Abstract
Complex patterns of morphogenesis require intricate coordination of multiple, regulatory processes that control cellular identities, shapes, and behaviors, both locally and over vast distances in the developing organism or tissue. Studying Drosophila oogenesis as a model for tissue morphogenesis, we have discovered extraovarian regulation of follicle formation. Clonal analysis and ovary transplantation have demonstrated that long-range control of follicle individualization requires stall gene function in cells outside of the ovary. Although tissue nonautonomous regulation has been shown to govern follicle maturation and survival, this is the first report of an extraovarian pathway involved in normal follicle formation.
FOLLICLE formation in Drosophila melanogaster takes place in the germarium, which is located at the anterior tip of each ovariole (Figure 1A; for a review, see Spradling 1993). Both germline stem cells (GSC) and somatic stem cells (SSC) reside in the germarium and receive cues from the anteriorly positioned terminal filament cells for their division and maintenance. A germline cyst is initiated when a GSC divides asymmetrically to produce a single cystoblast, while regenerating the GSC. The cystoblast undergoes four rounds of mitosis with incomplete cytokinesis to form a germline cyst, composed of 16 interconnected cystocytes. One of these cystocytes becomes specified as an oocyte, while the remaining cystocytes become nurse cells. As the cyst matures and moves posteriorly through the germarium, somatic cells produced by SSC divisions move to surround each cyst to form a single-layered cuboidal epithelium. The completed follicle consists of a single germline cyst enveloped by the somatic epithelium and, as it exits the germarium, a single column of 6–10 somatic cells separates it from neighboring follicles for continued maturation.
Figure 1.—
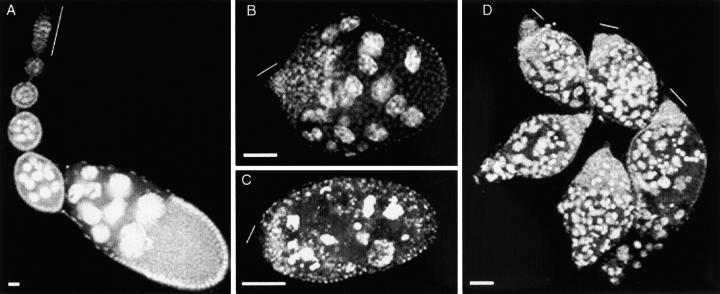
Disruption of follicle morphology in ovaries of stl mutant adults. (A) Normal ovarian morphology is illustrated by a wild-type ovariole. (B) In contrast, abnormal follicle formation is already apparent in stl homozygous ovarioles that were dissected 1 day posteclosion. Although morphology is disrupted, there is little evidence of cell degradation in these young ovarioles. (C) The defective ovariole structure of stl homozygous ovaries shows increasing amounts of cell degeneration with age, as illustrated by this 22-day posteclosion ovariole. Both germline and somatic cells appear to be degrading. (D) The heteroallelic combination of stla16/stlPA49 displays an ovarian phenotype that is identical to that of either homozygote: There is severe mispackaging, as well as degeneration in all five of the ovarioles shown here. All ovaries were stained with the nuclear dye DAPI, to visualize tissue organization. Thin lines indicate the positions of germarium regions in the ovarioles. In this and all subsequent figures, anterior is upward or to the left. Bars, 100 μm.
The complex array of cellular events that contribute to ovarian follicle formation is regulated by an equally complex assortment of regulatory mechanisms. Signal transduction cascades that act within the ovary during the earliest stages of follicle formation include the decapentaplegic and fs(1)Yb/piwi/hedgehog pathways for the maintenance of GSC and SSC fates, as well as for the regulation of mitotic divisions (Xie and Spradling 1998; King and Lin 1999; Cox et al. 2000; King et al. 2001). Interfollicular communication is mediated by the Notch/Delta and JAK/STAT pathways (Torres et al. 2003), and intrafollicular communication requires daughterless (Cummings and Cronmiller 1994) for the specification of cellular identities in the established follicle. Intracellular regulators have been shown to control the establishment and maintenance of the cystoblast fate [e.g., bag of marbles (Chen and McKearin 2003) and benign gonial cell neoplasm (Lavoie et al. 1999)] or to monitor the balance between germline and soma production (daughterless; Smith et al. 2002). Finally, extraovarian signaling via the insulin pathway regulates germline cyst production in response to the nutritional state of the fly (Drummond-Barbosa and Spradling 2001). We show here that long-range signaling regulates follicle formation as well and that stall (stl) function is an essential component of this morphogenetic control.
MATERIALS AND METHODS
Drosophila stocks:
Flies were maintained on molasses-cornmeal-yeast medium at 25°. Fly stocks used in this study are listed in Table 1.
TABLE 1.
