Abstract
Purpose
To evaluate the vitreoretinal relationship in eyes with idiopathic macular disorders putatively caused by vitreomacular traction associated with early stages of age-related posterior vitreous detachment (PVD).
Methods
Retrospective observational case series of 43 eyes of 40 patients diagnosed with one of several idiopathic vitreomacular conditions. Included patients had no biomicroscopic evidence for complete PVD on presentation and underwent a specific clinical assessment of the vitreoretinal relationship. Affected eyes were evaluated with slit-lamp biomicroscopy, B-scan ultrasonography, optical coherence tomography, and/or intraoperative examination of the posterior hyaloid.
Results
By one or more examination techniques, 41 (95.3%) of the 43 study eyes had evidence of vitreous detachment from the perifoveal macular region and the remaining two eyes had complete PVD. When measurable, the size of the vitreomacular adhesion varied by diagnosis. Of 31 eyes with perifoveal vitreous detachment seen in follow-up, only three (9.7%) showed progression to complete PVD over an average preoperative or total follow-up period of 30.0 months (range, 2 to 237 months). Surgical or spontaneous separation of the residual vitreomacular adhesion in 16 eyes was followed in 15 (93.8%) by partial or complete resolution of the symptoms and signs of macular traction.
Conclusions
Age-related PVD appears to be an insidious, chronic event that begins in the perifoveal macula and evolves over a prolonged period of time prior to vitreopapillary separation. Though usually asymptomatic, its early (perifoveal) stages may be complicated by one of several macular pathologies, determined in part by the size of the residual vitreomacular adhesion.
INTRODUCTION
The most important age-related change in the human vitreous gel is posterior vitreous detachment (PVD). Defined as a separation between the posterior vitreous cortex and the internal limiting membrane (ILM) of the retina,1 PVD represents the culmination of the aging and liquefaction of human vitreous and may induce several potentially serious pathologic events at the vitreoretinal interface. It is commonly believed that age-related PVD occurs as an acute event, precipitated by the abrupt development of a dehiscence in the thin posterior cortical vitreous layer overlying the macular region.1–8 This is thought to result in the sudden passage of synchetic or liquefied vitreous into the subhyaloid space, causing a rapid and smooth separation of the vitreous cortex from the retina, beginning posteriorly and progressing peripherally to the vitreous base region.7,8 Partial PVDs are thought generally to progress rapidly to complete PVD.1,3
The clinical complications of age-related PVD are generally considered to be those of complete PVD and typically occur subsequent to vitreopapillary separation. The most common complications include retinal or optic disc hemorrhage, vitreous hemorrhage, retinal tear, and rhegmatogenous retinal detachment.2,6,9 These are caused by dynamic traction on focal areas of firm vitreoretinal adhesion owing to large movements of vitreous gel induced by saccadic eye movements. These large excursions of the vitreous gel during ocular rotations are made possible by the release of the vitreous-tethering effect of vitreous attachment to the posterior retina. The vitreomacular traction syndrome has long been recognized as a complication of partial PVD, in which the vitreous is separated from the retina throughout the peripheral fundus, but remains adherent in a broad, often dumbbell-shaped region encompassing the macular area and optic nerve.10,11
THESIS PROPOSITION
Although the completion of age-related PVD at the time of vitreopapillary separation occurs in many patients as an acute symptomatic event, there is growing evidence that this terminal event is preceded by earlier stages that are typically chronic, occult, and asymptomatic, unless complicated by one of various macular pathologies. The present thesis argues for the proposition that, in contrast to the conventional view summarized above, age-related PVD begins insidiously as a localized separation of the vitreous from the perifoveal retina and progresses slowly over months or years until its completion at the time of vitreopapillary separation. In the majority of individuals, the early, or perifoveal, stage of PVD is asymptomatic and goes undetected clinically until separation from the optic disc margin produces the symptoms and signs of a Weiss ring. In a subset of patients, however, the tractional effects of perifoveal vitreous detachment cause or exacerbate at least seven distinct but related macular pathologies, including idiopathic epiretinal membrane (ERM), macular microhole, idiopathic macular hole (including macular pseudo-operculum and inner lamellar macular hole), tractional cystoid macular edema (CME), tractional diabetic macular edema (DME), vitreomacular traction syndrome, and myopic macular retinoschisis. The complication that occurs in a given eye with perifoveal vitreous detachment is likely determined in part by the size and strength of the residual vitreomacular adhesion and perhaps by the structural integrity of the foveal stroma.
VITREOUS COMPOSITION AND BIOCHEMISTRY
Collagen and hyaluronic acid are the major structural macromolecules of the vitreous. In young human eyes, the vitreous is a semisolid gel composed of a framework of randomly spaced collagen fibrils, 10 to 25 nm in diameter, suspended in a viscoelastic hyaluronic acid solution.12 Vitreous collagen is classified predominantly as type II collagen, similar to that found in cartilage but with distinctive characteristics that may be related to vitreous physiology.12
Hyaluronic acid is a polysaccharide composed of repeated disaccharide units and belongs to the glycosaminoglycan group of polysaccharides. Hyaluronic acid is the major glycosaminoglycan present in the vitreous. Vitreous hyaluronic acid levels remain relatively constant in the adult eye, due in part to a constant level of synthesis combined with escape of hyaluronic acid molecules through the anterior segment.12 The interpenetrating networks of vitreous hyaluronic acid molecules, collagen fibrils, and other macromolecules allow for important interactions, which are thought to influence the structure and function of these components.12 Hyaluronic acid has long been thought to be important in providing stability to the helical structure of collagen and to the collagen fibril network within vitreous, thus helping maintain the viscoelasticity of the vitreous gel.5,12,13 Comper and Laurent14 showed that if hyaluronic acid is removed from vitreous, the gel shrinks. Larsson and Osterlin13 demonstrated that vitreous hyaluronic acid concentration was higher in postmortem eyes without PVD than in eyes with complete PVD, supporting the concept that hyaluronic acid plays a role in stabilizing the collagen gel of the vitreous body. Recently, it has been suggested that other macromolecules in the vitreous matrix (such as various proteoglycans and a protein called opticin) play an important role as “spacers” between collagen fibrils, stabilizing the collagen framework by preventing fibrillar aggregation.15,16
The peripheral or outer layer of vitreous is known as the vitreous cortex. The posterior vitreous cortex, which lies along the inner retina, is approximately 100 μm thick, but is thinner over the macula and absent over the optic disc.12 In the vitreous cortex, there is less hyaluronic acid, the collagen fibrils are more densely packed, and the vitreous gel is correspondingly denser compared with the central vitreous cavity.5,17 Following vitreous separation from the retina, the collagen fibrils composing the outer surface of the vitreous cortex become further condensed and realigned to form a distinct membrane known as the posterior hyaloid membrane.8
Vitreous cells, termed hyalocytes, are mononuclear cells found widely scattered in the vitreous cortex, maximally concentrated within the vitreous base and near the posterior pole.12 These cells exhibit phagocytic properties, may be involved in the production and maintenance of several vitreous components, and may have a function in maintaining the vitreoretinal junction.18 They may also be stimulated to proliferate and form collagen, thus playing a possible role in the production of vitreous membranes in pathologic states.8 Other vitreous components include noncollagenous proteins derived from plasma (such as albumin and transferrin), glycoproteins, proteoglycans, low-molecular-weight substances such as electrolytes, and miscellaneous compounds (eg, ascorbic acid, lactic acid, amino acids, and lipids).12,15,16,19
THE VITREORETINAL JUNCTURE AND ADHESION
The condensed collagen fibrils that form the vitreous cortex insert superficially into the ILM of the retina (or basal lamina of Müller cells).20,21 The precise nature of the adhesion between the posterior vitreous cortex and the ILM is not known but is thought to result in part from physicochemical properties of extracellular matrix molecules.12,22 The vitreous is most firmly attached to the retina at those sites where the ILM is thinnest, including the vitreous base, along major retinal vessels, the optic disc margin, and the 500-μm-diameter foveola.8,12,20,23,24 The attachment between the vitreous gel and its surrounding tissues is greatest in the vitreous base, where the concentration of vitreous collagen fibrils is especially high.12,20 Following the vitreous base, the peripheral margin of the optic nerve head (and immediate peripapillary retina) is the zone of firmest adhesion between the cortical vitreous and retinal surface.12 Here, vitreous fibrils are intermeshed with microscopic gaps in the basal lamina and often with astroglial epipapillary membranes.25 Clinical evidence of this firm attachment zone is the ring of thickened tissue (Weiss ring) visible on the posterior hyaloid membrane overlying the optic nerve following vitreopapillary separation during PVD.
The ILM is thicker posteriorly than anteriorly, reaching its maximal thickness of approximately 25,000 Å in the posterior retina.20,21 However, at the crest of the foveal clivus, it begins to rapidly thin, becoming extremely thin (200 Å or less) at the foveola.20 In a scanning electron microscopic study of autopsy eyes with spontaneous PVD, Kishi and coworkers23 found remnants of vitreous cortex in the foveal area in 26 (44%) of 59 eyes. Most remnants had the configuration of a 500-μm-diameter plaque or ring of vitreous adherent to the foveola. In some eyes, a 1,500-μm-diameter ring of vitreous remnants was found adherent to the foveal margin. These observations strongly suggest that the central 500-μm foveolar area and the margin of the 1,500-μm fovea are sites of firm vitreoretinal adhesion. These observations are consistent with the thinness of the ILM in the foveal area and possibly with the distribution of ILM attachment plaques (hemidesmosomes) in the posterior retina.12,20
In addition to these zones of firm vitreoretinal adhesion in the posterior pole, there are several types of lesions in the peripheral fundus associated with abnormally strong vitreoretinal attachments. These include lattice degeneration, meridional complexes, cystic retinal tufts, firm paravascular attachments, and some chorioretinal scars.7 Such lesions play an important role in the complications of complete PVD.
AGE-RELATED VITREOUS LIQUEFACTION AND SYNERESIS
Aging of the vitreous gel (synchisis senilis) involves the irreversible reorganization or destruction of the collagen-hyaluronate complex of its macromolecular network. It is characterized in the human eye by gel liquefaction and the development of fluid-filled pockets, or lacunae, typically beginning in front of the macula or in the central vitreous cavity.1,3,5,8,26–30 Liquid vitreous appears in the human eye usually after the age of 4 years and fills approximately 20% of the total vitreous space by the middle to late teenage years.5,28 By the age of 40, the liquid vitreous pockets generally become large enough to be easily visible on slit-lamp biomicroscopy. As the volume of liquid vitreous continues to increase in subsequent years, there is a corresponding decrease in volume of the vitreous gel.5 The declining volume of vitreous gel is accompanied by an increase in collagen concentration within the gel and a collapse of the collagen fibrillar meshwork, a process called syneresis.27 As part of this process, there is aggregation of fine collagen fibrils into bundles of parallel fibrils that form larger fibers.31 With advancing age, the fibers become increasingly thickened and tortuous by dark-field slit microscopy.27 These changes resulting from dissolution of the collagen-hyaluronate complex are visible biomicroscopically and are measurable quantitatively in postmortem eyes.5
The pathophysiological mechanisms underlying vitreous synchisis and syneresis remain poorly understood. It is believed that a change in the conformation of hyaluronic acid and perhaps other matrix macromolecules could result in vitreous liquefaction and syneresis by altering collagen-hyaluronate interaction and triggering a dissociation of the two molecules, thereby destabilizing the vitreous matrix.1 Plausible candidates for inducing conformational changes in the tertiary structure of hyaluronic acid and collagen are singlet oxygen and other free radicals generated by metabolic and photosensitized reactions.1,32–34 Recently, Los and colleagues16 have provided ultrastructural evidence that collagen breakdown into smaller fragments (by unknown mechanisms) may also play a role in the pathogenesis of vitreous aging.
As hyaluronic acid macromolecules and the intervening matrix of collagen fibrils undergo progressive degeneration with advancing age, the liquefied lacunae increase in number, size, and degree of coalescence.1,3,13,16,26,28 In a large gross pathology study using autopsy eyes, at least 50% liquefaction of the vitreous gel was found in approximately 15% of subjects aged 20 to 29 years, and was present in more than 70% of subjects over age 70.3 Other pathology studies have similarly documented a progressive increase in vitreous liquefaction with advancing age, particularly after the age of 40.5,26,27
In their large study of autopsy eyes, Foos and Wheeler3 found that both the degree of vitreous liquefaction and the prevalence of PVD were age-related. Furthermore, they found a strong positive statistical correlation between increasing degrees of vitreous liquefaction and prevalence of PVD, noting a sharp upturn in the rate of PVD with gel liquefaction of 50% or more. PVD was present in less than 10% of individuals under the age of 50 but was found in 63% of patients over the age of 70 years. O’Malley,26 in a study of 800 consecutive autopsy eyes, found a similar relationship between age, vitreous liquidity, and PVD, noting likewise that eyes with PVD generally showed liquefaction of greater than 50% of the vitreous gel. Larsson and Osterlin13 studied postmortem eyes with clinical and physicochemical methods, finding greater amounts of liquid vitreous fluid with increasing degrees of PVD. A direct relationship between age and PVD has been confirmed in many large clinical studies of otherwise normal eyes, with a low incidence of PVD invariably found in age groups younger than 50 years.6,35–40
Vitreous liquefaction and PVD occur at an earlier age in myopic compared with emmetropic or hyperopic eyes.6,36,41 Clinically detectable PVD prevalence has been shown to be higher in moderate or highly myopic compared with emmetropic eyes.35,36 Recently, Yonemoto and coworkers37 found that the age at onset of PVD correlates inversely with the degree of myopia. In this large clinical study, the regression line for the calculation of PVD onset age estimates 61 years for emmetropic eyes and subtracts approximately 1 year of age for each diopter of myopia. By multivariate analysis, Morita and colleagues36 found a significant correlation between vitreous changes (lacuna formation and PVD) and axial length in highly myopic eyes. The vitreous alterations in myopia may relate in part to the lower concentration of hyaluronic acid in the vitreous of highly myopic compared with emmetropic eyes.42
Varying degrees of myopia accompanied by premature vitreous liquefaction and syneresis are known to occur in several developmental and hereditary vitreoretinal syndromes and connective tissue disorders.41,43 Maumenee43 suggested that the vitreous changes present in many of the arthro-ophthalmopathies represent developmental dysgenesis of the vitreous and serve as a sign of generalized connective tissue diseases involving type II collagen metabolism. Other factors known to accelerate vitreous liquefaction and PVD include trauma, aphakia, intraocular inflammation, retinal vascular disease, and vitreous hemorrhage.41,44,45
In a large clinical study, Lindner6 found that PVD was significantly more common in women than in men of comparable age. A female predominance in this condition, particularly after the age of 50, has subsequently been confirmed by other investigators.2,3,9 Yonemoto and coworkers37 found that the onset age of PVD in women with normal eyes was somewhat lower than that in men. Larsson and Osterlin13 reported that the concentration of hyaluronic acid in the vitreous was significantly lower in females than in males, an observation that offers a possible explanation for the sex-related difference in the prevalence of PVD. Several investigators1,13 point out that the synthesis of hyaluronic acid (and other glycosaminoglycans) is known to be influenced by gonadal hormones and may drop with declining estrogen levels in postmenopausal women. These observations lend further support to the concept that insufficient hyaluronic acid destabilizes the vitreous gel, contributing to liquefaction and PVD.
AGE-RELATED CHANGES IN VITREORETINAL ADHESION
In addition to vitreous rheologic changes that lead to liquefaction and syneresis, a second critical factor in the pathogenesis of PVD is the progressive age-related weakening of the adhesion between the posterior vitreous cortex and the ILM.22 Vitreous liquefaction alone is not sufficient to precipitate PVD, because autopsy studies have shown that among patients younger than 60 years, the vitreous remains attached to the retina in the majority of eyes with extensive liquefaction.3,26 In contrast, past the age of 60 there is a striking relationship between the degree of liquefaction and prevalence of PVD.3,26 Thus, although the vitreous gel appears to destabilize as it reaches approximately 50% liquefaction,3 this instability generally does not result in age-related PVD until the vitreoretinal adhesion is sufficiently weakened.
In a study of postmortem eyes, Sebag22 showed that in young eyes, the adhesion between the vitreous cortex and ILM is topographically extensive in the posterior pole and is very strong, exceeding in many cases the integrity of Müller cells. The strength of this adhesion was found to weaken significantly with advancing age. Kloti46 reported that Müller cell infarction resulted in dissolution of the adhesion of ILM to cortical vitreous, concluding that Müller cell metabolism plays a role in vitreoretinal adhesion. Sebag22 has postulated that the progressive thickening of the retinal ILM that occurs with advancing age adversely affects the ability of Müller cells to synthesize and maintain the components of the extracellular matrix at the ILM-vitreous cortex interface, thereby weakening the vitreoretinal adhesion. Others5 have similarly suggested that thickening of the ILM may be a prerequisite to age-related PVD.
