (43%) | 32/74 (43%) |
| UCVA ≤ 20/25 | 11/14 (79%) | 109/140 (78%) | 81/122 (66%) | 53/74 (72%) |
| UCVA ≤ 20/40 | 13/14 (93%) | 133/140 (95%) | 109/122 (89%) | 69/74 (93%) |
| MRSE ± 0.50 D | 13/14 (93%) | 97/140 (69%) | 69/122 (57%) | 43/74 (58%) |
| MRSE ± 1.00 D | 14/14 (100%) | 130/140 (93%) | 105/122 (86%) | 55/74 (74%) |
| MRSE ± 2.00 D | 14/14 (100%) | 139/140 (99%) | 119/122 (98%) | 73/74 (99%) |
CRSE = cycloplegic refractive spherical equivalent; MRSE = manifest refractive spherical equivalent; UCVA = uncorrected visual acuity.
Table 7 shows undercorrected and overcorrected eyes over time. The percentages of undercorrected eyes increased with time, whereas the percentages of overcorrected eyes decreased with time.
TABLE 7.
ACCURACY OF MANIFEST REFRACTIVE SPHERICAL EQUIVALENT: PERCENTAGES UNDERCORRECTED AND OVERCORRECTED
| ACCURACY | MONTH 1 n = 354 | MONTH 6 n = 352 | MONTH 12 n = 354 | MONTH 24 n = 285 |
|---|---|---|---|---|
| Undercorrected > +1.00 D | 3% | 6% | 10% | 15% |
| Undercorrected > +2.00 D | 0.28% | 0.28% | 1% | 2% |
| Overcorrected < −1.00 D | 23% | 6% | 2% | 1% |
| Overcorrected < −2.00 D | 6% | 1% | 0% | 0% |
Stability.
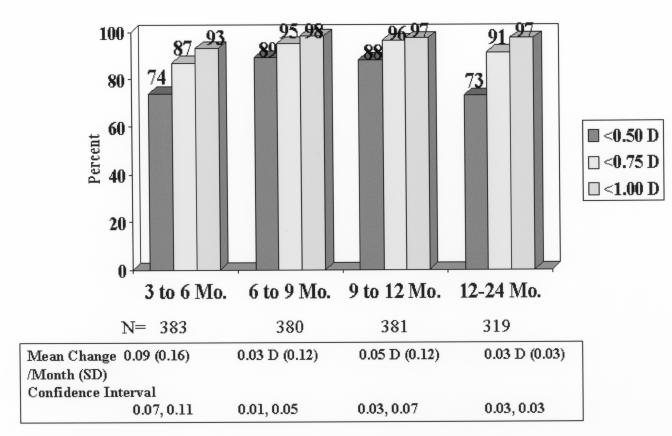
All eyes (including the early nomogram eyes) were evaluated for stability. In patients with consecutive follow-up visits, the MRSE changed ≤0.50 D in 283 of 383 (74%) between the 3- and 6-month visits, in 337 of 380 (89%) between the 6- and 9-month visits, in 335 of 381 (88%) between the 9- and 12-month visits, and in 234 of 319 (73%) between the 12- and 24-month visits (Figure 7). The mean change in MRSE per month was 0.09 D, 0.03 D, 0.05 D, and 0.03 D, between months 3 to 6, 6 to 9, 9 to12, and 12 to 24, respectively. Expressed as change per interval, the MRSE changed a mean of +0.25 ± 0.50 D between 3 and 6 months, +0.11 ± 0.41 D between 6 and 9 months, 0.13 ± 0.33 D between 9 and 12 months, and 0.32 ± 0.41 D between 12 and 24 months.
FIGURE 7.
Manifest refractive spherical equivalent (MRSE) stability shown by 3-month intervals. Patients with consecutive visits. The mean change in MRSE per month decreased with every interval. Reprinted with permission from Ophthalmology 2002; 109:1983.
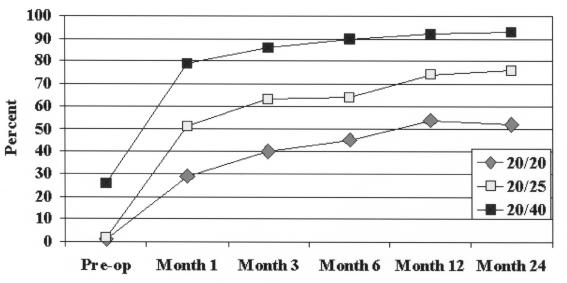
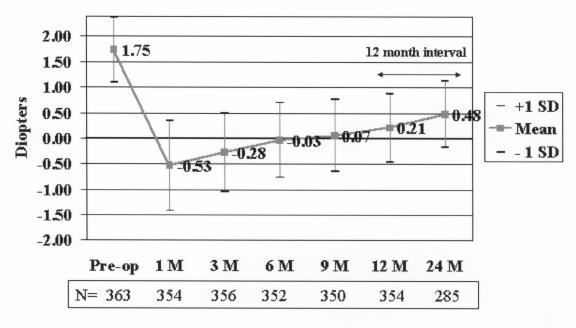
The plot of the mean MRSE (Figure 8) showed an overcorrection of approximately 0.5 D at month 1, followed by slight regression over the following months. At 6 months the eyes were essentially emmetropic (mean MRSE, −0.03 D). Some regression occurred after 6 months, with the mean MRSE of +0.21 D and +0.48 D at 12 and 24 months, respectively. Safety
FIGURE 8.
Mean manifest refractive spherical equivalent (MRSE) refraction over time. Eyes with a preoperative mean of +1.75 D showed a mean MRSE +0.48 D at 24 months. Reprinted with permission from Ophthalmology 2002;109:1984.
Best Spectacle-Corrected Visual Acuity.
Key safety results over time for the multicenter study are summarized in Table 8. At 1 month, eight of 390 (2%) lost more than 2 lines of BSCVA and 25 of 390 eyes (6%) lost 2 lines. Line losses decreased over time so that at 12 and 24 months, no eye had lost more than 2 lines, and 2% and 1% had lost 2 lines of BSCVA, respectively. All nine eyes that lost 2 lines at 12 months were left with very functional vision. Preoperatively, these nine eyes had 20/10 to 20/16 BSCVA. Postoperatively, one of nine eyes had 20/16 BSCVA, five of seven had 20/20, and three of seven eyes had 20/25. No eye had BSCVA worse than 20/40 at any follow-up visit, and no eye that had 20/20 or better BSCVA preoperatively had worse than 20/25 BSCVA at 12 or 24 months postoperatively.
TABLE 8.
SUMMARY SAFETY VARIABLES (ALL TREATED EYES): MULTICENTER CONDUCTIVE KERATOPLASTY STUDY
| SAFETY VARIABLE | 1 MONTH | 3 MONTHS | 6 MONTHS | 9 MONTHS | 12 MONTHS | 24 MONTHS |
|---|---|---|---|---|---|---|
| 2-Line loss BSCVA | 25/390 (6%) | 20/392 (5%) | 16/389 (4%) | 13/386 (3%) | 9/391* (2%) | 6/322 (1%) |
| > 2-Line loss BSCVA | 8/390 (2%) | 4/392 (1%) | 2/389 (0.5%) | 2/386 (0.5%) | 0/391 (0%) | 0/322 (0%) |
| BSCVA worse than 20/40 | 0/390 (0%) | 0/392 (0%) | 0/389 (0%) | 0/386 (0%) | 0/391 (0%) | 0/322 (0%) |
| Increase > 2.00 D cylinder | 13/390 (3%) | 8/392 (2%) | 3/389 (0.8%) | 1/386 (0.3%) | 1/391 (0.3%) | 0/322 (0%) |
| BSCVA worse than 20/25 if better than 20/20 preoperatively | 14/390 (4%) | 7/392 (2%) | 3/389 (0.8%) | 2/386 (0.5%) | 0/391 (0%) | 0/322 (0%) |
BSCVA = best spectacle-corrected visual acuity.
Preoperatively, these eyes had 20/10 to 20/16 BSCVA. Postoperatively, 1 of 9 eyes had 20/16 BSCVA, 5 of 7 had 20/20, and 3 of 7 eyes had 20/25. Thus all eyes with 2-line losses had functional vision.
Cylinder.
Postoperative absolute cylinder increases for the multicenter study are shown in Table 9. Percentages for all levels of cylinder were highest at the first month and declined thereafter. At 12 months, 87% had no change in cylinder, and at 24 months, 93% had no change. The effect of induced cylinder on BSCVA at 12 months is shown in Table 10. There appeared to be no correlation with induced cylinder and the change in BSCVA.
TABLE 9.
CYLINDER INCREASES THROUGH 24 MONTHS IN MULTICENTER CONDUCTIVE KERATOPLASTY STUDY
| CYLINDER INCREASE | MONTH 1 | MONTH 3 | MONTH 6 | MONTH 12 | MONTH 24 |
|---|---|---|---|---|---|
| >2.00 D | 13/390
3% |
8/392
2% |
3/389
0.8% |
1/391
0.3% |
0/322
0% |
| 2.00 D | 11/390
3% |
3/392
0.76% |
5/389
1% |
2/391
0.5% |
1/322
0.3% |
| 1.75 D | 12/390
3% |
9/392
2% |
9/389
2% |
2/391
0.5% |
0/322
0% |
| 1.50 D | 14/390
4% |
15/392
4% |
9/389
2% |
8/391
2% |
0/322
0% |
| 1.25 D | 31/390
8% |
25/392
6% |
29/389
7% |
9/391
2% |
8/322
2% |
| 1.00 D | 49/390
13% |
45/392
11% |
40/389
10% |
27/391
7% |
12/322
4% |
TABLE 10.