Drosophila stocks used in this study
| Stock genotype | Originally obtained from |
|---|---|
| Oregon-R (wild-type strain) | |
| cn stlPA49 bw/CyO | T. Schüpbach |
| cn stlPH57 bw/CyO | T. Schüpbach |
| stlWU40 bw/CyO | T. Schüpbach |
| c stlAWK26 bwD/CyO | T. Schüpbach |
| w/w; al b pr cn stla16/CyO | R. Nagoshi |
| w; P{w+mW.hs=FRT(whs)}G13 | Bloomington Stock Center |
| w1118; P{w+mW.hs=FRT(whs)}G13 P{w+mC=Ubi-GFP.nls}2R1 P{Ubi-GFP.nls}2R2 | Bloomington Stock Center |
| P{ry+t7.2=hsFLP}1, w1118; Adv1/CyO | Bloomington Stock Center |
| P{w+mW.hs=FRT(whs)}G13 P{w+mc=ovoD1-18}32X9a P{w+mc=ovoD1-18}32X9b/ Dp{?;2}bwD, S1, wgSp-1 Ms(2)M1bwD/In(2LR)O, Cy dplvl pr cn2 | Bloomington Stock Center |
| w/w; P{w+mW.hs=FRT(whs)}G13 stla16/CyO | |
| w/w; P{w+mW.hs=FRT(whs)}G13 stlPA49 bw/CyO |
Genetic analysis of stl:
Originally isolated as fs(2)A16 (Bakken 1973), the stla16 allele fails to complement all other known EMS-induced alleles of stl (Jones 1999). To aid in the interpretation of mutant phenotypes, we subjected several of the various stl mutant chromosomes to recombination to remove unrelated extraneous mutations in linked genes.
Staining and analysis of ovarian tissue:
Fixed ovarian tissue was stained with DAPI as described previously (Cummings and Cronmiller 1994). Stained ovaries were visualized in all cases on Zeiss Axiophot/Axioscope microscopes, and images were captured in black and white by either Pixera (germline clones) or Olympus Magnafire (all other experiments) digital cameras. Images were false-colored for GFP, fluorescein, and rhodamine in Adobe Photoshop.
Larval and pupal gonad preparation:
Gonads were dissected from either wandering third instar larvae or pharate adult pupae and fixed in 4% paraformaldehyde. Fixed gonads were stained with antibodies against Hts (1B1) and Vasa, as described previously (Smith and Cronmiller 2001).
Mosaic analysis of stl:
For germline clone analysis, progeny from an hs-FLP w/+; FRT G13 stla16 × w; FRT ovoD1/CyO cross were heat-shocked at 37° for 2 hr on each of 2 consecutive days. Progeny were allowed to mature at 25° until eclosion. At 3–5 days posteclosion, ovaries were dissected, fixed in 4% paraformaldehyde, and stained with DAPI.
To generate marked somatic clones of stlPA49 and stla16, w/w; FRT G13 stl/Cy virgin females were crossed to hs-FLP w; FRT G13 GFP males. Progeny were heat-shocked at 37° for 2 hr on each of 2 consecutive days, during first, second, or third instar larval stages. Progeny were raised at 25° and, after eclosion, w/w; FRT G13 stl/FRT G13 GFP flies were aged 4–7 days before ovary dissection. Tissue was fixed in 4% paraformaldehyde and stained with DAPI.
Ovary transplantation:
Transplantation of germarial tissue from donor females to male hosts was performed according to the protocol outlined in Lin and Spradling (1993) with the following modifications: Germaria were dissected in Shields and Sang M3 insect medium (Sigma, St. Louis) and held at room temperature for no more than 20 min before transfer to male host abdomens. Hosts were allowed to recover for 11 days before dissection and staining with DAPI.
RESULTS
The stl mutant phenotype:
Our analysis of the stl mutant phenotype endorsed initial assessments of this gene's essential role during oogenesis (Bakken 1973; Schüpbach and Wieschaus 1991). We found follicle formation in adults to be severely disrupted, even in newly eclosed females. For example, the interfollicular stalks that normally separate adjacent wild-type follicles were completely absent in stl ovarioles (Figure 1, B and C). In addition, these mutant ovarioles lacked the somatic epithelial layers that normally envelop individual germline cysts. As a consequence of these defects, each stl ovariole appeared essentially as a single irregular somatic epithelium that contained multiple germline cysts. This phenotype resulted from failed follicle individualization, rather than from persistent germline cell division, because mutant ovarioles contained germ cells at varying stages of maturation and because groups of 16 interconnected germ cells that would ordinarily have identified a single germline cyst were still recognizable. Moreover, this disruption of oogenesis in stl mutant adults was exacerbated by degeneration of both germline and somatic cells (Figure 1C). These ovary defects were identical not only in homozygotes of all five known alleles of stl, but also in all heteroallelic combinations of those alleles (Figure 1D, for example). We were unable to document unambiguously the stl hemizygous phenotype, since all of the putative deletion chromosomes that should have uncovered the stl locus proved to be structurally complex. Nevertheless, because of the similar nature of the stl homozygous and heteroallelic follicular defects and because none of the stl alleles has ever been observed to have any dominant effects (data not shown), the ovarian phenotype associated with these alleles most likely represents the loss of stl gene function.