POSTERIOR VITREOUS DETACHMENT
Age-related PVD ultimately results from progressive vitreous liquefaction (synchisis) in conjunction with progressive weakening of the adhesion between the vitreous cortex and the ILM.1 As the premacular vitreous liquefied pocket enlarges, its posterior wall, the vitreous cortical layer overlying the macular area, becomes very thin.29 Gradual dissolution and weakening of the adhesion between the vitreous cortex and the ILM in the posterior pole allow liquid vitreous to enter the retrohyaloid space through the prepapillary hole in the vitreous cortex or perhaps through a dehiscence in the thin layer of premacular cortical vitreous.1,3–6,8,27 Rotational eye movements likely contribute to the subsequent dissection of a plane between the vitreous cortex and the ILM, leading to progressive PVD. The apparent collapse (syneresis) of the vitreous gel is believed to result from displacement of fluid from the central vitreous cavity to the subhyaloid space.1 When PVD occurs, there is usually complete separation of all vitreous material from the retina, although thin portions of cortical vitreous remain attached to the ILM in select cases.23
Large holes in the premacular vitreous cortex, many of which may be artifactual, are seen after mechanical separation of the vitreous from the retina in the laboratory.12,22,27 Similar holes have been seen biomicroscopically in a subset of eyes with PVD and ERM47–49 and in selected eyes with atypical vitreomacular traction.10,47,50 In his study of 800 postmortem eyes, O’Malley26 was able to detect an opening between the large vitreous liquefied cavity and the retrohyaloid space in only a small minority of the eyes with PVD. Using fluorescein staining of the vitreous gel in autopsy eyes, Kishi and Shimizu28 detected oval defects in the posterior vitreous cortical layer overlying the macula in 10 (28%) of 36 eyes with complete PVD. Such breaks are generally not detected clinically or during vitrectomy in patients with complete PVD.7 In a prospective study of stage 1 and 2 macular holes, Johnson and coworkers51 examined the surgically detached posterior hyaloid and found a small, possibly iatrogenic premacular defect in only one of 24 eyes. Furthermore, optical coherence tomography (OCT) and ultrasound imaging of the partially detached posterior hyaloid membrane in various macular hole and vitreomacular traction conditions typically reveal a continuous membrane without breaks in its premacular portion.51–60 These clinical observations do not exclude the possibility of microscopic dehiscences in eyes without visible holes in the posterior vitreous cortex.
Initial Stages of PVD
The perception that age-related PVD occurs as an abrupt event is based in large part on the clinical observation that patients presenting with symptoms of complete PVD describe the acute onset of new floaters and/or photopsias.1,2,4,6 Clinical examination of such patients generally reveals the presence of a mobile prepapillary (Weiss) ring attached to the anteriorly displaced posterior hyaloid membrane, providing clinical confirmation of acute vitreopapillary separation. Historically, however, early stages of shallow, partial PVD that precede vitreopapillary separation have been difficult or impossible to detect clinically. Detailed and dynamic slit-lamp biomicroscopic examination with either a fundus contact lens or preset lens such as the 90-diopter (D) or El Bayadi-Kajiura lens50,61–63 has proven useful in ascertaining various states of PVD. However, localized shallow vitreous separations without posterior vitreous cortex thickening are invisible by these techniques. Similarly, because of the low reflectivity of the posterior hyaloid membrane, localized shallow separations of the vitreous from the retina have generally been difficult to detect with ultrasound.63
Recently, B-scan ultrasonography performed with the probe directly perpendicular to the macula (axial view) has been found to be a sensitive method for detecting partial posterior hyaloid separations from the retina.51,53,54,59 Additionally, the advent of OCT within the last decade has provided new capability for high-resolution (10 μm axially) cross-sectional imaging of vitreoretinal anatomy.63 This technology has proven to be of great value in the detection of shallow detachments of the posterior hyaloid membrane from the retina64,65 but is generally not useful for detecting more extensive vitreous detachments.
Thus, until recently, the true pattern and rate of PVD development have been impossible to determine because of the difficulty in identifying and imaging its early, asymptomatic stages. It has long been suspected that age-related PVD begins in the macular region,1,3,8,44 possibly related to the large premacular liquefied vitreous pocket that is universally present in older adult eyes.28–30 Preferential liquefaction in the posterior pole may result from the effect on vitreous collagen-hyaluronate structure of free radicals generated by focused light irradiation and/or macular metabolic processes.1,3,32–34 Gaudric and coworkers52 recently demonstrated by OCT the initial stages of PVD in fellow eyes of macular hole patients, beginning typically at the macular periphery and then spreading gradually throughout the entire perifoveal macular area while remaining focally attached at the foveola. Subsequently, Uchino and colleagues66 evaluated the initial stages of PVD by OCT in a large prospective study of healthy eyes in subjects aged 31 to 74 years (mean age, 52 years). In 60% of subjects, beginning as early as the fourth decade of life, they found varying degrees of localized vitreous detachment from the perifoveal retina with persistent attachment to the fovea, optic disc, and midperipheral retina. Partial vitreous detachment over the entire macula was present in 2% of eyes, and complete PVD was found in 9% of eyes (although the number of subjects older than 60 years was small). All eyes with partial PVD states were asymptomatic, and in none were the focal vitreous separations detectable on biomicroscopy. Although not longitudinal in design, the study demonstrated a significant age-related progression from no PVD through partial PVD to complete PVD.
In a study of aged autopsy eyes in which the vitreous was stained with fluorescein, photographs of eyes without complete PVD demonstrated shallow detachments of the thin layer of cortical vitreous from the perifoveal retina, with or without persistent foveolar attachment.28 This report and a laboratory study by Chauhan and coworkers56 provide evidence that the discrete linear signal detected by OCT in eyes with presumed perifoveal vitreous detachment does correspond to the posterior hyaloid membrane. Similarly, several investigators have found close correlation between ultrasonographic and intraoperative findings in cases of shallow, limited posterior hyaloid detachments in eyes with idiopathic macular holes, confirming the identity of these shallowly detached preretinal membranes imaged by meticulous, axial B-scan ultrasonography.51,59
That age-related PVD appears to begin as a localized vitreous separation from the perifoveal retina is not unexpected in view of the topographical variations in strength of the vitreoretinal adhesion reviewed previously. Ito and colleagues60 recently constructed maps of the partial PVD found by OCT in eyes with various stages of idiopathic macular hole. These maps showed correlation between the stage of macular hole and the extent of PVD, suggesting gradual progression of perifoveal vitreous detachment over time. In stage 1 holes, the vitreous detachment was limited to the perifoveal area, with persistent attachment to areas of strong adherence including the fovea, vascular arcades, and optic disc. Maps of the partial PVDs in more advanced macular hole stages suggest that perifoveal vitreous detachment usually extends first into the superior and temporal midperiphery, presumably due to gravitational effects, and then into the fovea, the inferior midperiphery, and finally the optic disc margin, resulting in complete PVD. The preterminal stage, in which there is complete PVD except for vitreopapillary attachment, is not commonly recognized clinically, because there are no symptoms or signs of a Weiss ring. However, this configuration, termed “funnel shaped” detachment, was noted in 29% of autopsy eyes with partial PVD,44 is sometimes visible on biomicroscopic examination, and occasionally causes optic disc hemorrhages.67
Study of the initial (perifoveal) stages of age-related PVD is hampered somewhat by the fact that in the majority of eyes, these stages are biomicroscopically invisible and asymptomatic until the vitreous finally separates from the optic disc margin, resulting in acute symptoms and signs. For example, the landmark study by Uchino and coworkers66 implies but does not prove that early PVD stages are chronic and slowly progressive. The investigation by Ito and coworkers60 illustrates that much can be learned about the evolution and tractional effects of age-related PVD by studying eyes affected by the complications of its early stages.
The purpose of the present investigation is to describe the vitreoretinal relationship in eyes with idiopathic macular disorders putatively caused or exacerbated by vitreomacular traction associated with early stages of age-related PVD. This report will present (1) evidence that each of the macular conditions evaluated herein is associated with some degree of PVD, (2) information suggesting that this association is causal rather than coincidental, (3) longitudinal data demonstrating that the early stages of PVD in these eyes persist chronically and progress slowly, and (4) data regarding the size of vitreomacular adhesions in the conditions studied. Using these clinical data and a review of pertinent literature, this thesis argues for the proposition that age-related PVD is insidious rather than abrupt, beginning as a shallow separation from the perifoveal retina, and extending slowly and asymptomatically for months or years until vitreous separation from the optic disc margin results in complete PVD with acute signs and symptoms. Just as complete PVD may be complicated by a variety of peripheral retinal pathologies, it appears that perifoveal PVD may be complicated by various disorders in the macular region, depending in part on the size and strength of the residual vitreomacular adhesion.
METHODS
This study is a retrospective analysis of patients diagnosed with one of several idiopathic macular conditions who (1) had no biomicroscopic evidence for complete PVD on presentation, and (2) underwent a specific and detailed clinical assessment of the vitreoretinal relationship. The idiopathic macular conditions studied were those suspected of being caused or exacerbated by vitreous traction, including idiopathic ERM, macular microhole, macular pseudo-operculum, inner lamellar macular hole, tractional CME, classic vitreomacular traction syndrome, and myopic macular retinoschisis. This study did not include patients with idiopathic macular hole or tractional DME, because the vitreomacular relationship in these disorders has been evaluated and reported previously.51,52,55,56,60,68–73
Macular microhole was defined as a tiny full-thickness foveolar defect with sharp, flat edges. It is distinguished from typical idiopathic macular hole by abrupt onset, tiny size, lack of obvious thickening or elevation of its edges, and spontaneous healing without surgical intervention. Macular pseudo-operculum was defined as a small, discrete, round opacity suspended immediately anterior to an intact foveola. Lamellar macular hole was defined as a small, partial-thickness defect in the inner aspect of the foveola, with or without an overlying operculum. Tractional CME (a subtle variant of the vitreomacular traction syndrome) was defined as the presence of foveal thickening with multiple cystoid spaces and no evidence on biomicroscopy or OCT of an evolving defect in the outer foveola typical of an evolving macular hole.55,68 The classic vitreomacular traction syndrome was defined as peripheral PVD with observable anteroposterior traction on a broad area of vitreoretinal adhesion encompassing the macular area and optic nerve.10,11 Myopic macular retinoschisis was defined as diffuse retinal thickening, typically with a microcystic appearance, throughout the area of posterior staphyloma, accompanied by the OCT finding of a wide hyporeflective space in the outer neurosensory retina.
Forty-six eyes of 43 patients from the author’s clinical practice who met the above inclusion criteria were identified through a diagnosis database. Medical records and images obtained by ultrasound and OCT evaluation were reviewed after approval of the hospital’s institutional review board. Standard informed consent was obtained from each patient undergoing vitreoretinal surgery. Patients were accrued retrospectively except for the cohort of patients with idiopathic ERM who were identified prospectively between January and July of 2003.
Each patient was evaluated with a complete ophthalmologic examination, including Snellen visual acuity testing and slit-lamp biomicroscopy of the posterior pole. In all but five eyes, the vitreomacular relationship was further evaluated by either B-scan ultrasonography alone (17 eyes), OCT alone (11 eyes), or both ultrasonography and OCT (13 eyes). In patients who underwent vitreoretinal surgery, information regarding the intraoperative assessment of the vitreoretinal relationship was obtained from the operative report. In the five eyes that were evaluated by neither ultrasonography nor OCT, the vitreoretinal relationship was clearly defined by the biomicroscopic and/or intraoperative findings.
Slit-lamp biomicroscopy was performed with either a fundus contact lens or a 78 D indirect biomicroscopy lens. A complete PVD was diagnosed when biomicroscopy revealed a mobile prepapillary (Weiss) ring continuous with the faintly visible and mobile posterior hyaloid membrane and associated with an optically empty subhyaloid space. When a well-defined and mobile Weiss ring was present, no further assessment of the vitreoretinal relationship was performed, because previous clinical and ultrasonographic experience has demonstrated that this finding is a highly specific sign of complete PVD.40 Such eyes were not included in the study cohort. Perifoveal vitreous detachment was diagnosed biomicroscopically when a faintly visible, typically taut, and often glistening membrane was noted in the perifoveal posterior pole, shallowly detached from the retinal surface, with or without a residual adhesion to the macula. A pseudo-operculum was diagnosed when a small round opacity was visible suspended on the detached posterior hyaloid membrane over an otherwise normal fovea. An operculum was diagnosed when a small round opacity was similarly suspended over a fovea with a tissue defect such as an inner lamellar or full-thickness macular hole.
