Abstract
Purpose
It is proposed that the anterior optic nerve is specifically susceptible to microcirculatory compromise contributing to the development of glaucomatous optic neuropathy.
Methods
Ischemic optic neuropathy was induced by delivering endothelin-1 (ET-1) to the retrobulbar space in one eye of 12 primates for 6 to 12 months. Regional ganglion cell axonal sizes and densities were compared with the normal, contralateral eyes.
Results
Without changes of intraocular pressure, mean axonal density was significantly decreased in ET-1 eyes compared to controls (P = .03, paired t test). Two-way matched-pair analysis of variance showed a significant effect of ET-1 on overall axonal density (P < .0001). Among the animals with significant axonal loss, the mean axonal loss was 11.6%, and loss varied from 4% to 21%. Axonal loss was commonly localized within specific quadrants. Five animals were examined for preferential axonal size loss. As a group, there appears to be a tendency toward preferential large axonal loss, but the mean axonal loss of large and small axons did not meet significant differences (P = .1) However, examination of individual animals with significant loss shows significantly greater loss of large axons as compared to the small axons in three of the animals.
Conclusions
Chronic optic nerve ischemia causes demonstrable and localized damage of the optic nerve, without intraocular pressure elevation. There is preferential loss of large retinal ganglion cell axons in animals with significant axonal loss. Ischemia-induced focal axonal loss is similar to human glaucoma and may represent a differential regional vulnerability.
HYPOTHESIS AND INTRODUCTION
The anterior optic nerve appears to be specifically susceptible to microcirculatory compromise, contributing to the development of glaucomatous optic neuropathy (GON). We believe chronic ischemia of the optic nerve creates patterns of retinal ganglion cell axonal loss within the optic nerve similar to glaucomatous optic nerve damage. Regional optic nerve damage, seen clinically in the development of localized neuroretinal rim notches, nerve fiber layer defects, and visual function defects, is the hallmark of human glaucoma. A novel experimental animal model of chronic optic nerve ischemia is used to examine the pathological sequelae of long-term and low-grade neural ischemia.
Vascular abnormalities that could contribute to the development of GON have been investigated for more than 150 years. Although the crucial role of intraocular pressure (IOP) in many glaucoma patients is recognized, glaucoma is more productively viewed as an optic neuropathy caused by a variety of factors, including, but not limited to, increased IOP. In the present study, another likely causative or contributory factor to GON is examined: chronic optic nerve ischemia. A model of chronic optic nerve ischemia has been developed using the vasoactive peptide endothelin-1 (ET-1) to create low-grade vasoconstriction and decreased blood flow to the anterior optic nerve in an animal eye. Retinal ganglion cell loss and glaucoma-like optic nerve cupping have previously been documented with this model in the rabbit eye, and the present study examines the effects of chronic ischemia in the nonhuman primate eye.
In this thesis, postmortem histology is presented, including examination of regional patterns of retinal ganglion cell loss and determination of preferential large versus small axonal loss. These findings are compared to human GON, because of the similarity of human and nonhuman primate optic nerves. This unique set of experiments will provide insight into the potential relationship between optic nerve ischemia and GON.
DEFINITION OF GLAUCOMATOUS OPTIC NEUROPATHY
Glaucoma has traditionally been defined as a disorder of IOP, which, when sufficiently elevated, produces characteristic damage to the optic nerve.1,2 Thus, glaucoma has been viewed as a disorder of aqueous humor dynamics with the optic nerve as an innocent, but vulnerable, bystander. This definition likely oversimplifies the clinical situation. Some glaucoma patients never exhibit elevation of IOP above statistical norms, others exhibit only mild elevation, and still others continue to deteriorate despite lowering of IOP.3 Conversely, many individuals have elevated IOP but never develop GON.4 In fact, in the recently published Ocular Hypertension Treatment Study, more than 90% of individuals with elevated IOP, without previously documented GON, and not taking IOP-lowering therapy, failed to progress to glaucoma over a 5-year period.5 While recognizing the crucial role of IOP in many glaucoma patients, many have argued that glaucoma would be more productively viewed as a progressive optic neuropathy caused by a variety of factors, including, but not limited to, increased IOP.6 In support of this argument, marked differences exist among glaucoma patients throughout the world.7 In North America, the prevalence of both glaucoma and ocular hypertension increases with advancing age, and most glaucoma patients exhibit IOP above the statistically normal range. In contrast, in Japan, GON increases but ocular hypertension diminishes in frequency with advancing age. Japanese glaucoma patients over 60 years are more likely to exhibit statistically normal IOP than elevated IOP.8 Thus factors other than, or in concert with, IOP must play a vital causative role in many glaucoma patients.
GLAUCOMA AND SYSTEMIC VASCULAR DISEASE
Abnormalities in the circulation of blood to the anterior optic nerve have been cited by many investigators as potential causative factors in the development of GON.9,10 There are many clinical reasons to believe that microvascular factors are important. Clinical microvascular diseases, including systemic hypertension, peripheral vascular disease, and possibly diabetes mellitus, are associated with glaucomatous damage.11–13 Studies have shown a higher incidence of systemic vascular diseases, including systemic hypotension,9,11 nocturnal hypotension,14–16 and systemic hypertension,12,13 in glaucoma patients. Epidemiologically, systemic hypertension, particularly in its late stages, has been related to glaucoma.17 Disorders associated with vasospasm, such as migraine headache or cold hands and feet, are more commonly seen in patients with glaucoma, both with and without elevated IOP.18,19 Other studies of glaucoma patients have shown abnormalities in blood rheology with increased blood viscosity and reduced erythrocyte deformability.7,20–24
In addition to the above clinical associations, a variety of experimental and clinical observations demonstrate circulatory aberrations of the anterior optic nerve, the peripapillary region,25–29 the choroid,27,30,31 and the retrobulbar vasculature32–40 in eyes with GON.33,40–43 These apparent alterations in the circulation of the glaucomatous optic nerve may implicate dysfunction of vascular regulatory mechanisms. Further support for vascular dysregulation has been suggested by the finding of elevation of ET-1, a potent vasoconstricting peptide, in the plasma39,44–46 and aqueous humor47,48 of patients with glaucoma. In the optic nerve, evidence of decreased blood flow correlating with visual field damage has been reported in glaucoma patients.40,41,49–52 Many of these studies remain controversial, as the validity of the various hemodynamic measurement techniques remains under investigation, but the hypothesis that circulatory abnormalities are related to the development of GON is well supported by these clinical associations. However, as attractive as this hypothesis may be, ischemia has never actually been definitively demonstrated to cause, or even contribute to, GON.
Is optic nerve ischemia alone sufficient to cause glaucoma-like neuropathy? The vascular hypothesis of GON is based upon three critically important, but unproven, assumptions53: (1) optic nerve ischemia can directly cause or increase the susceptibility of the optic nerve to glaucomatous damage, (2) our current knowledge of the vascular anatomy and physiology of the anterior optic nerve allows us to identify the regional vascular beds of importance in the development of GON, and (3) currently available techniques provide reliable measurements to monitor these vascular beds and measure blood flow alterations. Experimental investigations examining each of these assumptions will allow a better understanding of the potential role of vascular abnormalities in the development of GON. This project is primarily focused on the first assumption, by investigating the direct effects of chronic ischemia on the anterior optic nerve in the nonhuman primate and comparing them to other models and human glaucoma.
INTRINSIC VERSUS EXTRINSIC FACTORS AND MODELLING GLAUCOMATOUS OPTIC NEUROPATHY
Glaucomatous optic nerve damage may derive from factors intrinsic to the optic nerve (such as alterations of the extracellular matrix composition and abnormalities of the microvasculature) or from factors extrinsic to the optic nerve (such as elevated IOP and alterations in systemic cardiovascular status). Intraocular pressure obviously plays a major role in the development of GON in many subjects, but some nerves are more sensitive to pressure than others.54 Research has suggested that elevated IOP directly damages the optic nerve by distorting the extracellular matrix, glia, and support structures. The extrinsic force of IOP on the optic nerve causes backward bowing of the laminar support tissues, distortion of the laminar plates, misalignment of the laminar pores, and nerve cell damage by direct mechanical compression or interruption of axoplasmic flow.55–57 These proposed mechanisms of pressure damage are largely derived from animal models of high IOP. Elevated IOP is easily measured and modeled in laboratory animals; thus the extrinsic, pressure-induced hypothesis of GON has been extensively studied.56 However, in most animal models with elevated IOP, the pressure levels are much higher than levels characteristically seen in human glaucoma. As noted above, evidence suggests that elevated IOP alone is insufficient to explain the development of glaucomatous nerve damage in all individuals. The extreme pressure levels produced in these models may, in fact, mislead our interpretations of the role of IOP in the development of GON.
The development of an alternative model of intrinsic optic nerve damage has proven more problematic. Microvascular abnormalities and insufficient optic nerve vascular perfusion may result in neural ischemia and axonal damage,6,49,58 but creation of a chronic model of optic nerve ischemia has previously been lacking. Models of acute ischemia are easily produced via ligation of selected vessels or elevation of IOP above arterial perfusion pressure. In that chronic open-angle glaucoma is typically a slowly progressive disease, a model of chronic optic nerve ischemia is likely more clinically applicable.
OPTIC NERVE VASCULAR ANATOMY AND PHYSIOLOGY
Many investigators have previously enhanced the understanding of the vascular anatomy and physiology of the anterior optic nerve. Most believe that the anterior optic nerve is the site of insult in glaucoma. The anterior optic nerve may be anatomically divided into four regions: the superficial nerve fiber layer, the prelaminar region, the laminar cribrosa, and the retrolaminar region. The optic nerve vascular supply varies depending on the region.10,59–65 The superficial nerve fiber layer is supplied principally from the arterioles in the adjacent retina, which derive from the central retinal artery. The central retinal artery usually does not contribute to either the prelaminar or laminar region, although it may contribute minimally to the retrolaminar region. The prelaminar, the laminar, and the retrolaminar regions are supplied by branches of the pial arteries and the short posterior ciliary arteries, both originating from the posterior ciliary arteries. Our knowledge of the vascular anatomy of the anterior optic nerve has been clarified in recent decades; however, precise understanding of the anatomic distribution of the fine capillary beds within individual eyes and the regulation of blood flow within these regions remains somewhat limited. Studies of vascular anatomy and physiology demonstrate that most of the anterior optic nerve is supplied by the posterior ciliary circulation, and further studies will enhance our ability to evaluate the potential contribution of ischemia to GON.
ISCHEMIA AND NEURAL DAMAGE
With advances in molecular and cellular biology, the understanding of the mechanisms that may lead to neural damage has been greatly expanded.66 Apoptosis (programmed cell death without necrosis) has been cited as a potential pathway of retinal ganglion cell death in both human glaucoma and experimental primate glaucoma associated with elevated IOP.67–69 Apoptosis is a cell death pathway in which a complex cascade of cellular events occurs, including nuclear clumping, DNA condensation, cellular shrinkage and involution, and, finally, macrophage engulfment without inflammation.70–73 As the genetic information necessary for apoptosis exists in neural cells (including the retinal ganglion cells), the question becomes, What is the initiating signal that leads to this terminal cascade of events? Potential initiating signals of apoptosis in central nervous system diseases include growth factor deprivation, excitotoxicity, oxygen free radical production, nitric oxide synthesis, and abnormalities of calcium metabolism. In the eye, growth factor deprivation of retinal ganglion cells (the “neurotrophic hypothesis”) results from blockade of retrograde transport at the lamina cribrosa, preventing growth factors from reaching their site of action in the cell body.74,75 Calcium and oxygen free radicals influence biologic pathways that can damage the optic nerve. Increased levels of intracellular calcium may damage neurons by stimulating catabolic enzymes or oxygen free radical production.76 Oxygen free radicals, such as superoxide ion and hydroperoxyl radical, have unpaired electrons that make them highly reactive.77 Following transient ischemia, these molecules are commonly liberated, resulting in “re-perfusion injury.” Oxygen free radicals preferentially damage the mostly unsaturated lipid cell membranes of neural tissues. Aberrant nitric oxide synthesis has also been demonstrated within the glaucomatous anterior optic nerve.78
Neural ischemia could initiate any or all of these apoptotic triggers. Ischemia of the anterior optic nerve results in a blockade of axoplasmic flow, which would prevent normal transport of agents such as growth factors. Ischemia stimulates excessive glutamate release. Ischemia also enhances oxygen free radical and nitric oxide production. As well, intracellular calcium levels increase following neural ischemia. A model of chronic optic nerve ischemia allows us to evaluate the consequences of ischemia and to compare these consequences to other forms of optic nerve perturbation. The model will allow the comparison of neuronal cell death patterns following various forms of insult.