Since ovarian follicle formation was completely disrupted already in newly eclosed stl mutant females, we examined larval (third instar) and pupal gonads to determine the onset of the stl phenotype. On the basis of the organization of germline and somatic cells, larval gonads looked morphologically normal (Figure 2, compare A with B). Pupal gonads, however, lacked complete follicle individualization: Although adjacent germline cysts were mostly separated from each other by clusters/layers of somatic cells, there was no evidence of interfollicular stalks (Figure 2, compare C with D). Thus, wild-type stl function must be required at least as early as the pupal stage for normal follicle morphogenesis. To address the question of whether stl normally functions in the germline and/or somatic cells of the ovary, we carried out clonal analysis.
Figure 2.—
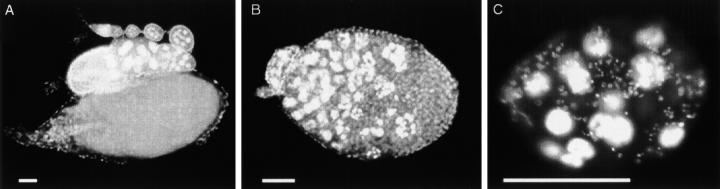
Disruption of follicle morphology in stl preadult ovaries. Larval and pupal gonads were stained with anti-Hts and anti-Vasa to mark somatic cells and germline cells, respectively. (A) Morphology of a normal larval gonad. (B) Gonads from stl larvae appear morphologically normal. (C) An ovariole from a normal pupal gonad, showing interfollicular somatic stalks between follicles (e.g., arrow). (D) In stl pupal gonads, interfollicular stalks do not form (arrows), resulting in a failure in the initial follicle individualization in mutant ovaries. Bars, 50 μm.
Mosaic analysis of stl:
Clonal analysis of stl function revealed unexpected and complex cellular requirements for the gene, failing to detect exclusive requirements for the gene in either the germline or the soma. We first used the dominant female sterile technique to generate stla16 germline clones that were characterized by the absence of the ovoD1 phenotype (DFS-FLP/FRT; Chou and Perrimon 1996). Since ovoD1 homozygous follicles arrest at stage 4, germline clones were recognizable as follicles that were more mature. We induced mitotic recombination during larval stages and recovered ovo+ clones in 19% of ovarioles (n = 279; Figure 3A). Of 54 stla16 clones, only 7 showed weak stl-like follicle formation defects (Figure 3B). Among the remaining 225 stl+ (ovoD1) ovarioles, 41 showed similar follicle formation defects, superimposed on the characteristic ovoD1 phenotype (Figure 3C). The frequency of defects in ovarioles with mutant germline clones (13%) was not statistically different from that in ovarioles without (18%; Student's t-test, P = 0.67), suggesting that stl function is not required in the germline for normal follicle morphogenesis. To determine whether the follicle defects observed in this experiment resulted from the perturbation of stl function in critical somatic cells, unrelated to the stl genotype in the germline, we generated stl mutant somatic clones that were identifiable by the absence of a GFP marker (Luschnig et al. 2000). Marked mutant clones were recovered in 14–84% of ovarioles, depending on the recombination induction protocol used; two independent stl alleles produced essentially indistinguishable results. Mutant clones were found in all somatic cell types, including the follicular epithelium, as well as the terminal filament (TF), cap (CAP), and inner sheath (IS) cells of the germarium. Clones restricted to the small population of specialized somatic cells in the germarium (TF, CAP, and IS) were not associated with any increase in the frequency of defective follicles (data not shown); however, we found that stl mutant clones in somatic epithelial cells were variably associated with follicular defects. To identify clone features that were most likely to result in follicular defects, we analyzed the stl somatic clones in greater detail.