Epiretinal membrane was graded according to biomicroscopic findings as follows:
Grade 0: No evidence of ERM
Grade 1: Irregular glistening (“cellophane”) light reflex from the retinal surface (no distortion of inner retina)
Grade 2: ERM with fine inner retinal striae ± retinal thickening
Grade 3: ERM causing retinal vascular distortion and/or retinal folds
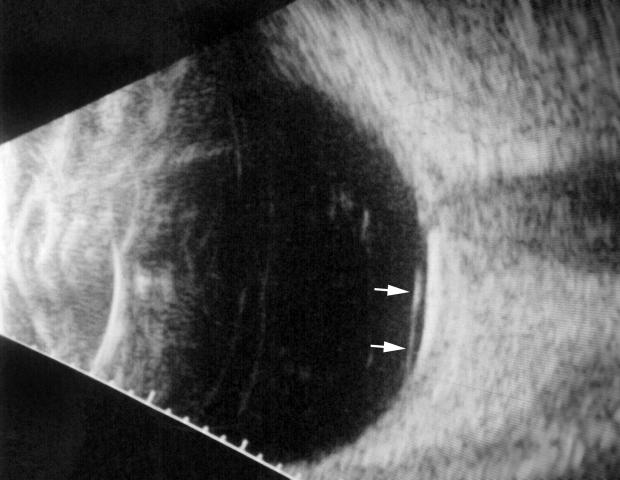
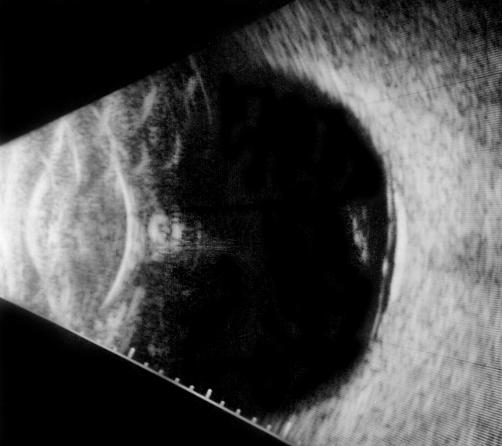
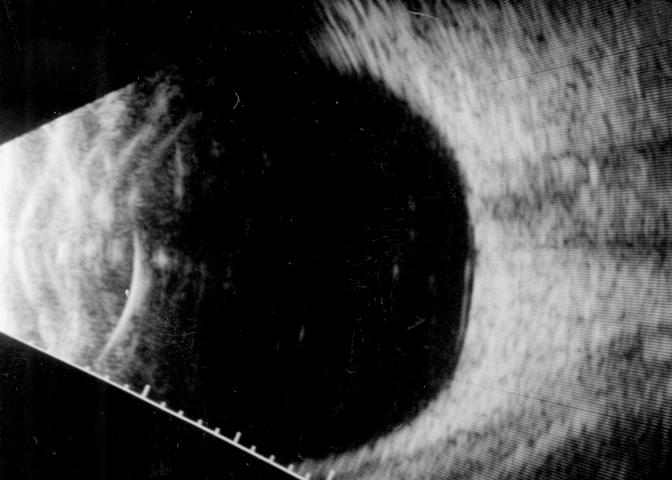
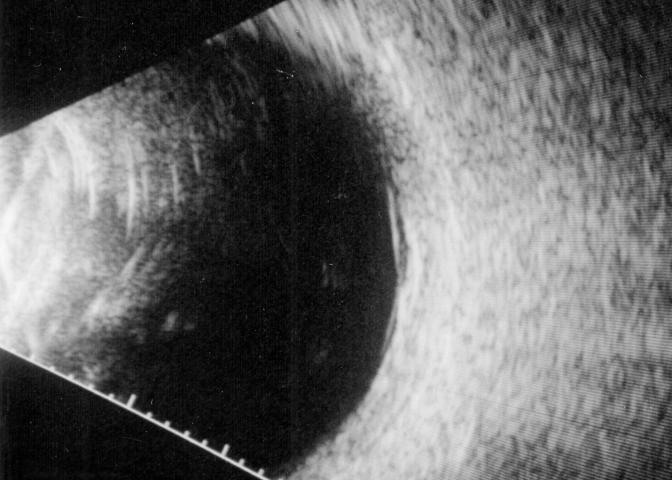
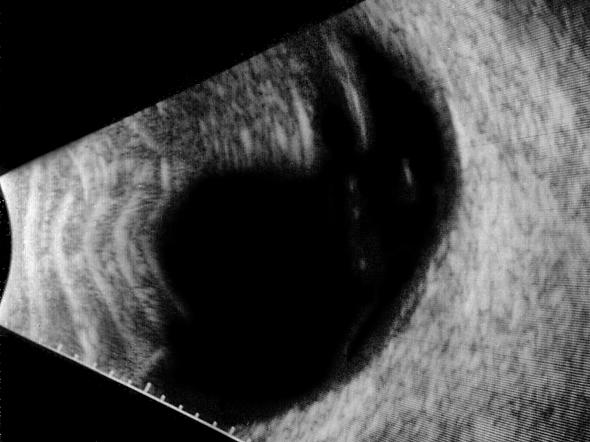
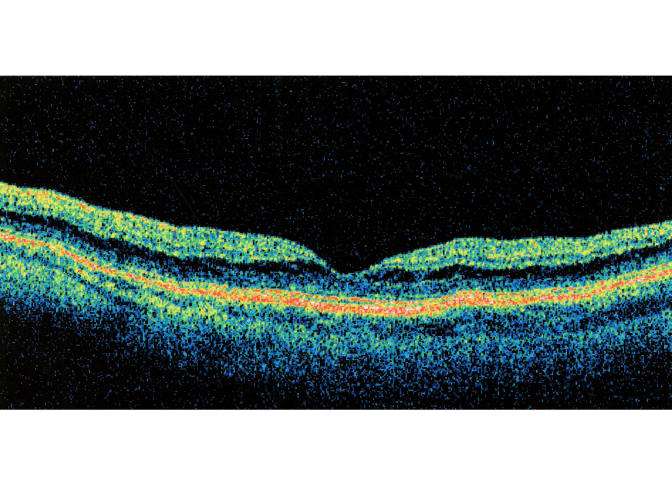
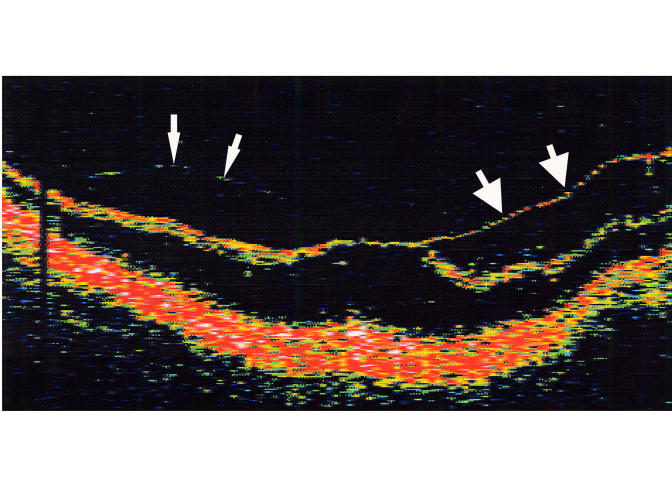
B-scan ultrasound examinations were performed under topical anesthesia with the eyelids open and the probe placed directly on the ocular surface using methylcellulose as a coupling gel. Ultrasound studies were performed with the I3System-ABD unit (Innovative Imaging, Inc, Sacramento, California) at medium and high gain settings. The studies consisted of longitudinal and transverse sections through the macula, obtained by positioning the probe on the nasal conjunctiva so as to avoid beam attenuation by the crystalline lens. Because absolute perpendicularity to the macula was necessary in many eyes for detecting shallow detachments of the posterior hyaloid membrane, horizontal and vertical axial views (through the cornea and lens) were included in the ultrasound examination as needed. Kinetic B-scan assessments were used to help define the vitreoretinal relationship when necessary. Localized vitreous detachment was diagnosed when a thin, smooth, continuous membrane with minimal aftermovement was detected anterior to the retinal surface (Figure 1A). Total PVD was diagnosed when a continuous, mildly echodense, undulating, and mobile membrane was found spanning the vitreous cavity, with no adhesions to the retina posterior to the vitreous base region (Figure 1B).
FIGURE 1A.
Horizontal axial B-scan ultrasonogram of a localized, shallow detachment of the posterior hyaloid membrane (arrows) from the macular area.
FIGURE 1B.
Transverse ultrasound image of total posterior vitreous detachment.
OCT was performed through a dilated pupil by a certified ophthalmic photographer using commercially available equipment (OCT II and Stratus OCT III; Humphrey Instruments, Carl Zeiss Division, San Leandro, California). The OCT studies were composed of scans 5 to 10 mm in length, oriented either in a cross-hair pattern or as six radial lines at intervals of 30 degrees. The study was centered on the subjective fixation point in patients with adequate visual acuity; otherwise, the intersection of the individual scans was placed at the center of the macula. Where necessary, the intensity of the incident light was set to the maximum (750 μW) available in order to detect the faintly reflective posterior hyaloid membrane. Quantitative measurements of the diameter of vitreomacular adhesions were made from prints of the OCT images. Where diameters differed between the several scans performed on a given eye, the maximum diameter was selected.
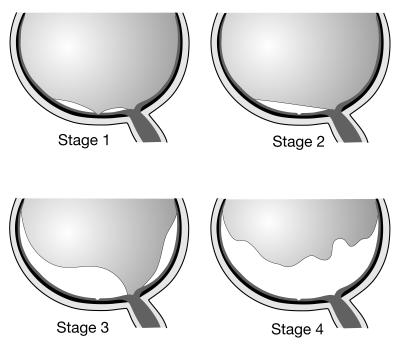
Using information gleaned from review of the medical record and imaging studies, the condition of the posterior vitreous was classified into one of the following five stages, modified from Uchino and coworkers (Figure 2).66
FIGURE 2.
Schematic illustration of the stages of posterior vitreous detachment. Stage 1, perifoveal vitreous detachment with residual vitreofoveal adhesion. Stage 2, perifoveal vitreous detachment with no vitreofoveal adhesion. Stage 3, near-complete posterior vitreous detachment (PVD) with only vitreopapillary adhesion remaining. Stage 4, complete PVD.
Stage 0: No evidence of PVD
Stage 1: Perifoveal vitreous detachment with vitreofoveal adhesion
Stage 2: Perifoveal vitreous detachment with no vitreofoveal adhesion
Stage 3: Complete PVD except for vitreopapillary adhesion
Stage 4: Complete PVD
The extent of the perifoveal vitreous detachment in stage 1 and 2 eyes was variable, typically extending to approximately the level of the vascular arcades, but sometimes extending into the midperipheral fundus. Eyes in which the perifoveal vitreous detachment appeared to extend throughout the entire periphery, with vitreous adhesions only at the macula and optic disc, were classified as having stage 1+ PVD.
For eyes with stage 1 PVD that had not undergone OCT evaluation, the size of the vitreomacular adhesion was estimated, where possible, based on available biomicroscopic, ultrasonographic, and surgical data.
RESULTS
Of the 46 eyes of 43 patients who met the study inclusion criteria, three eyes (of three patients) with tractional CME were excluded from further analysis. These three women, ranging in age from 58 to 86 years, presented with idiopathic multicystoid foveal thickening associated with perifoveal vitreous detachment on OCT examination. One patient was lost to follow-up after 1 month. The remaining two patients developed stage 1B macular holes within 3 and 8 months of follow-up, respectively, which ultimately progressed to stage 2 macular holes. On initial presentation, these eyes were indistinguishable clinically and by OCT from the 10 eyes with tractional CME who did not progress to macular hole over extended follow-up.
The study group consisted of 43 eyes of 40 patients, of whom 21 (52.5%) were women. The age at onset of symptoms or signs ranged from 36 to 83 years, with a median age of 67.5 years. The macular diagnosis was ERM (12 eyes), tractional CME (11 eyes), vitreomacular traction syndrome (eight eyes), macular microhole (four eyes), macular pseudo-operculum (four eyes), inner lamellar macular hole (two eyes), and myopic macular retinoschisis (two eyes).
Despite the absence of biomicroscopic evidence for complete PVD, two study eyes (4.7%) were found to have complete (stage 4) PVD on ultrasound evaluation. Earlier stages of PVD were found in all remaining study eyes and classified as stage 1 in 26 eyes (60.5%), stage 2 in 13 eyes (30.2%), and stage 3 in two eyes (4.7%). Each of the 31 eyes in this study with a primary diagnosis other than ERM presented with stage 1 or 2 perifoveal vitreous detachment and was seen in follow-up after initial presentation. Over an average preoperative or total follow-up period of 30.0 months (range, 2 to 237 months), only three (9.7%) of these eyes showed progression to complete PVD. All three eyes that progressed to complete PVD had a diagnosis of macular microhole.
Of the 37 patients with one study eye, biomicroscopic evidence for complete PVD was present in 17 fellow eyes (45.9%). Imaging studies to detect earlier stages of PVD had been performed in seven of the 20 fellow eyes without complete PVD. Asymptomatic and biomicroscopically invisible partial PVD states were found in five of these seven eyes, and were classified as stage 1 in three eyes, stage 2 in one eye, and stage 3 in one eye.
Additional results are presented in the following diagnostic categories.
Epiretinal Membrane
Twelve eyes of 11 patients with idiopathic ERM unaccompanied by biomicroscopic evidence for complete PVD were identified prospectively between January and July 2003. The age of symptom onset ranged from 36 to 76, with a median age of 68 years (Table 1). The visual acuity ranged from 20/20 to 20/200, with a median acuity of 20/30. The average ERM grade was 2.3. Despite the absence of a Weiss ring, two eyes (16.7%) were found on B-scan ultrasonography to have complete PVD. The remaining 10 eyes were each found to have an earlier (partial) stage of PVD. There was no apparent correlation between the grade of ERM and the stage of PVD.
TABLE 1.
CLINICAL DATA FOR PATIENTS WITH EPIRETINAL MEMBRANE
|
PHM VISIBILITY |
FELLOW EYE |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PATIENT NO. | ONSET AGE (YR)/SEX/EYE | SYMPTOM DURATION (MO) | VISUAL ACUITY | ERM GRADE | BIOMICROSCOPY | OCT | B-SCAN | PVD STAGE | ADHESION SIZE (μM) | WEISS RING | ERM GRADE |
| 1 | 72/M/OD | 12 | 20/50 | 3 | No | No | Yes | 1* | ND | Yes | 3 |
| 2 | 66/M/OS | 4 | 20/20 | 1 | No | Yes | Yes | 1 | 2,050 | Yes | 2‡ |
| 3 | 60/M/OS | 36 | 20/30 | 3 | No | No | Yes | 4 | N/A | Yes | 3 |
| 4 | 36/F/OS | 72 | 20/25 | 3 | No | Yes | Yes | 1 | 2,090 | No | 0 |
| 5 | 66/M/OS | 192 | 20/40 | 2 | No | ND | Yes | 3 | N/A | † | † |
| 6 | 76/F/OD | 4 | 20/100 | 3 | No | Yes | Yes | 3 | N/A | ||
| /OS | 1.5 | 20/30 | 1 | No | Yes | ND | 1 | 200 | |||
| 7 | 71/M/OD | 1 | 20/30 | 2 | No | Yes | ND | 2 | N/A | Yes | 3 |
| 8 | 69/M/OD | 18 | 20/200 | 3 | No | Yes | ND | 1 | 470 | Yes | 2‡ |
| 9 | 68/M/OD | 0 | 20/20 | 1 | Yes | ND | Yes | 4 | N/A | Yes | 2 |
| 10 | 69/M/OD | 5 | 20/50 | 3 | No | Yes | Yes | 2 | N/A | No | 0 |
| 11 | 56/F/OD | 60 | 20/80 | 3 | Yes | Yes | ND | 2 | N/A | No | 0 |
ERM = epiretinal membrane; N/A = not applicable; ND = not done; OCT = optical coherence tomography; PHM = posterior hyaloid membrane; PVD = posterior vitreous detachment.
Two separate vitreomacular adhesions were found at surgery.
Eye had previously undergone vitrectomy for ERM.
ERM was associated with peripheral retinal tear.
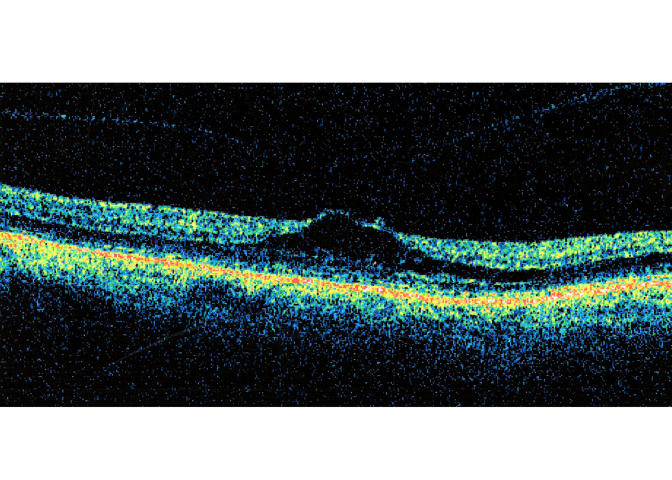
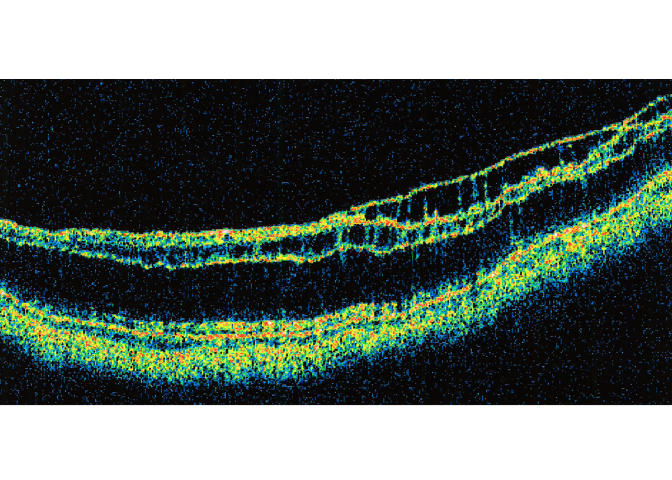
In the 10 eyes with partial PVD, the duration of symptoms or signs of ERM ranged from 1 to 192 months, with an average of 37 months. The partially detached posterior hyaloid membrane was visible on biomicroscopy in only one (10.0%) of these eyes. It was visible by both OCT and ultrasonography in all eyes tested except for one pseudophakic eye in which no posterior hyaloid membrane separation was visible on OCT (Table 1). The stage of PVD was confirmed intraoperatively in all five eyes that underwent vitrectomy and membrane peeling. The PVD was classified as stage 1 in five eyes, stage 2 in three eyes, and stage 3 in two eyes (Figures 3 through 5). Four of the eyes with stage 1 perifoveal vitreous detachment had a single vitreomacular adhesion at the fovea, ranging from 200 to 2,090 μm in diameter on OCT. The remaining eye had two focal vitreomacular adhesions in the superior macular area.
FIGURE 3.
Case 2. Optical coherence tomography scan of stage 1 perifoveal vitreous detachment associated with a small grade 1 epiretinal membrane (not seen in this meridian).