ENDOTHELIAL FACTORS (ENDOTHELINS)
In recent years, the vascular endothelium has been identified as an active participant in regulation of vascular tone. Perhaps the greatest advances in endothelial physiology have been the discovery and characterization of endothelium-derived vasoactive factors. Endothelial cells detect changes in their mechanical, chemical, and humoral environment, process these signals, and respond with the synthesis of various modulators in order to maintain a delicate balance between vasoconstriction and vasodilation.79 The endothelium synthesizes several vasoactive agents that participate in the maintenance of vascular tone and regulation of optic nerve blood flow.80–84 Endothelial-derived vasomodulators include prostanoids, nitric oxide, endothelins, smooth-muscle hyperpolarization factors, and angiotensin. Utilizing this set of regulatory mechanisms, the perfusion of most vascular beds throughout the body, including the anterior optic nerve, is maintained relatively constant under normal conditions. However, disorders of any part of the system can result in altered perfusion and create regional ischemia.
An example of a potential regulatory failure is found in glaucoma patients with abnormally high systemic concentrations of E-1. Endothelins are a family of short-chain polypeptides that are among the most potent vasoconstrictors known and are present in the ocular vasculature. Endothelin-1 is a very potent vasoconstrictor, produced by vascular endothelial cells, which plays a role in the regulation of blood flow throughout the cardiovascular system. It may also directly regulate the blood flow within the optic nerve.85 The ocular circulation is particularly sensitive to changes in local ET-1 concentration, even at doses that do not affect systemic hemodynamics or flow velocity in the ophthalmic artery.86 In some individuals with primary open-angle glaucoma, higher-than-normal plasma and aqueous humor concentrations of E-1 have been observed.44,45,47,87 This high concentration of E-1 has been associated with reduced blood flow in the posterior ciliary arteries that supply the optic nerve vasculature.39 Normal peripheral vascular response to the E-1 is also altered in some individuals with glaucoma.88 Intravitreal injections of E-1 into the rabbit eye produce marked effects on the anterior ciliary circulation, as well as constriction of the retinal vasculature.89 With E-1 administration to the retrobulbar perineural space, localized constriction in the posterior ciliary arterial circulation has been produced, in both the rabbit and primate eye.90,91
REGIONAL OPTIC NERVE DAMAGE IN GLAUCOMA
In glaucoma, there appears to be a differential susceptibility of various regions within the optic nerves of individual eyes. In fact, GON is characterized by regional patterns of retinal ganglion cell death associated with loss of visual function. Whereas some recent studies show that the initial damage in human glaucoma may be diffuse, distinct patterns of retinal ganglion cell loss and the consequential localized loss of visual function remain hallmarks of human glaucoma.92 Localized visual field scotomata and isolated nerve fiber layer defects are commonly seen clinically in individuals with glaucoma. Preferential regional damage of the optic nerve in human glaucoma is seen clinically as the development of localized neuroretinal rim notches,93 nerve fiber layer thinning,94,95 disc hemorrhages,96 and visual function defects.97 Jonas and colleagues93 and Mok and coworkers98 showed that glaucomatous optic nerve loss occurs in all neuroretinal rim sectors of the optic nerve, but in early glaucoma the most pronounced loss is in the inferior-temporal rim. Preferential regional damage in glaucoma has traditionally been attributed to differential mechanical forces and explained by anatomic features at the level of the anterior lamina cribrosa.99–101 That is, in the superior and inferior regions, there is less connective tissue around lamina cribrosa pores, and the pores are large in size compared to the nasal and temporal regions.99,100 In addition, lamina cribrosa pores in the peripheral region are longer than the central pores.101 Quigley and coworkers102 have further demonstrated that large optic nerve fibers, especially in the superior and inferior quadrants, are more specifically susceptible to damage in human GON. Recent biomechanical modeling of posterior segment of the eye and optic nerve head suggests that disproportionate stress and strain forces occur within regions of the optic nerve and may contribute to regional patterns of axon loss.103 These physical forces may cause the axons passing through specific regions of the lamina cribrosa to be more vulnerable to mechanical stress from elevated IOP.
Interestingly, in clinical studies, focal damage of the optic nerve has also been attributed to ischemia (so-called focal ischemic GON).104, 105 In one large study, four patterns of GON were examined: (1) focal ischemic, (2) myopic tilted, (3) senile sclerotic, and (4) generalized cup enlargement. Focal ischemic GON was typically seen in older individuals, was highly associated with optic nerve hemorrhages, was more frequently associated with migraines, and was almost always associated with localized functional loss that often threatened fixation.104 Others have contended that focal ischemia is a clinical form of GON that occurs at all levels of IOP.105 Local defects in the circulation have also been found in regions of peripapillary atrophy (beta-zone atrophy),106 and zones of nonperfusion have been demonstrated with fluorescein angiography.52 Beta-zone peripapillary atrophy is associated with localized neuroretinal rim thinning (focal axonal loss) and progressive glaucomatous damage. In addition, even in normal human subjects, fluorescein filling rates are significantly different among the four quadrants of the optic disc.107 The existence of regional watershed zones has been suggested to be important in the development of regional damage in the human GON.60,108,109 Approximately 60% of these watershed zones pass through the temporal half of the optic nerve in eyes with glaucoma.110 Because the area within a watershed zone is comparatively less perfused, especially during hemodynamic stress, these regions are thought to be more vulnerable to ischemic insult. Human GON, especially in early disease, is characterized by regional patterns of retinal ganglion cell death, and various regions of the optic nerve are more susceptible to localized damage in high IOP models. These patterns of regional loss have been attributed to both localized vascular and mechanical perturbations.
DIFFERENTIAL LOSS OF LARGE VERSUS SMALL AXONS IN GLAUCOMA
As cited above, large optic nerve fibers, especially in the superior and inferior quadrants, may be more susceptible to damage in human GON.102 Although the gradual loss of retinal ganglion cells is a characteristic of pathological changes in glaucoma, it has been proposed that retinal ganglion cells with large axons are more vulnerable than retinal ganglion cells with small axons. This observation was first reported in an experimental primate model of glaucoma111 and later demonstrated in human glaucoma.102 Similarly, large retinal ganglion cell loss was observed in human glaucoma, particularly in the inferior retina.112 This corresponds to reports of decreased magnocellular input at the level of the lateral geniculate nucleus in human glaucoma, as compared to normals.113 Additional experimental evidence of selective large cell loss has been seen in beagle dogs with hereditary open-angle glaucoma, although regional differences existed within these optic nerves.114 Despite these findings, the notion that the retinal ganglion cells with large axons are more vulnerable to glaucomatous damage has become controversial. As more extensive examinations of the magnocellular and parvocellular components within the higher levels of the visual system115–117 are performed, the simple division between large and small optic nerve fibers becomes blurred. As well, the possibility remains that the apparent preferential loss of large axons in glaucoma may result from cellular shrinkage prior to the cell death.118 In addition, classification of retinal ganglion cells based only on the size of their axons, while anatomically achievable, may have limited physiological relevance. Nevertheless, to further compare the optic neuropathy that results from chronic ischemia to glaucoma, examination of selective loss of various populations of retinal ganglion cells may be important.
PREVIOUS INVESTIGATIONS: MODEL OF CHRONIC OPTIC NERVE ISCHEMIA
A reproducible, in vivo model of chronic optic nerve ischemia was developed in the rabbit eye and later extended to the nonhuman primate eye, using ET-1 delivered to the optic nerve vasculature via osmotically driven minipumps. ET-1 is delivered to retrobulbar space adjacent to the optic nerve with this constant and controlled delivery system (see “Methods” section). The aim of the model is to study the effect of a single potential risk factor for GON. Without other confounding factors, such as elevated IOP, chronic optic nerve ischemia will be investigated. In addition, the model will determine if ischemia in isolation is sufficient to cause GON or render the optic nerve particularly susceptible to GON. As detailed above, the primary vascular supply to the anterior optic nerve is via the posterior ciliary arterial system. Therefore, vasoconstriction of the posterior ciliary arteries and their branches would result in decreased perfusion of the anterior optic nerve. In the model, decreased optic nerve blood flow has been previously documented with intravascular microsphere techniques, and focal vasoconstrictions of the ciliary arterial system were demonstrated using intraluminal corrosion castings. The dose-response characteristics for ET-1 were determined in the rabbit eye, and further experimentation has been done in the nonhuman primate eye. Neuronal death and even morphologic optic nerve cupping were seen in the rabbit. A spectrum of new and old technologies is used for evaluation of the primate eye during the experiments (ophthalmoscopy, tonometry, optic nerve photography, scanning laser ophthalmoscopy, laser doppler flowmetry, 3-D microsphere blood flow measurements, multifocal electroretinogram, optic nerve histology, and immunohistochemistry). The following is a brief summary of previously published observations in the rabbit and primate model, which have led to the present line of investigations.
Rabbit Model
The model of chronic optic nerve ischemia continuously delivers ET-1 to the perineural region of the optic nerve in the retrobulbar space and has been described in rabbits,91 rats,119 and nonhuman primates.120 The arterial supply of the anterior optic nerve in the rabbit is anatomically similar to the primate eye and demonstrates a dose-dependent vasoconstriction with chronic, local application of ET-1. Optic nerve blood flow was measured with microsphere blood flow determinations in two normal rabbits, five rabbits receiving ET-1 (daily dose: 0.1 mg), and two rabbits with saline sham pumps for 2 weeks. Blood flow was reduced approximately 38% (37.66% ± 7.22) in the ET-1 exposed optic nerves compared to the normal and sham optic nerves.120,121 To ascertain that consistent reduction of anterior optic nerve blood flow occurred over extended periods of time with ET-1 delivery, blood flow measurements were performed at various time intervals. In four rabbits at 1 week, 41.5% blood flow reduction was observed; in five rabbits at 2 weeks, 38.0% reduction was observed; in four rabbits at 4 weeks, 41.5 % reduction was observed; and in five rabbits at 8 weeks, 49.0% reduction was observed. These data show that significant tachyphylaxis does not occur in this model and that the blood flow reduction can be maintained for extended periods. In addition, in four rabbits receiving ET-1 (0.1 gm/day) for 2 months, there was a significant increase of the cup volume (Heidelberg Retinal Tomography, HRT, volume below surface, Heidelberg, Germany). Average cup volumes increased from a baseline of 1.08 mm3 to 1.27 mm3 at 1 month and 1.33 mm3 at 2 months in ET-1 eyes (analysis of variance [ANOVA]: P = .0064). There was no change of the cup size in the fellow eyes (ANOVA: P = .6). Histological examination of the optic nerves was performed following 60 days of ET-1 delivery, and there were no signs of acute infarction, such as coagulative necrosis, cystoid spaces, or proliferation of leukocytes or mononuclear cells. Among the ET-1 treated optic nerves, there was a suggestion of increased number of retinal ganglion cells with small, darkened nuclei (a potential sign of apoptotic cell death) as compared to the fellow eyes. Within the anterior optic nerve, there was loss of myelin and a decrease of axons. However, the extensive myelination of the rabbit anterior optic nerve and ultrastructural differences to the primate optic nerve limit the significance of further histological comparisons. In summary, in the rabbit model, a consistent reduction of blood flow to the anterior optic nerve of approximately 38% was achieved, resulting in loss of optic nerve axons, loss of neuroretinal tissues of the optic nerve, and enlargement of the optic nerve cup.
Primate Model
In the nonhuman primate eye (cynomolgus and rhesus monkey), ET-1 dosages between 0.2 and 0.34 mg per day produced marked vasoconstriction of the anterior optic nerve vasculature as compared to the fellow, untreated eye. In contrast, balanced saline solution delivered via the minipumps to the perineural optic nerve had no effect on the microvasculature. The effects of ET-1 and decreased perfusion in the primate eye were initially evaluated in six rhesus monkeys implanted with minipumps (four ET-1, daily dose: 0.2 mg, and two sham pumps) for a duration of 1 week.120 Average optic nerve blood flow values (mL/mg/min) were 0.53 in the sham pump eyes, 0.37 in the ET-1 treated eyes, and 0.57 in the untreated eyes. This represents an average reduction of 35% of the optic nerve blood flow in the ET-1 group, with infusion of 0.34 mg per day. The identical experiment was performed in two monkeys for 1 month with a 38% blood flow decrease. The blood flow in the sham procedure group was not significantly different from that in the untreated eyes. The normal optic nerve blood flow estimates in rhesus monkeys correlates well with our previous estimates in cynomolgus monkeys of 0.56 mL/mg/min. Therefore, similar to the rabbit model, the nonhuman primate model (both cynomologus and rhesus) showed approximately 35% to 38% blood flow reductions to the anterior optic nerve120 and significant loss of optic nerve axons after 3 to 6 months of continuous ischemia.122,123 Since nonhuman primates have a vascular architecture and tissue properties within the anterior optic nerve that are very similar to the human eye,124,125 primate models are often used to provide meaningful comparisons to human glaucoma.
Diffuse Versus Focal Retinal Ganglion Cell Axonal Loss
Very early in our investigations of primates, it was realized that retinal ganglion cell axonal loss was not uniform throughout the optic nerve and the effects of chronic ischemia of the anterior optic nerve were not limited to the retinal ganglion cell axons (Figure 1). Effects were also noted in the extracellular matrix and in the glial tissues of the optic nerve. It was realized that formal evaluation of the primate optic nerve for patterns of axonal loss was complex. Because the primate optic nerve contains between 800,000 and 1,500,000 retinal ganglion cell axons, counting all of the optic nerve fibers is time-consuming and impractical. Therefore, estimates have traditionally been based on partial counts, which are extrapolated. To detect early loss of axons and to compare optic nerves for regional loss, estimates of axonal numbers need to both be accurate and have high reproducibility. Therefore, a semiautomated system of estimating the number of axons in an optic nerve was developed (see below, “Estimating Axon Densities”).