Figure 3.—
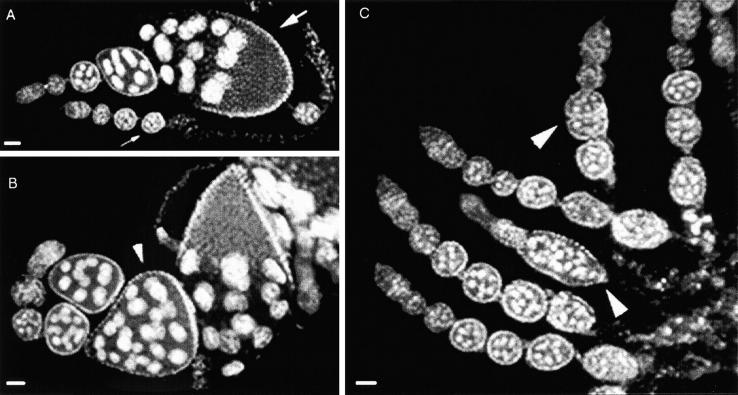
Clonal analysis of stl in the germline. Clones were initiated in first and second instar larvae; germline mosaics were identified by the loss of the ovoD1 phenotype. (A) Despite the stl clone in the germline, an ovo+ stla16 ovariole forms normally maturing, discrete follicles (e.g., large arrow). An ovoD1 stl+ ovariole in the same ovary (small arrow) shows the absence of maturing follicles that is typical of the ovoD1 mutant phenotype. (B) Follicular defects were observed occasionally in ovo+ stla16 ovarioles. In this example more than one germline cyst is enveloped by a single somatic epithelium (arrowhead). (C) Similar follicular defects (arrowheads) are shown in ovoD1 stl+ ovarioles. All ovaries were stained with DAPI to visualize tissue organization. Bars, 100 μm.
Severe stl-like follicle formation defects were most often associated with large stl clones in the somatic ovary; however, not every large somatic clone exhibited such severe defects. We classified follicular defects as severe, weak, or absent, while simultaneously categorizing the corresponding ovarioles according to whether they contained large, small, or no somatic clones. Severe morphological defects were found exclusively in ovarioles that contained mutant clones. Further, gross follicular disruptions were most often correlated with large clones that encompassed a majority of the somatic epithelium (Figure 4A), although they occasionally coincided with small mutant patches (Figure 4B). Phenotypic severity, however, was not strictly correlated with somatic clone size, since rare large clones were recovered that exhibited no visible defects (Figure 4C). Similarly, while weak follicular defects were often associated with small clones, they were just as often present in ovarioles that lacked clones altogether (Figure 4D). Thus, although clone size did influence phenotypic severity, it was not the only determinant; another critical factor of the stl phenotype appeared to originate outside the ovary. This interpretation was supported by a quantitative review of the ovarian phenotypes that were produced during our mitotic clonal analysis, even in the absence of ovarian clones. We calculated the percentage of defective ovarioles per female following induction of clones in either wild-type or stl genotypic backgrounds. If disruption of stl function in extraovarian cells increased the likelihood of follicle formation errors, we would expect to recover clusters of defective ovarioles within individual females, regardless of the presence and/or size of their ovarian clones. The highest per female percentage of defects in the controls was 7.9%; only two females exhibited percentages >5% (Figure 4E). In contrast, among experimental females the percentage of defective ovarioles per female was mostly >5% and reached as high as 30%. In addition to this clustering of ovarian defects, an increased incidence (contingency χ2, P < 0.1) of weak defects in experimental (6.0%) vs. control (2.9%) females suggests that weak defects often represent the impact of nonovarian stl clones. Overall, these mitotic clone data suggest a model in which stl normally contributes to ovarian follicle formation not only in somatic cells of the ovary itself, but also through a previously unidentified regulatory pathway that originates in cells outside of the ovary. Moreover, the extraovarian cells that express stl's function are probably no longer dividing in adults, since we did not recover significant ovarian defects (0.8% of ovarioles) when clones were initiated in adults, whereas ∼11% of ovarioles had defects when mutant clones were induced during larval stages. We tested our nonautonomy model by performing ovary transplantations.