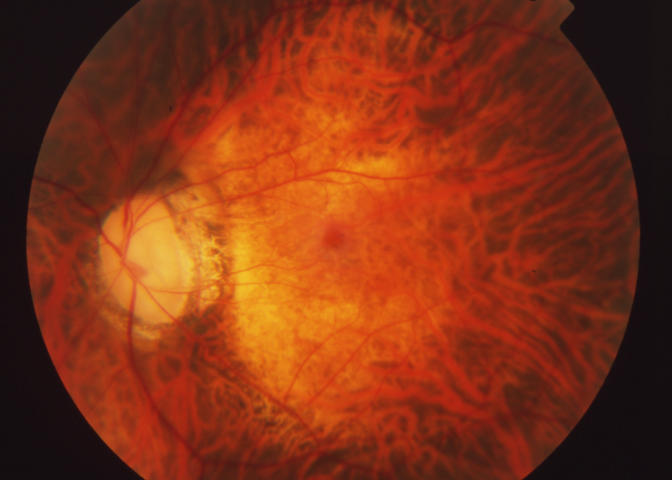
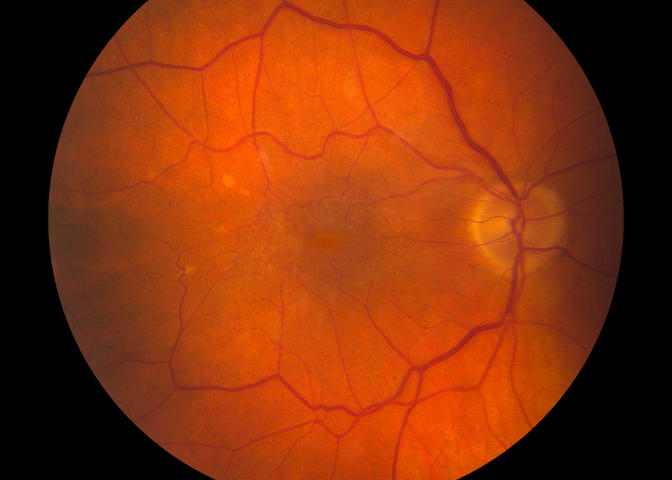
FIGURE 5A.
Case 10. Fundus photograph of right eye showing grade 3 epiretinal membrane with pseudohole and no biomicroscopic evidence for posterior vitreous detachment.
FIGURE 4B.
Case 8. Horizontal optical coherence tomography image showing stage 1 perifoveal vitreous detachment with vitreous attachment to prolapsing foveola.
FIGURE 5B.
Case 10. Vertical axial ultrasound image demonstrating shallow stage 2 vitreous detachment over the macular region.
FIGURE 5C.
Case 10. Optical coherence tomography confirms shallow separation of the posterior hyaloid overlying epiretinal membrane and cystoid pseudohole.
The ERM was located on the retinal surface in areas underlying detached posterior hyaloid in all eyes except case 4. In this 36-year-old woman with an opaque, atypical ERM, the posterior hyaloid appeared by OCT to be attached at the ERM and separated from the retina surrounding it.
During the same 6-month period, 11 eyes of 10 patients with idiopathic ERM accompanied by a Weiss ring were identified prospectively. Additionally, four fellow eyes in the study cohort had idiopathic ERM accompanied by a Weiss ring. Thus, of the 27 eyes with idiopathic ERM identified prospectively during this 6-month period, 17 eyes (63.0%) had complete PVD, and the remaining 10 eyes (37.0%) had various stages of partial PVD.
Conditions Related to Idiopathic Macular Hole
The group of eyes with macular hole–related conditions was composed of four eyes with pseudo-operculum, two eyes with inner lamellar macular hole, and four eyes with macular microhole (Table 2). Patient age at symptom onset ranged from 41 to 75, with a median age of 57. The two patients younger than 50 years in this group had spherical equivalent refractive errors of −3.00 and −7.50 D, respectively. All patients were symptomatic, with the nature of the visual symptoms differing by diagnosis. Patients with pseudo-operculum complained of a small, round, yellow or gray relative scotoma or blurred spot, which in two cases exhibited a small-amplitude quivering motion. Patients with lamellar hole were symptomatic only for mild metamorphopsia, whereas each eye with macular microhole had a tiny central scotoma without metamorphopsia.
TABLE 2.
CLINICAL DATA FOR PATIENTS WITH CONDITIONS RELATED TO IDIOPATHIC MACULAR HOLE
|
PHM VISIBILITY |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PATIENT NO. | ONSET AGE (YR)/SEX/EYE | DIAGNOSIS | VISUAL ACUITY | FOLLOW-UP SINCE ONSET (MO) | BIOMICROSCOPY | OCT | B-SCAN | INITIAL PVD STAGE | LESION SIZE (μM) | PROGRESSION TO COMPLETE PVD (TIME) |
| 12 | 64/M/OD | Pseudo-operculum | 20/30 | 2 | No | Yes | ND | 2 | 276 | No |
| 13 | 53/F/OD | Pseudo-operculum | 20/25 | 3 | No | Yes | ND | 2 | 305 | No |
| 14 | 69/F/OS | Pseudo-operculum | 20/30 | 24 | No | Yes | Yes | 2 | 296 | No |
| 15 | 75/M/OS | Pseudo-operculum | 20/30 | 2 | No | ND | Yes | 2 | ND | No |
| 16 | 41/F/OS | Lamellar hole | 20/30 | 9 | No | ND | Yes | 1 → 2* | ND | No |
| 17 | 57/F/OD | Lamellar hole | 20/30 | 6 | No | Yes | ND | 2 | 433 | No |
| 18 | 48/F/OD | Microhole | 20/20 | 68 | No | ND | Yes | 2 | ~50† | Yes (5 mo) |
| /OS | Microhole | 20/20 | 48 | No | Yes | Yes | 1 → 2* | ~50† | No | |
| 19 | 62/M/OS | Microhole | 20/20 | 19 | No | ND | Yes | 2 | ~50† | Yes (4 mo) |
| 20 | 55/F/OD | Microhole | 20/20 | 14 | No | Yes | ND | 2 | ~50† | Yes (12 mo) |
ND = not done; OCT = optical coherence tomography; PHM = posterior hyaloid membrane; PVD = posterior vitreous detachment.
Transient stage 1 perifoveal vitreous detachment with vitreofoveolar traction was the initial lesion in each case.
Diameter of microhole estimated on biomicroscopy.
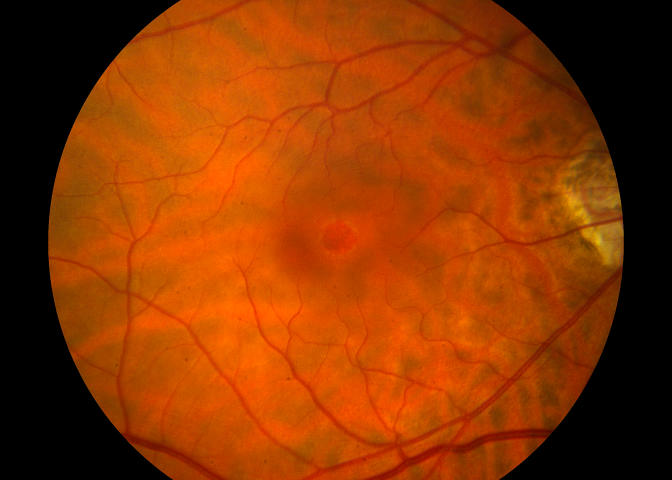
Pseudo-opercula were visible on biomicroscopy in all cases. A small operculum was visible in both eyes with inner lamellar macular holes and in one of four eyes with macular microhole. Although the detached posterior hyaloid membrane was biomicroscopically invisible in every eye, a stage 2 vitreous separation over the macular region was documented with OCT and/or B-scan in all 10 eyes (Figures 6 through 9). In two eyes, a transient stage 1 perifoveal vitreous detachment had been documented prior to the development of the macular lesion. After lesion development, none of the eyes showed biomicroscopic evidence for residual vitreofoveal traction, such as thickening, striae, or cystoid space formation. Measured or estimated lesion sizes ranged from 50 to 433 μm in diameter (Table 2).
FIGURE 6.
Case 12. Optical coherence tomography scan of pseudo-operculum suspended on detached posterior hyaloid over normal fovea.
FIGURE 9A.
Case 18. Fundus photograph of right eye showing macular microhole in patient with acute onset of a tiny central scotoma.
FIGURE 7.
Case 15. Vertical axial B-scan ultrasonogram shows shallow stage 2 vitreous detachment with pseudo-operculum. The underlying fovea was normal on biomicroscopy.
FIGURE 8A.
Case 17. Fundus photograph of right eye demonstrating lamellar macular hole with overlying operculum.
FIGURE 8B.
Case 17. Composite optical coherence tomography image showing small operculum suspended on minimally reflective posterior hyaloid over inner lamellar foveal defect.
FIGURE 9B.
Case 18. Horizontal axial ultrasound of right eye demonstrating shallow detachment of posterior hyaloid over macular region.
FIGURE 9C.
Case 18. Fundus photograph of right eye 3 months later shows spontaneously healed microhole.
The mean follow-up time for this group was 19.5 months (range, 2 to 68 months). None of the eyes with pseudo-operculum or lamellar macular hole developed complete PVD during follow-up. Three of four eyes with macular microhole developed complete PVD between 4 and 12 months after symptom onset, but the remaining eye had failed to progress beyond stage 2 PVD after 48 months of follow-up (Figure 9D and 9E). In three of four eyes with microhole, the subjective scotoma decreased in size over time. The foveal defect disappeared in all four eyes, leaving no visible foveal abnormality in two eyes and a tiny red spot in two eyes. OCT imaging of the tiny red spot in each eye showed no evidence of a residual foveal defect.
FIGURE 9D.
Case 18. Fundus photograph of left eye 48 months after the development of microhole shows tiny persistent red spot centrally with no apparent foveal defect. Patient still has tiny scotoma subjectively.
FIGURE 9E.
Case 18. Optical coherence tomography of left eye shows healed microhole and persistence of stage 2 vitreous detachment 48 months after onset.
Tractional CME
Of 14 eyes (of 13 patients) with CME presumed to be caused by vitreofoveolar traction, three patients were excluded from further analysis for the reasons noted above. The 10 patients composing the study cohort ranged in age of symptom onset from 48 to 81 years (median, 71 years) and had a female to male ratio of 3:2 (Table 3). The only patient less than 60 years old had a spherical equivalent refractive error of −8.00 D. The past ocular history was notable for mild nonproliferative diabetic retinopathy in four eyes, none of which had significant fluorescein leakage suggestive of DME. Four eyes were pseudophakic at symptom onset, but in only one of these eyes was there a temporal relationship with cataract surgery that suggested a possible component of postoperative CME. Two patients were using latanoprost in the study eye. Two patients had a macular hole in the fellow eye.
TABLE 3.
PRETREATMENT CLINICAL DATA FOR PATIENTS WITH TRACTIONAL CME
|
PHM VISIBILITY |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PATIENT NO. | ONSET AGE (YR)/SEX/EYE | SYMPTOM DURATION (MO)* | PRE-TREATMENT VISUAL ACUITY | VISIBLE PERIPAPIL LARY TRACTION | CLINICAL ASYMMETRY OF CME | BIOMICROSCOPY | OCT | B-SCAN | PVD STAGE | ADHESION SIZE, μM | FA LEAKAGE |
| 21 | 81/M/OD | 48 | 20/60 | No | Pagoda | Yes | ND | Yes | 1 | ~500† | No |
| 22 | 68/F/OD | 14 | 20/80 | Yes | Ridge | No | ND | Yes | 1 | ~400 to 500† | Yes‡ |
| 23 | 67/M/OD | 6 | 20/60 | Yes | Ridge | Yes | ND | Yes | 1 | ND | No |
| 24 | 72/F/OD | 14 | 20/80 | No | Ridge ×2 | Yes | ND | ND | 1 | ~500† | No |
| /OS | 3 | 20/60 | Yes | Ridge | Yes | ND | Yes | 1 | ~500† | No | |
| 25 | 70/M/OS | 52 | 20/100 | No | Pagoda | Yes | ND | ND | 1 | ~300 to 400† | Yes‡ |
| 26 | 79/M/OS | 22 | 20/70 | No | Ridge | Yes§ | Yes | Yes | 1 | 233 | No |
| 27 | 67/F/OS | 10 | 20/200 | Yes | Pagoda | Yes | Yes | Yes | 1 | 536 | Yes‡ |
| 28 | 78/F/OD | 3 | 20/30 | No | Pagoda | Yes | ND | Yes | 1 | ~400† | No |
| 29 | 48/F/OS | 0 | 20/25 | No | No | No | Yes | Yes | 1 | 394 | No |
| 30 | 77/F/OD | 24 | 20/30 | No | No | No | Yes | Yes | 1 | 489 | No |
CME = cystoid macular edema; FA = fluorescein angiogram; ND = not done; OCT = optical coherence tomography; PHM = posterior hyaloid membrane; PVD = posterior vitreous detachment.
Refers to symptom duration at the time of treatment (surgery or observation).
Estimated size by biomicroscopy and/or intraoperative observation.
Responded to anti-inflammatory medications with reduced fluorescein leakage but no improvement in cystoid foveal thickening or visual acuity.
PHM invisible on presentation and became visible over 2-year preoperative follow-up.
At the time of treatment (surgery or observation), the visual acuity ranged from 20/25 to 20/200 (median acuity, 20/60). In addition to visual blurring and reduced acuity, metamorphopsia was present in six of 11 eyes (54.5%). One patient was visually asymptomatic. In the remaining 10 eyes, the visual symptoms had been present for an average of 19.6 months (range, 3 to 52 months). Biomicroscopy revealed foveal thickening with multiple cystoid spaces in all eyes and mild (grade 1) ERM in seven eyes (63.6%). In addition, meticulous biomicroscopy revealed subtle clues to the tractional etiology of the CME in most eyes, including asymmetry of the foveolar thickening in nine eyes, a mild juxtapapillary traction ridge in four eyes, inner lamellar foveolar defect in three eyes, and mild inner retinal radial striae in two eyes. The asymmetry of the foveolar thickening appeared on biomicroscopy as either a subtle traction ridge along a portion of the foveolar border (five eyes) (Figure 10) or a pagoda-shaped elevation of the foveola without a visible ridge (four eyes) (Figures 11 and 12).
FIGURE 10A.
Case 22. Macular photograph of right eye showing cystoid macular thickening with shallow ridge of inner retinal traction along superonasal edge of foveola.
FIGURE 11A.
Case 26. Vertical optical coherence tomography scan of left eye shows perifoveal hyaloidal detachment with focal foveolar attachment causing tractional cystoid foveal thickening. On biomicroscopy, the detached posterior hyaloid was invisible initially and became visible over 2-year follow-up period.
FIGURE 12A.
Case 27. Vertical optical coherence tomography image of left eye shows pagoda-shaped, cystoid foveal thickening and mild epiretinal membrane associated with perifoveal vitreous detachment. Note the increased thickening of posterior hyaloid membrane near its attachment to the foveola.
FIGURE 10B.
Case 22. Vertical macular ultrasound image showing shallow perifoveal vitreous detachment with residual foveolar adhesion.
FIGURE 11B.
Case 26. Longitudinal ultrasound view of same eye, showing limited extent of perifoveal vitreous detachment.
FIGURE 12B.
Case 27. Vertical ultrasound image shows perifoveal vitreous separation, slightly more extensive superiorly.
FIGURE 12C.
Case 27. Optical coherence tomography scan 6 months postoperatively shows resolution of tractional cystoid macular edema.
All 11 eyes were found to have a shallow perifoveal vitreous detachment with adherence to the foveola and optic disc margin (stage 1 PVD). The detached and slightly taut posterior hyaloid membrane was visible as a glistening interface on biomicroscopic examination in eight eyes (72.7%) and as a smooth membrane on both ultrasonography and OCT in all eyes tested (Table 3). Although the perifoveal vitreous detachment typically extended to approximately the level of the vascular arcades, in three eyes it extended into the midperiphery in at least the superior quadrant. In eyes examined prior to OCT availability, the diameter of the vitreous adhesion to the foveola was estimated clinically to be in the range of 300 to 500 μm in all eyes. The mean adhesion size in the four eyes evaluated by OCT was 413 μm (range, 233 to 536 μm).
Fluorescein angiography revealed no leakage of perifoveal capillaries in eight eyes (72.7%) (Figures 13A and 13B) and showed mild to moderate angiographic CME in the remaining three eyes. In only one of these eyes did the relatively mild leakage appear to be caused by vitreofoveolar traction. Of the remaining two eyes, one was 2 weeks post–cataract surgery and the other was receiving glaucoma treatment with latanoprost. Medical treatment in the three eyes with angiographic CME resulted in decreased capillary leakage but no change in foveal thickening or visual acuity.
FIGURE 13A.
Case 29. Fundus photograph of left eye shows cystoid foveal thickening and radial inner retinal striae. The posterior hyaloid was not visible on biomicroscopy.
FIGURE 13B.
Case 29. Corresponding midphase fluorescein angiogram reveals no leakage from retinal capillaries.