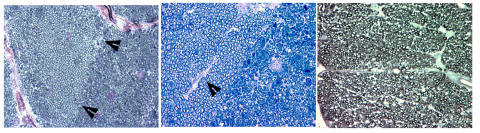
FIGURE 1.
Microphotographs of cross-sections from three different nonhuman primate optic nerves following 6 months of endothelin-1 (ET-1) induced ischemia. All three photographs show localized or nonuniform patterns of axonal loss. Left, boundary (arrows) between an area of relative axonal preservation and severe axonal damage. Middle, region of axonal preservation surrounding a septum (arrow) within the optic nerve, which contains a capillary. Right, nonuniform axonal loss with highly variable sizes of the remaining axons (left and middle, toluidine blue stain; right, phenylenediamine, ×100).
Figure 1 shows examples of axonal loss in the primate model following 6 months of ET-1 induced optic nerve ischemia. In these cross-sectional views of the retrobulbar optic nerves from three different animals, note the boundaries between regions with relatively preserved axons and severely damaged or absent axonal fibers. The recognition of this differential axonal susceptibility to ischemia led to the detailed evaluations of the patterns of axonal loss presented in this study. Since distinct patterns of regional retinal ganglion cell loss and the consequential localized loss of visual function are one of the hallmarks of human glaucoma,92 detailed regional measures were created.
Estimating Retinal Ganlion Cell Axon Densities in the Primate Optic Nerve
As discussed above, a variety of methods have been used to evaluate optic nerves and to determine the number of axons within the optic nerve in rats, dogs, monkeys, and human eyes.114,126–130 On account of the large number of axons (optic nerve fibers), often more than one million in the primate optic nerve (nonhuman and human), accurately estimating the number of axons is complex. A number of studies have estimated the total number of axons and axon density within the optic nerve by using a small random sample of between 3% and 5% of the total number of axons within the optic nerve.55,126,128 These studies have shown poor reproducibility as well as marked differences in the estimation of total numbers of axons within the optic nerve. This variability among counting techniques has led to a reexamination of this issue. Using an image analysis system, a novel sampling method for estimating the optic nerve counts within the optic nerve was developed (see “Methods” section).131 In this study, the axons from normal, nonhuman primates were counted, and methods to enhance the reproducibility of the estimation of the total number of axons were developed. As well, guidelines for minimizing the variability of axon estimates were established. This ultimately allows better statistical comparison between normal and damaged optic nerves. In this study, cross-sectional images of five optic nerves from five adult rhesus monkeys were analysed with an imaging system. The sections were each 1 mm thick, and the axon areas and x-y coordinates were recorded. The total axon number in each nerve was estimated by counting 1%, 2%, 3%, 4%, 5%, 10%, 20%, 40%, and 50% of the total optic nerve area. A Monte Carlo estimate and theoretical estimate of the standard deviation (SD) at each sampled area were computed. A SD as a percentage of the mean from the Monte Carlo estimate to the total axon count was calculated. The average number of axons for the five primate optic nerves was 1,320,438 ± 94,663 fibers. When sampling 5% or greater of the optic nerve, a SD as a percentage of the mean of the total number of axons is less than 1.4%.
In these five normal primate optic nerves, axon estimates of between 1.1 and 1.4 million are similar to what has been reported in previous studies in the macaque monkey.55,126,128 Some earlier investigations of optic nerve axon counts had axon number estimations as high as 1.7 million fibers127 and were probably overestimations. Potts and associates127 counted 100% of the optic nerve fibers of three monkeys using an early imaging system and obtained 1.5 to 1.8 million optic nerve fibers. However, they did not have an observer remove objects that may have been wrongly identified as axons by automated counts. This may explain the higher numbers that were reported. Ogden and Miller128 counted 4.3% of the nerve fiber area of only one monkey, resulting in an estimation of 1.4 million fibers in the optic nerve. Our novel technique for estimating axon numbers has shown that sampling a 5% or greater area of the optic nerve truly reflects the total number of axons within the normal optic nerve of the primate.131 Comparisons of the variability and accuracy of axonal estimates in our analyses have led to the recommendation that axons in at least 8% of the optic nerve area be counted in normal eyes and 20% in damaged eyes to allow detection of regional loss patterns in the primate eye.131
Patterns of Retinal Ganglion Cell Axonal Loss
The present study examines the susceptibility of the optic nerve retinal ganglion cell axons to chronic ischemia in the nonhuman primate eye. Histological assessment of the axonal loss within the optic nerve is compared between eyes with chronic ischemia from ET-1 treatment and contralateral control eyes.132 Further, differences in the axonal density in various subregions of the optic nerve are examined following experimental chronic ischemia, to detect regional susceptibility of the retinal ganglion cell axons.
METHODS
ET-1 INDUCED OPTIC NERVE ISCHEMIA MODEL (OSMOTIC MINIPUMP IMPLANTATION)
Twelve adult female rhesus (Macaca mulatta) monkeys, weighing 4 to 8 kg and between the ages of 8 and 19 years, were used in accordance with the Association for Research in Vision and Ophthalmology statement on the use of animals in ophthalmic and vision research. At the beginning of the study, animals had a complete eye examination, which included slit-lamp examination, IOP measurements, and dilated funduscopic examination followed by stereoscopic optic nerve photography. For these examinations, animals were sedated with ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, Iowa) administered intramuscularly (15 mg/kg) and intubated. Anesthesia of the animals was achieved with inhalation of 3% isofluorane in oxygen. Blood oxygen saturation and pulse rate were monitored via peripheral oxymetry, and core body temperature was maintained with a heating pad.
The surgical procedure for the implantation of the minipumps in primates has been described previously.91 In brief, an osmotic minipump (Alza Corp, Mountain View, California) preloaded with approximately 250 μL ET-1 (Peptides International, Louisville, Kentucky) solution (55 μM/mL) was implanted subcutaneously superior to the lateral rim of the orbit of the right eye. The outlet of the pump was connected to one end of a silicone delivery tube. The tube was passed subcutaneously into the superior-temporal orbit and under the bulbar conjunctiva and Tenon’s capsule. The tube was placed beneath the superior rectus muscle, and the end of the tube was secured in the retrobulbar space adjacent to the superior-nasal aspect of the optic nerve. The tube was fixed in place using a 9-0 nylon suture through the sclera. The minipump continually delivered the ET-1 solution to the retrobulbar region at a dose of 0.34 μg per day with a constant flow rate (6 μL/day) over 4 weeks. At the end of each 4-week period, the minipump was replaced with a new ET-1 loaded pump at the original site of placement, and the patency of the tube was ensured by irrigation of normal saline solution prior to the placement of the new pump. Tubes with a blocked opening due to scar tissue growth were replaced. Otherwise, the tube was left in place and the new pump attached. Sham implantation of minipumps loaded with vehicle without ET-1 demonstrated no short-term effect on blood flow and other parameters examined in a previous report.120,133 An additional sham animal was included in the present study, but unfortunately it died of unrelated causes 3 months following pump implantation, and tissue was not available for analysis. The contralateral eyes of all 12 animals served as the controls. In addition, both optic nerves of a control animal with normal eyes (no pump implantation and normal ocular examination) were analyzed to assess the normal intereye variability of axonal density.
The duration of ET-1 induced ischemia was approximately 6 months (23 ± 2 weeks) in eight monkeys, 9 months (38 weeks) in one monkey, and 12 months (52 ± 1 weeks) in three monkeys (Table). During this period, a variety of in vivo measurements were made in each animal at baseline prior to pump implantation and bimonthly during the study. These tests included IOP measurements in both eyes measured with tonometery (Tonopen; Mentor, Inc, Norwell, Massachusetts) immediately after the animals were anesthetized with ketamine. Three measurements were made for each eye, and an average IOP was recorded. Optic nerve appearance was monitored with stereoscopic optic nerve photography (3-DX; Nidek Co Ltd, Japan). Photographs of the optic nerves were evaluated in a masked fashion for indications of acute anterior ischemic optic neuropathy, such as focal or global pallor, edema, or hemorrhages.
TABLE.
AGE, DURATION OF ENDOTHELIN-1 (ET-1) TREATMENT, AND OVERALL AXON DENSITY STATISTICAL ANALYSIS (ANOVA) FOR EACH ANIMAL
|
AXONAL DENSITY (mm2) |
||||||
|---|---|---|---|---|---|---|
| MONKEY | AGE (YR) | DURATION OF ET-1 TREATMENT (mo) | ET-treated | Control | RELATIVE DENSITY (ET:CONTROL) | PVALUE |
| 1 | 17 | 6 | 188329 | 237385 | 0.79 | <.001 |
| 2 | 18 | 6 | 177837 | 219066 | 0.81 | <.001 |
| 3 | 18 | 6 | 216970 | 203618 | 1.07 | <.01 |
| 4 | 19 | 6 | 188463 | 170622 | 1.10 | <.001 |
| 5 | 8 | 6 | 196162 | 187568 | 1.05 | >.05 (ns) |
| 6 | 10 | 6 | 199160 | 215628 | 0.92 | <.001 |
| 7 | 10 | 6 | 204615 | 200046 | 1.02 | >.05 (ns) |
| 8 | 9 | 6 | 147487 | 174783 | 0.84 | <.001 |
| 9 | 15 | 9 | 233631 | 247583 | 0.94 | <.01 |
| 10 | 17 | 12 | 274855 | 286825 | 0.96 | <.05 |
| 11 | 11 | 12 | 240969 | 264584 | 0.91 | <.001 |
| 12 | 10 | 12 | 231248 | 240227 | 0.96 | >.05 (ns) |
| Mean (SD) | 13.5 (4.2) | 7.75 (2.7) | 208310 (33579) | 220661 (35855) | 0.95 (0.10) | P = .03 |
Test of ET-1 Stability in the Osmotic Minipumps
To test the chemical stability of ET-1 in the minipump during the 4-week implantation cycles, four minipumps were preloaded with the same ET-1 solution as described above and placed in saline solution at temperature of 37°C for 1, 2, 3 and 4 weeks, respectively. At the end of each time point, the ET-1 solution was withdrawn from the pumps. A sample of 5 μL was obtained from each minipump and compared to a freshly prepared control ET-1 solution (three tests for each sample). With use of high-performance liquid chromatography (HP 1100, Hewlett Packard), the test samples and control samples were analyzed. Compared to the control sample, the percentages of ET-1 detected in the samples at the end of 1, 2, 3, and 4 weeks were 110%, 83%, 83%, and 74%, respectively.
Optic Nerve Tissue Sampling and Staining
At the end of the experiments, the animals were euthanized with an intravenous injection of pentobarbital, 100 mg/kg (Euthasol; Delmarva Laboratories, Inc, Midlothian, Virginia). Perfusion fixation was accomplished with 4% formaldehyde via the carotid arteries. Both eyes were immediately enucleated and fixed in 4% formaldehyde for an additional 2 to 3 hours. The retrobulbar optic nerves were transected (approximately 2 to 3 mm behind the globe), and a 0.5-mm-thick section was obtained and processed for resin sectioning. All of the optic nerve tissue sections were fixed in 5% glutaraldehyde in phosphate buffer (pH 7.4) for 1 hour and then postfixed in 2% osmium tetroxide for 3.5 hours. The tissue was rinsed, dehydrated in an ethanol-acetone series, and embedded in Epon 812. Semithin sections (1 μm) were cut and mounted onto glass slides. The adjacent stump of each optic nerve still attached to the globe immediately opposite to the cut surface was marked with colored tissue dye (tissue marking dyes; Triangle Biomedical Sciences, Inc, Durham North Carolina) to preserve the orientation in situ for later anatomical orientation of the each section.
The sections on the slides were stained with 1% phenylenediamine (in 1:1 methanol/isopropanol) for 20 minutes, rinsed two or three times with isopropanol, and air-dried. If staining was incomplete with phenylenediamine at a temperature of approximately 80°C, adjacent sections were stained with 1% toluidine blue in the phosphate buffer (pH 7.0 to 7.4) for 3 minutes followed by adding a few drops of Sorensen’s buffer for another 2 minutes. The slides were rinsed with distilled water and air-dried. The orientation of the optic nerve sections was determined by matching certain landmarks (such as blood vessels and contour lines of axonal bundles) of the sections with the adjacent optic nerve stumps from where the sections were cut.
