Abstract
Objectives. We estimated the number and cost of syphilis-attributable HIV cases among African Americans.
Methods. A mathematical model of HIV transmission was used to estimate the number of partnerships consisting of HIV-discordant African Americans in which infectious syphilis was present and the number of new HIV cases attributable to syphilis in these partnerships.
Results. In 2000, an estimated 545 new cases of HIV infection among African Americans could be attributed to the facilitative effects of infectious syphilis, at a cost of about $113 million.
Conclusions. Syphilis prevention could reduce HIV incidence rates and the disproportionate burden of HIV/AIDS on the African American community, resulting in substantial reductions in future HIV/AIDS medical costs.
African Americans suffer disproportionate morbidity and mortality from a variety of preventable and treatable health conditions, including cancer, heart disease, and stroke.1,2 Elimination of racial health disparities is a major national priority, as is reflected in the federal government’s Healthy People 2010 goals.1 With regard to HIV and AIDS, African Americans have higher rates of incidence, prevalence, and mortality than any other racial or ethnic group in the United States.3 Of the 42 156 AIDS cases reported in the country in 2000, almost half (19 890) occurred among African Americans.4 The reported AIDS rate among African Americans in 2000 was almost 60 per 100 000 population, about 8 times the rate for non-Hispanic Whites.4 On the basis of recent estimates that African Americans account for about 54% of the approximately 40 000 new HIV infections that occur each year, 21 600 or more African Americans were infected with HIV in 2000.5
African Americans also are disproportionately affected by other sexually transmitted diseases (STDs), including syphilis.6,7 The 4231 new cases of primary and secondary syphilis among African Americans reported in 2000 represent more than 70% of all such cases.6 The rate of primary and secondary syphilis among African Americans (12.8 per 100 000) in 2000 was more than 20 times greater than the rate among non-Hispanic Whites.6 Addressing this racial disparity in syphilis is 1 of the primary goals of the national campaign to eliminate syphilis that was begun October 1999.8,9
Syphilis elimination efforts also might have an impact on HIV incidence rates. Ulcerative STDs such as syphilis can increase HIV infectiousness and susceptibility through a variety of biological processes, such as disruption of epithelial and mucosal barriers to infection.10–12 In this article, we estimate the number of HIV cases, and associated costs, attributable to the facilitative effects of infectious syphilis on HIV transmission and acquisition among African Americans. These syphilis-attributable HIV cases represent a potential reduction in HIV incidence among African Americans that could be achieved through syphilis prevention efforts.
METHODS
We adapted a simplified model of the effect of infectious syphilis on HIV transmission13,14 to estimate the number of new HIV cases among African Americans attributable to syphilis in 2000. If syphilis is to facilitate HIV transmission from 1 sex partner to another, the partners must initially be of discordant HIV status, and at least 1 of the partners must have infectious syphilis (for simplicity, we define a sexual partnership as 2 people engaged in at least 1 act of vaginal or anal intercourse). Therefore, the first step in our model was to estimate the number of HIV-discordant partnerships in which infectious syphilis was present. We multiplied this estimated number of partnerships by the estimated probability that a syphilis-attributable HIV transmission would occur in such partnerships to arrive at an estimate of the number of new HIV cases attributable to syphilis.
Specifically, the number of new cases (C) of HIV among African Americans attributable to syphilis in 2000 was estimated via the following equation:
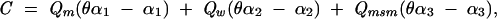 |
1 |
where Qm is the number of partnerships involving an HIV-infected man and an HIVuninfected woman in which infectious syphilis was present in 1 or both partners; θ, the “cofactor effect,” is the magnitude by which infectious syphilis increases the probability of HIV transmission; α1 is the per-partnership probability of male-to-female HIV transmission (the presence of infectious syphilis in 1 or both partners increases the probability of HIV transmission from α1 to θα1); Qw is the number of partnerships involving an HIVinfected woman and an HIV-uninfected man in which infectious syphilis was present in 1 or both partners; α2 is the per-partnership probability of female-to-male HIV transmission; Qmsm is the number of partnerships involving an HIV-infected man and an HIVuninfected man in which infectious syphilis was present in 1 or both partners; and α3 is the per-partnership probability of male-to-male HIV transmission. The terms within parentheses reflect the excess risk attributable to infectious syphilis (i.e., the probability of HIV transmission when infectious syphilis is present minus the probability of HIV transmission when syphilis is not present).