The three eyes maintaining visual acuities of 20/50 or better were managed by observation alone. After a mean follow-up period (beginning at symptom onset) of 59.7 months (range, 35 to 84 months), the final visual acuity in these eyes remained within 2 lines of the initial acuity (Table 4). One eye (case 28) had spontaneous vitreofoveal separation with lamellar macular hole formation at 15 months of follow-up but did not develop a Weiss ring over the remaining 45 months. The other two eyes showed no progression of PVD stage and little change in macular appearance during the extended follow-up period (Figures 13C and 13D).
TABLE 4.
POSTTREATMENT CLINICAL DATA FOR PATIENTS WITH TRACTIONAL CME
| PATIENT NO. | TREATMENT* | MIDPERIPHERAL VITREORETINAL ADHESIONS | SURGICAL COMPLICATIONS | POSTTREATMENT FOLLOW-UP (MO) | PROGRESSION OF PVD STAGE | FINAL MACULAR STATUS | FINAL VISUAL ACUITY |
|---|---|---|---|---|---|---|---|
| 21 | PPV | Yes | 14 | N/A | Foveal reflex | 20/40 | |
| 22 | PPV, MP | No | 28 | N/A | Foveal reflex | 20/30 | |
| 23 | PPV, MP | Yes | NS | 4 | N/A | Foveal reflex | 20/30 |
| 24/OD | CE, PPV, MP | Yes | VH | 53 | N/A | Foveal reflex, mild ERM | 20/30 |
| 24/OS | CE, PPV | Yes | Pseudophakic CME | 24 | N/A | Foveal reflex, mild ERM | 20/25 |
| 25 | PPV, MP, F/Gx | Yes | Macular hole | 67 | N/A | Foveal reflex | 20/30 |
| 26 | PPV | Yes | 3 | N/A | No foveal depression | 20/30 | |
| 27 | PPV, MP | Yes | NS | 15 | N/A | Foveal reflex | 20/25 |
| 28 | Observe | N/A | 57 | 1 → 2 | Lamellar hole | 20/40 | |
| 29 | Observe | N/A | 84 | No | Increased foveal thickening | 20/25 | |
| 30 | Observe | N/A | 11 | No | No apparent change | 20/50 |
CE = cataract extraction; CME = cystoid macular edema; ERM = epretinal membrane; F/Gx = intraoperative fluid-gas exchange; MP = epiretinal membrane peeling; N/A= not applicable; NS = nuclear sclerosis; PPV = pars plana vitrectomy, including peeling of vitreous cortex from all sites of residual vitreoretinal adhesion; PVD = posterior vitreous detachment; VH = postoperative vitreous hemorrhage.
FIGURE 13C.
Case 29. Optical coherence tomography scan demonstrates cystoid foveal thickening associated with perifoveal vitreous detachment.
FIGURE 13D.
Case 29. Optical coherence tomography scan 20 months later shows only slight progression of vitreous separation and associated tractional deformation of the fovea.
The eight eyes with visual acuities of 20/60 or worse were managed with pars plana vitrectomy, peeling of residual vitreoretinal adhesions, and ERM peeling when indicated (Table 4). Fluid-gas exchange was performed only in one eye that was noted to have a deep circular inner lamellar foveal defect preoperatively. The surgical technique differed from that used in the typical vitreomacular traction syndrome because of the absence of vitreous separation in the midperiphery. Access to the subhyaloid space was not trivial, because the localized perifoveal hyaloid detachment was shallow and sometimes invisible. After core vitrectomy, an opening was made in the perifoveal posterior hyaloid membrane using either a bent microvitreoretinal blade (when the posterior hyaloid was taut and visible) or a lighted pick in combination with a silicone cannula. In eyes without ERM centrally, the vitreous could then be separated atraumatically from the foveola with a pick. However, in the presence of ERM, the vitreous was firmly anchored to thefoveal center, precluding its simple separation with a pick (Figure 14). In these eyes, vitreofoveal separation was possible only upon peeling the ERM (usually after carefully inducing a Weiss ring and circumscribing the vitreous around the fovea).
FIGURE 14.
Schematic illustration of vitreomacular interface encountered in cases of tractional cystoid macular edema accompanied by mild epiretinal membrane (ERM). A, The vitreous is firmly anchored to the fovea by extension of ERM onto the posterior hyaloid. This prevents intraoperative separation of vitreous from the fovea from the surgical plane shown in frame B. C, Vitreofoveal separation occurs readily upon peeling the associated ERM.
In such cases, the vitreous remnant was firmly adherent to the peeled ERM specimen. No attempts were made specifically to peel the ILM. Once the vitreous was separated from the posterior pole, it was separated from other areas of vitreoretinal adhesion evident in the midperiphery in seven (87.5%) of eight operated eyes.
After a mean postoperative follow-up of 26.0 months (range, 3 to 67 months), the final visual acuity had improved by 2 or more lines and measured 20/40 or better in eight (100%) of eight operated eyes (Table 4). On final examination, the cystoid spaces had resolved in all eyes, and the foveal depression/reflex was restored in seven eyes (87.5%). The macular thickening often resolved within weeks of surgery.
Vitreomacular Traction Syndrome
The eight patients with the vitreomacular traction syndrome (Table 5) had a median age at symptom onset of 71.5 years (range, 61 to 83 years). No patient had bilateral involvement, although five patients had partial or complete PVD in the fellow eye, with an associated mild ERM in three eyes. At the time of treatment (surgery or observation), visual symptoms had been present for an average of 24.4 months (range, 2 to 96 months) and included blurred vision and decreased acuity in all patients, with metamorphopsia in seven patients.
TABLE 5.
CLINICAL DATA FOR PATIENTS WITH THE VITREOMACULAR TRACTION SYNDROME
| PATIENT NO. | ONSET AGE (YR)/SEX/EYE | SYMPTOM DURATION (MO)* | PRE-TREATMENT VISUAL ACUITY | PVD STAGE | ESTIMATED ADHESION SIZE (μm)† | MACULAR TRACTION RETINAL DETACHMENT | TREATMENT | POST-TREATMENT FOLLOW-UP (MO) | PROGRESSION OF PVD STAGE | FINAL MACULAR STATUS | FINAL VISUAL ACUITY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 31 | 75/M/OS | 24 | 20/30 | 1+ | ~1500 | Yes | Observe | 30 | No | Same | 20/40 |
| 32 | 73/F/OD | 24 | 20/400 | 1+ | ~2250 | Yes | Observe‡ | 12 | No | Same | 20/300 |
| 33 | 61/M/OS | 96 | 20/400 | 1+ | ~2250 × 3000 | Yes | Observe§ | 141 | No | Same | 5/200 |
| 34 | 67/F/OD | 12 | 20/80 | 1+ | ~750 × 3000 | Yes | PPV, MP | 13 | N/A | Flat, LMH | 20/30 |
| 35 | 64/F/OS | 2 | 20/60 | 1 | ~1500 × 2250 | Yes | PPV, MP | 18 | N/A | Flat | 20/20 |
| 36 | 71/F/OS | 5 | 20/60 | 1+ | ~2250 × 3000 | Yes | PPV, F/Gx, laser | 35 | N/A | Flat, LMH | 20/40 |
| 37 | 72/F/OS | 24 | 20/200 | 1++# | ~500 × 2000 | Yes | PPV, MP | 11 | N/A | Flat | 20/50 |
| 38 | 83/M/OS | 8 | 20/60 | 1+ | ~500 | Yes | PPV | 2 | N/A | Flat | 20/60 |
F/Gx = fluid-gas exchange; LMH = lamellar macular hole; MP = epiretinal membrane peeling; N/A = not applicable; PPV = pars plana vitrectomy, including peeling of vitreous cortex from all sites of residual vitreoretinal adhesion; PVD = posterior vitreous detachment.
Refers to symptom duration at the time of treatment (surgery or observation).
Estimated size by biomicroscopy and/or intraoperative observation.
Poor visual potential due to concomitant age-related macular degeneration with geographic atrophy.
Poor visual potential due to amblyopia.
Had additional vitreoretinal adhesions along arcade vessels.
Indicates total PVD (including disc) except for macular adhesion.
The detached posterior hyaloid membrane, which was often thickened and taut, was visible by meticulous biomicroscopy in all eight eyes and by B-scan ultrasonography in each of the five eyes tested. In the three eyes not evaluated by ultrasound, the clinical PVD stage was confirmed at surgery. Seven eyes had stage 1+ vitreous detachment, extending throughout the periphery (Figure 15), and a single eye had a more limited stage 1 perifoveal detachment extending into the midperiphery. All eyes had residual vitreoretinal attachments in the macular and immediate peripapillary areas except for case 37, in which only a macular adhesion was present. The size and shape of the macular vitreous adhesion were variable and irregular, ranging from circular to linear, sometimes eccentric along retinal vessels, and occasionally multiple. In seven of the eight eyes, the longest diameter of the vitreomacular adhesion was estimated to be in the range of 1 to 2 disc diameters (Table 5). By biomicroscopy, a tractional macular detachment of varying severity was diagnosed in all eyes, accompanied by a visible ERM in four of the eight eyes (Figure 16).
FIGURE 15A.
Ultrasound image of vitreomacular traction syndrome case showing peripheral vitreous detachment with broad area of vitreomacular adhesion. Vertical transverse scan of right eye, case 32.
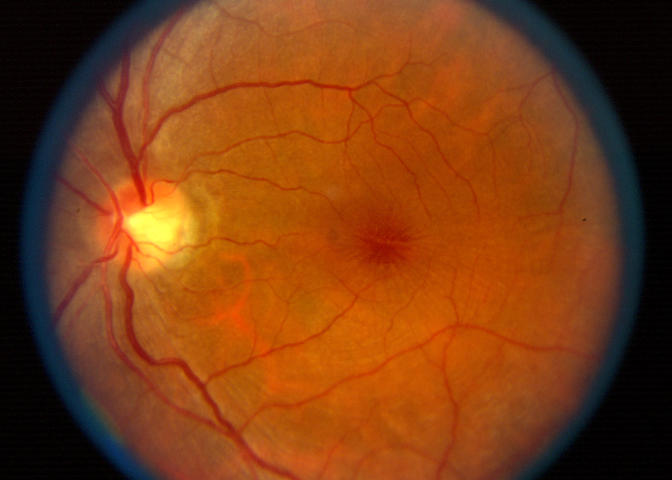
FIGURE 16.
Case 33. Fundus photograph of left eye with classic vitreomacular traction syndrome. There is vitreous attachment to a broad area involving the macula and optic disc margin, associated with shallow traction macular detachment and epiretinal membrane.
FIGURE 15B.
Ultrasound image of vitreomacular traction syndrome case showing peripheral vitreous detachment with broad area of vitreomacular adhesion. Longitudinal scan of right eye, case 34.
The three eyes managed by observation showed no apparent change in either PVD grade or macular status over an average total follow-up period (from symptom onset) of 109 months (range, 36 to 237 months). The five operated eyes underwent standard pars plana vitrectomy, peeling of vitreoretinal adhesions, and peeling of ERM as needed. When ERM was present, atraumatic separation of the vitreous from sites of macular adhesion was possible only upon peeling the ERM. In each case, the circumscribed vitreous was firmly adherent to the peeled ERM specimen. Over an average postoperative follow-up period of 15.8 months (range, 2 to 35 months), the macula flattened in all five eyes and visual acuity improved by 2 or more Snellen lines in four eyes.
Myopic Macular Retinoschisis
Five eyes of five patients with macular retinoschisis complicating high myopia were identified retrospectively. Three eyes with complete PVD were excluded from further analysis. Data from the two patients included in the study are listed in Table 6. By OCT evaluation, neither patient had retinoschisis in the fellow eye. Both patients had biomicroscopic and OCT evidence for diffuse outer macular retinoschisis. In addition, patient 39 had evidence for a stage 1B macular hole (Figure 17), and patient 40 had a component of inner retinoschisis in the temporal macula (Figure 18). Although not visible biomicroscopically, both patients demonstrated stage 1 perifoveal vitreous detachment by OCT, which extended into the superior and nasal periphery in patient 40. The vitreomacular adhesion was smaller in the patient with the early macular hole (381 versus 893 μm).
TABLE 6.
CLINICAL DATA FOR PATIENTS WITH MYOPIC MACULAR RETINOSCHISIS
| PATIENT NO. | ONSET AGE(YR)/SEX/EYE | SYMPTOM DURATION(MO)* | RE | PRETREATMENT VISUAL ACUITY | POSTERIOR STAPHYLOMA | PVD STAGE | ADHESION SIZE(μm) | TREATMENT | POST-TREATMENT FOLLOWUP (MO) | FINAL MACULAR STATUS | FINAL VISUAL ACUITY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 39 | 52/F/OD | 2 | −5.50 | 20/200 | No | 1 | 381 | PPV, F/Gx† | 4 | Minimal residual schisis | 20/40 |
| 40 | 59/M/OS | 7 | −16.50 | 20/100 | Yes | 1 | 893 | PPV‡ | 10 | LMH, schisis unchanged | 20/80 |
F/Gx = fluid-gas exchange; LMH = lamellar macular hole; PPV = pars plana vitrectomy, including peeling of vitreous cortex from all sites of residual vitreoretinal adhesion; PVD = posterior vitreous detachment; RE = spherical equivalent refractive error.
Refers to symptom duration at the time of treatment.
Patient had stage 1B macular hole in addition to diffuse outer retinoschisis.
Patient had additional vitreoretinal adhesion in superior midperiphery with focal tractional retinoschisis.
FIGURE 17A.
Case 39. Fundus photograph reveals deep yellow foveal ring suggesting outer foveolar defect (stage 1B hole) associated with schisis-like retinal thickening throughout the macular region.
FIGURE 18A.
Case 40. Fundus photograph shows posterior staphyloma in high myopia. Biomicroscopy revealed retinal thickening throughout the area of the staphyloma.
FIGURE 17B.
Case 39. Corresponding optical coherence tomography image shows perifoveal vitreous detachment, break in the outer foveola, and outer retinoschisis throughout the macula.
FIGURE 17C.
Case 39. Three-month postoperative fundus photograph demonstrates resolution of both macular hole and retinoschisis.
FIGURE 17D.
Case 39. Postoperative optical coherence tomography demonstrates resolution of both macular hole and retinoschisis.
FIGURE 18B.
Case 40. Preoperative horizontal optical coherence tomography shows extensive outer retinoschisis, with an area of inner retinoschisis temporally (large arrows). Detachment of the posterior hyaloid is seen nasally (small arrows).
FIGURE 18C.
Case 40. One year postoperatively, optical coherence tomography shows persistence of both inner and outer retinoschisis.
Both patients underwent pars plana vitrectomy with peeling of vitreoretinal adhesions, using gas tamponade only in patient 39. In this patient, surgery resulted in complete resolution of the stage 1 hole and near-complete resolution of the outer macular retinoschisis, with significant visual benefit. In patient 40, however, neither the outer retinoschisis nor the visual acuity was substantially improved by surgery.
DISCUSSION
The findings presented in this study support the thesis that age-related PVD begins invisibly as a localized perifoveal vitreous detachment that progresses in a chronic rather than an acute fashion and that may be complicated by several distinct but related macular disorders. The findings further illustrate the type of vitreous traction involved in these macular pathologies and suggest that the specific disorder seen in association with perifoveal vitreous detachment appears to depend at least in part on the size and strength of the residual vitreomacular adhesion.
The patients evaluated in this study had macular conditions putatively caused by vitreous traction and had no biomicroscopic evidence of complete PVD on presentation. The age demographics of this cohort proved to be similar to that found in large clinical series of symptomatic complete PVD.2,6,37 Specific evaluation of the vitreoretinal status revealed that 39 (90.7%) of the 43 eyes in this series had early, perifoveal stages of PVD. The remaining four eyes each had a diagnosis of ERM and were found to have either complete (stage 4) or near-complete (stage 3) PVD (Figure 2). Thus, in this study, the macular conditions evaluated were universally associated with some degree of PVD, usually an early perifoveal stage.
Several lines of evidence suggest that this association is causal rather than coincidental. First, the majority of eyes in this series with perifoveal vitreous detachment and a persistent vitreomacular adhesion (stage 1) exhibited a tractional profile on OCT evaluation or slit-lamp biomicroscopy. In most cases, this profile resembles a pagoda, with a steeply sloping inner foveal or macular surface and a sharp angulation where the partially detached posterior hyaloid remains attached to the top of the inner macular elevation (Figure 12).71,74 A biomicroscopic clue to this profile is sometimes the presence of a subtle tractional ridge, best seen with a fundus contact lens.