Optic Nerve Retinal Ganglion Cell Axonal Density Estimates
Our methods of retinal ganglion cell axonal density quantification are described in a previous publication.131 In brief, with an image analysis system (Bioquant; R&M Biometrics, Inc, Nashville, Tennessee), a composite of the entire area of the optic nerve cross-section was created. The area was then divided with a grid such that each square of the grid had a size of 2,025 μm2 (45 μm × 45 μm). Eight percent of the grid-squares in normal controls and 20% in ET-1 treated optic nerves were randomly selected, and all of the axons within each of the randomly selected grid-squares were counted using the image analysis system. These percentages of the total area to be counted were chosen to minimize variance while maximizing efficiency.131 The density of axons in each of the grid-squares was calculated by dividing the number of axons by the area of the grid-square, and the density of each region was estimated by averaging the densities of all of the counted grid-squares within the region of interest. This technique allowed for the variance to be calculated for the mean axonal densities in each region and for statistical comparison of the individual regions. In addition, the area of the entire optic nerve was automatically calculated by the Bioquant system by thresholding the outer edges of the optic nerve sections. This was done to assess for swelling or shrinkage of the optic nerves and its effect on density calculations.
COMPARISON OF LARGE VERSUS SMALL RETINAL GANGLION CELL AXONAL LOSS
To determine if preferential loss of large or small retinal ganglion cell axons occurs in the model of chronic ischemia, the size and numbers of axons were calculated and compared between the ET-1 treated and control eyes. Accurate determinations of the axon sizes depend upon uniform staining of the entire optic nerve cross-section from both eyes. Whereas the total axon numbers can be accurately estimated in eyes with nonuniform staining, the automated determination of axonal sizes depends on a thresholding algorithm that incorrectly underestimates axonal size in lightly stained sections and overestimates axonal size in darkly stained sections. Five animals (M1, M4, M5, M7, and M11) had uniformity of staining intensity of both eyes and were therefore included in this analysis. An arbitrary dichotomy between large and small axons was determined a priori. The axons within the largest 20th percentile of the axon size distributions for the normal control eyes of each pair were defined as “large” axons. The remaining axons were defined as “small” axons. This method was used to compare pairs of eyes from individual animals, as well as for group comparison of the entire population (n = 5). Since the total area counted in the normal (8%) and the ET-1 treated (20%) optic nerves is different, the axonal frequencies for the two optic nerve populations were normalized for comparison.
STATISTICAL ANALYSIS
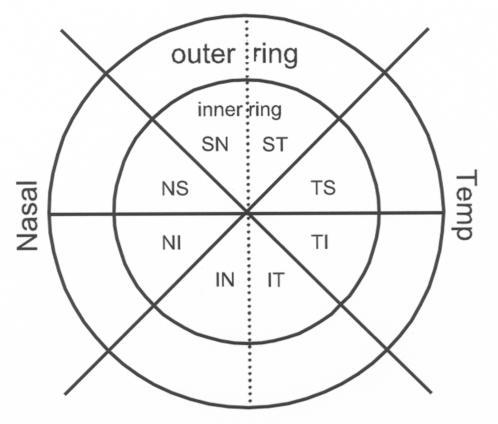
For the purpose of statistical analysis and assessment of regional axonal loss, the optic nerves were divided into 16 subregions consisting of an inner and outer segment within each of eight wedge-shaped, radial sectors, as shown in Figure 2 (ST, TS, TI, IT, IN, NI, NS, SN; T = temporal, N = nasal, S = superior, I = inferior). All 16 subregions had approximately equal areas. The axonal density for each region was calculated as the average of all grid-square samples within the region. Similarly, the total overall axonal density for an entire optic nerve was calculated as the average of all density samples (ie, all counted grid-squares) for each optic nerve.
FIGURE 2.
Schematic showing the topography of the regional divisions on an optic nerve cross-section. A central point was determined by an image analysis system. Through the central point, eight subregions were divided. In addition, each of the subregions was further divided into inner and outer portions with approximately equal-sized areas.
The effect of ET-1 induced chronic ischemia was evaluated by comparison of ET-1 treated and control eye group averages for overall axonal density using a paired t test (n = 12 pairs). Two-way, matched-pair ANOVA (treatment × animal) was used to further evaluate the effect of ET-1 treatment on overall axonal density while controlling for variation due to differences between individual animals. Bonferroni post hoc tests were used to evaluate the significance of ET-1 effects for individual animals (pairs of eyes). Potential regional effects of ET-1 induced ischemia were first evaluated across the whole group (n = 12) using two-way, matched-pair ANOVA (treatment × region). Regional effects of ET-1 treatment were further explored within individual animals using a similar two-way matched-pair ANOVA (treatment × region). This analysis was possible because the variance of each observation (mean of each optic nerve region) could be calculated based on the numerous samples obtained (grid-squares counted) in each optic nerve region. For this regional analysis of each individual animal, the effect of ET-1 treatment was considered significant if P < .0042; thus, α was adjusted for 12 comparisons. If there was also a significant interaction between ET-1 treatment and optic nerve region, Bonferroni post hoc tests were used to evaluate the significance of axonal density differences among individual pairs of regions (ET-1 versus control eye). Finally, the optic nerve areas were compared using a paired t test, to assess for optic nerve swelling or shrinkage and preferential loss. Intraocular pressure measurements at baseline, the midpoint, and the end of the experiment were analyzed with repeated measures ANOVA.
RESULTS
Intraocular Pressure
The average IOP (±SD) for the 12 ET-1 treated eyes at baseline (pretreatment) was 15.5 (±4.3) mm Hg. At the midpoint of each animal’s experimental period, the average IOP in ET-1 treated eyes was 17.1 (±3.0) mm Hg. At the final examination in the ET-1 treated eyes, the IOP was 14.9 (±4.5) mm Hg. The average IOP in the group of contralateral control eyes was 15.1 (±3.6) mm Hg at baseline, 16.7 (±3.8) mm Hg during the experimental period, and 14.6 (±4.0) mm Hg at the final examination. There was no significant difference between the IOPs in the control and ET-1 treated eyes (P = .61). Likewise, the IOP did not differ significantly between baseline and final measurements in either the control eyes or the ET-1 treated eyes. (P > .05, ANOVA).
In Vivo Optic Nerve Appearance
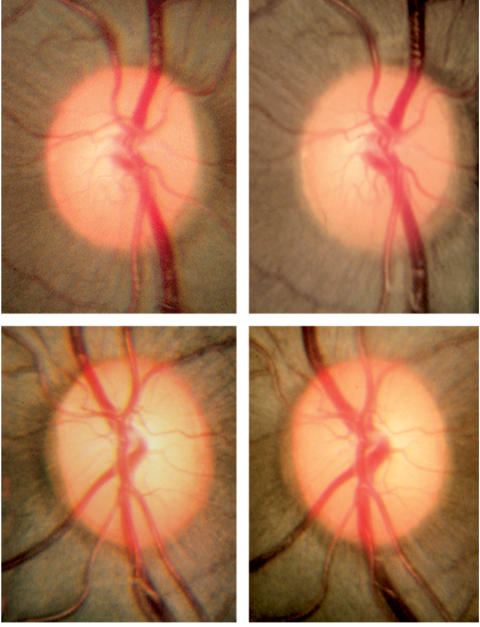
The stereophotographs of the optic nerves were examined and compared between baseline and subsequent examinations in a masked fashion. There was no evidence of pallor, edema, telangiectasias, or other changes commonly associated with anterior ischemic optic neuropathy in any of the photos (Figure 3). On account of the relatively small numbers of animals and the high variability associated with clinical assessment of stereoscopic optic nerve photographs for progressive change, we have not included such an analysis in this report. However, automated optic nerve head analysis (Heidelberg retinal tomographs; Heidelberg Engineering, Heidelberg, Germany) was performed and is presently being analyzed.
FIGURE 3.
Photomicrographs of the endothelin-1 treated (top panels) and contralateral control (bottom panels) optic nerves in one monkey (monkey 8) taken at baseline and at the end of 6 months (final, top right and bottom right panels) of ischemia. This monkey has 16% axonal loss of the entire optic nerve, with the most severe loss in the nasal and superior nasal regions. There is no evidence of diffuse or focal pallor or optic disc edema, findings that are commonly associated with anterior ischemic optic neuropathy. Reprinted with permission from Archives of Ophthalmology.132
Light Microscopy: Optic Nerve and Retinal Ganglion Cell Axon Size
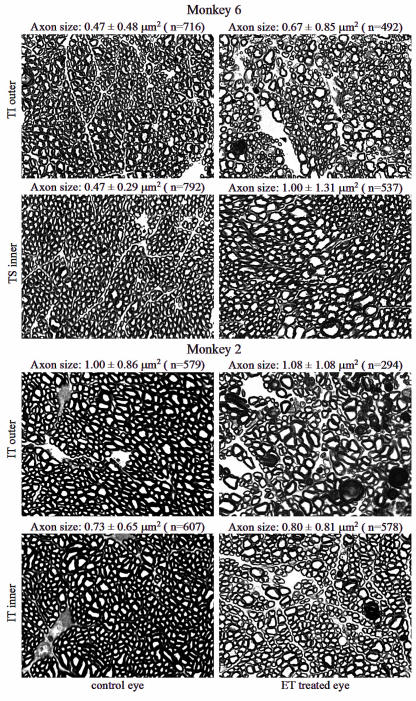
Under the light microscope, the optic nerves of the ET-1 treated eyes showed a variety of morphological changes in the retinal ganglion cell axons and connective tissues compared to the normal, contralateral eyes (Figure 4). These changes included axonal demyelination, axonal swelling, axonal shrinkage, and axonal fragmentation similar to earlier experiments (Figure 1). These changes may represent the various stages in the continuum of axonal degeneration. Connective tissue changes included increased thickness of the perineurium and enlarged extra-axonal spaces associated with the loss of axons. In areas with more severe damage, neuroglial cells appeared to be larger and increased in number, and the sizes of the axons appeared to be highly variable. Figure 4 shows comparisons between two different optic nerve regions in two different individual animals (monkeys 2 and 6). The optic nerve subregions were chosen because they represent areas of significant axonal loss in the ET-1 treated eyes compared to the normal contralateral eyes (see section below, “Regional Retinal Ganglion Cell Axonal Density Comparison,” and Figure 6). The mean and SD of the axon sizes are given for each region in Figure 4, and additional data regarding axon size distributions in damaged eyes are described below. On average in regions of axonal damage in the ET-1 treated eyes, the variability of the axon size was greater and there appears to be a selective loss of large axons.
FIGURE 4.
Representative microphotographs of the optic nerve cross-sections of the two regions of endothelin-1 (ET-1) treated eyes (right column), compared to the matching regions of the contralateral eyes (left column), from two animals (monkeys 2 and 6). The optic nerve regions were chosen because they represent areas of significant axonal loss in the ET-1 treated eyes (see Figure 6). Average axon sizes (mean ± SD) are given for each image. Changes in the ET-1 treated optic nerves include axonal demyelination, axonal swelling, axonal shrinkage, and axonal fragmentation. In regions of axonal damage, retinal ganglion cell axons appeared generally enlarged or swollen, the variability of the axon size appeared greater, and the glial cells appeared hypertrophic (toluidine blue stain, ×100). Reprinted with permission from Archives of Ophthalmology.132
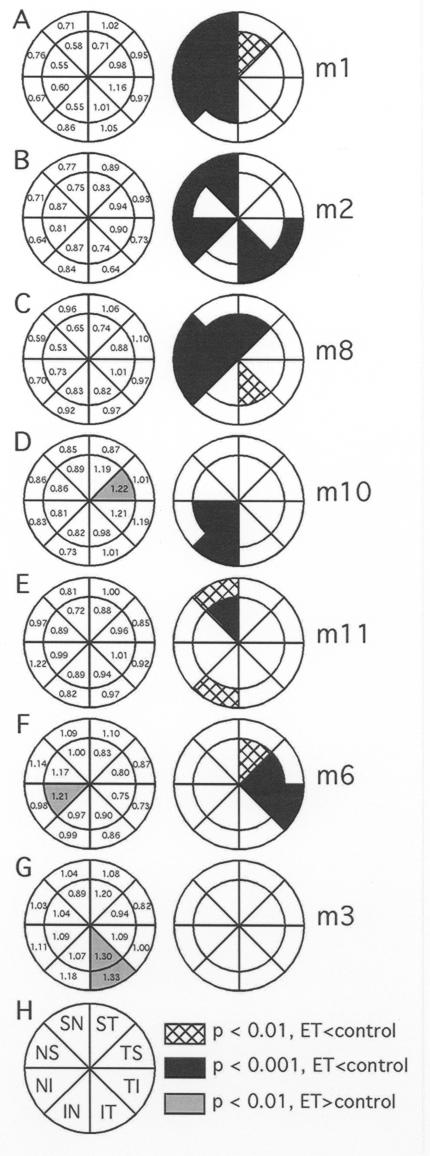
FIGURE 6.