HIV-Discordant Partnerships
Estimates of the number of HIV-discordant partnerships (Qm, Qw, and Qmsm) in which infectious syphilis was present in at least 1 partner were based on the number of reported cases of syphilis. Each new case of syphilis occurring in a woman, for example, results from sexual activity with a partner who had infectious syphilis. Thus, the number of new syphilis cases reported in women (Nw) represents a lower-bound estimate of the number of partnerships in which a woman’s sex partner introduced infectious syphilis into the partnership (either by having syphilis at the onset of the partnership or by acquiring syphilis from a concurrent sex partner).
New cases of syphilis reported in women (Nw) were calculated as the number of reported cases of primary, secondary, and early latent syphilis; these reported cases represent new syphilis infections acquired within the previous year. If the percentage of new syphilis cases that are not reported is U, and if the percentage of syphilis cases in women that were acquired from sexual contact with another woman is λw, then the term Nw(1 − λw)/(1 − U) represents the estimated number of heterosexual partnerships in which the man introduced infectious syphilis into the partnership, after adjustment for underreporting of new syphilis cases. Not all such partnerships will result in syphilis transmission to the woman. To account for the partnerships in which infectious syphilis was present in the man but not acquired by the woman, we multiplied the term Nw(1 − λw)/(1 − U) by 1/S, where S is the per-partnership probability of syphilis transmission.
The probability that the partnership consisted of a woman without HIV and a man with HIV was calculated as Hm(1 − Δhw), where Hm and hw are HIV prevalence rates among men with syphilis and women without syphilis, respectively. The HIV prevalence rate in women was multiplied by Δ to indicate that HIV-infected persons are Δ times more likely to choose HIV-infected partners than would be expected by chance alone (“assortative partner selection”).
Thus, estimates of the number of HIVdiscordant partnerships (Qm, Qw, and Qmsm) in which infectious syphilis was present in at least 1 partner were calculated as follows:
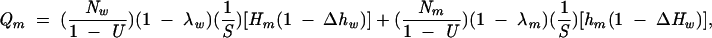 |
2 |
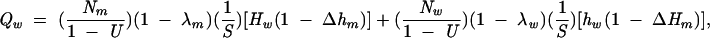 |
3 |
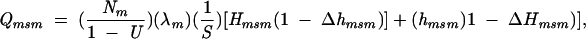 |
4 |
where Nm and Nw are the number of primary, secondary, and early latent syphilis cases in African American men and women, respectively, reported in 2000 to the Centers for Disease Control and Prevention (CDC)6; U is the proportion of syphilis cases not reported; λm and λw are the percentages of syphilis cases in men and women that were acquired from a same-sex partner; S is the per-partnership probability of syphilis transmission; Hm, Hw, and Hmsm are HIV prevalence rates among heterosexual men with syphilis, heterosexual women with syphilis, and men who have sex with men (MSM) with syphilis; Δ is an adjustment factor for assortative partner selection; and hm, hw, and hmsm are HIV prevalence rates among heterosexual men, heterosexual women, and MSM without syphilis (Table 1 ▶). In the model, the ΔH and Δh terms were not allowed to exceed a value of 1.
