Abstract
In 1998, treatment completion was low among chlamydia and gonorrhea cases reported to the San Francisco Department of Public Health and assigned for treatment follow-up.
To improve treatment completion among growing numbers of chlamydia and gonorrhea cases, the department implemented field-delivered therapy (FDT), a single-dose, directly observed therapy protocol for uncomplicated chlamydial and gonococcal infections. After the protocol was implemented in March 1999, the proportion of cases completing treatment increased significantly, from 61.8% in 1998 to 81.0% in 2000. The greatest increases in treatment completion were observed for females and individuals younger than 20 years old.
FDT is an effective, feasible, and convenient way to reach and treat individuals who are unlikely to return for chlamydia and gonorrhea treatment.
CHLAMYDIA AND GONORRHEA are the most commonly reported diseases in the United States.1 In women, these sexually transmitted diseases (STDs) have common and severe consequences if left untreated: pelvic inflammatory disease, infertility, ectopic pregnancy, and chronic pelvic pain.2,3 In both men and women, chlamydia and gonorrhea are associated with a two- to fivefold increase in risk for HIV infection.4 While these infections may cause symptoms of discharge and dysuria, most chlamydial and gonococcal infections are asymptomatic.5 Screening programs are therefore critical for their detection and control.
However, a proportion of detected, asymptomatic infections remain untreated either because people cannot be found after their initial screening for treatment follow-up or because some people are not motivated to make a clinical treatment visit for asymptomatic infection. During 1998, 38% (165 of 432) of people assigned for chlamydia and gonorrhea treatment follow-up by the STD program staff of the San Francisco Department of Public Health were not treated. In addition, new noninvasive urine-based nucleic acid amplification tests allowed us to expand screening in nonclinical settings in 1999, further increasing the number of chlamydial and gonococcal infections detected and needing treatment follow-up.
Given the high proportion of untreated cases and increased case detection, innovative approaches to increasing treatment were needed. Field delivery of medication was an integral part of directly observed therapy for tuberculosis6 but no similar protocol was in place for STD treatment. Because single-dose, orally administered treatments were available for chlamydia and gonorrhea and because directly observed therapy had been successful in ensuring treatment for tuberculosis,6 the San Francisco Department of Public Health’s STD program implemented a similar protocol in March 1999. We called our program fielddelivered therapy (FDT).
The objective of FDT was to allow STD program field staff, under the medical license of the STD controller, to treat uncomplicated chlamydial and gonococcal infections in persons who were unable, unwilling, or unlikely to come into the municipal STD clinic for treatment. We evaluated the FDT protocol by measuring the number of individuals accepting medication in the field, determining the proportion of individuals treated, and characterizing the population receiving field treatment.
THE PROTOCOL OF FIELD-DELIVERED THERAPY
Development of the FDT protocol included designing a field pack, staff training, and implementation. The field pack contained all of the items needed for obtaining consent for treatment, administering medication, and educating patients about STDs and safer sex practices (first box). Before implementing the FDT protocol, field staff received two 1-hour training sessions from the STD controller, a licensed physician, on how to evaluate whether a patient should be treated in the field or referred to the STD clinic, administer antibiotics, and recognize adverse reactions. After completing the training, staff expanded their regular field activities to include FDT. Patients who did not receive treatment at the time of their initial medical visit were assigned for treatment follow-up. If a patient was unable or unwilling to come to the STD clinic for treatment, staff would go into the field to meet the patient and deliver therapy following the FTD protocol (second box).
KEY FINDINGS.
Oral, single-dose medications for uncomplicated chlamydial and gonococcal infections can be safely administered in the field.
Field-delivered therapy is an effective means to increase the proportion of patients treated, especially young persons and women.
Field-delivered therapy is a feasible new tool for STD control that can be adopted by local health departments.