The spectrum of regional effects observed following 6 to 12 months of endothelin-1 (ET-1) induced ischemia. The pair of pie-charts in each panel shows the data for a single animal. Left column represents the ratio of axonal density in the ET-1 treated eye relative to the corresponding region from the fellow eye (ET/control). Right column shows the probability plot of the observed regional differences (two-way analysis of variance). Regions where the density in the ET-1 treated eye were significantly reduced were common (black or cross-hatched subregions, ET-1 axonal density < control). Panels A through F each show that significant loss of axonal density occurred in multiple subregions. Most commonly, areas of axonal loss were found in clusters of contiguous subregions. Panels D, F, and G demonstrate significantly lower axonal densities within isolated subregions of the control eyes (gray shaded subregions, ET-1 axonal density > control) (T = temporal, S = superior, I = inferior, N = nasal). Reprinted with permission from Archives of Ophthalmology.132
COMPARISON OF AXONAL DENSITY BETWEEN ISCHEMIC EYES AND CONTROLS
Global Retinal Ganglion Cell Axonal Density Comparison
Following 6 to 12 months of continuous ET-1 delivery in one eye, the group average axonal density for the entire optic nerve among the 12 ET-1 treated eyes was 208,310/mm2, whereas the control eye group average was 220,661/mm2 (t = 2.04, P = .03; see Table). On average, this change represents a 5.1% ± 10.0% decrease of axonal density due to ET-1 induced chronic ischemia. However, Table also shows that the effect of ET-1 treatment was not uniform across all 12 animals; individual animals varied greatly in response to chronic ischemia. There was no significant effect of duration of exposure to ET-1 (Kruskal-Wallis statistic = 0.04, P = .98). Because the number of animals treated for 9 and 12 months is small, detection of a small effect may not have been possible. Axonal density also varied substantially among control eyes, ranging from 170,622 to 286,825 axons/mm2 (220,661 ± 35,855, mean ± SD). Therefore, a two-way ANOVA (treatment × animal) was applied and Bonferroni post hoc tests were used to evaluate the effect of ET-1 on overall axonal density for the whole experimental group and for each individual, respectively (Table). This allowed for control of the variation due to differences between individual animals. As expected, axonal density varied significantly between individual animals (F = 129.9; P < .0001). Two-way matched-pair ANOVA showed a significant effect of ET-1 on overall axonal density for the whole experimental group (P < .0001), and there was a significant interaction between treatment and animal effects (F = 31.4, P < .0001). Bonferroni post hoc testing revealed that nine of the 12 animals had significant axonal density differences between their ET-1 treated and control eyes, but two of these had a greater density in the ET-1 treated eye (see Table). Significant axonal loss was seen in seven animals and varied from 4% to 21% loss, with a mean loss of 11.6 ± 6.7%.
Regional Retinal Ganglion Cell Axonal Density Comparison
Several studies have demonstrated regional susceptibility within the optic nerve in glaucoma and other forms of optic neuropathy in humans, as well as in experimental glaucoma in nonhuman primates and rodents. In visual evaluation of most optic nerve cross-sections, it was immediately evident that some subregions sustained more damage than others. To evaluate the potential regional susceptibility in this experimental model of chronic ischemia, the axonal density was also assessed for 16 optic nerve subregions (see “Methods” section). Figure 5 shows regional differences in axonal densities for the group of 12 experimental animals, ET-1 treated versus contralateral, untreated eyes. Regional analysis showed a significant group effect of ET-1 induced ischemia (F = 19.9; P < .0001), but there was no significant interaction observed between treatment and region (F = 1.2; P > .05). As most of the variance (71%) in axonal density was attributable to differences between individual animals, comparisons between the two eyes (ET-1 treated and control) of each animal would allow a better measure of regional axonal density change.
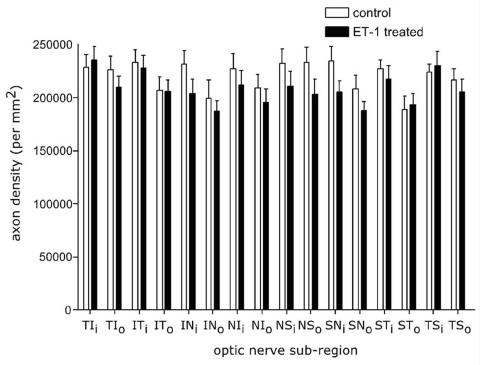
FIGURE 5.
The histogram demonstrates the average regional retinal ganglion cell axonal density differences (axons/mm2) between the endothelin-1 (ET-1) treated eye and the contralateral eyes of 12 animals. Although the ET-1 treated eyes had significant loss of axons for the entire optic nerve, for the entire population regionally the differences were not significant (T = temporal, S = superior, I = inferior, N = nasal, i = inner, o = outer). Reprinted with permission from Archives of Ophthalmology.132
Figure 6 displays the spectrum of regional effects observed across the group. The pair of pie charts in each panel shows the data for a single animal. The numbers within each region in the left column represent the axonal density in the ET-1 treated eye relative to the density in the corresponding region of the optic nerve from the fellow control eye (ET/control). The right column for each pair shows the probability plot determined by two-way ANOVA with post hoc testing of the observed regional differences between the treated and control eye for each individual animal. There were very few regions where the axonal density in the ET-1 treated eye was significantly greater than the density in corresponding region of the control eye. For example, panels D, F, and G demonstrate significantly lower axonal densities within single subregions of the control eyes (Gray Shaded Sub-Regions, ET-1 Axonal Density > Control). In contrast, regions where the density in the ET-1 treated eye was significantly reduced as compared with the density in corresponding region of the control eye were common. Panels A through F show that significant loss of axonal density occurred in multiple subregions of ET-1 treated eyes. Most commonly, areas of axonal loss were found in clusters of contiguous subregions. In panel B, there is significant loss in both the superior nasal and inferior temporal regions, whereas panels A, C, D, E, and F show more focal deficits in clusters of two to seven subregions. Regional mapping of the damage showed that axonal loss was not uniformly distributed within the nerve and the axonal loss was unrelated to the site of delivery of the ET-1. Regional losses in axonal density were observed in eight of the 12 ET-1 treated eyes relative to the corresponding region of the optic nerve from each animal’s control eye. Two of the 12 experimental eyes (monkeys 3 and 4) had differences in regional axonal density where the density was actually significantly greater in the ET-1 treated eye. The remaining two experimental eyes (monkeys 5 and 7) had no significant effect of ET-1 treatment, and thus analysis of regional differences (treatment by region interactions) was not applicable. Axonal loss often clustered within specific quadrants, with many regions showing greater than 20% loss and some regions with 40% to 45% loss of axons. In summary, chronic ischemia induced by local administration of ET-1 causes significant global loss of optic nerve axons in primate eyes, with varying regional loss (0 to 47%).
Optic Nerve Swelling and Axonal Density Calculations
The mean cross-sectional area of the retrobulbar optic nerves in the ET-1 treated eyes was 6.27 ± 0.96 mm2, whereas the area of the contralateral, control eyes was 5.91 ± 0.89 mm2. As noted above, the ET-1 treated optic nerves did not demonstrate clinically apparent swelling in serial fundus photography. This average size difference of the retrobulbar optic nerves represents an overall increase of 6% in the ET-1 treated eyes (P = .03, paired t test). However, the contribution of optic nerve swelling to axonal density calculations is not uniform, as some ET-1 treated optic nerves were larger and some were unchanged. To test this relationship, a linear correlation was calculated for the percentage of axonal loss compared to the percentage of optic nerve swelling in animals with significant loss. As expected, the correlation was inversely related, but quite weak (R2 = 0.021). As an example, monkey 6 had highly statistically significant overall axonal loss of 8% (P < .001), as well as regional axonal loss varying from 17% to 27%. However, this primate did not exhibit any optic nerve swelling in the ET-1 treated eye (ET-1 eye optic nerve size 7.3 mm2, control eye 7.2 mm2). In another example, monkey 2 had significant regional (19% to 36%) and overall (19%) axonal loss with optic nerve enlargement (ET-1 eye optic nerve size 7.4 mm2, control eye 6.3 mm2). Finally, in some animals the increase in the size of the optic nerve may have contributed to the decrease in axonal density, whereas it did not contribute to others. In regions of severe axonal loss, the small amount of optic nerve swelling does not significantly change the estimated amounts of loss.
The increase in the average size of the ET-1 treated optic nerves was contributed to by increases in the extracellular space, apparent increases in the optic nerve glial populations, and generalized thickening of the perineurium of the entire optic nerve. As well, regional or localized loss of axons is the most significant finding in this study. Figures 1 and 4 provide examples of regions with very significant localized axonal loss and multiple other histological changes, described above. As previously shown, some regions of individual optic nerves treated with ET-1 had axonal loss of greater than 40%, as compared to the contralateral eye. In summary, modest retrobulbar optic nerve swelling occurs in the chronic ischemia model, but this swelling is not strongly correlated with axonal loss, does not account for significant decreases of the overall axonal density in individual animals, and is unlikely to account for the significant regional losses that exceeded 20% in many animals.
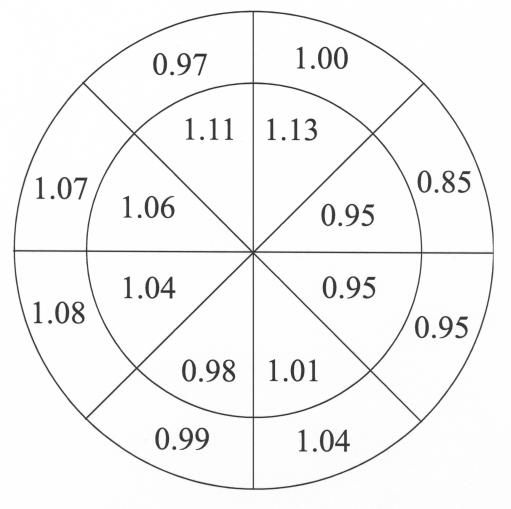
Intereye Variability in a Control Animal
To further investigate the variability of axonal density in normal eyes and between the two eyes of a normal primate, two eyes of a single monkey without minipump implantation were examined and analyzed in an identical statistical fashion (Figure 7). The left column in Figure 7 shows the relative densities in the various regions between two normal eyes of the single monkey. The right panel is the probability plot derived by identical statistical methods as were used for the ET-1 monkeys. As expected, there was some variability of the intereye ratio of axonal density for various optic nerve regions, although none of the intereye regional differences were statistically significant (F = 0.1, P = .79). The effect of region was significant (F = 11.3, P < .0001), but the effect of eye was not significant (F = 0.1, P = .79). The global density difference between these two normal eyes was less than 1% and not significantly different (right eye 233,118 axons/mm2, and left eye 221,470 axons/mm2, P = .78, paired t test).
FIGURE 7.
As in Figure 6, the diagram shows the relative axon density within the various regions of the right eye versus the left eye of a normal monkey. Probability plots were calculated, and there were no significant differences for any region (all P > .05). The global density difference between these two normal eyes was less than 1% and not significantly different (right eye 233,118 axons/mm2 and left eye 221,470 axons/mm2, P = .78, paired t test). Reprinted with permission from Arch Ophthalmol.132
Comparison of Large Versus Small Axon Loss
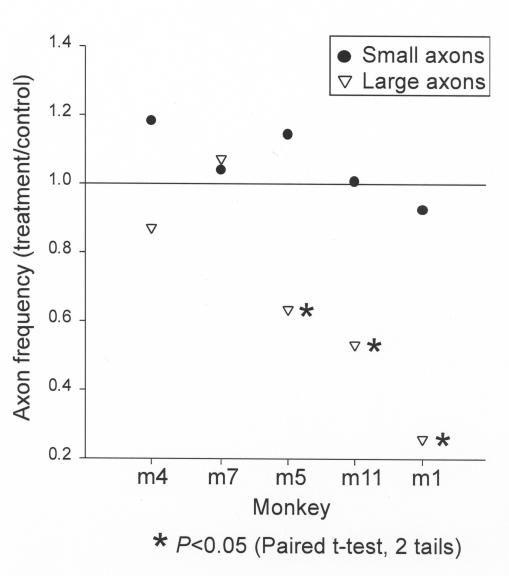
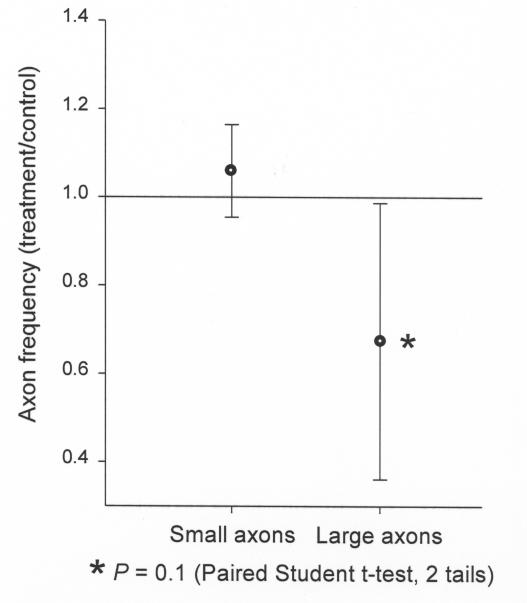
To determine if preferential loss of large or small retinal ganglion cell axons occurs in the model of chronic ischemia, the size and numbers of axons were calculated and compared between the ET-1 treated and control eyes. Five animals (M1, M4, M5, M7, and M11) had uniformity of staining intensity of both eyes and were therefore included in this analysis. As a group, these animals demonstrated variable global loss of axons from 0 to 21% (see Table), and three (M1, M5, and M11) had significant regional loss, with selected regions experiencing greater than 40% loss (Figure 6). Figure 8 demonstrates the comparison of large versus small retinal ganglion cell axonal loss in ET-1 treated eyes as compared to the contralateral control eyes. The larger axons show significantly greater loss in three of the animals (M1, M5, and M11) as compared to the small axons (solid dots). Of note, M1 and M11 demonstrate significant global decrease of axonal density, 21% and 9% loss, respectively (see Table), and M5 lacks significant global axonal loss but does demonstrate significant inferior regional loss. M4 and M7 do not show preferential loss of large axons and did not demonstrate significant global or regional axonal loss. Figure 9 illustrates the mean large versus small axonal changes for the entire group of five animals. As a group, the mean axonal frequency changes of large and small axons between the ET-1 treated and the controlled optic nerves were compared and do not show significant differences (P = .1) However, there appears to be a tendency toward preferential large axonal loss.