TABLE 1—
Parameter Values, Ranges, and Sources
| Parameter | Base Case Value | Range | Source for Base Case Value |
| Per-partnership probability of HIV transmission, male to female (α1) | 0.08a | 0.04–0.12 | Mastro and de Vincenzi16 |
| Per-partnership probability of HIV transmission, female to male (α2) | 0.04a | 0.02–0.06 | Mastro and de Vincenzi16 |
| Per-partnership probability of HIV transmission, male to male (α3) | 0.07b | 0.04–0.12 | Grant et al.35 |
| Syphilis cofactor effect (magnitude of increase in HIV transmission probability due to infectious syphilis), per partnership (θ) | 2.6 | 1.4–3.8 | Padian et al.17 |
| Probability of syphilis transmission, per partnership (S) | 0.55 | 0.3–0.8 | Cates et al.36; Hook37; Garnett et al.38 |
| HIV prevalence in men with syphilis (Hm) | 23%c | 12%–30% | Blocker et al.11 |
| HIV prevalence in women with syphilis (Hw) | 12% | 6%–20% | Blocker et al.11 |
| HIV prevalence in MSM with syphilis (Hmsm) | 64%c | 12%–90% | Blocker et al.11 |
| HIV prevalence in men without syphilis (hm) | 4.5% | 0%–9% | Blocker et al.11 |
| HIV prevalence in women without syphilis (hw) | 2.7% | 0%–5% | Blocker et al.11 |
| HIV prevalence in African American MSM without syphilis (hmsm) | 14% | 5%–30% | CDC39 |
| No. of reported primary, secondary, and early latent syphilis cases in African American men (Nm) | 6124d | . . . | Division of STD Prevention6 |
| No. of reported primary, secondary, and early latent syphilis cases in African American women (Nw) | 4818d | . . . | Division of STD Prevention6 |
| Percentage of syphilis cases not reported (U) | 28%e | 0%–50% | Hook and Marra40 |
| Percentage of syphilis cases in men acquired from male sex partner (λm) | 11%f | 0%–30% | Assumed |
| Percentage of syphilis cases in women acquired from female sex partner (λw) | 1%g | 0%–2% | Assumed |
| Assortative matching by HIV status (Δ) | 3h | 1–5 | Dow and Philipson41 |
Note. MSM = men who have sex with men.
aMastro and de Vincenzi’s review16 of studies of stable partnerships suggests average per-partnership HIV transmission probabilities of 0.23 (male to female) and 0.12 (female to male). Although these HIV transmission probabilities are not adjusted for the possible presence of syphilis in the partnerships, any arising bias is probably small, because syphilis prevalence is likely low in these partnerships. As described in the text, we applied lower transmission probabilities because partnerships in which syphilis is present may be of shorter duration than the stable partnerships on which these HIV transmission probability estimates were based.
bHIV transmission probabilities for MSM were calculated as the average for the receptive and the insertive partners. The per-partnership risk associated with receptive anal intercourse has been estimated at 0.10.35 As a result of a scarcity of data, we used the base case female-to-male HIV transmission probability (0.04) to estimate the per-partnership risk for the insertive partner.
cMedian HIV seroprevalence rates were 27.5% (interquartile range: 23.1%–29.6%) among men with syphilis and ranged from 64.3% to 90% among MSM with syphilis.11 We applied the lower values of these ranges as the base case values. Estimated HIV prevalence rates among heterosexual men and women without syphilis are not general-population estimates but are for persons at high risk of acquiring syphilis (by virtue of having a sex partner with infectious syphilis) and were based on HIV prevalence rates observed in high-risk settings such as STD clinics. HIV prevalence among MSM without infectious syphilis was based on a recent seroprevalence study of African American MSM aged 15–22 years in 7 US cities. 39 With this exception (HIV prevalence among MSM without syphilis), no HIV prevalence estimate was based on data specific to African Americans.
dWe assumed that the distribution of reported early latent cases by sex and race/ethnicity would be the same as the distribution of primary and secondary cases.
eAs many as half of all syphilis cases might not be reported.40 We assumed that 28% of primary, secondary, and early latent syphilis cases are not reported on the basis of the distribution of reported syphilis cases from 1980 to 1999. Over this period, reported late and late latent syphilis cases represented 28% of the total number of reported cases of primary, secondary, early latent, late, and late latent syphilis. 42
fReported syphilis cases in men exceed reported cases in women by about 1306. In the base case, we assumed that half of these excess cases (roughly 11% of the total cases in men) were attributable to same-sex contact.
gApproximately 1% of women in the United States report having had same-sex contact in the previous year.43,44 We therefore assumed that 1% of syphilis cases in women could be attributed to same-sex contact. For simplicity, we did not consider the potential effect of syphilis on HIV transmission between women.
hThe assortative matching factor Δ indicates that HIV prevalence in partners of HIV-infected persons is Δ times higher than would be expected by chance alone.