DISCUSSION AND EVALUATION
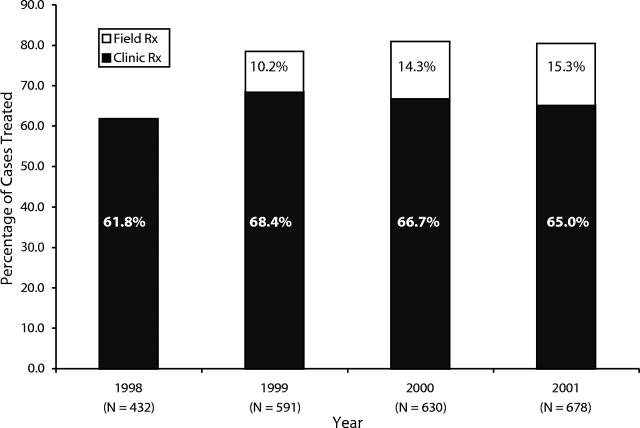
In 1998, before FDT was implemented, 61.8% of assigned cases had documented treatment. In 2000, the first full calendar year that FDT was in place, treatment completion was 81.0%, a 31.0% relative increase over 1998. This increase in the proportion of assigned cases that were treated occurred despite a 46% rise in the number of assigned chlamydia and gonorrhea cases. The increase in treatment completion was maintained in 2001, with 80.3% of assigned cases treated and 15.3% of cases receiving FDT (Figure 1 ▶).
FIGURE 1—
Percentage of chlamydia and gonorrhea cases treated before and after implementation of field- delivered therapy: San Francisco, 1998 to 2001.
FIELD-DELIVERED THERAPY PACK.
- Single-dose medication packets
- Chlamydia (Azithromycin, 1 g)
- Gonorrhea (Cefixime, 400 mg)
- 3 × 5 medication card
- Medication name
- Lot number
- Expiration
Medication instructions
Dosing cup (to mix medication)
8 oz bottle of water
Consent forms (English and Spanish)
STD fact sheets (English, Spanish, Chinese, and Russian)
Condoms
Field staff identification badge
Stationery
Cellular phone
The percentage of cases receiving treatment increased across all age, race, and gender/ sexual orientation categories, with the greatest increases observed for women and persons younger than 20 years (Table 1 ▶). Among men who have sex with men (MSM), treatment completion was very high, above 90% in 1998 and 2000. We believe this is because MSM in San Francisco are more conscientious of their sexual health as a result of the HIV epidemic. Excluding MSM, further stratification by gender showed large increases in the percentage of White women (123.1% increase, P < .001) and Hispanic women (67.9% increase, P = .001) treated. Among non-MSM males, increases were seen for individuals younger than 20 (76% increase, P = .002) and for African Americans (40.8% increase, P = .003).
TABLE 1—
Demographic Characteristics of Chlamydia and Gonorrhea Cases Receiving Treatment Before and After Implementation of Field-Delivered Therapy (FDT): San Francisco, 1998 and 2000
| Cases Receiving Treatment, % | Relative Increase in Cases Treated | |||||
| Characteristic | Before FDT (1998) (n=432) | After FDT (2000) (n=630) | Percentage | P | ||
| Age, y | ||||||
| 12–19 | 53.6 | (59/110) | 81.3 | (113/139) | 51.6 | <.001 |
| 20–24 | 61.9 | (78/126) | 75.0 | (135/180) | 21.2 | .014 |
| 25–29 | 60.6 | (57/94) | 81.5 | (110/135) | 34.4 | <.001 |
| 30–34 | 70.2 | (40/57) | 82.9 | (58/70) | 18.1 | .090 |
| ≥ 35 | 73.3 | (33/45) | 88.7 | (94/106) | 20.9 | .018 |
| Race/ethnicity | ||||||
| African American | 58.8 | (120/204) | 77.3 | (187/242) | 31.4 | <.001 |
| Hispanic | 65.5 | (55/84) | 80.1 | (113/141) | 22.4 | .014 |
| White | 62.1 | (59/95) | 86.7 | (143/165) | 39.5 | <.001 |
| Asian/Pacific Islander | 74.3 | (26/35) | 89.2 | (58/65) | 20.1 | .052 |
| Gender/sexual orientation | ||||||
| Female | 57.1 | (121/212) | 80.4 | (221/275) | 40.8 | <.001 |
| MSM | 92.9 | (39/42) | 94.8 | (128/135) | 2.1 | .631 |
| Other male | 59.9 | (106/177) | 73.9 | (161/218) | 23.3 | .003 |
| Overall | 61.8 | (267/432) | 81.0 | (510/630) | 31.0 | <.001 |
Note. MSM=men who have sex with men.