Figure 4.—
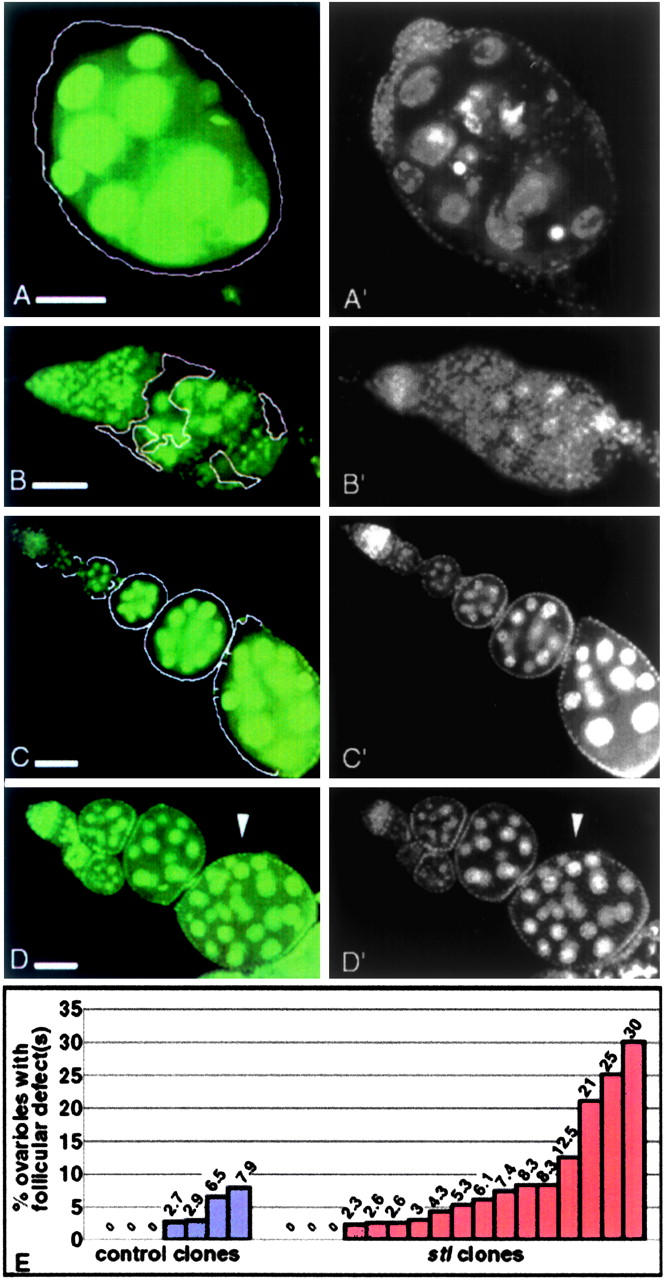
Variation in phenotypic severity among marked stl mutant clones. stl mutant clones in an otherwise wild-type background were initiated in first and second instar larvae and identified by the absence of a GFP marker (A, B, C, and D); DAPI staining reveals the structure of these ovarioles (A′, B′, C′, and D′). (A and A′) A large somatic clone that encompasses an entire ovariole (GFP− stla16 is outlined) exhibits follicle formation defects that are as severe as those observed in homozygous stl ovaries. (B and B′) An ovariole with relatively small patches of stla16 mutant soma (outlined) shows severe follicular defects. All other somatic cells, including TF, IS, and CAP, are wild type in this ovariole. (C and C′) In another ovariole, however, follicles are formed properly, in spite of a large area of stla16 soma (outlined). (D and D′) Weak follicular defects (arrowhead) are present in an ovariole that contains no stl mutant clones. Bars, 100 μm. (E) Increased incidence of multiple follicular defects among mutant (FRT GFP/FRT stlA16 or FRT GFP/FRT stlPA49) vs. wild-type (control, FRT GFP/FRT stl+) females, following clone induction. Each bar represents a single female; the percentage of follicle defects is indicated above each bar. Follicular defects were scored independently of clone frequencies.
Ovarian transplantation analysis:
We carried out reciprocal ovary transplantations between genetically wild-type and homozygous stl mutant adults and confirmed an extrinsic, i.e., extraovarian, role for stl during ovarian follicle formation. Wild-type control transplants confirmed that oogenesis proceeded normally following transfer of germarial tissue to the abdomens of male hosts (Figure 5A; Lin and Spradling 1993). Transplantation of wild-type germaria into two different stl host genotypes, however, resulted in morphologically abnormal ovarioles that were similar to, but not as severe as, those of homozygous mutant females. In some cases, recovered ovarioles did contain portions of interfollicular somatic epithelium, which has never been observed in stl homozygous ovaries (Figure 5B). However, all cases showed one or more typical stl ovarian defects, including germline cyst mispackaging, absence of interfollicular stalks, and widespread cellular degeneration (Figure 5, B and C). Reciprocal transplantations, namely stl mutant ovary tissue into wild-type hosts, were problematic, since transplanted tissue was never recovered in these experiments. However, the number of transplants performed and the host survival rates were comparable in both transplant operations, suggesting that the stl mutant tissue may have been too fragile to survive the stringent transplantation procedure. Although our transplant experiments could not address the sufficiency of extraovarian stl function for normal oogenesis, they do directly demonstrate the necessity for such a function.