Parameter Values
Parameter values were based on published sources (Table 1 ▶). We used lower per-partnership transmission probabilities than suggested by the literature because partnerships in which syphilis is present may be of shorter duration than the stable partnerships upon which most per-partnership transmission probabilities are based. HIV transmission might be less likely in partnerships of shorter duration, because there may be fewer opportunities (sex acts) for transmission to occur.
In 1 relevant study, HIV transmission was 57% less likely in partnerships of shorter duration (fewer than 10 contacts) than in partnerships of longer duration (2000 contacts).15 In our analysis, we conservatively applied base case per-partnership HIV transmission values of 8% (male to female) and 4% (female to male), roughly one third the average estimates of 23% and 12% found in studies of stable partnerships.16
Estimates of HIV prevalence rates among persons with syphilis (Hm, Hw, and Hmsm) were based on a recent review of more than 30 US studies of syphilis and HIV coinfection rates.11 We assumed HIV prevalence rates of 4.5% and 2.7% for heterosexual men and women without syphilis but at high risk for syphilis, on the basis of median HIV seroprevalence rates observed in high-risk settings such as STD clinics.11
The base case value of the per-partnership cofactor effect of infectious syphilis on HIV transmission was based on a large study of heterosexual transmission of HIV in the United States showing that a history of STD (the most important risk factor identified) was associated with an adjusted odds ratio of 2.6 for male-to-female HIV transmission in stable partnerships.17
Cost
To estimate the cost of syphilis-attributable HIV cases among African Americans, we multiplied the estimated number of new HIV cases attributable to infectious syphilis by the estimated discounted lifetime direct medical treatment cost per case of HIV ($207 000 in 1999 dollars18). Because HIV treatments continue to evolve at a rapid pace, there is considerable uncertainty in the estimated lifetime cost of HIV care. We therefore varied the cost per case of HIV from $125 000 to $275 000.
Sensitivity Analyses
We performed 3 sensitivity analyses. First, we performed a univariate sensitivity analysis to examine how our estimates would change when we varied each parameter individually, holding other parameters at their base case values. Second, we conducted a multivariate (“Monte Carlo”) sensitivity analysis in which we randomly assigned to each parameter a value between its lower- and upper-bound estimates and then recalculated the number of syphilis-attributable HIV cases. We repeated this procedure 10 000 times to obtain a distribution of the estimated number of HIV cases attributable to syphilis.
Third, we addressed the uncertainty in partnership duration by assuming that each partnership consisted of exactly 1 act of unprotected intercourse. Specifically, we repeated the base case analysis using per-act estimates of HIV transmission (0.001, male to female; 0.0006, female to male; 0.0044, male to male), syphilis transmission (0.25), and the cofactor effect of syphilis on HIV transmission (30, with a range of 10 to 50). The estimated per-act cofactor effect is substantially higher than the per-partnership cofactor effect because as the length of the partnership increases, the proportion of the partnership duration in which infectious syphilis is present decreases.19
RESULTS
Under base case assumptions, we estimated that about 545 new cases of HIV among African Americans in 2000 could be attributed to the facilitative effects of infectious syphilis on HIV transmission (Table 2 ▶). These 545 cases represent about 3% to 5% of all new HIV cases among African Americans in 2000, assuming that there are 11 200 to 21 600 new HIV infections among African Americans each year.5,20 We estimated the future treatment cost of these 545 syphilis-attributable HIV cases among African Americans to be approximately $113 million (at $207 000 per case), with a range of $68 million ($125 000 per case) to $150 million ($275 000 per case).