FIELD-DELIVERED THERAPY PROTOCOL.
Arrange time/place to meet patient
Confirm identity of patient
Assure confidentiality
Counsel patient about chlamydial or gonococcal infection
Discuss need for treatment
Offer field treatment
- Assess patient for:
- Allergies to antibiotics
- Current medications
- Serious medical conditions
- Symptoms of STDs
- Indications of current substance use
Obtain written consent
Administer medication
Observe for adverse reactions (15 minutes)
- Counsel patient
- Partner notification
- STD prevention
Provide fact sheets and condoms
Refer to STD clinic if cannot treat in field
There was an increase in the number of staff workers treating follow-up cases during this time; however, since these people also had other duties, the total amount of staff time spent on treatment follow-up remained roughly the same. Thus, it is unlikely that the increase in treatment is entirely explained by these staff changes.
FDT is not only effective in increasing treatment completion, but it is feasible and convenient. As staff were already working in the field to locate patients, FDT was a natural extension of these activities at little additional cost. Although a formal cost analysis has not been done, we expect that FDT is cost saving since it saves clinician time and prevents complications associated with untreated infections. FDT is also convenient for patients, eliminating the need for a return trip to the clinic.
NEXT STEPS
Health departments must continually devise new and innovative approaches to detect and treat STDs, especially among hard-to-reach populations and persons at risk for complications. Our program found that before FDT was implemented, the proportion of assigned chlamydia and gonorrhea cases receiving treatment was low, especially among young persons, who are often difficult to motivate to return for treatment, and women, who are at risk for sequelae from untreated infections. After FDT became available in 1999, the percentage of cases completing treatment increased significantly. We will continue to monitor trends in treatment completion to see if the percentage of cases treated continues to increase or reaches a plateau.
FDT is an effective, feasible, and convenient means to ensure treatment completion in these demographic groups. As many health departments already have staff that work in the field, other localities should evaluate FDT as a new tool for STD control.
Acknowledgments
We thank the field staff—Christine Geoghegan, Anna Branzuela, Felipe Acosta, Sharon Penn, Terrance Sha, Ilene Zolt, and Rosito Bartolini—for their commitment to patient care and STD control by delivering therapy in the field. Thanks are also extended to Gloria Calero and Bettye Spears for packaging the field-treatment therapy and for other support of this program.
Human Participant Protection
We collected data during routine public health activities of disease control and analyzed data for program evaluation. This activity was therefore designated as public health practice and non-research. In accordance with the Code of Federal Regulations, Title 45, Part 46, The Public Service Act, human subjects review is not required for public health non-research activities.
Contributors
K. C. Steiner conducted the data analysis and led the writing. C. K. Kent directed the analysis and assisted with writing and editing the manuscript. V. Davila and L. Fischer were responsible for drafting the protocol, training staff, and implementing field-delivered therapy. J. K. Chaw contributed to the data analysis. J. D. Klausner conceived the protocol and supervised its implementation. All authors reviewed drafts of the manuscript.
Peer Reviewed
References
- 1.Sexually Transmitted Diseases Surveillance, 2001. Atlanta, Ga: Centers for Disease Control and Prevention; September2002.
- 2.Cates W, Wasserheit JN. Genital chalmydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991;164:1771–1781. [DOI] [PubMed] [Google Scholar]
- 3.Sweet RL. Pelvic Inflammatory disease: etiology, diagnosis, and treatment. Sex Transm Dis. 1981;8(suppl 4): 308–315. [PubMed] [Google Scholar]
- 4.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korenromp EL, Sudaryo MK, de Vlas SJ, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS. 2002;13:91–101. [DOI] [PubMed] [Google Scholar]
- 6.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis, consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–948. [DOI] [PubMed] [Google Scholar]