FIGURE 8.
Comparison of large versus small retinal ganglion cell axonal loss in the optic nerve of five endothelin-1 treated eyes as compared to the contralateral control eyes. On average, the larger axons (triangles) show significantly greater loss in three of the animals (M1, M5, and M11) as compared to the small axons (solid dots). Of note, M1 and M11 demonstrate significant global decrease of axonal density, 21% and 9% loss, respectively (see Table 1), and M5 lacks significant global axonal loss, but does demonstrate significant inferior regional loss. M4 and M7 do not show preferential loss of large axons and did not demonstrate significant global or regional axonal loss.
FIGURE 9.
Mean large versus small axonal changes for the entire group of five animals illustrated in Figure 8 (M1, M4, M5, M7 and M11). As a group, the mean axonal frequency changes of large and small axons between the endothelin-1 treated and the control optic nerves were compared and do not show significant differences (P = .1) This is likely due to the high variability in the axonal sizes, the varying degrees of axonal loss in each animal, and the small sample size. However, there appears to be a tendency toward preferential large axonal loss.
DISCUSSION
Using an established model of optic nerve ischemia that employs ET-1 delivered to the retrobulbar space in the primate eye, we show that chronic ischemia results in demonstrable damage of the optic nerve without elevation of IOP. Eyes in which ischemia was induced for 6 to 12 months showed significant decrease in axonal density. Whereas the axonal loss was significant overall for the population, the damage represents early loss with an average of approximately 5%. However, some individuals have global loss exceeding 15% to 20%, with even greater amounts of regional loss. This ischemia-induced axonal loss was commonly focal in nature, but the location of loss within the optic nerve varied among individual animals. The axonal loss was often seen in clusters of contiguous subregions of the optic nerve of an individual animal. Clinical findings typical for anterior ischemic optic neuropathy, such as generalized or focal optic nerve pallor, optic nerve and retinal edema, and telangiectatic vessels, were not seen. Although, on average, the retrobulbar optic nerve area was increased in the ischemic eyes on account of axonal enlargement and extracellular space enlargement from axonal loss, there was no clinical evidence of anterior optic nerve swelling. This finding may imply a difference between severe acute ischemia associated with anterior ischemic optic neuropathy and milder, chronic ischemia associated with the hemodynamic alterations produced in our model.
Among the most important findings was that variability in the response to ischemia was seen both between animals and among regions of the optic nerve. These findings suggest that optic nerves differ in their susceptibility to ischemia and that individual optic nerves have varied regional susceptibility. Regional axonal loss and structural damage within the optic nerve are found in various forms of optic neuropathy, including glaucoma.93,134 Interestingly, focal damage of the optic nerve has been attributed to localized ischemia and has been referred to as “focal ischemic” GON.104 This subtype of glaucoma has been statistically associated with systemic vascular abnormalities, such as migraine. In addition, preferential loss of large axons was demonstrated in animals with significant global and/or regional axonal loss secondary to chronic ischemia. These findings pose two important questions: Why are certain optic nerves and certain optic nerve regions of an individual optic nerve more susceptible than others to damage from noxious stimuli? and Why are large retinal ganglion cell axons preferentially damaged in both high IOP and ischemic models of glaucoma?
Although elevated IOP and chronic ischemia are presumably disparate insults, both result in regional axonal injury and preferential large axon loss. Potential hypotheses should be investigated. However, before considering potential hypotheses for the regional loss of axons in this model of ischemia, one should consider if the model itself is responsible for a particular pattern of loss. The delivery tubes for the ET-1 within the retrobulbar space were located to the nasal or superior-nasal side of the optic nerve in all animals. Although there is no way to know if the decrease in blood flow to the optic nerve was uniform, the highly varied susceptibility to damage is somewhat surprising. If the location of the delivery of the ET-1 is an important factor in the pattern of axonal loss, one would anticipate a greater loss of axons in the superior-nasal region due to the relatively greater exposure. However, this pattern was not uniformly seen. Instead, Figures 4 and 6 demonstrate that regional susceptibility was quite varied among individuals. Varying the placement of the delivery tube in future experiments will provide additional information regarding this matter. It is also possible that optic nerves of different animals may experience different levels of induced ischemia. This could explain the apparent differences in susceptibility between the optic nerves of different animals. However, blood flow experiments with intravascular microspheres indicated a fairly uniform decrease of anterior optic nerve blood flow between animals.120 Whereas microsphere experiments provide reliable information about vascular perfusion, it is not possible to perform these experiments in the same eyes that are evaluated for axonal loss. In the future, validation of in vivo blood flow measurement techniques may allow assessment of optic nerve perfusion in eyes predestined for histological evaluation.
Preferential superior and inferior regional damage of the optic nerve in human glaucoma correlates clinically with the development of localized neuroretinal rim notches, disc hemorrhages, and visual function defects. Jonas and colleagues93 and Mok and associates98 showed that glaucomatous optic nerve loss occurs in all neuroretinal rim sectors of the optic nerve, but in early glaucoma the most pronounced loss is in the inferior-temporal rim. Preferential regional damage in glaucoma has traditionally been attributed to differential mechanical forces and explained by anatomic features at the level of the anterior lamina cribrosa.99–101 That is, in the superior and inferior regions, there is less connective tissue around lamina cribrosa pores, and the pores are large in size compared to the nasal and temporal regions.99,100 In addition, lamina cribrosa pores in the peripheral region are longer than the central pores.101 Recent biomechanical modeling of posterior segment of the eye and optic nerve suggests that disproportionate stress and strain within the optic nerve may contribute to regional loss of axons.103 These anatomic features may cause the axons passing through the lamina cribrosa within these regions to be more vulnerable to mechanical stress, such as an increase in IOP.
Realization that chronic optic nerve ischemia, like chronic mechanical compression, produces regional neural damage forces reexamination of the topic. Several hypothetical explanations based on regional differences in the anatomic, metabolic, and/or vascular profiles of the anterior optic nerve can be considered. First, regional anatomic differences that exist within the lamina cribrosa, such as differences in the constitutive glial cell populations and in the retinal ganglion cell types, may account for varied susceptibility. Second, the metabolic demand may vary between different regions of the optic nerve. Under normal physiological conditions, the metabolic demand of certain regions of the optic nerve is accommodated, but when perturbed, the system is overwhelmed and highly metabolically active regions would be more vulnerable to damage, such as perfusion deficits or localized mechanical stress. Third, differences in the vascular anatomy, such as the number and caliber of supply arteries or the distribution of capillaries within the optic nerve, may play a role in regional variations. There may be differences in the patterns of capillary beds within the anterior optic nerves. Perhaps the total number of capillaries subserving a set number of retinal ganglion cell axons in various regions of the optic nerve is borderline but becomes inadequate and exhibits increased susceptibility when stressed due to differential regional autoregulatory capabilities.135,136 Regional functional inadequacy may not be accompanied by a structural decrease in the vasculature, as demonstrated previously.137 Instead of a measurable decrease in the number of capillaries, loss of autoregulatory capacity may be the underlying deficiency.
The existence of regional watershed zones has been suggested to be important in the development of regional damage in the human glaucomatous optic nerve.60,108,109 Approximately 60% of these watershed zones pass through the temporal half of the optic nerve in eyes with glaucoma.110 Since the area within a watershed zone is comparatively less perfused, especially during hemodynamic stress, these regions are more vulnerable to ischemic damage. Given the similar optic nerve structure and vasculature of nonhuman primates to the human eye, perhaps the varied severity and regional location of axonal damage seen in this experiment are the result of uneven perfusion of the optic nerve caused by watershed zones. In the future, this could be investigated with in vivo angiography in this primate model. This hypothesis might also explain the variability in the response between different animals.
Finally, differential regional response or sensitivity to the vasoconstrictive effects of ET-1 could also explain the findings of the present study. This model assumes that the axonal damage is the direct result of ischemia, which was induced by ET-1.91,120 Endothelins are a family of short-chain polypeptides that have been identified and described over the last two decades. As the most potent vasoconstrictor known, ET-1 was selected in this model in order to create significant vasoconstriction over a prolonged period of time. This peptide affects the vasculature through two major receptors, ET a and b receptors. Recent studies have indicated that the ET b receptor, which is largely located on glial cells in central nervous system, can also participate in the pathological mechanism of neuronal damage.138–141 This concept may be particularly interesting because patients with primary open-angle glaucoma may have higher ET-1 levels in the plasma and vitreous humor, as compared to normal controls.39,44,45,47,48 Thus, ET-1 may cause blood flow insufficiencies in the optic nerve due to its potent vasoconstrictive effect or, on the other hand, may cause neuronal damage mediated by ET b receptors on the glial cells via mechanisms not yet fully understood. This also provides the potential opportunity of developing therapeutic approaches for the treatment of glaucoma, via blockade of endothelin activity or otherwise enhancing the blood flow to the anterior optic nerve. Examining the distribution of ET receptors and their activity in the optic nerve may provide insight into these issues.
As cited in the “Introduction,” large optic nerve fibers may be more susceptible to damage in human GON.102 It has been proposed that retinal ganglion cells with large axons are more vulnerable than retinal ganglion cells with small axons. This observation has been shown in both the experimental high IOP primate model of glaucoma111 and in human glaucoma.102 The present study demonstrates selective loss of large axons in our model of chronic optic nerve ischemia. Whereas this finding is interesting and extends to the concept that ischemia is involved in human glaucoma, the notion that the retinal ganglion cells with large axons are more vulnerable to glaucomatous damage remains controversial. The possibility remains that the apparent preferential loss of large axons in glaucoma and our model may result from cellular shrinkage prior to the cell death.118 In addition, classification of retinal ganglion cells based only on the size of their axons, while anatomically achievable, may have limited physiological relevance.
CONCLUSIONS
Chronic optic nerve ischemia in the nonhuman primate eye leads to demonstrable damage of the optic nerve, without elevation of IOP. Whereas the average loss of retinal ganglion cell axons for all the animals was significant, it was small. However, localized loss of axons was significant in most of the animals and extensive in many regions. Ischemia-induced axonal loss usually was focal in nature but variable in location within the optic nerve of individual animals. In addition to this variation in regional axonal loss, individual animals showed marked differences in axonal damage. Also, there appears to be preferential loss of large retinal ganglion cell axons as compared to small axons. These findings suggest that optic nerves differ in their susceptibility to ischemia and that individual optic nerves have varied regional susceptibility.
Clinically, glaucoma is a slowly progressive optic neuropathy that is caused by a variety of factors, including but not limited to elevated IOP. Other factors either alone or in concert with IOP likely result in glaucomatous damage. Regional axonal loss and structural damage within the optic nerve are common in GON, and focal damage of the optic nerve has previously been attributed to localized ischemia. Glaucoma has been associated with many systemic vascular disorders through epidemiologic studies and through experimentation. However, optic nerve ischemia has not been proven to be a causative factor in the development of GON. The present study demonstrates that focal optic neuropathy, similar to that seen in glaucoma, results from chronic ischemia alone. Potential regional differences in the anatomy, metabolism, or vasculature of the optic nerve may underlie the differential susceptibility of the various regions. Finally, whereas preferential regional damage in glaucoma has traditionally been attributed to differential mechanical forces, it is possible that ischemic perturbations of the optic nerve result in regional increases in susceptibility that render parts of the anterior optic nerve more susceptible to mechanical damage.
ACKNOWLEDGMENTS
I wish to acknowledge the many contributions of my collaborators, E. Michael Van Buskirk, MD, Lin Wang, PhD, Grant Cull, and Brad Fortune, PhD.
Footnotes
Supported in part by grant RO1-EY05231 from the National Institutes of Health and by Legacy Good Samaritan Foundation, Portland, Oregon.
REFERENCES
- 1.Hoskins HD, Kass MA. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas 6th ed. St Louis: CV Mosby; 1989.
- 2.Chandler PA, Grant WM. Glaucoma 2nd ed. Philadelpia: Lea & Febiger; 1979.