EFFECT OF INDUCED CYLINDER ON BCVA AT 12 MONTHS
| CHANGE IN BSCVA | EYES WITH ≤ 1.00 D INDUCED CYLINDER (n = 369/391) | EYES WITH >1.00 D INDUCED CYLINDER (n = 22/391) |
|---|---|---|
| Loss of >2 lines | 0/391 (0%) | 0/391 (0%) |
| Loss of 2 lines | 9/391 (2%) | 0/391 (0%) |
| Loss of 1 line | 80/391 (22%) | 5/391 (23%) |
| No change | 200/391 (54%) | 13/391 (59%) |
| Increase of 1 line | 66/391 (18%) | 3/391 (14%) |
| Increase of 2 lines | 14/391 (4%) | 1/391 (5%) |
| Increase of >2 lines | 0/391 (0%) | 0/391 (0%) |
BSCVA = best spectacle-corrected visual acuity.
Complications and Adverse Events.
A summary of adverse events is shown in Table 11. There were no treatment-related adverse events, such as peripheral corneal defect, corneal edema later than 1 week postoperatively, recurrent corneal erosion at 1 month or later, double or ghosting images at any time, foreign body sensation at 1 month or later, or pain at 1 month or later. There were three events related to device or procedure. In one case, a corneal perforation occurred during the procedure. Investigation revealed that the glue bond attaching the Teflon stop to the CK tip was fractured from a lateral force, which may have occurred during removal from packaging, allowing the stop to separate from the tip. This patient was subsequently treated and had an excellent outcome, with uncorrected acuity of 20/16 at 12 months. The other two cases had no energy applied during initial treatment; one of these patients did not receive treatment because of narrow angles, and the other was subsequently successfully treated.
TABLE 11.
SUMMARY OF ADVERSE EVENTS THROUGH MONTH 12: MULTICENTER CONDUCTIVE KERATOPLASTY STUDY
| ADVERSE EVENT | OUTCOME |
|---|---|
| IOP > 25 mm Hg in 3 eyes of 2 patients (<1%) | One eye had baseline IOP of 25 mm Hg
Two eyes had resolution of IOP increase without |
| Mild iritis in 1 eye of 1 patient (<1%) | Resolved without sequelae |
| Retinal break in 1 eye of 1 patient | 18 months after CK, successfully treated with argon laser |
| Decrease of BSCVA > 2 lines | Optic atrophy 1 eye of 1 patient; secondary to spinal surgery |
BSCVA = best spectacle-corrected visual acuity; IOP = intraocular pressure.
Corneal Haze.
The investigational sites were expected to measure corneal haze at the central cornea only. However, the evaluator at one investigational center measured and reported haze at the CK treatment site, and the results in this section reflect all reports. No haze was seen in 384 of 390 eyes (98%) at 1 month postoperatively, in 96% of eyes at 3 months, in 97% at 6 months, and in 100% at 12 and 24 months. The highest level of haze was a level of mild seen in 4 of 390 (1%) at month 1 and in 1 of 394 (0.25%) at months 3 and 6.
Intraocular Pressure.
There were no occurrences of an uncontrolled IOP increase of greater than 5 mm Hg above baseline. An IOP reading greater than 25 mm Hg was measured in two of 387 eyes at 6 months and in one eye at 9 and 12 months.
Specular Microscopy
Table 12 shows the mean endothelial cell density counts over time, measured at the corneal center, the midperiphery, and the periphery. Mean endothelial cell density showed values of approximately 2,700 cells/mm2 for preoperative and postoperative measurements for the central, midperipheral, and peripheral corneal regions. No clinically or statistically significant changes appeared in the mean cell count over time for any of the corneal regions. Changes in cell density (Table 13) were within the error of testing.
TABLE 12.
MEAN ENDOTHELIAL CELL DENSITY OVER TIME
| REGION | PREOP | 3 MONTHS | 6 MONTHS | 12 MONTHS |
|---|---|---|---|---|
| Central | (n = 162) | (n = 127) | (n = 123) | (n = 42) |
| Mean | 2,686 | 2,730 | 2,727 | 2,683 |
| SD | 160.9 | 163.7 | 153.6 | 163.2 |
| Midperiphery | (n = 162) | (n = 111) | (n = 108) | (n = 31) |
| Mean | 2,722 | 2,734 | 2,727 | 2,691 |
| SD | 162 | 141.3 | 134.4 | 158.4 |
| Periphery | (n = 159) | (n = 107) | (n = 104) | (n = 28) |
| Mean | 2,724 | 2,727 | 2,724 | 2,716 |
| SD | 150.9 | 140.2 | 138.4 | 145.3 |
SD = standard deviation.
TABLE 13.
CHANGES IN ENDOTHELIAL CELL DENSITY FROM PREOPERATIVE LEVELS
| REGION | 3 MONTHS | 6 MONTHS | 12 MONTHS |
|---|---|---|---|
| Central | (n = 127) | (n = 123) | (n = 42) |
| Mean (SD) | +0.31% (4.5) | +1.4% (4.2) | +1.0% (3.9) |
| CI | −0.57, 0.99 | 0.66, 2.1 | −0.19 to +2.2 |
| Mid Periphery | (n = 111) | (n = 108) | (n = 31) |
| Mean (SD) | −0.61% (3.0) | −0.23% (3.05) | −0.6% (3.6) |
| CI | −1.2, −0.08 | −0.8, 0.35 | −1.87 (0.69) |
| Periphery | (n = 107) | (n = 104) | (n = 28) |
| Mean (SD) | −0.76% (2.9) | −0.41% (3.2) | 0.2% (3.5) |
| CI | −1.3, −0.28 | −1.03, 0.21 | −1.1, 1.5 |
CI = confidence interval; SD = standard deviation.
Contrast Sensitivity
A comparison of the preoperative and 6-month postoperative mesopic contrast sensitivity values showed no differences for 1.5 cycles per degree (cpd), 3 cpd, 6 cpd, 12 cpd, and 18 cpd, both with and without glare.
Subjective Evaluations
Extreme, marked, or moderate improvement in postoperative vision was noted by a total of 300 of 353 patients (85%) at 1 month and by 340 of 377 (91%) at 12 months (Table 14). Eleven patients (3%) thought they had no improvement at 12 months. Patients who were very satisfied or satisfied with the results of their surgery numbered 76% at 1 month and 82% at 12 months (Table 15).
TABLE 14.
PATIENT EVALUATION OF VISION QUALITY AFTER SURGERY
| IMPROVEMENT | MONTH 1 | MONTH 6 | MONTH 12 | MONTH 24 |
|---|---|---|---|---|
| Extreme/marked | 232/353 (66%) | 273/370 (73%) | 280/377 (75%) | 218/313 (70%) |
| Moderate | 68/353 (19%) | 57/370 (15%) | 60/377 (16%) | 50/313 (16%) |
| Slight | 37/353 (10%) | 28/370 (8%) | 26/377 (7%) | 28/313 (9%) |
| None | 16/353 (5%) | 12/370 (3%) | 11/377 (3%) | 17/313 (5%) |
TABLE 15.
PATIENT SATISFACTION WITH SURGERY RESULTS
| PATIENT RESPONSE | MONTH 1 | MONTH 6 | MONTH 12 | MONTH 24 |
|---|---|---|---|---|
| Very satisfied/satisfied | 273/356 (76%) | 306/371 (82%) | 319/377 (82%) | 240/313 (77%) |
| Neutral | 57/356 (16%) | 34/371 (9%) | 33/377 (9%) | 45/313 (14%) |
| Dissatisfied | 16/356 (4%) | 20/371 (5%) | 27/377 (7%) | 19/313 (6%) |
| Very dissatisfied | 10/356 (3%) | 11/371 (3%) | 8/377 (2%) | 9/313 (3%) |
Effects on the Cornea
Corneal Topography.
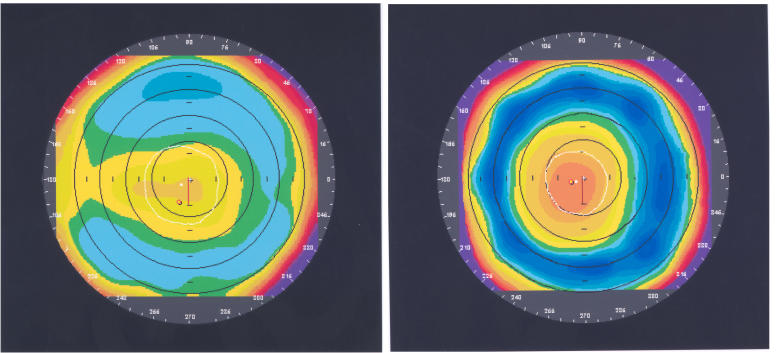
Orbscan corneal topography, with a preoperative view (Figure 9, left) and the same cornea 1 year after CK treatment (Figure 9, right) shows postoperative central corneal steepening surrounded by midperipheral flattening.
FIGURE 9.
Orbscan corneal topography before (left) and 12 months after (right) CK treatment with 16 spots. The manifest refractive spherical equivalent was +1.625 D before CK treatment and −0.375 D after CK. Reprinted with permission from Ophthalmology 2002;109:1986.