TABLE 2—
Estimates of the Number of New Syphilis Cases, the Number of HIV-Discordant Partnerships in Which Infectious Syphilis Is Present, and the Number of New HIV Cases Attributable to Syphilis Among African Americans in 2000: Heterosexual Men, Heterosexual Women, and Men Who Have Sex With Men (MSM)
| Heterosexual Men | Heterosexual Women | MSM | Totala | |
| No. of primary, secondary, and early latent syphilis cases, adjusted for underreporting | 7 570 | 6 625 | 936 | 15 130 |
| No. of HIV-discordant partnerships in which infectious syphilis is present, HIV-uninfected index partnerb | 1 529 | 2 942 | 631 | 5 103 |
| No. of new HIV cases among index partners | 159 | 612 | 115 | 886 |
| No. of new HIV cases among index partners if syphilis had not been present | 61 | 235 | 44 | 341 |
| No. of new HIV cases attributable to syphilis | 98 | 377 | 71 | 545 |
aTotals may differ from sums of individual columns owing to rounding.
bIndex partner is the member of the group noted in the column.
In the 1-way sensitivity analysis (Table 3 ▶), the estimated number of new cases of HIV attributable to syphilis ranged from 136 to 1000. The results were most sensitive to the transmission probability of HIV and syphilis, the estimated cofactor effect of syphilis on HIV transmission, and HIV prevalence rates. In the multivariate (Monte Carlo) sensitivity analysis, the mean number of syphilis-attributable HIV cases ranged from 133 to 1330 in 90% of the simulations, with a mean of 568.
TABLE 3—
Estimated Number of New HIV Infections Attributable to Syphilis Among African Americans After Application of Lower- and Upper-Bound Values of Model Parameters: Sensitivity Analyses
| Parameter Varied | Lower Value of Parameter Applied | Upper Value of Parameter Applied |
| Univariate sensitivity analysis | ||
| Probability of HIV transmission (α1, α2, α3) | 278 | 833 |
| Probability of syphilis transmission (S) | 1000a | 375 |
| Syphilis cofactor effect (θ) | 136 | 954 |
| HIV prevalence rates (Hm, Hw, Hmsm, hm, hw, hmsm) | 263 | 606 |
| Percentage of syphilis cases not reported (U) | 393 | 785 |
| Percentage of syphilis cases acquired from same-sex partner (λm, λw) | 495 | 634 |
| Assortative matching factor (Δ) | 646a | 457 |
| Multivariate (Monte Carlo) sensitivity analysis, mean and rangeb | 568 | 133–1330 |
| Per-act sensitivity analysis, mean and rangeb | 424 | 131–716 |
aLower values of the probability of syphilis transmission and the assortative matching factor lead to higher estimates of the number of syphilis-attributable HIV cases.
bFor the multivariate sensitivity analysis, the range represents the 5th and 95th percentiles of estimates in the Monte Carlo simulation. For the per-act sensitivity analysis, the mean represents the base case results, and the lower and upper bounds of the range were calculated using values of 10 and 50, respectively, for the per-act cofactor effect of syphilis.
In the per-act sensitivity analysis, we estimated that 424 new cases of HIV among African Americans could be attributable to syphilis in 2000. This estimate, based on a per-act cofactor effect of 30, ranged from 131 (cofactor = 10) to 716 (cofactor = 50).
DISCUSSION
We estimated that 545 new cases of HIV infection among African Americans in 2000 could be attributed to the facilitative effects of infectious syphilis on HIV transmission. The discounted, lifetime cost of HIV-related medical care associated with these 545 cases is about $113 million. In comparison, nationwide syphilis elimination efforts will require an estimated $60 million annually in federal, state, and local funds.8 These program costs are considerably less than the base case estimate of the syphilis-attributable HIV treatment costs that could be averted through syphilis prevention. In addition, syphilis prevention can avert substantial syphilis treatment costs, such as those associated with congenital syphilis and related complications.