Figure 5.—
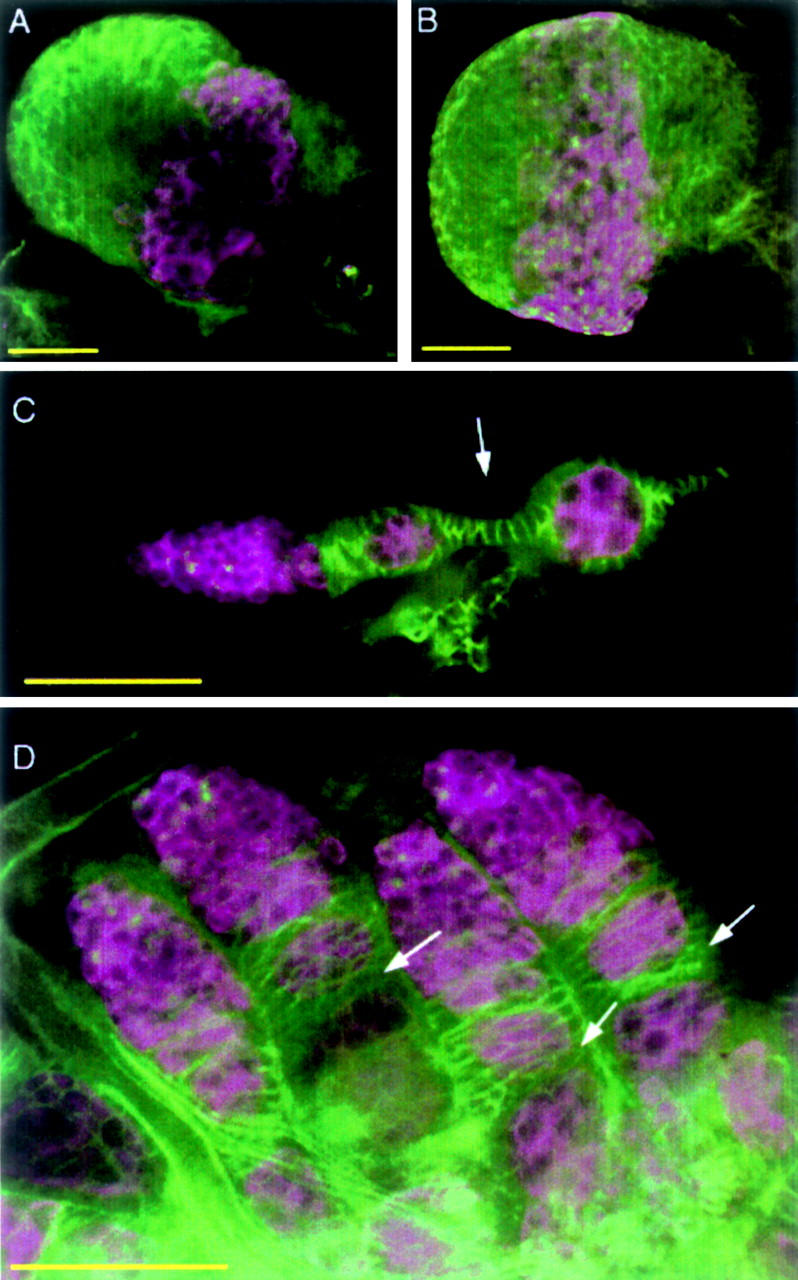
Ovary transplantation. (A) A control ovariole that was recovered following transplantation of a wild-type donor germarium into a wild-type adult male host is morphologically normal. Because the transplanted tissue did not include its associated sheath or muscle layers, the ovariole doubled over as it grew in the host. A total of 220 control transplants were performed with a host survival rate of 10.9%. Wild-type morphogenesis was observed in all eight ovarioles recovered. (B) An ovariole that was recovered following transplantation of a wild-type donor germarium into a stla16/stla16 adult male host exhibits severe morphological defects. (C) This grossly abnormal follicle that was recovered following transfer of a wild-type germarium into a stlPA49/stlPA49 host exhibits substantial somatic degeneration. A total of 362 wild-type to mutant (stla16 or stlPA49) transplants were performed with a host survival rate of 10.5%. Aberrant morphogenesis was observed in all 5 donor ovarioles recovered. All ovarioles were stained with DAPI to visualize tissue morphology. Bars, 100 μm.
DISCUSSION
Our results collectively support a model in which stl function is essential for proper ovarian follicle formation both in somatic cells in the ovary and in extraovarian somatic cells. Indeed, although hormone regulation has been linked to a germline survival checkpoint in flies (Drummond-Barbosa and Spradling 2001), our discovery of stl's extraovarian contribution to oogenesis provides the first evidence for a tissue nonautonomous pathway that regulates follicle individualization. While our transplantation experiments definitively demonstrate the requirement for stl function outside of the ovary, our interpretation that the gene is simultaneously required within the ovary is supported by several observations. First, our inability to recover stl tissue from wild-type transplant hosts could indicate an essential stl ovarian function, especially if that function were required prior to the patterning of the adult ovariole during pupal gonadogenesis, since stl mutants already show follicle defects in pupal ovaries. However, an alternative interpretation could be that the transplanted stl tissue simply did not survive our transplantation procedure. More significantly, the phenotypic defects exhibited by the wild-type ovarian tissue grown in stl mutant hosts were less severe than those associated with the ovaries of homozygous mutant females. Finally, in our clone generation experiment, weak defects were observed even in the absence of ovarian clones, and severe defects were most often observed when large ovarian clones were present. We propose that weak follicular defects arising from clones outside of the ovary were exacerbated by the simultaneous disruption of stl function within the ovarian soma. Thus, we conclude that both the somatic ovarian and the extraovarian roles for stl are essential for proper folliculogenesis, but neither is sufficient, indicating that these functions are nonredundant.