- 3.Levene RZ. Low tension glaucoma: a critical review and new material. Surv Ophthalmol. 1980;24:621–664. doi: 10.1016/0039-6257(80)90123-x. [DOI] [PubMed] [Google Scholar]
- 4.Hollows FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–586. doi: 10.1136/bjo.50.10.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 6.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–452. doi: 10.1016/s0002-9394(14)76171-9. [DOI] [PubMed] [Google Scholar]
- 7.Carter CJ, Brooks DE, Doyle DL, et al. Investigations into a vascular etiology for low-tension glaucoma. Ophthalmology. 1990;97:49–55. doi: 10.1016/s0161-6420(90)32627-1. [DOI] [PubMed] [Google Scholar]
- 8.Shiose Y, Kitazawa Y, Tsukahara S, et al. Epidemiology of glaucoma in Japan—a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35:133–155. [PubMed] [Google Scholar]
- 9.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38:S3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 10.Hayreh SS. Progress in the understanding of the vascular etiology of glaucoma. Curr Opin Ophthalmol. 1994;5:26–35. [Google Scholar]
- 11.Leighton DA, Phillips CI. Systemic blood pressure in open-angle glaucoma, low tension glaucoma, and the normal eye. Br J Ophthalmol. 1972;56:447–453. doi: 10.1136/bjo.56.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod SD, West SK, Quigley HA, et al. A longitudinal study of the relationship between intraocular and blood pressures. Invest Ophthalmol Vis Sci. 1990;31:2361–2366. [PubMed] [Google Scholar]
- 13.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113:216–221. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Zimmerman MB, Podhajsky P, et al. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–624. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 15.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–867. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham SL, Drance SM, Wijsman K, et al. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 17.Sommer A. Doyne Lecture. Glaucoma: facts and fancies. Eye. 1996;10(pt 3):295–301. doi: 10.1038/eye.1996.63. [DOI] [PubMed] [Google Scholar]
- 18.Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology. 1997;104:1714–1719. doi: 10.1016/s0161-6420(97)30075-x. [DOI] [PubMed] [Google Scholar]
- 19.Gasser P, Flammer J, Guthauser U, et al. Do vasospasms provoke ocular diseases? Angiology. 1990;41:213–220. doi: 10.1177/000331979004100306. [DOI] [PubMed] [Google Scholar]
- 20.Klaver JH, Greve EL, Goslinga H, et al. Blood and plasma viscosity measurements in patients with glaucoma. Br J Ophthalmol. 1985;69:765–770. doi: 10.1136/bjo.69.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trope GE, Salinas RG, Glynn M. Blood viscosity in primary open-angle glaucoma. Can J Ophthalmol. 1987;22:202–204. [PubMed] [Google Scholar]
- 22.Foulds WS. 50th Bowman lecture. “Blood is thicker than water.” Some haemorheological aspects of ocular disease. Eye. 1987;1(pt 3):343–363. doi: 10.1038/eye.1987.55. [DOI] [PubMed] [Google Scholar]
- 23.Weinreb RN. Blood rheology and glaucoma. J Glaucoma. 1993;2:153–154. [PubMed] [Google Scholar]
- 24.O’Brien C, Butt Z, Ludlam C, et al. Activation of the coagulation cascade in untreated primary open-angle glaucoma. Ophthalmology. 1997;104:725–730. doi: 10.1016/s0161-6420(97)30245-0. [DOI] [PubMed] [Google Scholar]
- 25.Park KH, Tomita G, Liou SY, et al. Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996;103:1899–1906. doi: 10.1016/s0161-6420(96)30409-0. [DOI] [PubMed] [Google Scholar]
- 26.Tezel G, Kass MA, Kolker AE, et al. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology. 1996;103:2105–2113. doi: 10.1016/s0161-6420(96)30382-5. [DOI] [PubMed] [Google Scholar]
- 27.Yin ZQ, Vaegan, Millar TJ, et al. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6:23–32. [PubMed] [Google Scholar]
- 28.Michelson G, Groh MJ, Langhans M. Perfusion of the juxtapapillary retina and optic nerve head in acute ocular hypertension. Ger J Ophthalmol. 1996;5:315–321. [PubMed] [Google Scholar]
- 29.Hollo G, Greve EL, van den Berg TJ, et al. Evaluation of the peripapillary circulation in healthy and glaucoma eyes with scanning laser Doppler flowmetry. Int Ophthalmol. 1997;20:71–77. doi: 10.1007/BF00212949. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki S, Inoue Y, Yoshikawa K. Peripapillary fluorescein angiographic findings in primary open angle glaucoma. Br J Ophthalmol. 1996;80:812–817. doi: 10.1136/bjo.80.9.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brart DP, de Souza Lima M, Bartsch DU, et al. Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am J Ophthalmol. 1997;123:657–666. doi: 10.1016/s0002-9394(14)71078-5. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki Y, Hayamizu F. Comparison of flow velocity of ophthalmic artery between primary open angle glaucoma and normal tension glaucoma. Br J Ophthalmol. 1995;79:732–734. doi: 10.1136/bjo.79.8.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolela MT, Walman BE, Buckley AR, et al. Ocular hypertension and primary open-angle glaucoma: a comparative study of their retrobulbar blood flow velocity. J Glaucoma. 1996;5:308–310. [PubMed] [Google Scholar]
- 34.Harris A, Spaeth GL, Sergott RC, et al. Retrobulbar arterial hemodynamic effects of betaxolol and timolol in normal-tension glaucoma. Am J Ophthalmol. 1995;120:168–175. doi: 10.1016/s0002-9394(14)72604-2. [DOI] [PubMed] [Google Scholar]
- 35.Rankin SJ, Walman BE, Buckley AR, et al. Color Doppler imaging and spectral analysis of the optic nerve vasculature in glaucoma. Am J Ophthalmol. 1995;119:685–693. doi: 10.1016/s0002-9394(14)72771-0. [DOI] [PubMed] [Google Scholar]
- 36.Rojanapongpun P, Drance SM, Morrison BJ. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993;77:25–29. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butt Z, McKillop G, O’Brien C, et al. Measurement of ocular blood flow velocity using colour Doppler imaging in low tension glaucoma. Eye. 1995;9(pt 1):29–33. doi: 10.1038/eye.1995.4. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser HJ, Schoetzau A, Stumpfig D, et al. Blood-flow velocities of the extraocular vessels in patients with high- tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997;123:320–327. doi: 10.1016/s0002-9394(14)70127-8. [DOI] [PubMed] [Google Scholar]
- 39.Cellini M, Possati GL, Profazio V, et al. Color Doppler imaging and plasma levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol Scand Suppl. 1997:11–13. doi: 10.1111/j.1600-0420.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 40.Grunwald JE, Piltz J, Hariprasad SM, et al. Optic nerve and choroidal circulation in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2329–2336. [PubMed] [Google Scholar]
- 41.Michelson G, Langhans MJ, Harazny J, et al. Visual field defect and perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1998;236:80–85. doi: 10.1007/s004170050046. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz B. Circulatory defects of the optic disk and retina in ocular hypertension and high pressure open-angle glaucoma. Surv Ophthalmol. 1994;38:S23–34. doi: 10.1016/0039-6257(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 43.Adam G, Schwartz B. Increased fluorescein filling defects in the wall of the optic disc cup in glaucoma. Arch Ophthalmol. 1980;98:1590–1592. doi: 10.1001/archopht.1980.01020040442009. [DOI] [PubMed] [Google Scholar]
- 44.Sugiyama T, Moriya S, Oku H, et al. Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol. 1995;39(Suppl 1):S49–56. doi: 10.1016/s0039-6257(05)80073-6. [DOI] [PubMed] [Google Scholar]
- 45.Kaiser HJ, Flammer J, Wenk M, et al. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol. 1995;233:484–488. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- 46.Nicolela MT, Ferrier SN, Morrison CA, et al. Effects of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci. 2003;44:2565–2572. doi: 10.1167/iovs.02-0913. [DOI] [PubMed] [Google Scholar]
- 47.Noske W, Hensen J, Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch Clin Exp Ophthalmol. 1997;235:551–552. doi: 10.1007/BF00947082. [DOI] [PubMed] [Google Scholar]
- 48.Tezel G, Kass MA, Kolker AE, et al. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6:83–89. [PubMed] [Google Scholar]
- 49.Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54:461–472. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaoeda K, Shirakashi M, Fukushima A, et al. Relationship between optic nerve head microcirculation and visual field loss in glaucoma. Acta Ophthalmol Scand. 2003;81:253–259. doi: 10.1034/j.1600-0420.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 51.Nicolela MT, Drance SM, Rankin SJ, et al. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121:502–510. doi: 10.1016/s0002-9394(14)75424-8. [DOI] [PubMed] [Google Scholar]
- 52.Yamazaki Y, Drance SM. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am J Ophthalmol. 1997;124:287–295. doi: 10.1016/s0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- 53.Cioffi GA. Three assumptions: ocular blood flow and glaucoma. J Glaucoma. 1998;7:299–300. [PubMed] [Google Scholar]
- 54.Perkins ES. The Bedford glaucoma survey. I. Long-term follow-up of borderline cases. Br J Ophthalmol. 1973;57:179–185. doi: 10.1136/bjo.57.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison JC, Dorman-Pease ME, Dunkelberger GR, et al. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990;108:1020–1024. doi: 10.1001/archopht.1990.01070090122053. [DOI] [PubMed] [Google Scholar]
- 56.Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. [PubMed] [Google Scholar]
- 57.Minckler DS, Bunt AH, Klock IB. Radioautographic and cytochemical ultrastructural studies of axoplasmic transport in the monkey optic nerve head. Invest Ophthalmol Vis Sci. 1978;17:33–50. [PubMed] [Google Scholar]
- 58.Flammer J, Gasser P, Prunte C, et al. The probable invovement of factors other than intraocular pressure in the pathogenesis of glaucoma. In: Drance SM, Van Buskirk EM, Neufeld AH, eds. Pharmacology of Glaucoma Baltimore: Williams & Wilkins; 1992:273–283.
- 59.Hayreh SS. Blood supply of the optic nerve head. Ophthalmologica. 1996;210:285–295. doi: 10.1159/000310727. [DOI] [PubMed] [Google Scholar]
- 60.Hayreh SS. The 1994 Von Sallman Lecture. The optic nerve head circulation in health and disease. Exp Eye Res. 1995;61:259–272. doi: 10.1016/s0014-4835(05)80121-6. [DOI] [PubMed] [Google Scholar]
- 61.Hayreh SS. Vascular factors in the pathogenesis of glaucomatous optic neuropathy. In: Drance SM, ed. International Symposium on Glaucoma, Ocular Blood Flow, and Drug Treatment. Baltimore: William & Wilkins; 1992:33–41.