Despite the overall decline in syphilis in the United States from 1990 to 2000, increases have occurred in several states and cities in recent years, particularly among MSM.9 Recent reports of outbreaks of syphilis among MSM across the country highlight the importance of enhanced syphilis prevention efforts.9,21 Our results indicate that syphilis prevention can have a discernible impact on HIV incidence and HIV-related costs, particularly in areas with increasing syphilis incidence rates.
Our estimate of the reduction in HIV incidence that could be achieved through syphilis prevention is based solely on the potential reduction in syphilis-attributable HIV infections and ignores the possible impact of syphilis prevention efforts on sexual behaviors. Because these efforts also might promote healthy sexual behaviors (e.g., increased condom usage or decreases in number of sex partners), the impact of syphilis prevention on HIV incidence might be far greater than we have estimated.
Our analysis is subject to several limitations. Some parameter values were based on limited information, including the percentage of syphilis cases acquired from a same-sex partner, the percentage of syphilis cases not reported, the per-partnership probability of transmission, and the cofactor effect of syphilis on HIV transmission.
The magnitude of the cofactor effect of syphilis on HIV transmission is particularly difficult to estimate, because studies that attempt to quantify the association between syphilis and HIV must control for numerous confounding factors related to an individual’s risk of acquiring syphilis and HIV, such as his or her number of sex partners.12,22–26 As a result of limited data, we assumed that the syphilis cofactor effect for male-to-male HIV transmission was the same as that for heterosexual transmission. However, syphilis might not play as important a role in male-to-male HIV transmission, because MSM without HIV who have unprotected anal intercourse with partners of unknown HIV status are at high risk for acquiring HIV even in the absence of syphilis.24
Our model omitted secondary transmission of HIV. Although we focused on the effect of syphilis on HIV incidence in 2000, these syphilis-attributable HIV cases in 2000 could lead to more HIV cases in subsequent years. The importance of preventing secondary infections has been demonstrated by morecomplex transmission models examining the subsequent spread of HIV in at-risk populations.27–31
For simplicity, we ignored the possibility that some of the syphilis cases occurring among African Americans might have been acquired from partners of another race. However, such instances are probably balanced out by instances in which syphilis cases in partners of other races (which were not included in our analysis) were acquired from African American partners.
Measures of condom use and the relative frequency of oral, anal, or vaginal sex acts were not included as inputs for our model. Instead, the transmission probabilities we used were based on studies of numerous partnerships and therefore represent an average “mix” of oral, anal, and vaginal sex acts as well as average rates of condom use and effectiveness. Our estimate of the number of partnerships in which infectious syphilis was present was based on cases of syphilis reported to the CDC, and it is unlikely that a substantial number of these syphilis cases were acquired by persons who used condoms consistently and correctly. Additional limitations of our approach and limitations of similar models of HIV transmission have been discussed elsewhere.13–15,32–34
Despite these limitations, our model provides useful estimates of the number of HIV cases among African Americans attributable to infectious syphilis and of the potential benefits of syphilis prevention in the United States. A successful national syphilis elimination program could reduce HIV incidence among African Americans by 3% to 5% and could avert as much as $113 million or more annually in lifetime HIV-related medical care costs. Because our analysis did not consider secondary transmission of HIV or the potential reduction in risky sexual behaviors that might result from syphilis prevention activities, the potential impact of syphilis prevention on HIV incidence could be even more substantial than we estimated.
Acknowledgments
Steven D. Pinkerton was supported, in part, by grants K02-MH01919 and P30-MH52776 from the National Institute of Mental Health.
We thank Kathleen Irwin, MD, and John Blandford, PhD, of the Division of STD Prevention, Centers for Disease Control and Prevention, and two anonymous reviewers for helpful comments.
Human Participant Protection
No protocol approval was needed for this study.
Contributors
H. W. Chesson and S. D. Pinkerton developed the mathematical model with input from G. W. Counts and R. Voigt. H. W. Chesson performed all calculations. All the authors contributed to the interpretation of the results and the writing of the article.
Peer Reviewed
References
- 1.Healthy People 2010. Washington, DC: US Dept of Health and Human Services; 2000. .