Our evidence for stl's extraovarian participation in ovarian follicle formation raises a number of intriguing questions about the nature of such a regulatory process. Since stl males appear morphologically normal and yet perturbing stl function in males (homozygous stl) was enough to disrupt oogenesis in transplanted ovarioles, the stl-mediated signaling pathway could be functionally female specific under normal conditions, but inducible in males by transplanted ovarian tissue. Alternatively, it is possible that stl contributes to a universal signal that is normally present in both males and females. In this case, at least one of its primary targets would be ovary specific and thereby female specific. Of the five extant alleles of stl, four were recovered from an exclusively female sterile mutagenesis screen (Schüpbach and Wieschaus 1991), while the remaining allele was isolated in a nonsaturating sex nonspecific sterility screen (Bakken 1973). It is possible, therefore, that any or all of these alleles of stl are female-specific lesions in a gene with broader functions. Regardless of the specificity of the signal's origin, no particular cellular mechanism during follicle formation that requires the long-range signal has yet been discovered; however, it has been shown that stl is not required for expression of the polar or interfollicular stalk cell fates in the soma (Tworoger et al. 1999). Molecular characterization of stl, together with genetic interaction studies, should provide clues to the identities of ovarian targets of the long-range signaling, defined by this gene's function. One intriguing possibility is the transcriptional regulatory gene, daughterless, on the basis of its strong dominant mutant interaction with stl (Smith and Cronmiller 2001). Finally, since hormones are known to control checkpoints in oogenesis both early (Drummond-Barbosa and Spradling 2001) and late (Soller et al. 1999), it is possible that stl is directly involved in generating or sending a similar hormonal signal to control follicle formation. Such a signal could originate in the nervous system, given the numerous peptides and hormones that are synthesized in specialized cells in the brain and consistent with the paucity of ovarian defects observed following clone induction in adults.
The discovery of extraovarian control of Drosophila follicle formation prompts the question of whether similar long-range signaling is involved in mammalian folliculogenesis. There are certainly similarities between Drosophila and mammals with respect to follicle maturation and survival, given that those stages of oogenesis in both systems are regulated indirectly via the alteration of ecdysone levels (in response to juvenile hormone) (Jowett and Postlethwait 1980; Soller et al. 1999) and progesterone synthesis (in response to follicle stimulating hormone) (Chun et al. 1996; Makrigiannakis et al. 2000), respectively. Another striking similarity is the importance of intraovarian regulation that mediates extensive cell-cell communication between the oocyte/germline and the surrounding somatic cells in control of cell proliferation or tissue organization; such regulation involves a number of signal transduction pathways, including Notch or EGFR (in Drosophila) (Goode et al. 1992; Torres et al. 2003) and GDF-9 or bFGF (in mammals) (Fortune 2003). Little is known, however, about the control of mammalian oogenesis prior to follicle activation and growth, and it is the early events, including the migration of granulosa cells to encapsulate individual oocytes, that are most analogous to Drosophila follicle formation, during which somatic cells migrate to surround individual germline cysts. Finally, Drosophila and human follicle formation share the same practical restrictions with respect to in vitro culturing. Only Drosophila follicles that are fully formed at the time of explant complete maturation during ovary in vitro culturing; this experimental limitation has hindered our progress toward understanding how follicle formation works at the cellular level. Similarly, cultured preantral human follicles exhibit low meiotic competence, uncoordinated granulosa cell and oocyte growth, and failed maturation (Smitz and Cortvrindt 2002); the inability to culture these early stage follicles has eliminated them as a source of oocytes for alternative reproductive strategies. It is an exciting possibility that preindividualized Drosophila follicle stages and preantral human follicles represent comparable premeiotic stages of oogenesis. If they involve comparable extrinsic physiologic controls, the stall-mediated signaling function could identify a more universal regulatory mechanism; it could be the missing ingredient that is needed for successful in vitro culturing of both tissues. The molecular identification of stall will allow this possibility to be explored further.
Acknowledgments
We are indebted to Yvonne Mowery for antibody staining of larval gonads and to John E. Smith, III and Helen Salz for helpful comments on the manuscript. We also thank Haifan Lin for advice on ovary transplantation and Paul Lasko for anti-Vasa antibody. This work was supported by grants to C.C. from the National Science Foundation and the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust. S.S.W. and E.F.O. were supported by a National Institute of Child Health and Human Development training grant (HD07528-01); S.S.W. received further support from a Ruth L. Kirschstein Memorial National Research Service Award (1 F31 DA06063-01).