Slit Lamp.
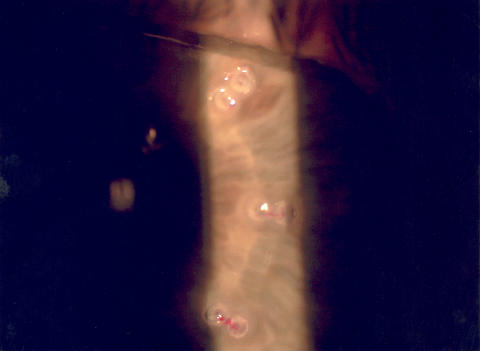
Figure 10 shows a slit-lamp photograph of the cornea 1 hour after CK treatment. The paired treatment spots of thermal coagulation at the 6- and 7-mm optical zones are visible as small surface leukomas, with lines of tension or striae connecting the treatment spots. These lines of tension are responsible for the tightening of the peripheral cornea and the subsequent steepening of the central cornea.
FIGURE 10.
Slit-lamp view of surface leukomas 1 hour after conductive keratoplasty treatment. The leukomas are very small because the radiofrequency energy is delivered deep within the stroma. Bands of striae between the treatment spots are visible. Reprinted with permission from Ophthalmology 2002;109:1986.
Histology.
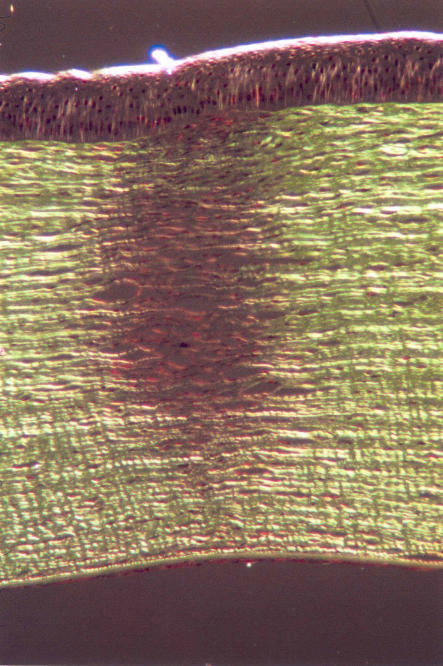
Histology views of the footprint in the corneal stroma of a pig’s eye 1 week after treatment show the treatment effect penetrating to approximately 80% of the depth of the midperipheral cornea (Figure 11).
FIGURE 11.
Transmission polarized micrograph of a conductive keratoplasty treatment spot in a pig cornea with a corneal thickness of about 650 μm, 7 days postoperatively. The footprint is cylindrical and approximately 80% of corneal depth. Reprinted with permission from Ophthalmology 2002;109:1987.
Confocal Microscopy.
Confocal microscopy of a patient’s eye 12 months after CK treatment showed a prominent stromal fold in the striae between the two treatment spots. Deep stromal penetration without damage to the corneal endothelium was apparent.
DISCUSSION
Despite the 60 million people in the United States who are hyperopic, surgical procedures for hyperopic correction have not advanced to the level of procedures for correction of myopia. Early procedures for hyperopia, such as hexagonal keratotomy, corneal lamellar procedures, and automated lamellar keratoplasty, were abandoned for reasons of safety, technical difficulties and complications, or lack of predictability. Holmium:YAG laser thermal keratoplasty (Sunrise LTK) appeared promising, but longer-term data showed disappointing efficacy, predictability, and stability. And finally, clear lens extraction and lens insertion are intraocular procedures that surgeons and patients do not undertake lightly and are generally reserved for high levels of hyperopia.
The success with LASIK in the treatment of myopia stimulated efforts to achieve the same for hyperopia. Hyperopic excimer laser procedures proved to be more challenging, however, because central corneal steepening is more difficult to achieve than central flattening. For a hyperopic correction, the cornea is ablated with the excimer laser in the periphery in an annular-shaped pattern. However, when this ablation pattern was used in PRK procedures, the wound healing process tended to restore the former corneal profile by filling in the ablated stroma with epithelium and decreasing or negating the refractive correction. Corneal haze that could last many months was also a postoperative problem.
Performing the ablation under a corneal flap with peripheral transition zones avoided many PRK-associated downsides, and a few investigators are now reporting success with H-LASIK for different levels of hyperopia, with and without astigmatism.64–75 But, since the reports describe procedures with different excimer laser systems, different ablation and transition zones, various levels of hyperopia treated, the presence or absence of astigmatism, different follow-up periods, and various numbers of treated patients (small studies are more likely to err in their conclusions), comparisons between reports and general statements about hyperopic LASIK technology must be made cautiously.
Furthermore, because reports of successful hyperopic LASIK procedures are not numerous, and flap diameters, algorithms, and ablation and transition zone diameters are still being developed, many surgeons are reluctant to perform H-LASIK until further refinements are made. A nonlaser procedure for hyperopia, such as CK, is therefore appealing at this time as an alternative for hyperopic patients and reluctant LASIK surgeons.
MULTICENTER CK RESULTS
The multicenter CK clinical trial was a rigorously designed and executed study that satisfied the requirements of the FDA for approval for clinical use in the correction of low to moderate spherical hyperopia in patients 55 years old or older. A large, prospective, consecutive, cohort series such as this, with surgeries conducted in various settings by a number of different surgeons, is likely to reduce biased outcomes and reflect more accurately the safety and efficacy profile of this technology. Cohort follow-up was excellent: 98% at 12 months and 80% at 24 months.
Conductive keratoplasty in the multicenter trial demonstrated a high degree of efficacy and safety in the correction of mild spherical hyperopia. At 1-year after treatment, 54% of the study eyes showed 20/20 or better UCVA and 92% showed 20/40 or better. This contrasts with 37% with LTK at 20/20 or better and 85% at 20/40 or better.48 Examination of the efficacy variables stratified by diopter group showed that eyes treated with CK for lower amounts of correction (eight and 16 spots) showed mostly better visual results than eyes treated for higher corrections (24 and 32 spots). This may reflect increased difficulty in correcting higher levels of a hyperopia, an observation that has been reported in a number of studies with different hyperopic procedures.
The FDA targets were exceeded for all accuracy levels (within ±0.50 D, within ±1.00 D) at all follow-up dates in the CK multicenter study. At 1 year, mean MRSE was within ±0.50 D in 61% after CK and in 57% after LTK.48 For percentages within ±1.00 D, it was 88% after CK and 83% after LTK.48 Thus both the UCVA and accuracy results with CK exceeded the results of noncontact LTK. As with the CK visual acuity results, eyes treated with CK for lower amounts of correction (eight and 16 spots) showed better accuracy results than eyes treated for higher corrections (24 and 32 spots).
The percentages of CK eyes undercorrected >1.0 D increased with time, suggesting some regression, whereas the percentages of overcorrected eyes decreased with time. Compared with LTK, however, the undercorrections with CK at all time points were lower. With LTK, 16% were undercorrected >1.0 D at 1 year, compared with 10% for CK.48
After CK, eyes were approximately 0.50 D myopic at month 1 and effectively emmetropic at 6 months. The CRSE refraction change per month for CK was 0.08 D, 0.03 D, 0.04 D, and 0.03 D for the 3- to 6-month, 6- to 9-month, 9- to 12-month, and 12- to 24-month intervals, respectively. Thus, loss of effect diminished with time. At 24 months, CK-treated eyes had a mean MRSE of +0.50 D from a mean of +1.80 D at baseline, which represents approximately a 0.50 D loss of effect from the intended plano. This is a 20% loss of effect after 2 years. The Beaver Dam Eye Study showed that the natural mean rate of hyperopic progression in patients older than 40 is approximately 0.03 D per year.78 This is markedly less than the change after CK treatment.
The reported follow-up periods for CK and LTK were different (6 to 9 months, and 9 to 12 months are not reported for LTK), making it difficult to make stability comparisons. However, MRSE mean change of ≤0.50 D for CK over 24 months is lower than that for LTK.48 Mean changes per interval were also lower with CK.
The CK and LTK study data differ most in the initial postoperative overcorrection and the subsequent long-term loss of effect or regression. These two variables are closely related in that the initial overcorrection must be adjusted to achieve a stable, long-term refractive outcome. The current LTK technique appears to have poorer long-term stability48 than CK, thus requiring a larger initial overcorrection to achieve a more stable long-term refractive effect. As a result, LTK nomograms are complex, because during surgical planning the surgeon must take into account patient age, attempted correction, and desired time point to achieve emmetropia.
Stability after LTK is also affected by patient age. Alio and associates45 found that younger patients (age 30 or younger) had significantly more regression than older patients and that half of patients under age 40 experienced 75% to 100% regression at 15 months. They also observed increased regression in corneas thicker centrally (>525 μm). The investigators proposed that the tissue returns to its original shape because of the higher elasticity of Bowman’s membrane and stromal collagen in younger corneas and the stronger mechanical integrity of thicker corneas.
The CK technique, on the other hand, appears to be stable over a range of attempted corrections and age groups. It provides greater long-term stability with a smaller initial overcorrection, typically 0.5 D of myopia at 1 month to achieve emmetropia at 6 months.