- 2.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. [PMC free article] [PubMed]
- 3.Smith DK, Gwinn M, Selik RM, et al. HIV/AIDS among African Americans: progress or progression? AIDS. 2000;14:1237–1248. [DOI] [PubMed] [Google Scholar]
- 4.Division of HIV Prevention. HIV/AIDS Among African Americans. Atlanta, Ga: Centers for Disease Control and Prevention; 2002.
- 5.Division of HIV Prevention. A Glance at the HIV Epidemic. Atlanta, Ga: Centers for Disease Control and Prevention; 2002.
- 6.Division of STD Prevention. Sexually Transmitted Disease Surveillance, 2000. Atlanta, Ga: Centers for Disease Control and Prevention; 2001.
- 7.Gayle HD, Counts GW. Syphilis elimination: a unique time in history. J Am Med Womens Assoc. 2001;56:2–3. [PubMed] [Google Scholar]
- 8.Division of STD Prevention. The National Plan to Eliminate Syphilis From the United States. Atlanta, Ga: Centers for Disease Control and Prevention; 1999.
- 9.Centers for Disease Control and Prevention. Primary and secondary syphilis—United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:113–117. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. HIV prevention through early detection and treatment of other sexually transmitted diseases—United States. MMWR Morb Mortal Wkly Rep. 1998;47:1–24.9450721 [Google Scholar]
- 11.Blocker ME, Levine WC, St. Louis ME. HIV prevalence in patients with syphilis, United States. Sex Transm Dis. 2000;27:53–59. [DOI] [PubMed] [Google Scholar]
- 12.Wasserheit JN. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 13.Chesson HW, Pinkerton SD, Irwin KL, Rein D, Kassler WJ. New HIV cases attributable to syphilis in the USA: estimates from a simplified transmission model. AIDS. 1999;13:1387–1396. [DOI] [PubMed] [Google Scholar]
- 14.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr. 2000;24:48–56. [DOI] [PubMed] [Google Scholar]
- 15.Downs AM, de Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:388–395. [DOI] [PubMed] [Google Scholar]
- 16.Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS. 1996;10(suppl A):S75–S82. [DOI] [PubMed] [Google Scholar]
- 17.Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–357. [DOI] [PubMed] [Google Scholar]
- 18.Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:54–62. [DOI] [PubMed] [Google Scholar]
- 19.Hayes RJ, Schulz KF, Plummer FA. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J Trop Med Hyg. 1995;98:1–8. [PubMed] [Google Scholar]
- 20.Division of HIV Prevention. HIV/AIDS Surveillance Report, 2001. Atlanta, Ga: Centers for Disease Control and Prevention; 2002.
- 21.Wolitski RJ, Valdiserri RO, Denning PH, Levine WC. Are we headed for a resurgence of the HIV epidemic among men who have sex with men? Am J Public Health. 2001;91:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boily MC, Anderson RM. Human immunodeficiency virus transmission and the role of other sexually transmitted diseases: measures of association and study design. Sex Transm Dis. 1996;23:312–332. [DOI] [PubMed] [Google Scholar]
- 23.Mertens TE, Hayes RJ, Smith PG. Epidemiological methods to study the interaction between HIV infection and other sexually transmitted diseases. AIDS. 1990;4:57–65. [DOI] [PubMed] [Google Scholar]
- 24.Bonell C, Weatherburn P, Hickson F. Sexually transmitted infection as a risk factor for homosexual HIV transmission: a systematic review of epidemiological studies. Int J STD AIDS. 2000;11:697–700. [DOI] [PubMed] [Google Scholar]
- 25.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28:579–597. [DOI] [PubMed] [Google Scholar]
- 26.Korenromp EL, de Vlass SJ, Nagelkerke NJ, Habbema JD. Estimating the magnitude of STD cofactor effects on HIV transmission: how well can it be done? Sex Transm Dis. 2001;28:613–621. [DOI] [PubMed] [Google Scholar]