Second, there was correlation in this study between increasing extent of stage 1 perifoveal vitreous detachment and the degree of macular traction. For example, seven (87.5%) of eight eyes with classic vitreomacular traction syndrome, all of which had tractional macular detachment, were found to have stage 1+ PVD extending throughout the fundus periphery. In contrast, all eyes with tractional CME, a subtle variant of vitreomacular traction syndrome without detachment, had more limited degrees of perifoveal vitreous separation.
Third, eyes without a persistent tractional macular profile (such as those with macular hole–related conditions) were typically found to have PVD stage 2 or higher, with no vitreofoveal attachment. Such eyes were presumed (and in two cases observed) to have had a preceding vitreofoveal adhesion that caused the macular pathology prior to or coincident with its release.
Finally, surgical or spontaneous separation of vitreomacular adhesions in this series was invariably followed by partial or complete resolution of the signs and symptoms of macular traction, usually with improvement in visual acuity.
The current study provides substantial new evidence that the early stages of PVD in most eyes persist chronically and progress slowly. In eyes with ERM associated with partial PVD, the average symptom duration was 37 months, suggesting that a period of years typically elapses between the onset of perifoveal vitreous detachment and the completion of PVD. Each of the remaining 31 eyes with primary diagnoses other than ERM presented with perifoveal vitreous detachment stage 1 or 2. Over an average preoperative or total follow-up period of 30.0 months (range, 2 to 237 months), only three (9.7%) of these eyes showed progression to complete PVD. The slow progression of early PVD stages is well illustrated by case 28, a patient who had documented progression from stage 1 to stage 2 PVD at 15 months of follow-up, then failed to progress to complete PVD over the subsequent 45 months (Tables 3 and 4).
Further evidence for the chronicity of early PVD stages is found in the observations reported by Van Newkirk and coworkers53 on a series of patients with macular pseudo-operculum. In this study, ultrasonography revealed a broad macular (stage 2) PVD in 22 (100%) of 22 eyes with pseudo-operculum. Over a mean follow-up period of 24 months (range, 6 to 65 months), only 7% of these eyes showed evidence for progression to complete PVD. Similarly, a longitudinal study of unoperated idiopathic macular holes found that one third of eyes with stage 2 or 3 macular hole at baseline had not progressed to complete PVD at 5 years of follow-up.75 It appears, then, that in the vast majority of eyes in which perifoveal vitreous detachment causes macular complications, the early stages of PVD are chronic, progressing slowly for many months or years until vitreopapillary separation yields a complete PVD. It is reasonable to assume that the rate of progression is similar in asymptomatic eyes, in which early PVD stages evolve smoothly and without macular morbidity.
Among the eyes evaluated in this study, there were obvious variations in the size of the vitreomacular adhesion associated with perifoveal vitreous detachment. Among eyes with a primary diagnosis of ERM, there was significant variability in the size, number, and presence or absence of vitreomacular adhesions. Eyes with tractional CME and those with macular hole–related conditions (in which lesion size was used as a surrogate measure of vitreomacular adhesion size) all had focal vitreous adhesions limited to the foveola, measuring approximately 500 μm or less. In eyes with the vitreomacular traction syndrome, estimated adhesion sizes were substantially larger and variable in shape, typically measuring between 1,500 and 3,000 μm in greatest linear dimension. The adhesion size in the two eyes with myopic macular retinoschisis was variable. Although quantitative information on vitreomacular adhesion size has not previously been reported for this disorder, review of published OCT images of myopic retinoschisis with associated perifoveal vitreous detachment suggests adhesions often extending beyond the 500 μm foveola.76,77
In addition to the macular disorders evaluated in the present study, there is strong evidence for a causal relationship between perifoveal vitreous detachment and at least two other macular disorders: idiopathic macular hole and tractional DME. Johnson and coworkers51 recently described a series of 26 consecutive patients with stage 1 or 2 idiopathic macular hole evaluated by contact lens biomicroscopy in addition to B-scan ultrasonography and/or intraoperative examination of the posterior vitreous cortex prior to inducing a PVD. In 96% of eyes, one or more examination techniques revealed a localized perifoveal vitreous detachment. Other investigators using similar ultrasound instrumentation and scanning techniques have also demonstrated localized separations of the posterior vitreous face in eyes with full-thickness macular holes.54,59
Haouchine and coworkers55 recently reported a prospective OCT study demonstrating stage 1 perifoveal vitreous detachment in 22 (100%) of 22 eyes with a foveal pseudocyst (stage 1 macular hole) on biomicroscopy. Numerous other investigators have similarly reported OCT imaging of localized perifoveal vitreous detachment in all or the majority of eyes with early-stage macular holes.52,56–58,60,64,69,78,79 The OCT maps constructed by Ito and coworkers60 showed close correlation between the stage of macular hole and the extent of PVD, suggesting that progression of idiopathic macular hole is related to increasing vitreofoveolar traction associated with progressive enlargement of the perifoveal vitreous detachment. Other investigators have similarly observed a correlation between progression of the perifoveal hyaloid detachment and macular hole progression.55 Adding to the evidence supporting a causal relationship between perifoveal vitreous detachment and idiopathic macular hole is the clinical observation that when a macular pseudocyst (impending hole) resolves or evolves into a lamellar macular hole, the change occurs after (or coincident with) separation of the hyaloid from the foveola.55,80 Taken together, these recent ultrasound and OCT observations have necessitated a reassessment and modification70,81 of the theory of macular hole pathogenesis proposed by Gass.68,82
Although the pathophysiology of DME is complex and multifactorial,83 previous investigators have suggested that the vitreous may play a pathogenic role in some eyes.84,85 Several groups have reported that biomicroscopic examination of select eyes with diffuse DME shows a thickened, taut, and glistening posterior hyaloid,86 and that vitrectomy and peeling of the posterior hyaloid to relieve the associated traction is often beneficial.86–89 OCT imaging has recently clarified that the posterior hyaloid in such eyes is not only taut and slightly thickened but also partially detached from the retina in a perifoveal geography.71–73 DME exacerbated by the vitreomacular traction effects of perifoveal vitreous detachment has recently been termed tractional DME.71 OCT findings in eyes with tractional DME include stage 1 perifoveal vitreous detachment with a thick and hyperreflective posterior hyaloid, steeply sloping cystoid macular thickening with a pagoda-shaped profile, and the variable presence of shallow macular detachment.71–73 Electron immunocytochemical analysis90 has shown that the thickened posterior hyaloid is infiltrated by cells of glial and epithelial origin, suggesting that cellular migration and contraction may contribute both to the strength of the vitreomacular adhesion and to the tautness of the detached hyaloid. The cellular component likely accounts for the glistening reflex on the shallowly detached posterior hyaloid membrane that is typically, but not universally, visible on biomicroscopic examination.71,73 There is recent evidence that even subclinical perifoveal vitreous detachment appears to play a contributing role in DME.91 Although quantitative information regarding the size of the vitreomacular adhesion in such eyes has not been published, clinical experience and review of published tomograms suggest that it is variable, ranging from a focal foveolar adhesion similar to that seen in tractional CME and idiopathic macular hole states, to a broader adhesion typical of the vitreomacular traction syndrome.
In a retrospective comparative case series, Massin and colleagues71 recently reported that vitrectomy was beneficial in eyes with tractional DME but not in eyes with diffuse DME without evidence of traction. That vitreous traction plays an important role in such eyes is further suggested by the report of Yamaguchi and coworkers73 demonstrating resolution of tractional DME following spontaneous vitreofoveal separation in three eyes. However, reports that vitrectomy may sometimes be beneficial in eyes without apparent posterior hyaloid abnormalities92–99 and even in eyes with complete PVD97,98,100,101 underscore the complexity of the underlying disease and the possibility that vitrectomy in these eyes has beneficial effects in addition to relieving vitreoretinal traction.83,94,96,102 It should be noted that in many of these studies, neither OCT nor ultrasound was used to determine the precise vitreoretinal relationship.
Perifoveal vitreous detachment appears to have a relationship with myopic macular retinoschisis that is similar to its relationship with DME. That is, vitreous traction from early-stage PVD appears to play a contributing or exacerbating role in at least a subset of eyes with each of these disorders. Retinoschisis within the area of myopic posterior staphyloma is a newly described disorder, identified and characterized largely since the advent of OCT.76,77,103–106 It is not an uncommon condition, occurring in 11 (34%) of 32 eyes in a prospective study of consecutive patients with high myopia and posterior staphyloma.103 Biomicroscopically, there is shallow retinal elevation, typically with a microcystic appearance, throughout the area of posterior staphyloma. OCT imaging demonstrates extensive schisis in the outer neurosensory retina, sometimes accompanied by separate areas of inner retinoschisis, foveal detachment, and/or lamellar or full-thickness macular hole.
The cause of myopic macular retinoschisis is not fully understood. Vitreous traction associated with stage 1 perifoveal vitreous detachment was evident in both affected eyes in the present study and was detected by OCT in seven (23%) of 30 eyes in two recent reports that provided information about the vitreoretinal status.76,77 Eyes with perifoveal vitreous detachment appear to be at especially high risk for developing secondary macular hole.76 The near-complete resolution of retinoschisis after vitrectomy in one of our patients (Figure 17) suggests that vitreous traction from perifoveal vitreous detachment causes or exacerbates macular retinoschisis in at least a subset of eyes. However, myopic retinoschisis is commonly seen in eyes with complete PVD76,77 and does not always resolve after the surgical relief of vitreomacular traction, suggesting that other pathogenic mechanisms may be involved. Such mechanisms might include “stretch retinoschisis” over the posterior staphyloma,76,103 a retinal degenerative process within the staphyloma,76,103 or tangential traction by ERM or residual vitreous cortex on the retina following PVD.77,103 In support of the latter concept, recent reports suggest that vitrectomy with ILM peeling and gas tamponade may be beneficial in eyes with myopic macular retinoschisis, especially when the condition is complicated by macular detachment and/or macular hole.77,105,106
The clinical data presented in this thesis and the literature cited above suggest that the macular disorders that may complicate the early, perifoveal stages of age-related PVD include at least those conditions listed in Table 7. The disorders more commonly considered as complications of PVD are those that develop coincident with or shortly after the completion of PVD at the time of vitreopapillary separation (Table 7). As a rule, then, complications of perifoveal (early-stage) PVD occur in the macula and begin insidiously, whereas complications of complete (late-stage) PVD occur in the retinal periphery and present with acute symptoms and signs.
TABLE 7.
COMPLICATIONS OF AGE-RELATED POSTERIOR VITREOUS DETACHMENT
| EARLY-STAGE (PERIFOVEAL) | LATE-STAGE (COMPLETE) |
|---|---|
| Epiretinal membrane | Retinal or optic disc hemorrhage |
| Macular microhole | Vitreous hemorrhage |
| Idiopathic macular hole, pseudo-operculum, and inner lamellar macular hole | Retinal tear |
| Tractional cystoid macular edema | Rhegmatogenous retinal detachment |
| Tractional diabetic macular edema | |
| Vitreomacular traction syndrome | |
| Tractional myopic macular retinoschisis |
As discussed by previous investigators,29,51,70,78 separation of the posterior vitreous cortex from the perifoveal retina could be expected to exert traction on the foveola in at least two important ways. First, the elastic properties of a trampoline-like posterior hyaloid detachment with focal adherence at the foveola should exert anterior traction on the foveola toward the plane of the detachment. Because the three-dimensional plane of posterior hyaloid detachment is anterior to the plane of the inner retinal surface, cross-sectional images (such as OCT and ultrasound) show the hyaloid to have a biconvex configuration where it is tethered posteriorly at the foveola.51 In contrast to pure tangential traction along the inner retinal surface, static traction from perifoveal vitreous detachment can pull the inner portion of the fovea above the plane of the perifoveal macula, which is the configuration commonly seen in early macular hole states, tractional CME, and tractional DME.51,55,58,71,72,78
Second, perifoveal vitreous detachment should localize to the fovea the dynamic vitreous tractional forces generated during ocular rotations. Although ocular rotations occur throughout life, associated tractional forces should be distributed evenly across the posterior retina until perifoveal vitreous detachment allows them to act focally on the fovea. This is analogous to peripheral retinal break formation, which occurs at a focal vitreoretinal adhesion after vitreous detachment from the surrounding retina. Although static and dynamic tractional forces likely both play a role in causing macular pathology, dynamic forces are probably more important, particularly in eyes without abnormal thickening or tightness of the detached posterior hyaloid.
It is plausible that the anatomical variation placing certain eyes at risk for macular complications of perifoveal vitreous detachment is an especially strong vitreofoveal adhesion. This prevents or delays the smooth vitreofoveal separation that normally occurs during the progression of uncomplicated age-related PVD, allowing the static and dynamic tractional forces associated with perifoveal hyaloid detachment to act on the fovea over time. Other factors, such as the size of the posterior precortical vitreous pocket, may influence the degree of force concentration on the fovea.30 The specific macular disorder that develops in a given eye with perifoveal vitreous detachment likely depends in large part on the size of the persistent vitreomacular adhesion. Spaide and coworkers78 recently found that in eyes with perifoveal vitreous detachment, there was a statistically significant correlation between smaller diameters of vitreous attachment and the amount of foveal deformation observed. These investigators suggested that the tractional stress (force per unit area) acting on the fovea would be expected to increase with decreasing area of vitreous attachment. The resulting foveal deformation, or strain, should vary with both the amount of stress and the amount of time the stress is applied. The formation of a macular cavitation or pseudocyst as the initial stage of macular hole development is considered to represent a type of internal fracture and mechanical failure of the foveola secondary to excessive stress.78
The data presented and reviewed in this thesis suggest that small vitreous adhesions (500 μm or less), by imparting greater tractional stress to the foveola, typically result in one of several macular hole conditions (Table 8). It appears that macular microholes develop in eyes with the highest tractional stress (force per unit area) by virtue of the smallest vitreofoveolar adhesion size (50 to 100 μm). Patients with microholes present with the abrupt onset of a tiny central scotoma without metamorphopsia, and are found to have stage 2 PVD overlying a tiny full-thickness macular defect with sharp edges and no residual traction.107 These acutely forming breaks routinely heal without intervention and likely account for at least a subset of patients with the foveal red spot syndrome.108
TABLE 8.
MACULAR COMPLICATIONS OF PERIFOVEAL VITREOUS DETACHMENT
| VARIABLE VITREOMACULAR ADHESION | ADHESION SIZE ≤ 500 μM | ADHESION SIZE ± 1,500 μM |
|---|---|---|
| Epiretinal membrane | Macular microhole
Idiopathic macular hole, pseudo-operculum, and inner lamellar macular hole Tractional cystoid macular edema |
Tractional diabetic macular edema
Vitreomacular traction syndrome Tractional myopic macular retinoschisis |
Typical idiopathic macular holes develop less acutely than microholes,51,55,82 suggesting that the tractional stress imparted to the foveola by vitreous adhesions in the range of 250 to 500 μm requires more time to cause mechanical failure of the fovea.55,78 Other factors, such as the strength of the vitreofoveal attachment and the lateral adhesion strength of the outer foveal layer, may play a role in determining whether a foveal pseudocyst evolves into a full-thickness defect or resolves (upon vitreofoveal separation) into a pseudo-operculum or inner lamellar macular hole.55,81 For reasons that remain unclear, another subset of eyes with a similar-sized vitreofoveolar adhesion (Table 3) develops chronic tractional cystoid foveal thickening and deformation that does not evolve into a macular hole over extended follow-up.
The lower tractional stress associated with larger vitreomacular adhesions is less likely to cause a macular dehiscence and more likely to result in chronic traction macular detachment, diffuse macular thickening, or exacerbation of myopic macular retinoschisis (Table 8). Even within a diagnostic category, variations in vitreous attachment size likely influence the specific pathological configuration that develops. For example, of the two patients in the present study with myopic macular retinoschisis, the patient with the smaller adhesion size developed an associated stage 1 macular hole (Table 6).
PVD is widely believed to play a critical role in the pathogenesis of idiopathic ERM through one of two mechanisms.41 First, transient vitreoretinal traction during the development of PVD is thought to create dehiscences in the ILM through which retinal glial cells can migrate and proliferate on the inner retinal surface.109,110 Alternatively, a subset of ERMs may result from the proliferation and fibrous metaplasia of hyalocytes contained within vitreous cortical remnants left on the retinal surface after PVD.8,23,48,49,111,112 In large clinical series, partial or complete PVD is typically present in 80% to 95% of eyes with idiopathic ERM.48,113–116 Several investigators have suggested that at least 10% to 25% of idiopathic ERMs develop in eyes with no PVD.8,48,113,117,118 A clear explanation for the pathogenesis of ERM in eyes with attached posterior cortical vitreous was not offered, and in none of these cases was the vitreous status evaluated with either OCT or ultrasound.