Thermal Modeling Studies
A finite-difference thermal model was created by researchers (J. Pearce, A. J. Welch, S. Thomsen) at the University of Texas at Austin to elucidate the thermal effects of CK treatment. Actual temperatures in human tissue during treatment cannot be measured, and a mathematical model along with animal studies must be used. Primarily this is because of the small area to be measured and because heating occurs below the surface of the cornea.
Temporal and spatial temperature profiles achieved during treatment were calculated and used to predict tissue damage, including collagen denaturation/birefringence loss. (Birefringence loss is a method to measure lesion formation in the stroma.) The information provided by the model led to a hypothesis that supports the observation of greater stability after CK treatment as compared to LTK treatment.
The default CK treatment parameters are 0.6 W for 0.6 seconds at 350 kHz. In response to the high-power density created at the end of the Keratoplast tip, the tissue surrounding the tip undergoes a temperature increase. As heated tissue dehydrates, its resistance to radiofrequency current increases. Because current seeks the path of least resistance, the current path moves up the shaft of the Keratoplast tip. Consequently, with CK, the tissue heats from bottom to top, and thermal conductivity creates a cylindrical thermal profile. It must be noted that the Keratoplast tip only delivers radiofrequency energy and heats only in response to the increase in tissue temperature. The tip acts as a heat sink (takes heat away) rather than a heat source (supplies heat).
In the model, the lesion formed as a result of treatment—the area with a loss of birefringence—is similar to that seen with histology after CK treatment. The polarized light micrograph of a histologic section from a 7-day post-CK pig cornea shows the birefringence effects of treating pig corneas with CK. The dark region shows the loss of birefringence characteristic of thermally damaged tissue and reveals formation of a cylindrical footprint, approximately 80% deep, that corresponds to the shape and size of the CK lesion as predicted by the model.
The thermal model was also used to describe the thermal effects of LTK. Laser thermal keratoplasty delivers laser energy at the surface of the eye, where it is absorbed by water in the tissue. Thermal diffusion drives the heat into the cornea, where it shrinks collagen. In contrast to CK, the noncontact LTK technique generates the greatest amount of heat at the surface of the cornea because of the high absorption of light energy in water. Heat energy diffuses radially and axially into the tissue. Additionally, the Ho:YAG beam is attenuated as it passes through the cornea, causing temperature variations over the treatment time. The result is corneal denaturation decreasing from top to bottom, forming a cone-shaped zone of collagen shrinkage5 of a depth that appears to be more shallow than that of a CK-treated spot.
The depth and the relatively straight cylinder of the CK lesion may be responsible for its superior clinical outcome. The deep and cylindrical lesion tends to produce forces through the thickness of the cornea that are more parallel to the corneal surface. With LTK, the shallow conical lesion provides a dramatic initial effect but may not be deep enough to maintain the effect over time.
In summary, CK treatment produces a deep, narrow thermal profile in tissue that has coagulated to form a deep cylindrical lesion. LTK, on the other hand, produces a shallow, conical thermal profile that results in a shallow conical lesion. Heating of corneal tissue with CK proceeds from the bottom of the probe to the top, producing a deep coagulation of corneal collagen, versus heating from the surface of the cornea downward with LTK, producing a more shallow coagulation. The CK footprint is cylindrical, whereas the LTK footprint is conical, with less effect in deeper parts of the cornea. The tension lines on the corneal surface created by the treatment are parallel to the corneal surface with CK, but they are not parallel after LTK.
Safety
Best spectacle-corrected visual acuity was generally preserved after the CK procedure, and no eye had BSCVA worse than 20/40. The incidence of induced cylinder of 2.00 D or greater was below 1% (the FDA target is 5%) and was nearly identical to that of LTK in which thermal spots are applied simultaneously to the cornea. One year after CK, 7% showed an increase of 1.00 D in cylinder, and 4.5% showed an increase of more than 1.00 D but less than 2.00 D. These percentages are clinically acceptable, especially because induced cylinder after CK tends to decrease markedly with time. Most important, the effect on BSCVA (ie, loss or gain of lines) did not appear to correlate with the amount of induced cylinder (≤1.00 D and >1.00 D). However, induced cylinder appeared to have a minor effect on UCVA. In eyes with CK-induced cylinder, there was an average of 1 line less improvement in UCVA; however, this effect improved over time as the astigmatism resolved. Results of the key safety variables after CK were generally comparable to those after LTK. The percentage with a loss of 2 lines of BSCVA at 1 year was nearly identical with the two modalities (CK, 2%; LTK, 2.3%).
The endothelial cell study at three investigational sites performed with specular microscopy showed that the corneal endothelium was not damaged following deep thermal penetration in the stroma by CK treatment and appear to indicate that the CK procedure is safe for the corneal endothelium. Postoperative cell counts showed less than 1% loss in endothelial cell density in any region (central, midperipheral, and peripheral cornea). Morphologic changes (polymegathism or polymorphism) were not observed.
Confocal microscopy views also showed deep penetration without damage to the endothelium. Striae between treatment zones were visible by slit lamp at 12 months postoperatively, suggesting that the effect of treatment on the stroma is long-lasting. Leukomas visible by slit lamp postoperatively were small because CK delivers energy deep into the stroma rather than on the surface. It appears that radiofrequency energy can be safely delivered to the cornea via the Keratoplast tip with little or no effect on the endothelium. No spikes were seen in postoperative IOP data, suggesting that flattening of the cornea by the procedure did not cause shallowing of the peripheral chamber angle. The incidence of adverse events was very low, and all resolved without sequelae. Contrast sensitivity was not changed by the procedure.
Subjective Evaluations
Subjective (patient) evaluations on satisfaction showed 76% and 82% very satisfied or satisfied at 1 month and 1 year, respectively. The percentage of patients who were dissatisfied or very dissatisfied was mostly constant throughout the study. For example, 7% and 9% were dissatisfied or very dissatisfied at 1 month and at 12 and 24 months, respectively. No retreatments were permitted in this study; thus dissatisfied patients could not be enhanced. Patient satisfaction results were not available from the Sunrise LTK studies, so comparisons cannot be made.
Subjective evaluations on the level of improvement in visual quality showed 91% with extreme, marked, or moderate improvement at 12 months. The percentage of patients expressing no improvement was fairly constant throughout the study (5% at 1 month and at 24 months). These patients may represent a group that could have benefited from a retreatment.
CK may also be compared with nonthermal modalities for the correction of hyperopia to determine its value as a refractive surgery procedure. Recently published H-LASIK studies64–75 were carried out for different levels of hyperopia, with and without astigmatism, using different excimer lasers, beam profiles, ablation zone diameters, nomograms, and retreatment rates. Follow-up ranged from 6 to 12 months. These variations make it difficult to evaluate the current performance of H-LASIK and to compare results with those of CK. However, certain trends do emerge.
Argento and Cosentino64 stratified their LASIK results by level of hyperopia, allowing for a valid comparison of their low to moderate hyperopia groups (group A, ≤+2.00; group B, +2.00 to +3.00 D) with the CK multicenter study in previously untreated eyes. However, their last follow-up was at 6 months. At that time, UCVA was 20/25 or better in 70% (group A) to 75% (group B) of the cases and 20/40 or better in 94% to 100%, respectively. Accuracy was within ±1.00 D in 95% and 100% (±0.50 D values not available). Their visual results are comparable to those of CK, but their accuracy results are higher. One eye (2%) lost 2 lines of BSCVA, which was attributed to decentered ablation. The trend toward initial overcorrection followed by regression was proportional to the level of hyperopia treated. The investigators state that “stability was achieved between three and six months,” but also state that “from 6 to 12 months postoperatively, refraction ranged from 0 to +1.00 D.” This suggests that stability was not achieved between 3 and 6 months postoperatively. Approximately 50% of the patients had mild to moderate dry eye symptoms postoperatively.
Ditzen and associates65 evaluated eyes with hyperopia ranging from +1.00 to +4.00 D with a 1-year follow-up after LASIK treatment. Postoperative UCVA was 20/40 or better in 95%, and 85% were within ±1.00 D of intended correction. This is comparable to results with CK. Epithelial ingrowth was the most common complication postoperatively. Regression was less than 1.00 D at 1 year. The investigators concluded that LASIK was superior to PRK for the correction of hyperopia but recommended LTK instead of LASIK for eyes with less than +3.00 D of hyperopia, because it is a less invasive procedure.
Williams66 reported on results of 52 eyes with primary and secondary hyperopia with and without cylinder treated with the Visx Star laser. The mean MRSE was +3.03 D preoperatively and −0.13 at 12 months. Uncorrected VA of 20/20 or better was achieved in 39% and 20/40 or better in 95%. A total of 79% were ±0.50 D of intended refraction. Thus, visual results were better with CK, and accuracy results were better with H-LASIK. No eye lost 2 or more lines of BSCVA. Refractive stability was not demonstrated at 6 months.
Zadok and associates67 performed H-LASIK on 45 eyes with a mean of 2.0 D of primary spherical hyperopia, which approximates the refractive status of eyes treated with CK. Follow-up was 6 months. The laser software in this study was modified to allow a 7.5-mm ablation within an 8.5-mm stromal bed. Uncorrected VA was achieved in 42% compared with 54% with CK. The MRSE within ± 1.0 D of intended was almost identical to that of CK (89%, 88%, respectively). The safety results were very good with no losses of 2 or more lines of BSCVA. Predictability decreased with increasing hyperopia; however, significant regression did occur in low-hyperopia eyes. The mean MRSE was +0.06 D at 1 month, +0.26 D at 3 months, and +0.30 D at 6 months.