- 27.Over M, Piot P. Human immunodeficiency virus infection and other sexually transmitted diseases in developing countries: public health importance and priorities for resource allocation. J Infect Dis. 1996;174(suppl 2):S162–S175. [DOI] [PubMed] [Google Scholar]
- 28.Boily MC, Brunham RC. The impact of HIV and other STDs on human populations: are predictions possible? Infect Dis Clin North Am. 1993;7:771–792. [PubMed] [Google Scholar]
- 29.Garnett GP, Anderson RM. Strategies for limiting the spread of HIV in developing countries: conclusions based on studies of the transmission dynamics of the virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:500–513. [PubMed] [Google Scholar]
- 30.Robinson NJ, Mulder DW, Auvert B, Hayes RJ. Proportion of HIV infections attributable to other sexually transmitted diseases in a rural Ugandan population: simulation model estimates. Int J Epidemiol. 1997;26:180–189. [DOI] [PubMed] [Google Scholar]
- 31.Renton AM, Whitaker L, Riddlesdell M. Heterosexual HIV transmission and STD prevalence: predictions of a theoretical model. Sex Transm Infect. 1998;74:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtgrave DR, Leviton LC, Wagstaff DA, Pinkerton SD. Cumulative probability of HIV infection: a summary risk measure for HIV prevention intervention studies. AIDS Behav. 1997;1:169–172. [Google Scholar]
- 33.Rehle TM, Saidel TJ, Hassig SE, Bouey PD, Gaillard EM, Sokal DC. AVERT: a user-friendly model to estimate the impact of HIV/sexually transmitted disease prevention interventions on HIV transmission. AIDS. 1998;12(suppl 2):S27–S35. [PubMed] [Google Scholar]
- 34.Kaplan EH. Modeling HIV infectivity: must sex acts be counted? J Acquir Immune Defic Syndr. 1990;3:55–61. [PubMed] [Google Scholar]
- 35.Grant RM, Wiley JA, Winkelstein W. Infectivity of the human immunodeficiency virus: estimates from a prospective study of homosexual men. J Infect Dis. 1987;156:189–193. [DOI] [PubMed] [Google Scholar]
- 36.Cates W Jr, Rothenberg RB, Blount JH. Syphilis control: the historic context and epidemiologic basis for interrupting sexual transmission of Treponema pallidum. Sex Transm Dis. 1996;23:68–75. [DOI] [PubMed] [Google Scholar]
- 37.Hook EW III. Biomedical issues in syphilis control. Sex Transm Dis. 1996;23:5–8. [DOI] [PubMed] [Google Scholar]
- 38.Garnett GP, Aral SO, Hoyle DV, Cates W Jr, Anderson RM. The natural history of syphilis: implications for the transmission dynamics and control of infection. Sex Transm Dis. 1997;24:185–200. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. HIV incidence among young men who have sex with men—seven US cities, 1994–2000. MMWR Morb Mortal Wkly Rep. 2001;50:440–444. [PubMed] [Google Scholar]
- 40.Hook EW III, Marra CM. Acquired syphilis in adults. N Engl J Med. 1992;326:1060–1069. [DOI] [PubMed] [Google Scholar]
- 41.Dow WH, Philipson T. An empirical examination of the implications of assortative matching on the incidence of HIV. J Health Econ. 1996;15:735–749. [DOI] [PubMed] [Google Scholar]
- 42.Division of STD Prevention. Sexually Transmitted Disease Surveillance, 1999. Atlanta, Ga: Centers for Disease Control and Prevention; 2000.
- 43.Turner CF, Danella RD, Rogers SM. Sexual behavior in the United States 1930–1990: trends and methodological problems. Sex Transm Dis. 1995;22:173–190. [DOI] [PubMed] [Google Scholar]
- 44.Michael RT, Wadsworth J, Feinlab J, Johnson AM, Laumann EO, Wellings K. Private sexual behavior, public opinion, and public health policy related to sexually transmitted diseases: a US-British comparison. Am J Public Health. 1998;88:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]