In the present study, all eyes with idiopathic ERM were found by prospective OCT and/or ultrasonographic evaluation to have partial or complete PVD, despite the lack of biomicroscopic evidence for PVD. In eight (67%) of the 12 eyes evaluated, the associated PVD was in the early perifoveal stages 1 or 2. Gallemore and coworkers65 similarly found partial PVD with vitreomacular adhesions in 52% of eyes with ERM evaluated by OCT. It is therefore probable that the association between idiopathic ERM and PVD is universal and that eyes previously reported as having no PVD actually had subtle perifoveal vitreous detachment that could not be discerned clinically.
The observation that ERM formation frequently begins during the early perifoveal stages of age-related PVD has several pathogenic implications. In the eyes reported herein, idiopathic age-related ERM was found on the retinal surface in areas underlying detached posterior hyaloid. Thus, the development of ERM in eyes with perifoveal vitreous detachment and persistent vitreofoveal attachment (stage 1) could help explain such clinical features as macular pseudohole and foveal prolapse through a pseudohole (Figure 4). In eyes that subsequently develop spontaneous vitreofoveal separation, a central defect in the ERM may persist as a pseudohole, marking the area of previous focal vitreous attachment.
FIGURE 4A.
Case 8. Fundus photograph of right eye showing grade 3 epiretinal membrane (ERM) associated with mild vascular distortion and mild foveal prolapse through central defect in ERM.
In eyes with an even firmer vitreofoveal adhesion, spontaneous vitreofoveal separation is further delayed. Several lines of evidence suggest that in such eyes, epiretinal fibroglial membranes may proliferate from the retinal surface onto the detached posterior hyaloid membrane (Figure 14). This configuration was noted intraoperatively in most of the eyes in this series in which tractional CME or the vitreomacular traction syndrome was accompanied by ERM. Glial migration onto the detached posterior hyaloid is also suggested by the observation with biomicroscopy and OCT that the posterior hyaloid membrane in these conditions is thicker and more reflective than normal, particularly as it approaches its point of attachment with the fovea (Figure 12) (Haouchine B, ARVO Meeting, 2001, Abstract).11 It would also explain the rare clinical occurrence of spontaneous separation of ERM from the retinal surface in conjunction with detachment of the posterior vitreous.119 Most pathological studies of the vitreomacular traction syndrome are not able to clarify this relationship, because the material chosen for analysis has been epiretinal tissue rather than detached posterior hyaloid.111,120 However, ultrastructural examination of both ERM and posterior hyaloid specimens from two eyes with the vitreomacular traction syndrome found cellular proliferation on the detached posterior hyaloid with a composition (principally fibrous astrocytes) similar to that found on the retinal surface.121 Similarly, cells of glial and epithelial origin were found in cortical vitreous specimens removed from patients with vitreofoveal traction122 and in the thickened posterior hyaloid in patients with tractional DME.90
The foregoing suggests that idiopathic ERM plays a role in chronic tractional vitreomaculopathies in at least two important ways. First, ERM likely increases the strength of the residual vitreomacular attachment by effectively anchoring the posterior hyaloid to the surrounding retinal surface, a possibility suggested by previous investigators.47,90,111,122,123 Support for this concept comes from the intraoperative observation in eyes with tractional CME and vitreomacular traction syndrome in the current study that it was usually not possible to separate the vitreous from the macula without simultaneously peeling the associated ERM (Figure 14). By preventing spontaneous separation of the vitreous from the macula,124 ERM bonding increases the duration of the vitreomacular tractional stress. Furthermore, the static vitreous traction component increases over time as fibrocellular organization and contraction cause thickening and tightening of the detached posterior hyaloid.90,121,122 This phenomenon was observed directly in case 26, in which the posterior hyaloid was initially invisible on biomicroscopy and then became visible over many months, coincident with increased traction and decreased visual acuity. In contrast to tractional CME and vitreomacular traction syndrome cases, eyes with idiopathic macular hole rarely develop fibrocellular infiltration of the detached posterior hyaloid sufficient to allow its biomicroscopic visibility.51,55 This may be due to the more rapid progression of macular hole and earlier separation of the vitreous from the fovea in this condition.
As defined in this study, tractional CME is a subtle variant of the vitreomacular traction syndrome that can easily be confused with postoperative or uveitic CME.125 The typical lack of fluorescein leakage and the subtle asymmetry of the cystoid foveal thickening are important clinical clues to its tractional etiology. It differs from the more commonly described vitreomacular traction syndrome10,11 primarily by the small size of its vitreofoveolar adhesion and by the more limited extent of the associated perifoveal vitreous detachment. A similar vitreoretinal relationship is seen in eyes that develop idiopathic macular hole. Most idiopathic macular holes begin as a single foveal cavitation or pseudocyst and progress rapidly to a break in the outer retinal layer at the umbo, signified by a deep yellow spot or ring.55 However, a smaller subset of patients present with multicystoid foveal thickening and no evidence of a break in the outer foveal layers,126 initial findings that are indistinguishable from those of the tractional CME cases described herein. It remains unclear why some eyes progress to macular hole and others exhibit stable or progressive tractional CME without hole formation over extended follow-up.
Because the Müller cell cone provides critical structural support for the fovea, its tractional separation from the photoreceptor and Henle fiber layers (pseudocyst formation) is thought to destabilize the outer foveola.52,55,127 Mechanical failure of the posterior wall of the foveal pseudocyst is a critical pathophysiologic step in macular hole formation and may result to a greater degree from the dynamic rather than static tractional forces associated with perifoveal vitreous detachment.81 Thus, the balance between static and dynamic vitreous tractional forces may be a factor in determining whether a given eye develops a macular hole or chronic tractional CME. In this regard, it is interesting to note that firm midperipheral vitreous adhesions (which might limit vitreous movement during ocular rotations) were present in seven of eight operated eyes with tractional CME in the present study, but were rarely found during surgery on eyes with idiopathic macular hole.51 Another determinant might be the relative strength of the adhesion between the Müller cell cone and the outer foveal layer. That is, eyes in which vitreous traction causes multicystoid foveal thickening rather than complete tractional separation of the Müller cell cone (pseudocyst formation) may be more likely to avoid the destabilization and breakdown of the outer fovea that leads to full-thickness macular hole formation.
This study suffers from several specific limitations in addition to the weaknesses inherent in all retrospective investigations. First, histological examination of surgically excised tissue was not performed and may have further clarified the pathobiology of these vitreoretinal disorders. Second, the study groups are small, in part because of the relative rarity of several of the conditions evaluated. Because the denominator (uncomplicated age-related PVD) is huge, the incidence of these disorders appears to be very low but cannot be calculated using data from this retrospective investigation. Third, as with most of the previous reports cited in this thesis, cross-sectional data are relied upon to support the longitudinal development of macular pathologies associated with perifoveal vitreous detachment. Unfortunately, a prospective longitudinal study of the development of these macular disorders is impractical, given their low incidence in the general population, the lack of established risk factors, the absence of premonitory signs and symptoms, and the occult and asymptomatic nature of the perifoveal vitreous detachment that precedes them. Although a causal relationship must therefore be inferred from the association between a given macular disorder and partial PVD, other observations, such as the resolution of tractional signs and symptoms after vitreomacular separation, confirm the relationship in many cases. Importantly, the longitudinal data presented in this study (follow-up of 31 eyes with stage 1 or 2 PVD over a mean period of 30 months) provide substantial new evidence regarding the chronic persistence and slow progression of early PVD stages. This underscores that much can be learned about the otherwise occult process of early PVD evolution through observation of its relatively uncommon macular complications.
SUMMARY
The findings of this study, combined with a review of pertinent literature, strongly suggest that in contrast to the prevailing clinical perception, age-related PVD is insidious in onset, beginning as a shallow separation from the perifoveal retina, and extending slowly and asymptomatically for months or years until vitreous separation from the optic disc margin results in complete PVD with acute signs and symptoms. In most individuals, the early perifoveal stages of age-related PVD are occult and asymptomatic, occurring smoothly and without pathological consequences. However, just as complete PVD may be complicated in select patients by a variety of peripheral retinal pathologies, perifoveal PVD, through its static and dynamic tractional effects, may be complicated by various disorders in the macular region. The disorders caused by perifoveal vitreous detachment are determined in part by the size and strength of the vitreomacular adhesion and appear to include idiopathic ERM, macular microhole, idiopathic macular hole (including pseudo-operculum and inner lamellar macular hole), tractional CME, tractional DME, vitreomacular traction syndrome, and tractional myopic macular retinoschisis.
REFERENCES
- 1.Sebag J. The Vitreous: Structure, Function, and Pathobiology. New York: Springer-Verlag; 1989:80–95.
- 2.Jaffe NS. Complications of acute posterior vitreous detachment. Arch Ophthalmol. 1968;79:568–571. doi: 10.1001/archopht.1968.03850040570012. [DOI] [PubMed] [Google Scholar]
- 3.Foos RY, Wheeler NC. Vitreoretinal juncture: synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89:1502–1512. doi: 10.1016/s0161-6420(82)34610-2. [DOI] [PubMed] [Google Scholar]
- 4.Eisner G. Posterior vitreous detachment. Klin Monatsbl Augenheilkd. 1989;194:389–392. doi: 10.1055/s-2008-1046391. [DOI] [PubMed] [Google Scholar]
- 5.Balazs EA, Denlinger JL. Aging changes in the vitreous. In: Sekuler R, Kline D, Dismukes K, eds. Aging and Human Visual Function. New York: Liss; 1982:45–57.
- 6.Lindner B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol. 1966;87(suppl):1–108. [Google Scholar]
- 7.Wilkinson CP, Rice TA. Michels Retinal Detachment. 2nd ed. St Louis: Mosby; 1990:30–34.
- 8.Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4th ed. St Louis: Mosby; 1997:904–951.
- 9.Novak MA, Welch RB. Complications of acute symptomatic posterior vitreous detachment. Am J Ophthalmol. 1984;97:308–314. doi: 10.1016/0002-9394(84)90628-7. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe NS. Vitreous traction at the posterior pole of the fundus due to alternations in the vitreous posterior. Trans Am Acad Ophthalmol Otolaryngol. 1967;71:642–652. [PubMed] [Google Scholar]
- 11.Smiddy WE. Vitreomacular traction syndrome. In: Yanoff M, Duker JS, eds. Ophthalmology. 2nd ed. St Louis: Mosby; 2004:951–955.
- 12.Sebag J. The Vitreous: Structure, Function, and Pathobiology. New York: Springer-Verlag; 1989:17–58.
- 13.Larsson L, Osterlin S. Posterior vitreous detachment: a combined clinical and physiochemical study. Graefes Arch Clin Exp Ophthalmol. 1985;223:92–95. doi: 10.1007/BF02150952. [DOI] [PubMed] [Google Scholar]
- 14.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 15.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 16.Los LI, van der Worp RJ, van Luyn MJA, et al. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44:2828–2833. doi: 10.1167/iovs.02-0588. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa S, Tamai M. Ultrastructure of hyaluronic acid and collagen in the human vitreous. Curr Eye Res. 1996;15:37–43. doi: 10.3109/02713689609017609. [DOI] [PubMed] [Google Scholar]
- 18.Snead MP, Snead DRJ, Richards AJ, et al. Clinical, histological and ultrastructural studies of the posterior hyaloid membrane. Eye. 2002;16:447–453. doi: 10.1038/sj.eye.6700198. [DOI] [PubMed] [Google Scholar]
- 19.Scott JE. The chemical morphology of the vitreous. Eye. 1992;6:553–555. doi: 10.1038/eye.1992.120. [DOI] [PubMed] [Google Scholar]
- 20.Foos RY. Vitreoretinal juncture; topographical variations. Invest Ophthalmol. 1972;11:801–808. [PubMed] [Google Scholar]
- 21.Heegaard S. Structure of the human vitreoretinal border region. Ophthalmologica. 1994;208:82–91. doi: 10.1159/000310458. [DOI] [PubMed] [Google Scholar]
- 22.Sebag J. Age-related differences in the human vitreoretinal interface. Arch Ophthalmol. 1991;109:966–971. doi: 10.1001/archopht.1991.01080070078039. [DOI] [PubMed] [Google Scholar]
- 23.Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9:253–260. doi: 10.1007/BF00137539. [DOI] [PubMed] [Google Scholar]
- 24.Nork TM, Gioia VM, Hobson RR, Kessel RH. Subhyaloid hemorrhage illustrating a mechanism of macular hole formation. Arch Ophthalmol. 1991;109:884–885. doi: 10.1001/archopht.1991.01080060148045. [DOI] [PubMed] [Google Scholar]
- 25.Foos RY, Roth AM. Surface structure of the optic nerve head. 2. Vitreopapillary attachments and posterior vitreous detachment. Am J Ophthalmol. 1973;76:662–671. doi: 10.1016/0002-9394(73)90560-6. [DOI] [PubMed] [Google Scholar]
- 26.O’Malley P. The pattern of vitreous syneresis: a study of 800 autopsy eyes. In: Irvine AR, O’Malley C, eds. Advances in Vitreous Surgery. Springfield, Illinois: Charles C Thomas; 1976:17–33.
- 27.Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225:89–93. doi: 10.1007/BF02160337. [DOI] [PubMed] [Google Scholar]
- 28.Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108:979–982. doi: 10.1001/archopht.1990.01070090081044. [DOI] [PubMed] [Google Scholar]
- 29.Kishi S, Hagimura N, Shimizu K. The role of the premacular liquefied pocket and premacular vitreous cortex in idiopathic macular hole development. Am J Ophthalmol. 1996;122:622–628. doi: 10.1016/s0002-9394(14)70480-5. [DOI] [PubMed] [Google Scholar]
- 30.Spaide RF. Measurement of the posterior precortical vitreous pocket in fellow eyes with posterior vitreous detachment and macular holes. Retina. 2003;23:481–485. doi: 10.1097/00006982-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Sebag J, Balazs EA. Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci. 1989;30:1867–1871. [PubMed] [Google Scholar]
- 32.Ueno N, Sebag J, Hirokawa H, et al. Effects of visible-light irradiation on vitreous structure in the presence of a photosensitizer. Exp Eye Res. 1987;44:863–70. doi: 10.1016/s0014-4835(87)80048-9. [DOI] [PubMed] [Google Scholar]
- 33.Akiba J, Ueno N, Chakrabarti B. Mechanisms of photo-induced vitreous liquefaction. Curr Eye Res. 1994;13:505–512. doi: 10.3109/02713689408999882. [DOI] [PubMed] [Google Scholar]
- 34.Kakehashi A, Ueno N, Chakrabarti B. Molecular mechanisms of photochemically induced posterior vitreous detachment. Ophthalmic Res. 1994;26:51–59. doi: 10.1159/000267374. [DOI] [PubMed] [Google Scholar]
- 35.Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100:1384–1388. doi: 10.1016/s0161-6420(93)31471-5. [DOI] [PubMed] [Google Scholar]
- 36.Morita H, Funata M, Tokoro T. A clinical study of the development of posterior vitreous detachment in high myopia. Retina. 1995;15:117–124. doi: 10.1097/00006982-199515020-00005. [DOI] [PubMed] [Google Scholar]
- 37.Yonemoto J, Ideta H, Sasaki K, et al. The age of onset of posterior vitreous detachment. Graefes Arch Clin Exp Ophthalmol. 1994;232:67–70. doi: 10.1007/BF00171665. [DOI] [PubMed] [Google Scholar]
- 38.Hikichi T, Hirokawa H, Kado M, et al. Comparison of the prevalence of posterior vitreous detachment in whites and Japanese. Ophthalmic Surg. 1995;26:39–43. [PubMed] [Google Scholar]
- 39.Weber-Krause B, Eckardt C. Frequency of posterior vitreous detachment in the elderly. Ophthalmologe. 1997;94:619–623. doi: 10.1007/s003470050170. [DOI] [PubMed] [Google Scholar]
- 40.Perichon JY, Brasseur G, Uzzan J. Ultrasonographic study of posterior vitreous detachment in emmetropic eyes. J Fr Ophtalmol. 1993;16:538–544. [PubMed] [Google Scholar]
- 41.Sebag J. The Vitreous: Structure, Function, and Pathobiology. New York: Springer-Verlag; 1989:97–160.