In 85 eyes with primary hyperopia with and without cylinder and a mean preoperative MRSE of +3.31 D, Rashad68 reported UCVA of 20/20 or better in 25% and 20/40 or better in 93% at 1 year postoperatively. The mean MRSE was within ±0.50 D of emmetropia in 61% and within ±1.00 D in 89%. The visual results with CK were better (UCVA of 20/20 or better in 54%), and the accuracy results were nearly identical. One eye in Rashad’s study lost 2 lines of BSCVA. Stability results showed a mean MRSE of −0.29 D at 1 week postoperatively, +0.22 D at 3 months, and +0.43 D at 12 months, demonstrating regression and lack of stability at 1 year postoperatively.
In a study by Tabbara and associates69 of hyperopic LASIK used for the correction of eyes with and without cylinder with a mean preoperative SE of +3.4 D, 44% had UCVA of 20/20 or better and 97% had 20/40 or better 6 months postoperatively. A total of 58% had a mean MRSE of ±0.50 D, and 84% were within ±1.00 D of intended correction. The investigators stated that all eyes showed regression 6 months postoperatively. One week after the procedure, the mean SE was +0.11 D and after 6 months, +0.25 D.
In a subset of a study of 92 eyes, Pineda-Fernandez and associates70 retrospectively evaluated results of 22 eyes with low spherical hyperopia (+1.00 to +3.00 D; mean, +2.16) that had undergone LASIK with the Nidek EC-5000. Six months postoperatively, 14% had UCVA of 20/20 or better and 73% had 20/40 or better. The mean SE at 6 months was within ±0.50 D in 54% and within ±1.00 D in 82%. These visual acuity results and level of accuracy for low hyperopia treatments without astigmatism were inferior to those obtained with CK treatment. At 3 months, the mean MRSE for the low hyperopia subgroup was +0.05 D, and at 6 months, +0.41 D. Thus substantial regression occurred postoperatively. The mean surgically induced astigmatism was +0.38 D. No eyes lost lines of BSCVA. The investigators concluded that nomogram adjustment was needed for better results and less regression.
Lian and associates71 studied the outcome of 54 hyperopic eyes undergoing LASIK with the Technolas 117C excimer laser. The mean preoperative MRSE was +3.12 D, with a range of +1.0 to +5.75 D. At 12 months, 63% had UCVA of 20/20 or better and 93% had 20/40 or better. The mean residual refraction at 12 months was +0.29 ± 0.78 D; 83% were within ±1.00 D and 61% were within ±0.50 D of emmetropia. No eye lost more than 2 lines of BSCVA, but 1.9% lost 2 lines. Halos and glare were reported by 3.7%. Except for a higher percentage for 20/20 or better UCVA with the LASIK treatment, the results of Lian and associates approximated those of CK treatment. The investigators concluded that primary and secondary hyperopia required different nomograms.
One hundred and seven eyes with mean preoperative primary hyperopia of +2.0 ± 0.50 D were treated by Cobo-Soriano and associates72 with the Technolas Keracor 217C (a later version than the 117) and followed up for a mean of 8.2 months. They found that favorable outcome of H-LASIK decreased with increasing levels of hyperopia. Their results with the low hyperopia group for percentages with 20/20 or better UCVA and within ±1.0 D of intended were similar to those obtained with CK.
Using the Meditec MEL 160, Ditzen and associates73 evaluated 23 eyes with a mean primary spherical hyperopia of +4.88 ± 2.13 D, a markedly higher mean of hyperopia than in CK treatment. Uncorrected VA was 20/20 or better and 20/40 or better in 39% and 83%, respectively, which is lower than obtained with CK. Predictability within ±0.50 D was 78%. The investigators considered stability to be good after 3 months, but the mean SE over time was −0.20 D, +0.17 D, +0.14 D, and +0.30 D at 1, 3, 6, and 12 months, respectively, which indicates lack of stability at the last follow-up.
The largest and most rigorously designed study of H-LASIK was the Summit-Autonomous Pre-Market Approval multicenter study conducted with LADARVision Excimer Laser System, an active-tracking, narrow-beam laser system, for LASIK treatment of up to +6.00 D of sphere and up to −6.00 D of cylinder.74 This study was later published.75 The primary spherical hyperopia group consisted of 110 eyes with a mean SE of +2.56 D ± 1.16 and a range of +0.875 to +6.0 D. Follow-up was 12 months. Uncorrected VA values of 20/20 or better and 20/40 or better were similar. Predictability results were slightly better with LADARVision (MRSE within ± 1.0 D: 91.4% with LADARVision, 88% with CK).
Safety results showed a 2-line loss of BSCVA in 4 of 88 (4.5%) of the LADARVision-treated eyes (the 12-month cohort) and 2% of the CK-treated eyes. No eye lost more than 2 lines with either technique. Glare was reported in 0.8% and halos in 2.3% at 6 months after LADARVision treatment. No eye had halos or glare after treatment with CK, and any haze was mild in severity.
Complications observed after LADARVision H-LASIK at any time during the 12 postoperative months included 1.8% with corneal edema, 2.7% with double or ghost images, 2.7% with epithelium in the interface, and 1.8% with foreign body sensation occurring later than 1 month postoperatively.77 With CK, the above complications were 0% at all time points, except for double/ghost images, which occurred at a level of 1% at months 1, 3, 9, 12, and 24, and 2% at month 6. Fluctuation in vision was reported by 6% of LADARVision patients at 6 months and 2.6% at 12 months. Dryness was reported by 3% at 6 months and 6.1% at 12 months.
In the LADARVision H-LASIK study, refractive stability was evaluated by assessing the percentage of eyes with a change in MRSE of 1.00 D or less between 3-month postoperative intervals, as well as by mean diopter change per interval.75 The mean MRSE changes of 1.00 D or less for all three intervals (3 to 6 months, 6 to 9 months, and 9 to 12 months) were similar between LADARVision and CK. However, 1.00 D is a rather coarse measurement for corrections of less than +3.00 D. With CK, changes of 0.50 D or less were 79%, 89%, and 88%, respectively, for the three intervals. Changes of 0.50 D or less were not reported for LADARVision. The mean diopter changes per interval were larger for CK than for LADARVision, especially for the 9- to 12-month interval, which differed by a factor of 10.
The stability outcome with LADARVision H-LASIK was better than that after CK and after all of the previously mentioned H-LASIK studies.64–75 However, patient populations and preoperative refractive status were varied in the H-LASIK studies, and some included patients with astigmatism and secondary hyperopia. Unlike many of the other H-LASIK studies, stability with LADARVision was established within 6 months.75 These results may be unique to LADARVision technology and may not be representative of results with other excimer laser systems.
At 1 and 2 years, CK-treated eyes had a mean MRSE of +0.21 and +0.50 D, respectively, from a mean of +1.80 D at baseline. This represents approximately a 0.50 D loss of effect from the intended plano at 2 years. Thus, most patients would still enjoy a notable decrease in hyperopia 2 years after surgery, and because the loss of effect diminishes with time, they could expect a change of approximately 0.03 D per 3-month interval. Most of the H-LASIK studies did not provide a firm definition of stability nor continue evaluating a cohort population for stability beyond 6 or 12 months as the CK study did. Many did not show stability at either the 6- or 12-month end point.64–75
Furthermore, haze and glare were frequent problems after H-LASIK, whereas after CK, 98% of the eyes had no haze at 1 month postoperatively, despite the erroneous inclusion of treatment site haze data into the overall data by one investigator. Loss of contrast sensitivity, epithelial ingrowth and other flap-related complications, fluctuation in vision, and dryness were also associated with the H-LASIK procedure.
A retrospective study was conducted to compare outcomes of LASIK with those of LTK (84 eyes) in the treatment of spherical hyperopia up to 3.0 D.79 LASIK was performed with the LaserSight LSX (81 eyes) or the Nidek EC-5000 laser (69 eyes). Laser thermal keratoplasty was performed with the Sunrise system. Follow-up was up to 18 months. All three treatments decreased hyperopia. Uncorrected VA at 3 months postoperatively was 41% with Nidek, 50% with LaserSight, and 21% with LTK. No eye lost more than 2 lines of BSCVA. The rate of surgically induced astigmatism in the LTK group was high, with a mean postoperative astigmatism of −0.81 D ± 0.51 (range, 0 to 2.25 D). One or more enhancements were performed in 38.3% of the LTK cases.
Comparison of BSCVA Line Losses
Loss of lines of BSCVA is a major concern in all refractive procedures. In the H-LASIK studies mentioned above, the loss of 2 lines of BSCVA at various follow-up periods ranged from 0% to 2%, with the exception of the H-LASIK studies reported by Salz and associates75 and Hill.79 Salz and associates reported 4.5% with a loss of 2 lines of BSCVA at 12 months following the treatment of low spherical hyperopia with LADARVision, and Hill reported 3% with a 2-line loss at 3 months after H-LASIK with the Nidek EC5000 laser.
These results can be compared with those of CK, which showed nine of 391 eyes (2%) with a 2-line loss of BSCVA at 12 months. All eyes that lost 2 lines at 12 months were left with very functional vision. Preoperatively, these nine eyes had 20/10 to 20/16 BSCVA. Postoperatively, one of nine eyes had 20/16 BSCVA, five of seven had 20/20, and three of seven eyes had 20/25. No eye had BSCVA worse than 20/40 at any follow-up visit, and no eye that had 20/20 or better BSCVA preoperatively had worse than 20/25 BSCVA at 12 or 24 months postoperatively.