- 62.Lieberman MF, Maumenee AE, Green WR. Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol. 1976;82:405–423. doi: 10.1016/0002-9394(76)90489-x. [DOI] [PubMed] [Google Scholar]
- 63.Olver JM, Spalton DJ, McCartney AC. Quantitative morphology of human retrolaminar optic nerve vasculature. Invest Ophthalmol Vis Sci. 1994;35:3858–3866. [PubMed] [Google Scholar]
- 64.Olver JM, Spalton DJ, McCartney AC. Microvascular study of the retrolaminar optic nerve in man: the possible significance in anterior ischaemic optic neuropathy. Eye. 1990;4(Pt 1):7–24. doi: 10.1038/eye.1990.3. [DOI] [PubMed] [Google Scholar]
- 65.Onda E, Cioffi GA, Bacon DR, et al. Microvasculature of the human optic nerve. Am J Ophthalmol. 1995;120(pt 1):92–102. doi: 10.1016/s0002-9394(14)73763-8. [DOI] [PubMed] [Google Scholar]
- 66.Schumer RA, Podos SM. The nerve of glaucoma! Arch Ophthalmol. 1994;112:37–44. doi: 10.1001/archopht.1994.01090130047015. [DOI] [PubMed] [Google Scholar]
- 67.Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 68.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 69.Kerrigan LA, Zack DJ, Quigley HA, et al. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 70.Kerr J, Nelson P, O’Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126:42–51. doi: 10.1016/s0002-9394(98)00074-9. [DOI] [PubMed] [Google Scholar]
- 71.Kerr JF, Gobe GC, Winterford CM, et al. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- 72.Wyllie AH. Apoptosis: an overview. Br Med Bull. 1997;53:451–465. doi: 10.1093/oxfordjournals.bmb.a011623. [DOI] [PubMed] [Google Scholar]
- 73.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 74.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166:386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- 75.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 76.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 77.Kontos HA. Oxygen radicals in CNS damage. Chem Biol Interact. 1989;72:229–255. doi: 10.1016/0009-2797(89)90001-x. [DOI] [PubMed] [Google Scholar]
- 78.Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- 79.Vanhoutte PM. The endothelium—modulator of vascular smooth-muscle tone. N Engl J Med. 1988;319:512–513. doi: 10.1056/NEJM198808253190809. [DOI] [PubMed] [Google Scholar]
- 80.Haefliger IO, Dettmann E, Liu R, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S51–58. doi: 10.1016/s0039-6257(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 81.Kondo M, Wang L, Bill A. Vascular responses in retina and optic nerve to L-NAME, a nitric oxide blocker, and flickering light in monkeys. Invest Ophthalmol Vis Sci. 1995:S116. [Google Scholar]
- 82.Donati G, Pournaras CJ, Munoz JL, et al. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci. 1995;36:2228–2237. [PubMed] [Google Scholar]
- 83.Buckley CH, Hadoke PW, O’Brien CJ. Role of the endothelium in modulating functional responses of isolated bovine anterior ciliary arteries to vasoconstrictor agonists. Br J Ophthalmol. 1998;82:826–829. doi: 10.1136/bjo.82.7.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu P, Beny JL, Flammer J, et al. Relaxation by bradykinin in porcine ciliary artery. Role of nitric oxide and K(+)-channels. Invest Ophthalmol Vis Sci. 1997;38:1761–1767. [PubMed] [Google Scholar]
- 85.Nishimura K, Riva CE, Harino S, et al. Effects of endothelin-1 on optic nerve head blood flow in cats. J Ocul Pharmacol Ther. 1996;12:75–83. doi: 10.1089/jop.1996.12.75. [DOI] [PubMed] [Google Scholar]
- 86.Schmetterer L, Findl O, Strenn K, et al. Effects of endothelin-1 (ET-1) on ocular hemodynamics. Curr Eye Res. 1997;16:687–692. doi: 10.1076/ceyr.16.7.687.5065. [DOI] [PubMed] [Google Scholar]
- 87.Nagata A, Mishima H, Choshi K, et al. [Fluorescein fundus angiography of optic nerve head in primary open angle glaucoma and low tension glaucoma] Nippon Ganka Gakkai Zasshi. 1992;96:1423–1428. [PubMed] [Google Scholar]
- 88.Gass A, Flammer J, Linder L, et al. Inverse correlation between endothelin-1-induced peripheral microvascular vasoconstriction and blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 1997;235:634–638. doi: 10.1007/BF00946939. [DOI] [PubMed] [Google Scholar]
- 89.MacCumber MW, Jampel HD, Snyder SH. Ocular effects of the endothelins. Abundant peptides in the eye. Arch Ophthalmol. 1991;109:705–709. doi: 10.1001/archopht.1991.01080050121041. [DOI] [PubMed] [Google Scholar]
- 90.Cioffi GA, Van Buskirk EM. Microvasculature of the anterior optic nerve. Surv Ophthalmol. 1994;38:S107–116. doi: 10.1016/0039-6257(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 91.Cioffi GA, Orgul S, Onda E, et al. An in vivo model of chronic optic nerve ischemia: the dose-dependent effects of endothelin-1 on the optic nerve microvasculature. Curr Eye Res. 1995;14:1147–1153. doi: 10.3109/02713689508995821. [DOI] [PubMed] [Google Scholar]
- 92.Emdadi A, Zangwill L, Sample PA, et al. Patterns of optic disk damage in patients with early focal visual field loss. Am J Ophthalmol. 1998;126:763–771. doi: 10.1016/s0002-9394(98)00281-5. [DOI] [PubMed] [Google Scholar]
- 93.Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–68. doi: 10.1016/s0161-6420(13)31694-7. [DOI] [PubMed] [Google Scholar]
- 94.Bagga H, Greenfield DS. Quantitative assessment of structural damage in eyes with localized visual field abnormalities. Am J Ophthalmol. 2004;137:797–805. doi: 10.1016/j.ajo.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 95.Mok KH, Lee VW, So KF. Retinal nerve fiber loss in high- and normal-tension glaucoma by optical coherence tomography. Optom Vis Sci. 2004;81:369–372. doi: 10.1097/01.opx.0000134911.36521.b4. [DOI] [PubMed] [Google Scholar]
- 96.Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996;103:1014–1024. doi: 10.1016/s0161-6420(96)30572-1. [DOI] [PubMed] [Google Scholar]
- 97.Johnson CA, Cioffi GA, Liebmann JM, et al. The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol. 2000;15:221–233. doi: 10.3109/08820530009037873. [DOI] [PubMed] [Google Scholar]
- 98.Mok KH, Lee VW, So KF. Retinal nerve fiber loss pattern in high-tension glaucoma by optical coherence tomography. J Glaucoma. 2003;12:255–259. doi: 10.1097/00061198-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Dandona L, Quigley HA, Brown AE, et al. Quantitative regional structure of the normal human lamina cribrosa. A racial comparison. Arch Ophthalmol. 1990;108:393–398. doi: 10.1001/archopht.1990.01070050091039. [DOI] [PubMed] [Google Scholar]
- 100.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- 101.Dichtl A, Jonas JB, Naumann GO. Course of the optic nerve fibers through the lamina cibrosa in human eyes. Graefes Arch Clin Exp Ophthalmol. 1996;234:581–585. doi: 10.1007/BF00448803. [DOI] [PubMed] [Google Scholar]
- 102.Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 103.Downs JC, Suh JK, Thomas KA, et al. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J Biomech Eng. 2003;125:124–131. doi: 10.1115/1.1536930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology. 1996;103:640–649. doi: 10.1016/s0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- 105.Spaeth GL. A new classification of glaucoma including focal glaucoma. Surv Ophthalmol. 1994;38:S9–17. doi: 10.1016/0039-6257(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 106.Emdadi A, Kono Y, Sample PA, et al. Parapapillary atrophy in patients with focal visual field loss. Am J Ophthalmol. 1999;128:595–600. doi: 10.1016/s0002-9394(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz B, Harris A, Takamoto T, et al. Regional differences in optic disc and retinal circulation. Acta Ophthalmol Scand. 2000;78:627–631. doi: 10.1034/j.1600-0420.2000.078006627.x. [DOI] [PubMed] [Google Scholar]
- 108.Sato Y, Tomita G, Onda E, et al. Association between watershed zone and visual field defect in normal tension glaucoma. Jpn J Ophthalmol. 2000;44:39–45. doi: 10.1016/s0021-5155(99)00148-3. [DOI] [PubMed] [Google Scholar]
- 109.Hayreh SS. Inter-individual variation in blood supply of the optic nerve head. Its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985;59:217–246. doi: 10.1007/BF00159262. [DOI] [PubMed] [Google Scholar]
- 110.Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye. 1990;4(pt 2):273–289. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- 111.Quigley HA, Sanchez RM, Dunkelberger GR, et al. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28:913–920. [PubMed] [Google Scholar]
- 112.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–491. [PubMed] [Google Scholar]
- 113.Chaturvedi N, Hedley-Whyte ET, Dreyer EB. Lateral geniculate nucleus in glaucoma. Am J Ophthalmol. 1993;116:182–188. doi: 10.1016/s0002-9394(14)71283-8. [DOI] [PubMed] [Google Scholar]
- 114.Brooks DE, Strubbe DT, Kubilis PS, et al. Histomorphometry of the optic nerves of normal dogs and dogs with hereditary glaucoma. Exp Eye Res. 1995;60:71–89. doi: 10.1016/s0014-4835(05)80085-5. [DOI] [PubMed] [Google Scholar]
- 115.Yucel YH, Zhang Q, Gupta N, et al. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- 116.Crawford ML, Harwerth RS, Smith EL, 3rd, et al. Glaucoma in primates: cytochrome oxidase reactivity in parvo- and magnocellular pathways. Invest Ophthalmol Vis Sci. 2000;41:1791–1802. [PubMed] [Google Scholar]
- 117.Vickers JC, Schumer RA, Podos SM, et al. Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res. 1995;680:23–35. doi: 10.1016/0006-8993(95)00211-8. [DOI] [PubMed] [Google Scholar]
- 118.Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 119.Chauhan BC, LeVatte TL, Jollimore CA, et al. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci. 2004;45:144–152. doi: 10.1167/iovs.03-0687. [DOI] [PubMed] [Google Scholar]
- 120.Orgul S, Cioffi GA, Bacon DR, et al. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J Glaucoma. 1996;5:135–138. [PubMed] [Google Scholar]
- 121.Orgul S, Cioffi GA, Wilson DJ, et al. An endothelin-1 induced model of optic nerve ischemia in the rabbit. Invest Ophthalmol Vis Sci. 1996;37:1860–1869. [PubMed] [Google Scholar]
- 122.Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9:S34–36. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- 123.Cioffi GA, Sullivan P, Van Buskirk EM, et al. The primate optic nerve following chronic ischemia. In: Krieglstein GK, ed. Glaucoma Update VI Vol. 6 Berlin: Springer-Verlag; 2000:103.
- 124.Radius RL. Regional specificity in anatomy at the lamina cribrosa. Arch Ophthalmol. 1981;99:478–480. doi: 10.1001/archopht.1981.03930010480020. [DOI] [PubMed] [Google Scholar]
- 125.Radius RL, Gonzales M. Anatomy of the lamina cribrosa in human eyes. Arch Ophthalmol. 1981;99:2159–2162. doi: 10.1001/archopht.1981.03930021035010. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez RM, Dunkelberger GR, Quigley HA. The number and diameter distribution of axons in the monkey optic nerve. Invest Ophthalmol Vis Sci. 1986;27:1342–1350. [PubMed] [Google Scholar]
- 127.Potts AM, Hodges D, Shelman CB, et al. Morphology of the primate optic nerve. 3. Fiber characteristics of the foveal outflow. Invest Ophthalmol. 1972;11:1004–1016. [PubMed] [Google Scholar]
- 128.Ogden TE, Miller RF. Studies of the optic nerve of the rhesus monkey: nerve fiber spectrum and physiological properties. Vision Res. 1966;6:485–506. [PubMed] [Google Scholar]
- 129.Mikelberg FS, Drance SM, Schulzer M, et al. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- 130.Kupfer C, Chumbley L, Downer JC. Quantitative histology of optic nerve, optic tract and lateral geniculate nucleus of man. J Anat. 1967;101:393–401. [PMC free article] [PubMed] [Google Scholar]
- 131.Cull G, Cioffi GA, Dong J, et al. Estimating normal optic nerve axon numbers in non-human primate eyes. J Glaucoma. 2003;12:301–306. doi: 10.1097/00061198-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 132.Cioffi GA, Wang L, Fortune B, et al. Chronic ischemia induces regional axonal damage in experimental primate optic neuropathy. Arch Ophthalmol. 2004;122:1517–1525. doi: 10.1001/archopht.122.10.1517. [DOI] [PubMed] [Google Scholar]
- 133.Cioffi GA, Orgul S. The effects of chronic optic nerve ischemia in the rabbit. In: Drance SM, ed. Vascular Risk Factors and Neuroprotection in Glaucoma-Update 1996 Amsterdam: Kugler ; 1997:115–122.
- 134.Piltz-Seymour JR, Grunwald JE, Hariprasad SM, et al. Optic nerve blood flow is diminished in eyes of primary open-angle glaucoma suspects. Am J Ophthalmol. 2001;132:63–69. doi: 10.1016/s0002-9394(01)00871-6. [DOI] [PubMed] [Google Scholar]
- 135.Boehm AG, Pillunat LE, Koeller U, et al. Regional distribution of optic nerve head blood flow. Graefes Arch Clin Exp Ophthalmol. 1999;237:484–488. doi: 10.1007/s004170050266. [DOI] [PubMed] [Google Scholar]
- 136.Pillunat LE, Anderson DR, Knighton RW, et al. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997;64:737–744. doi: 10.1006/exer.1996.0263. [DOI] [PubMed] [Google Scholar]
- 137.Quigley HA, Hohman RM, Addicks EM. Quantitative study of optic nerve head capillaries in experimental optic disk pallor. Am J Ophthalmol. 1982;93:689–699. doi: 10.1016/0002-9394(82)90461-5. [DOI] [PubMed] [Google Scholar]
- 138.Rogers SD, Demaster E, Catton M, et al. Expression of endothelin-B receptors by glia in vivo is increased after CNS injury in rats, rabbits, and humans. Exp Neurol. 1997;145:180–195. doi: 10.1006/exnr.1997.6468. [DOI] [PubMed] [Google Scholar]
- 139.Uesugi M, Kasuya Y, Hayashi K, et al. SB209670, a potent endothelin receptor antagonist, prevents or delays axonal degeneration after spinal cord injury. Brain Res. 1998;786:235–239. doi: 10.1016/s0006-8993(97)01431-5. [DOI] [PubMed] [Google Scholar]
- 140.Yamashita K, Niwa M, Kataoka Y, et al. Microglia with an endothelin ETB receptor aggregate in rat hippocampus CA1 subfields following transient forebrain ischemia. J Neurochem. 1994;63:1042–1051. doi: 10.1046/j.1471-4159.1994.63031042.x. [DOI] [PubMed] [Google Scholar]
- 141.Koyama Y, Takemura M, Fujiki K, et al. BQ788, an endothelin ET(B) receptor antagonist, attenuates stab wound injury-induced reactive astrocytes in rat brain. Glia. 1999;26:268–271. doi: 10.1002/(sici)1098-1136(199905)26:3<268::aid-glia8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]