References
- Bakken, A. H., 1973. A cytological and genetic study of oogenesis in Drosophila melanogaster. Dev. Biol. 33: 100–122. [DOI] [PubMed] [Google Scholar]
- Chen, D., and D. M. McKearin, 2003. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Chou, T.-B., and N. Perrimon, 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, S. Y., K. M. Eisenhauer, S. Minami, H. Billig, E. Perlas et al., 1996. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 137: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Cox, D. N., A. Chao and H. Lin, 2000. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127: 503–514. [DOI] [PubMed] [Google Scholar]
- Cummings, C. A., and C. Cronmiller, 1994. The daughterless gene functions together with Notch and Delta in the control of ovarian follicle development in Drosophila. Development 120: 381–394. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa, D., and A. C. Spradling, 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231: 265–278. [DOI] [PubMed] [Google Scholar]
- Fortune, J. E., 2003. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci. 78: 135–163. [DOI] [PubMed] [Google Scholar]
- Goode, S., D. Wright and A. P. Mahowald, 1992. The neurogenic locus brainiac cooperates with the Drosophila EGF receptor to establish the ovarian follicle and to determine its dorsal-ventral polarity. Development 116: 177–192. [DOI] [PubMed] [Google Scholar]
- Jones, N. A., 1999 The function of stall in Drosophila oogenesis. M.S. Thesis, University of Virginia, Charlottesville, VA.
- Jowett, T., and J. Postlethwait, 1980. The regulation of yolk polypeptide synthesis in Drosophila ovaries and fat bodies by 20-hydroxyecdysone and a juvenile hormone analogue. Dev. Biol. 80: 225–234. [DOI] [PubMed] [Google Scholar]
- King, F. J., and H. Lin, 1999. Somatic signaling mediated by fs(1)Yb is essential for germline stem cell maintenance during Drosophila oogenesis. Development 126: 1833–1844. [DOI] [PubMed] [Google Scholar]
- King, F. J., A. Szakmary, D. N. Cox and H. Lin, 2001. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Mol. Cell 7: 497–508. [DOI] [PubMed] [Google Scholar]
- Lavoie, C. A., B. Ohlstein and D. M. McKearin, 1999. Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev. Biol. 212: 405–413. [DOI] [PubMed] [Google Scholar]
- Lin, H., and A. C. Spradling, 1993. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159: 140–152. [DOI] [PubMed] [Google Scholar]
- Luschnig, S., J. Krauss, K. Bohmann, I. Desjeux and C. Nusslein-Volhard, 2000. The Drosophila SHC adaptor protein is required for signaling by a subset of receptor tyrosine kinases. Mol. Cell 5: 231–241. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis, A., G. Coukos, M. Christofidou-Solomidou, S. Montas and C. Coutifaris, 2000. Progesterone is an autocrine/paracrine regulator of human granulosa cell survival in vitro. Ann. NY Acad. Sci. 900: 16–25. [DOI] [PubMed] [Google Scholar]
- Schüpbach, T., and E. Wieschaus, 1991. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics 129: 1119–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. E., III, and C. Cronmiller, 2001. The Drosophila daughterless gene autoregulates and is controlled by both positive and negative cis regulation. Development 128: 4705–4714. [DOI] [PubMed] [Google Scholar]
- Smith, J. E., III, C. A. Cummings and C. Cronmiller, 2002. daughterless coordinates somatic cell proliferation, differentiation and germline cyst survival during follicle formation in Drosophila. Development 129: 3255–3267. [DOI] [PubMed] [Google Scholar]
- Smitz, J. E., and R. G. Cortvrindt, 2002. The earliest stages of folliculogenesis in vitro. Reproduction 123: 185–202. [DOI] [PubMed] [Google Scholar]
- Soller, M., M. Bownes and E. Kubli, 1999. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208: 337–351. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., 1993 Developmental genetics of oogenesis, pp. 1–70 in The Development of Drosophila melanogaster, edited by M. Bate and A. M. Arias. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Torres, I. L., H. Lopez-Schier and D. St Johnston, 2003. A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev Cell 5: 547–558. [DOI] [PubMed] [Google Scholar]
- Tworoger, M., M. K. Larkin, Z. Bryant and H. Ruohola-Baker, 1999. Mosaic analysis in the Drosophila ovary reveals a common Hedgehog-inducible precursor stage for stalk and polar cells. Genetics 151: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, T., and A. C. Spradling, 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94: 251–260. [DOI] [PubMed] [Google Scholar]