Subjective Evaluations
Patient satisfaction has traditionally been regarded as an indicator of treatment success. Satisfaction studies can thus provide important information about treatment outcome apart from the objective measurements. Only one published study (Salz and associates75) provides patient satisfaction information following H-LASIK. At 12 months, 79.8% of patients in the spherical hyperopia group treated with the LADARVision system were satisfied or extremely satisfied with their results.75 This is comparable to CK results, which showed 82% of patients very satisfied or satisfied 1 year after CK.
The percentage of CK patients who were dissatisfied or very dissatisfied was mostly constant throughout the study. For example, 7% and 9% were dissatisfied or very dissatisfied at 1 month and 12 and 24 months, respectively. No retreatments were permitted in this study; thus dissatisfied patients could not be enhanced. No enhancement was also the case with the H-LASIK study by Salz and associates,75 which showed very similar patient satisfaction values at 1 year.
Given the relatively low rate of BSCVA line losses after CK, higher satisfaction rates could have been expected. Some patients had induced cylinder early in the postoperative period, which compromised their vision, but could not be addressed through an enhancement. Although the induced cylinder eventually resolved, the patient remained dissatisfied.
In addition, despite preoperative explanations, many patients undergoing a procedure to correct hyperopia do not understand that plano is the intended refraction and that accommodation is necessary for near vision. Their expectation for improved distance and near vision postoperatively may have resulted in dissatisfaction. And finally, among patients undergoing CK or any other refractive surgery, some will have the expected response to treatment and others will be undercorrected. Again, undercorrection could not be treated with an enhancement under the study protocol.
Regarding the level of improvement in visual quality, 95% of CK patients reported extreme/marked, moderate, or slight improvement at 12 months. The percentage of patients expressing no improvement was fairly constant throughout the study (5% at 1 month and at 24 months). These patients may represent a group that could have benefited from a retreatment. The H-LASIK study75 showed 96.5% of spherical hyperopes reporting quality of vision as unchanged, better, or significantly better at 12 months postoperatively. The values from this study could be better compared with CK quality of vision values if their report excluded the percentage of patients who reported their vision as “unchanged” after LASIK.
DRY EYE AND OTHER CORNEAL EFFECTS
Patients who do not tolerate or are uncomfortable with contact lenses because of tear film or ocular surface disorders frequently seek LASIK surgery. However, severe dry eye and other abnormalities have been reported after LASIK procedures for both myopia and hyperopia.80–88 Decreases in tear secretion, corneal and conjunctival sensitivity, Schirmer test values, tear breakup time, and basal tear secretion were frequently reported during postoperative follow-up. Similarly, punctate corneal staining and ocular irritation symptoms, including dryness, soreness, and eyelid sticking to eyeball upon awakening, have been reported high in incidence and severity.
Albietz and associates80 performed an extensive evaluation of the effects of H-LASIK on tear film and the ocular surface. Patients had a mean age of 49 and a mean preoperative MRSE of +2.35 ± 1.41 D. They found chronic dry eye (lasting 6 months or longer) in 32% of patients, and an association with female gender (50% in female patients and 16% in male). Symptoms persisted at 12 months postoperatively in 23%. Patients with chronic dry eye had decreased fluorescein tear breakup time, ocular surface staining, decreased tear volume, and decreased goblet cell density. Mean values for these variables had not returned to preoperative levels 12 months after the LASIK procedure.
A significant association between chronic dry eye and refractive regression was observed. In the first 12 months postoperatively, eyes with chronic symptoms had a 50% regression rate compared with 22% in those without chronic symptoms. Overall, 32% of patients demonstrated refractive regression. The investigators believe that the adverse tear film and ocular surface effects are worse after LASIK for the treatment of hyperopia than after LASIK for the treatment of myopia. The proposed reasons are that H-LASIK patients are older, that the large flaps required for hyperopia treatments may have a greater effect on sensitivity loss, and that the steeper central cornea may adversely affect tear film stability and blinking patterns.
Battat and associates81 have proposed that sensory denervation of the ocular surface after LASIK disrupts ocular surface tear dynamic and causes irritation symptoms, whereas Patel and associates82 attributed dry eye symptoms after LASIK partly to poor-quality tear lipid layer following the procedure. Wilson and Ambrosio88 proposed neurotrophic epitheliopathy as the likely cause for LASIK-induced dry eye signs.
In addition to dry eye and ocular surface disorders, diffuse lamellar keratitis, a serious complication that decreases vision and causes patient dissatisfaction, has been reported.88 A study of 1,569 eyes that had LASIK for myopia or hyperopia showed 0.9% having postoperative diffus lamellar keratitis of stage 1 or 2. The proposed trigger was the release of cytokines that cause inflammatory cells to accumulate at the flap interface. Although an incidence of 0.9% is not particularly high, less experienced surgeons may have a higher incidence. Because CK does not involve the creation of a corneal flap, this complication cannot occur.
Patient Satisfaction
Patient satisfaction at 12 months postoperatively with CK was comparable to that after H-LASIK with LADARVision.75 A total of 82% of CK-treated patients were satisfied or very satisfied compared with 79.8% of spherical hyperopia patients treated with LADARVision. For improvement in vision quality, 95% of CK-treated patients expressed a range of improvement, compared with 95.6% of the spherical hyperopia LADARVision patients.
CONCLUSIONS
The CK technique appears to correct low to moderate hyperopia effectively and safely and produce more stable results than other hyperopic procedures. This may be due to the deeper stromal penetration and cylindrical footprint of CK (80% corneal depth) compared with the more shallow, cone-shaped penetration of the LTK treatment. CK spares the visual axis and does not involve removal of any corneal tissue or use of a microkeratome. There is thus far no evidence of recurrent erosions, infections, visually significant corneal scarring, or corneal haze following the CK procedure. Irregular astigmatism may result from asymmetric application of the high-frequency energy, but such occurrences have been very low. The 20% loss of initial effect at 24 months was acceptable. The 2% loss of 2 lines of BSCVA may be diminished with refinement of technique.
REFERENCES
- 1.Mendez A, Mendez Noble A. Conductive keratoplasty for the correction of hyperopia. In: Sher N, ed. Surgery for Hyperopia and Presbyopia Philadelphia: Williams & Wilkins; 1997;163–171.
- 2.Privalov PL. Stability of proteins: proteins which do not present a single cooperative system. In: Advances in Protein Chemistry. New York: Academic Press; 1982;55–104. [PubMed]
- 3.Pearce J, Thomsen S. Rate process analysis of thermal damage. In: Welch AJ, van Gemert MJC, eds. Optical-Thermal Response of Laser-Irradiated Tissue New York: Plenum Press; 1995; 561–605.