- 42.Berman ER, Michaelson IC. The chemical composition of the human vitreous body as related to age and myopia. Exp Eye Res. 1964;89:9–15. doi: 10.1016/s0014-4835(64)80003-8. [DOI] [PubMed] [Google Scholar]
- 43.Maumenee IH. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol. 1979;88:432–449. doi: 10.1016/0002-9394(79)90645-7. [DOI] [PubMed] [Google Scholar]
- 44.Foos RY. Posterior vitreous detachment. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:480–497. [PubMed] [Google Scholar]
- 45.Hikichi T, Trempe CL. Ocular condition associated with posterior vitreous detachment in young patients. Ophthalmic Surg Lasers. 1996;27:782–786. [PubMed] [Google Scholar]
- 46.Kloti R. Experimental occlusion of retinal and ciliary vessels in owl monkeys. I. Technique and clinical observations of selective embolism of the central retinal artery system. Exp Eye Res. 1967;6:393–399. doi: 10.1016/s0014-4835(67)80014-9. [DOI] [PubMed] [Google Scholar]
- 47.Kakehashi A, Schepens CL, Trempe CL. Vitreomacular observations: I. Vitreomacular adhesion and hole in the premacular hyaloid. Ophthalmology. 1994;101:1515–1521. doi: 10.1016/s0161-6420(94)31140-7. [DOI] [PubMed] [Google Scholar]
- 48.Kishi S, Schimizu K. Oval defect in detached posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1994;118:451–456. doi: 10.1016/s0002-9394(14)75795-2. [DOI] [PubMed] [Google Scholar]
- 49.Hikichi T, Takahashi M, Trempe CL, et al. Relationship between premacular cortical vitreous defects and idiopathic premacular fibrosis. Retina. 1995;15:413–416. doi: 10.1097/00006982-199515050-00007. [DOI] [PubMed] [Google Scholar]
- 50.Kakehashi A, Kado M, Akiba J, et al. Variations of posterior vitreous detachment. Br J Ophthalmol. 1997;81:527–532. doi: 10.1136/bjo.81.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson MW, Van Newkirk MR, Meyer KA. Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol. 2001;119:215–222. [PubMed] [Google Scholar]
- 52.Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999;117:744–751. doi: 10.1001/archopht.117.6.744. [DOI] [PubMed] [Google Scholar]
- 53.Van Newkirk MR, Gass JDM, Callanan D, et al. Follow-up and ultrasonographic examination of patients with macular pseudo-operculum. Am J Ophthalmol. 1994;117:13–18. doi: 10.1016/s0002-9394(14)73009-0. [DOI] [PubMed] [Google Scholar]
- 54.Van Newkirk MR, Johnson MW, Hughes JR, et al. B-scan ultrasonographic findings in the stages of idiopathic macular hole. Trans Am Ophthalmol Soc. 2000;98:163–171. [PMC free article] [PubMed] [Google Scholar]
- 55.Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation. Ophthalmology. 2001;108:15–22. doi: 10.1016/s0161-6420(00)00519-4. [DOI] [PubMed] [Google Scholar]
- 56.Chauhan DS, Antcliff RJ, Rai PA, et al. Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol. 2000;118:32–38. doi: 10.1001/archopht.118.1.32. [DOI] [PubMed] [Google Scholar]
- 57.Tanner V, Chauhan DS, Jackson TL, et al. Optical coherence tomography of the vitreoretinal interface in macular hole formation. Br J Ophthalmol. 2001;85:1092–1097. doi: 10.1136/bjo.85.9.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori K, Abe T, Yoneya S. Dome-shaped detachment of premacular vitreous cortex in macular hole development. Ophthalmic Surg Lasers. 2000;31:203–209. [PubMed] [Google Scholar]
- 59.Dugel PU, Smiddy WE, Byrne SF, et al. Macular hole syndromes: echographic findings with clinical correlation. Ophthalmology. 1994;101:815–821. [PubMed] [Google Scholar]
- 60.Ito Y, Terasaki H, Suzuki T, et al. Mapping posterior vitreous detachment by optical coherence tomography in eyes with idiopathic macular hole. Am J Ophthalmol. 2003;135:351–355. doi: 10.1016/s0002-9394(02)01944-x. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Trempe CL, Schepens CL. Biomicroscopic evaluation and photography of posterior vitreous detachment. Arch Ophthalmol. 1980;98:665–668. doi: 10.1001/archopht.1980.01020030659002. [DOI] [PubMed] [Google Scholar]
- 62.Sebag J. Classifying posterior vitreous detachment: a new way to look at the invisible. Br J Ophthalmol. 1997;81:521. doi: 10.1136/bjo.81.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sebag J. Imaging vitreous. Eye. 2002;16:429–439. doi: 10.1038/sj.eye.6700201. [DOI] [PubMed] [Google Scholar]
- 64.Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–756. doi: 10.1016/s0161-6420(95)30959-1. [DOI] [PubMed] [Google Scholar]
- 65.Gallemore RP, Jumper JM, McCune BW, II, et al. Diagnosis of vitreoretinal adhesions in macular disease with optical coherence tomography. Retina. 2000;20:115–120. [PubMed] [Google Scholar]
- 66.Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–1479. doi: 10.1001/archopht.119.10.1475. [DOI] [PubMed] [Google Scholar]
- 67.Katz B, Hoyt WF. Intrapapillary and peripapillary hemorrhage in young patients with incomplete posterior vitreous detachment. Ophthalmology. 1995;102:349–354. doi: 10.1016/s0161-6420(95)31018-4. [DOI] [PubMed] [Google Scholar]
- 68.Gass JDM. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–759. doi: 10.1016/s0002-9394(14)72781-3. [DOI] [PubMed] [Google Scholar]
- 69.Kishi S, Takahashi H. Three-dimensional observations of developing macular holes. Am J Ophthalmol. 2000;130:65–75. doi: 10.1016/s0002-9394(00)00383-4. [DOI] [PubMed] [Google Scholar]
- 70.Johnson MW. Improvements in the understanding and treatment of macular hole. Curr Opin Ophthalmol. 2002;13:152–160. doi: 10.1097/00055735-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–177. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 72.Kaiser P, Riemann CD, Sears JE, et al. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol. 2001;131:44–49. doi: 10.1016/s0002-9394(00)00872-2. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi Y, Otani T, Kishi S. Resolution of diabetic cystoid macular edema associated with spontaneous vitreofoveal separation. Am J Ophthalmol. 2003;135:116–118. doi: 10.1016/s0002-9394(02)01855-x. [DOI] [PubMed] [Google Scholar]
- 74.Uchino E, Uemura A, Doi N, et al. Postsurgical evaluation of idiopathic vitreomacular traction syndrome by optical coherence tomography. Am J Ophthalmol. 2001;132:122–123. doi: 10.1016/s0002-9394(00)00957-0. [DOI] [PubMed] [Google Scholar]
- 75.Casuso LA, Scott IU, Flynn HW, et al. Long-term follow-up of unoperated macular holes. Ophthalmology. 2001;108:1150–1155. doi: 10.1016/s0161-6420(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 76.Benhamou N, Massin P, Haouchine B, et al. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133:794–800. doi: 10.1016/s0002-9394(02)01394-6. [DOI] [PubMed] [Google Scholar]
- 77.Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003;110:1702–1707. doi: 10.1016/S0161-6420(03)00714-0. [DOI] [PubMed] [Google Scholar]
- 78.Spaide RF, Wong D, Fisher Y, et al. Correlation of vitreous attachment and foveal deformation in early macular hole states. Am J Ophthalmol. 2002;133:226–229. doi: 10.1016/s0002-9394(01)01377-0. [DOI] [PubMed] [Google Scholar]
- 79.Azzolini C, Patelli F, Brancato R. Correlation between optical coherence tomography data and biomicroscopic interpretation of idiopathic macular hole. Am J Ophthalmol. 2001;132:348–355. doi: 10.1016/s0002-9394(01)01005-4. [DOI] [PubMed] [Google Scholar]
- 80.Wiznia RA. Reversibility of the early stages of idiopathic macular holes. Am J Ophthalmol. 1989;107:241–245. doi: 10.1016/0002-9394(89)90306-1. [DOI] [PubMed] [Google Scholar]
- 81.Spaide RE. Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina. 2000;20:587–590. doi: 10.1097/00006982-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Gass JDM. Idiopathic senile macular hole: its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 83.Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol. 2001;131:123–125. doi: 10.1016/s0002-9394(00)00660-7. [DOI] [PubMed] [Google Scholar]
- 84.Hikichi T, Fujio N, Akiba J, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104:473–478. doi: 10.1016/s0161-6420(97)30289-9. [DOI] [PubMed] [Google Scholar]
- 85.Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–1339. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 86.Lewis H, Abrams GW, Blumenkranz MS, et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–759. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 87.Harbour JW, Smiddy WE, Flynn HW, et al. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–413. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 88.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–186. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 89.Gandorfer A, Messmer EM, Ulbig MW, et al. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–133. [PubMed] [Google Scholar]
- 90.Jumper JM, Embabi SN, Toth CA, et al. Electron immunocytochemical analysis of posterior hyaloid associated with diabetic macular edema. Retina. 2000;20:63–68. doi: 10.1097/00006982-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Gaucher D, Tadayoni R, Erginay A, et al. Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am J Ophthalmol. 2005;139:807–813. doi: 10.1016/j.ajo.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 92.Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122:258–260. doi: 10.1016/s0002-9394(14)72018-5. [DOI] [PubMed] [Google Scholar]
- 93.Ikeda T, Sato K, Katano T, et al. Vitrectomy for cystoid macular oedema with attached posterior hyaloid in patients with diabetes. Br J Ophthalmol. 1999;83:12–14. doi: 10.1136/bjo.83.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kadonosono K, Itoh N, Ohno S. Perifoveal microcirculation before and after vitrectomy for diabetic cystoid macular edema. Am J Ophthalmol. 2000;130:740–744. doi: 10.1016/s0002-9394(00)00575-4. [DOI] [PubMed] [Google Scholar]
- 95.Otani T, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol. 2000;129:487–494. doi: 10.1016/s0002-9394(99)00409-2. [DOI] [PubMed] [Google Scholar]
- 96.Otani T, Kishi S. A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol. 2002;134:214–219. doi: 10.1016/s0002-9394(02)01548-9. [DOI] [PubMed] [Google Scholar]
- 97.Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132:369–377. doi: 10.1016/s0002-9394(01)01050-9. [DOI] [PubMed] [Google Scholar]
- 98.Terasaki H, Kojima T, Niwa H, et al. Changes in focal macular electroretinograms and foveal thickness after vitrectomy for diabetic macular edema. Invest Ophthalmol Vis Sci. 2003;44:4465–4472. doi: 10.1167/iovs.02-1313. [DOI] [PubMed] [Google Scholar]
- 99.Le Heij EC, Hendrikse F, Kessels AG, et al. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol. 2001;239:264–270. doi: 10.1007/s004170000251. [DOI] [PubMed] [Google Scholar]
- 100.Ikeda T, Sato K, Katano T, et al. Improved visual acuity following pars plana vitrectomy for diabetic cystoid macular edema and detached posterior hyaloid. Retina. 2000;20:220–222. doi: 10.1097/00006982-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 101.Yamamoto T, Hitani K, Tsukahara I, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol. 2003;135:14–19. doi: 10.1016/s0002-9394(02)01819-6. [DOI] [PubMed] [Google Scholar]
- 102.Hoerle S, Poestgens H, Schmidt J, et al. Effect of pars plana vitrectomy for proliferative diabetic vitreoretinopathy on preexisting diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol. 2002;240:197–201. doi: 10.1007/s00417-002-0432-8. [DOI] [PubMed] [Google Scholar]
- 103.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–476. doi: 10.1016/s0002-9394(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 104.Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135:338–342. doi: 10.1016/s0002-9394(02)01937-2. [DOI] [PubMed] [Google Scholar]
- 105.Ikuno Y, Tano Y. Early macular holes with retinoschisis in highly myopic eyes. Am J Ophthalmol. 2003;136:741–744. doi: 10.1016/s0002-9394(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 106.Kanda S, Uemura A, Sakamoto Y, et al. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136:177–180. doi: 10.1016/s0002-9394(03)00243-5. [DOI] [PubMed] [Google Scholar]
- 107.Reddy CV, Folk JC, Feist RM. Microholes of the macula. Arch Ophthalmol. 1996;114:413–416. doi: 10.1001/archopht.1996.01100130409007. [DOI] [PubMed] [Google Scholar]
- 108.Douglas RS, Duncan J, Brucker A, et al. Foveal spot: a report of thirteen patients. Retina. 2003;23:348–353. doi: 10.1097/00006982-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 109.Bellhorn MB, Friedman AH, Wise GN, et al. Ultrastructure and clinicopathologic correlation of idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1975;79:366–373. doi: 10.1016/0002-9394(75)90608-x. [DOI] [PubMed] [Google Scholar]
- 110.Hiscott PS, Grierson I, Trombetta CJ, et al. Retinal and epiretinal glia—an immunohistochemical study. Br J Ophthalmol. 1984;68:698–707. doi: 10.1136/bjo.68.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86:902–909. doi: 10.1136/bjo.86.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaffe NS. Macular retinopathy after separation of vitreoretinal adherence. Arch Ophthalmol. 1967;78:585–591. doi: 10.1001/archopht.1967.00980030587005. [DOI] [PubMed] [Google Scholar]
- 113.Wise GN. Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol. 1975;79:349–357. doi: 10.1016/0002-9394(75)90605-4. [DOI] [PubMed] [Google Scholar]
- 114.Wiznia RA. Posterior vitreous detachment and idiopathic preretinal macular gliosis. Am J Ophthalmol. 1986;102:196–198. doi: 10.1016/0002-9394(86)90144-3. [DOI] [PubMed] [Google Scholar]
- 115.Appiah AP, Hirose T, Kado M. A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol. 1988;106:533–535. doi: 10.1016/0002-9394(88)90581-8. [DOI] [PubMed] [Google Scholar]
- 116.Sidd RJ, Fine SL, Owens SL, et al. Idiopathic preretinal gliosis. Am J Ophthalmol. 1982;94:44–48. doi: 10.1016/0002-9394(82)90189-1. [DOI] [PubMed] [Google Scholar]
- 117.Hirokawa H, Jalkh AE, Takahashi M, et al. Role of the vitreous in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1986;101:166–169. doi: 10.1016/0002-9394(86)90589-1. [DOI] [PubMed] [Google Scholar]
- 118.Heilskov TW, Massicotte SJ, Folk JC. Epiretinal macular membranes in eyes with attached posterior cortical vitreous. Retina. 1996;16:279–284. doi: 10.1097/00006982-199616040-00001. [DOI] [PubMed] [Google Scholar]
- 119.Greven CM, Slusher MM, Weaver RG. Epiretinal membrane release and posterior vitreous detachment. Ophthalmology. 1988;95:902–905. doi: 10.1016/s0161-6420(88)33077-0. [DOI] [PubMed] [Google Scholar]
- 120.Shinoda K, Hirakata A, Hida T, et al. Ultrastructural and immunohistochemical findings in five patients with vitreomacular traction syndrome. Retina. 2000;20:289–293. doi: 10.1097/00006982-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Gastaud P, Bétis F, Rouhette H, et al. Ultrastructural findings of epimacular membrane and posterior hyaloid in vitreo-macular traction syndrome. J Fr Ophthalmol. 2000;23:587–593. [PubMed] [Google Scholar]
- 122.Campochiaro PA, Van Neil E, Vinores SA. Immunocytochemical labeling of cells in cortical vitreous from patients with premacular hole lesions. Arch Ophthalmol. 1992;110:371–377. doi: 10.1001/archopht.1992.01080150069031. [DOI] [PubMed] [Google Scholar]
- 123.Smiddy WE, Michels RG, Green WR. Morphology, pathology, and surgery of idiopathic vitreoretinal macular disorders. Retina. 1990;10:288–296. doi: 10.1097/00006982-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 124.Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1994;119:55–61. doi: 10.1016/s0002-9394(14)73813-9. [DOI] [PubMed] [Google Scholar]
- 125.Falcone PM. Vitreomacular traction syndrome confused with pseudophakic cystoid macular edema. Ophthalmic Surg Lasers. 1996;27:392–394. [PubMed] [Google Scholar]
- 126.Folk JC, Boldt HC, Keenum DG. Foveal cysts: a premacular hole condition associated with vitreous traction. Arch Ophthalmol. 1998;116:1177–1183. doi: 10.1001/archopht.116.9.1177. [DOI] [PubMed] [Google Scholar]
- 127.Gass JDM. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol. 1999;117:821–823. doi: 10.1001/archopht.117.6.821. [DOI] [PubMed] [Google Scholar]