- 4.Chen SS, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: pseudoelastic behavior at 37° C. J Biomech. 1998;31:211–216. doi: 10.1016/s0021-9290(97)00121-8. [DOI] [PubMed] [Google Scholar]
- 5.Koch DD, Kohnen T, Anderson JA, et al. Histologic changes and wound healing response following 10-pulse noncontact holmium: YAG laser thermal keratoplasty. J Refract Surg. 1996;12:623–634. doi: 10.3928/1081-597X-19960701-16. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Soderberg PG, Denham D, et al. Temperature induced corneal shrinkage. In: Parel JM, ed. Ophthalmic Technologies VI. Proc SPIE. 1996;2673:70–76. [Google Scholar]
- 7.Kampmeier J, Radt B, Birngruber R, et al. Thermal and biochemical parameters of porcine cornea. Cornea. 2000;19:355–363. doi: 10.1097/00003226-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Sporl E, Genth U, Schmalfuss K, et al. Thermomechanical behavior of the cornea. Ger J Ophthalmol. 1997;5:322–327. [PubMed] [Google Scholar]
- 9.Brinkmann R, Radt B, Flamm C, et al. Influence of temperature and time on thermally induced forces in corneal collagen and the effect of laser thermokeratoplasty. J Cataract Refract Surg. 2000;26:744–754. doi: 10.1016/s0886-3350(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 10.Stringer H, Parr J. Shrinkage temperature of eye collagen. Nature. 1964;4965:1307. doi: 10.1038/2041307a0. [DOI] [PubMed] [Google Scholar]
- 11.Lans LJ. Experimentelle Untersuchungen uber Entstehung von Astigmatismus durch nicht-perforirende Corneawunden. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1889;44:117–152. [Google Scholar]
- 12.Gassett AR, Kaufman HE. Thermokeratoplasty in the treatment of keratoconus. Am J Ophthalmol. 1975;79:226–232. doi: 10.1016/0002-9394(75)90076-8. [DOI] [PubMed] [Google Scholar]
- 13.Keates RH, Dingle J. Thermokeratoplasty for keratoconus. Ophthalmic Surg. 1975;6:89–92. [PubMed] [Google Scholar]
- 14.Aquavella JV, Smith RS, Shaw EL. Alterations in corneal morphology following thermokeratoplasty. Arch Ophthalmol. 1976;94:2082–2085. doi: 10.1001/archopht.1976.03910040742008. [DOI] [PubMed] [Google Scholar]
- 15.Arentsen J, Rodriquez M, Laibson P. Histopathological changes after thermokeratoplasty for keratoconus. Invest Ophthalmol Vis Sci. 1977;16:32–38. [PubMed] [Google Scholar]
- 16.Aquavella J, Buxton J, Shaw E. Thermokeratoplasty in the treatment of persistent corneal hydrops. Arch Ophthalmol. 1977;95:81–84. doi: 10.1001/archopht.1977.04450010081007. [DOI] [PubMed] [Google Scholar]
- 17.Fogle JA, Kenyon KR, Stark WJ. Damage to the epithelial basement membrane by thermokeratoplasty. Am J Ophthalmol. 1977;83:392–401. doi: 10.1016/0002-9394(77)90739-5. [DOI] [PubMed] [Google Scholar]
- 18.Caster AI. The Fyodorov technique of hyperopia correction by thermal coagulation: a preliminary report. J Refract Surg. 1988;4:105–108. [Google Scholar]
- 19.Neumann A, Sanders D, Salz J. Radial thermokeratoplasty for hyperopia. J Refract Corneal Surg. 1989;5:50–54. [PubMed] [Google Scholar]
- 20.Neumann A, Fyodorov S, Sanders D. Radial thermokeratoplasty for the correction of hyperopia. J Refract Corneal Surg. 1990;6:404–412. [PubMed] [Google Scholar]
- 21.Neumann A, Sanders D, Raanan M, et al. Hyperopic thermokeratoplasty: clinical evaluation. J Cataract Refract Surg. 1991;17:830–838. doi: 10.1016/s0886-3350(13)80419-1. [DOI] [PubMed] [Google Scholar]
- 22.Feldman S, Ellis W, Frucht-Pery J, et al. Regression of effect following radial thermokeratoplasty in humans. J Refract Surg. 1995;18:288–291. [PubMed] [Google Scholar]
- 23.Charpentier D, Nguyen-Khoa J, Duplessix M, et al. Intrastromal thermokeratoplasty for correction of spherical hyperopia: a 1-year prospective study. J Fr Ophthalmol. 1995;18:200–206. [PubMed] [Google Scholar]
- 24.McDonnell PJ. Radial thermokeratoplasty for hyperopia. I. The need for prompt investigation. Refract Corneal Surg. 1989;5:50–52. [PubMed] [Google Scholar]
- 25.Rowsey JJ, Doss JD. Preliminary report of Los Alamos keratoplasty techniques. Ophthalmology. 1981;88:755–760. doi: 10.1016/s0161-6420(81)34946-x. [DOI] [PubMed] [Google Scholar]
- 26.Rowsey JJ, Gaylor JR, Dahlstrom R, et al. Contact Intraocul Lens Med J. 1980;6:1–12. [Google Scholar]
- 27.McDonnell PJ, Garbus J, Romero JL, et al. Electrosurgical keratoplasty: clinicopathologic correlation. Arch Ophthalmol. 1988;106:235–238. doi: 10.1001/archopht.1988.01060130245037. [DOI] [PubMed] [Google Scholar]
- 28.Horn G, Spears KG, Lopez O, et al. New refractive method for laser thermal keratoplasty with the Co:MgF2 laser. J Cataract Refract Surg. 1990;16:611–616. doi: 10.1016/s0886-3350(13)80779-1. [DOI] [PubMed] [Google Scholar]
- 29.Seiler T, Matallana M, Bende T. Laser thermokeratoplasty by means of a pulsed holmium:YAG laser for hyperopic correction. Refract Corneal Surg. 1990;6:355–359. [PubMed] [Google Scholar]
- 30.Mainster MA. Ophthalmic applications of infrared lasers—thermal considerations. Invest Ophthalmol Vis Sci. 1979;18:414–420. [PubMed] [Google Scholar]
- 31.Durrie DS, Seiler T, King MC, et al. Application of the holmium:YAG laser for refractive surgery. In: Parel J-M, ed. Ophthalmic Technologies II. Proc SPIE. 1992;1644:56–60. [Google Scholar]
- 32.Thompson V, Seiler T, Hunkeler JD, et al. Application of the holmium:YAG laser for refractive surgery: an update of clinical progress. In: Parel J-M A, Ren Q, eds. Ophthalmic Technologies III. Proc SPIE. 1993;1877:52–56. [Google Scholar]
- 33.Durrie DS, Schumer JD, Cavanaugh TB. Holmium:YAG laser thermokeratoplasty for hyperopia. J Refract Corneal Surg. 1994;10:S277–280. [PubMed] [Google Scholar]
- 34.Tutton MK, Cherry PMH. Holmium:YAG laser thermal keratoplasty to correct hyperopia: two years of follow-up. Ophthalmic Surg Lasers. 1996;27(suppl):S521–S524. [PubMed] [Google Scholar]
- 35.Parel JM, Ren Q, Simon G. Noncontact photothermal keratoplasty. I. Biophysical principles and laser beam delivery system. J Refract Corneal Surg. 1994;10:511–518. [PubMed] [Google Scholar]
- 36.Simon G, Ren Q, Parel J-M. Noncontact laser thermal keratoplasty. II. Refractive effects and treatment parameters in cadaver eyes. J Refract Corneal Surg. 1994;10:519–528. [PubMed] [Google Scholar]
- 37.Ren Q, Simon G, Parel JM. Noncontact laser thermal keratoplasty. III. Histological study in animal eyes. J Refract Corneal Surg. 1994;10:529–539. [PubMed] [Google Scholar]
- 38.Moreira H, Campos M, Sawusch MR, et al. Holmium laser thermokeratoplasty. Ophthalmology. 1993;100:752–761. doi: 10.1016/s0161-6420(93)31579-4. [DOI] [PubMed] [Google Scholar]
- 39.Ariyasu RG, Sand B, Menefee R, et al. Holmium laser thermal keratoplasty of 10 poorly sighted eyes. J Refract Surg. 1995;11:358–365. doi: 10.3928/1081-597X-19950901-12. [DOI] [PubMed] [Google Scholar]
- 40.Koch DD, Abarca A, Villareal R, et al. Hyperopia correction by non-contact holmium:YAG laser thermokeratoplasty: clinical study with two-year follow-up. Ophthalmology. 1996;103:731–740. doi: 10.1016/s0161-6420(96)30622-2. [DOI] [PubMed] [Google Scholar]
- 41.Koch DD, Kohnen T, McDonnell PJ, et al. Hyperopia correction by non-contact holmium:YAG laser thermokeratoplasty; United States phase IIA clinical study with a 1-year follow-up. Ophthalmology. 1996;103:1525–1536. [PubMed] [Google Scholar]
- 42.Koch D, Kohnen T, McDonnell P, et al. Hyperopia correction by noncontact holmium:YAG laser thermal keratoplasty. U.S. Phase IIA Clinical Study with 2-year follow-up. Ophthalmology. 1997;104:1938–1947. doi: 10.1016/s0161-6420(97)30003-7. [DOI] [PubMed] [Google Scholar]
- 43.Kohnen T, Koch DD, McDonnell PJ, et al. Noncontact holmium:YAG laser thermal keratoplast y to correct hyperopia:18-month follow-up. Ophthalmologica. 1997;211:274–282. doi: 10.1159/000310808. [DOI] [PubMed] [Google Scholar]
- 44.Kohnen T, Villareal R, Menefee R, et al. Hyperopia correction by noncontact holmium:YAG laser thermal keratoplasty: five-pulse treatments with 1-year follow-up. Graefes Arch Clin Exp Ophthalmol. 1997;235:702–708. doi: 10.1007/BF01880669. [DOI] [PubMed] [Google Scholar]
- 45.Alio JL, Ismail MM, Sanchez Pego JL. Correction of hyperopia with non-contact Ho:YAG laser thermal keratoplasty. J Refract Surg. 1997;13:17–22. doi: 10.3928/1081-597X-19970101-07. [DOI] [PubMed] [Google Scholar]
- 46.Eggink CA, Bardak Y, Cuypers MHM, et al. Treatment of hyperopia with contact Ho:YAG laser thermal keratoplasty. J Refract Surg. 1999;15:16–22. doi: 10.3928/1081-597X-19990101-04. [DOI] [PubMed] [Google Scholar]
- 47.Eggink CA, Meurs P, Bardak Y, et al. Holmium laser thermal keratoplasty for hyperopia and astigmatism after photorefractive keratectomy. J Refract Surg. 2000;16:317–322. doi: 10.3928/1081-597X-20000501-04. [DOI] [PubMed] [Google Scholar]
- 48.Hyperion LTK System Device Labeling, PMA P990078, SunriseTechnologies, Fremont, California, May 2000. Available atwww.fda.gov/cdrh/pdf/p990078.html
- 49.Brinkmann R, Droge G, Koop N, et al. Investigations on laser thermokeratoplasty. Lasers Light Ophthalmol. 1994;6:259–269. [Google Scholar]
- 50.Bende T, Jean B, Oltrup T. Laser thermal keratoplasty using a continuous wave diode laser. J Refract Surg. 1999;15:154–158. doi: 10.3928/1081-597X-19990301-13. [DOI] [PubMed] [Google Scholar]
- 51.Koop N, Borcherding S, Brinkmann R, et al. Laserthermokeratoplasik mit IR-Laserdioden bei 1.87 μm. In: Waidelich W, ed. Lasers in Medicine. Proceedings of Laser 95. Munich: Springer Verlag; 1996:393–396.
- 52.Brinkmann R, Koop N, Geerling G, et al. Diode laser thermokeratoplasty: application strategy and dosimetry. J Cataract Refract Surg. 1998;24:1192–1207. doi: 10.1016/s0886-3350(98)80011-4. [DOI] [PubMed] [Google Scholar]
- 53.Geerling G, Koop N, Brinkmann R, et al. Continuous-wave diode laser thermokeratoplasty in blind human eyes. J Refract Surg. 1999;25:32–40. doi: 10.1016/s0886-3350(99)80008-x. [DOI] [PubMed] [Google Scholar]
- 54.Wirbelauer C, Koop N, Tuengler A, et al. Corneal endothelial cell damage after experimental diode laser thermal keratoplasty. J Refract Surg. 2000;16:323–329. doi: 10.3928/1081-597X-20000501-05. [DOI] [PubMed] [Google Scholar]
- 55.Rad AS, Jabbarvand M, Farahvash MM, et al. Laser in situ keratomileusis and diode thermal keratoplasty for correction of hyperopia from +5.00 to +10.00 diopters. J Refract Surg. 2002;18(suppl):S318–20. doi: 10.3928/1081-597X-20020502-05. [DOI] [PubMed] [Google Scholar]
- 56.Jackson WB, Mintsioulis G, Agapitos PJ, et al. Excimer laser photorefractive keratectomy for low hyperopia: safety and efficacy. J Cataract Refract Surg. 1997;23:480–487. doi: 10.1016/s0886-3350(97)80203-9. [DOI] [PubMed] [Google Scholar]
- 57.Daya SM, Tappouni FR, Habib NE. Photorefractive keratectomy for hyperopia. Six month results in 45 eyes. Ophthalmology. 1997;104:1952–1958. doi: 10.1016/s0161-6420(97)30001-3. [DOI] [PubMed] [Google Scholar]
- 58.Vincinguerra P, Epstein D, Radice P, et al. Long-term results of photorefractive keratectomy for hyperopia and hyperopic astigmatism. J Refract Surg. 1998;14:S183–185. doi: 10.3928/1081-597X-19980401-08. [DOI] [PubMed] [Google Scholar]
- 59.Pietila J, Makinen P, Pajari S, et al. Excimer laser photorefractive keratectomy for hyperopia. J Refract Surg. 1997;13:504–510. doi: 10.3928/1081-597X-19970901-06. [DOI] [PubMed] [Google Scholar]
- 60.O’Brart DPS, Stephenson CG, Oliver K, et al. Excimer laser photorefractive keratectomy for the correction of hyperopia using an erodible mask and axicon system. Ophthalmology. 1997;104:1959–1970. doi: 10.1016/s0161-6420(97)30000-1. [DOI] [PubMed] [Google Scholar]
- 61.Dausch D, Klein R, Schroder E. Excimer laser photorefractive keratectomy for hyperopia. J Refract Surg. 1993;9:20–28. [PubMed] [Google Scholar]
- 62.Davidorf JM, Eghbali F, Onclinx T, et al. Effect of varying the optical zone diameter on the results of hyperopic laser in-situ keratomileusis. Ophthalmology. 2001;108:1261–1265. doi: 10.1016/s0161-6420(01)00588-7. [DOI] [PubMed] [Google Scholar]
- 63.Anschutz T. Laser correction of hyperopia and presbyopia. Int Ophthalmol Clin. 1994;10:S277–S280. [PubMed] [Google Scholar]
- 64.Argento CJ, Cosentino MJ. Laser in situ keratomileusis for hyperopia. J Cataract Refract Surg. 1998;24:1050–1058. doi: 10.1016/s0886-3350(98)80097-7. [DOI] [PubMed] [Google Scholar]
- 65.Ditzen K, Huschka H, Pieger S. Laser in situ keratomileusis for hyperopia. J Cataract Refract Surg. 1998;24:42–47. doi: 10.1016/s0886-3350(98)80073-4. [DOI] [PubMed] [Google Scholar]
- 66.Williams DK. One year results of laser vision correction for low to moderate hyperopia. Ophthalmology. 2000;107:72–75. doi: 10.1016/s0161-6420(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 67.Zadok D, Maskaleris G, Montes M, et al. Hyperopic laser in situ keratomileusis with the Nidek EC-5000 excimer laser. Ophthalmology. 2000;107:1132–1137. doi: 10.1016/s0161-6420(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 68.Rashad KM. Laser in situ keratomileusis for the correction of hyperopia from +1.25 to 5.00 diopters with the Keracor 117C laser. J Refract Surg. 2001;17:123–128. doi: 10.3928/1081-597X-20010301-04. [DOI] [PubMed] [Google Scholar]
- 69.Tabbara KF, El-Sheikh HF, Islam SM. Laser in situ keratomileusis for the correction of hyperopia from +0.50 to 11.50 diopters with the Technolas Keracor 117C laser. J Refract Surg. 2001;17:123–128. doi: 10.3928/1081-597X-20010301-05. [DOI] [PubMed] [Google Scholar]
- 70.Pineda-Fernandez A, Rueda L, Huang D, et al. Laser in situ keratomileusis for hyperopia and hyperopic astigmatism with the Nidek EC-5000 Excimer laser. J Refract Surg. 2001;17:670–675. doi: 10.3928/1081-597X-20011101-06. [DOI] [PubMed] [Google Scholar]
- 71.Lian J, Ye W, Zhou D, et al. Laser in situ keratomileusis for correction of hyperopia and hyperopic astigmatism with the Technolas 117C. J Refract Surg. 2002;18:435–438. doi: 10.3928/1081-597X-20020701-03. [DOI] [PubMed] [Google Scholar]
- 72.Cobo-Soriano R, Llovet F, Gonzalez-Lopez F, et al. Factors that influence outcomes of hyperopic laser in situ keratomileusis. Cataract Refract Surg. 2002;28:1530–1538. doi: 10.1016/s0886-3350(02)01367-6. [DOI] [PubMed] [Google Scholar]
- 73.Ditzen K, Fiedler J, Pieger S. Laser in situ keratomileusis for hyperopia and hyperopic astigmatism using the Meditec MEL 70 spot scanner. J Refract Surg. 2002;18:430–434. doi: 10.3928/1081-597X-20020701-02. [DOI] [PubMed] [Google Scholar]
- 74.LADARVision Excimer Laser System, PMA P970043/7, Supplement for LASIKtreatment of up to +6.00 D of sphere and up to −6.00 D of cylinder. Summit Autonomous, Inc, Orlando, Florida. October 13, 1999. Available atwww.fda.gov/cdrh/pdf/p970043.html
- 75.Salz JJ, Stevens CA LADARVision LASIK Hyperopia Study Group. LASIK correction of spherical hyperopia, hyperopic astigmatism, and mixed astigmatism with the LADARVision excimer laser system. Ophthalmology. 2002;109:1647–1656. doi: 10.1016/s0161-6420(02)01133-8. [DOI] [PubMed] [Google Scholar]
- 76.McDonald MB, Davidorf J, Maloney RK, et al. Conductive keratoplasty for the correction of low to moderate hyperopia: 1-year results on the first 54 eyes. Ophthalmology. 2002;109:637–649. doi: 10.1016/s0161-6420(01)01022-3. [DOI] [PubMed] [Google Scholar]
- 77.McDonald MB, Hersh PS, Manche EE, et al. Conductive keratoplasty for the correction of low to moderate hyperopia: U.S. clinical trial 1-year results on 355 eyes. Ophthalmology. 2002;109:1978–1989. doi: 10.1016/s0161-6420(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 78.Lee KE, Klein BEK, Klein R. Changes in refractive error over a 5-year interval in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1999;40:1645–1649. [PubMed] [Google Scholar]
- 79.Hill JC. Treatment of simple hyperopia: comparison of laser in situ keratomileusis and laser thermal keratoplasty. J Cataract Refract Surg. 2003;29:912–917. doi: 10.1016/s0886-3350(02)01990-9. [DOI] [PubMed] [Google Scholar]
- 80.Albietz JM, Lenton LM, McLennan SG. Effect of laser in situ keratomileusis for hyperopia on tear film and ocular surface. J Refract Surg. 2002;18:113–123. doi: 10.3928/1081-597X-20020301-02. [DOI] [PubMed] [Google Scholar]
- 81.Battat L, Macri A, Dursun D, et al. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108:1230–1235. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 82.Patel S, Perez-Santonja JJ, Alio JL, et al. Corneal sensitivity and some properties of the tear film after laser in situ keratomileusis. J Refract Surg. 2001;17:17–24. doi: 10.3928/1081-597X-20010101-02. [DOI] [PubMed] [Google Scholar]
- 83.Benitez-del-Castillo JM, del Rio T, Iradier T, et al. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20:30–32. doi: 10.1097/00003226-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27:577–584. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 85.Yu EY, Leung A, Rao S, et al. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. 2000;107:2131–2135. doi: 10.1016/s0161-6420(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 86.Lindstrom RL, Linebarger EJ, Hardten DR, et al. Early results of hyperopic and astigmatic laser in situ keratomileusis in eyes with secondary hyperopia. Ophthalmology. 2000;107:1858–1863. doi: 10.1016/s0161-6420(00)00271-2. [DOI] [PubMed] [Google Scholar]
- 87.Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001;108:1082–1087. doi: 10.1016/s0161-6420(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 88.Wilson SE, Ambrosio R., Jr Sporadic keratitis (DLK) after LASIK. Cornea. 2002;21:560–563. doi: 10.1097/00003226-200208000-00005. [DOI] [PubMed] [Google Scholar]