Abstract
Objective
Placement in baboons of a distal femoral arteriovenous fistula increases shear stress through aortoiliac polytetrafluoroethylene (PTFE) grafts and induces regression of a preformed neointima. Atrophy of the neointima might be controlled by shear stress-induced genes, including the bone morphogenetic proteins (BMPs). We have investigated the expression and function of BMPs 2, 4, and 5 in the graft neointima and in cultured baboon smooth muscle cells (SMCs).
Methods
Baboons received bilateral aortoiliac PTFE grafts and 8 weeks later, a unilateral femoral arteriovenous fistula.
Results
Quantitative polymerase chain reaction showed that high shear stress increased BMP2, 4, and 5 messenger RNA (mRNA) in graft intima between 1 and 7 days, while noggin (a BMP inhibitor) mRNA was decreased. BMP4 most potently (60% inhibition) inhibited platelet-derived growth factor-stimulated SMC proliferation compared with BMP2 and BMP5 (31% and 26%, respectively). BMP4 also increased SMC death by 190% ±10%. Noggin reversed the antiproliferative and proapoptotic effects of BMP4. Finally, Western blotting confirmed BMP4 protein upregulation by high shear stress at 4 days. BMP4 expression demonstrated by in situ hybridization was confined to endothelial cells.
Conclusions
Increased BMPs (particularly BMP4) coupled with decreased noggin may promote high shear stress-mediated graft neointimal atrophy by inhibiting SMC proliferation and increasing SMC death.
Clinical Relevance
Pharmacologic therapy to prevent luminal stenosis or restenosis after vascular reconstruction is directed at inhibiting intimal hyperplasia and smooth muscle cell growth. An alternative approach might be to induce intimal atrophy after luminal narrowing has developed. This approach would be particularly useful for treating stenosis in stented vessels or synthetic bypass grafts because intimal hyperplasia is the only mechanism for luminal narrowing. Furthermore, it would permit the physician to treat the population of patients (about 30%) who actually develop a problem with stenosis or restenosis. We have previously provided proof of principle that an established neointima can be induced to atrophy in baboon polytetrafluoroethylene grafts, but not in normal artery, by simply switching from normal to high blood flow and shear stress. In this study, we provide evidence that members of the bone morphogenetic protein family may play a role in this neointimal atrophy.
Exuberant growth of smooth muscle cells (SMCs) is a characteristic of vascular diseases such as pulmonary hypertension, in-stent restenosis, transplant atherosclerosis, and vein graft failure.1 To date, the pharmacologic strategies employed for these diseases have been aimed at preventing neointimal hyperplasia, which is the primary mechanism of stent and graft stenosis.2 However, since less than one third of all stents and grafts fail because of intimal hyperplasia, an alternative strategy is to develop therapies that reverse wall thickening in only those patients with hyperplasia.
Proof of principle comes from two studies from our laboratory that used a polytetrafluoroethylene (PTFE) aortoiliac graft model in baboons. In this model, bilateral, unwrapped 60 μm internodal distance PTFE grafts are placed into the aortoiliac position. Unlike wrapped 30-μm internodal distance PTFE grafts used clinically, these grafts uniformly heal by the ingrowth of capillaries through the interstices of the PTFE graft. Complete endothelialization is achieved by 2 weeks, and maximal, even neointimal thickening is completed by 2 months.3–5 Atrophy of this established neointima occurs when either blocking antibodies to platelet-derived growth factor receptors (PDGF)-α and -β are administered6 or when blood flow and shear stress are increased by the creation of a distal femoral arteriovenous fistula.5 The loss of intima is associated with decreased proliferation and increased apoptosis of neointimal SMCs as well as loss of matrix.7–9 These results are consistent with the association of increased blood flow and shear stress with decreased intimal thickening in atherosclerotic arteries and vein grafts and injured vessels.5
Of greater interest with regards to the regression of an established neointima are several similarities of this PTFE model to arteries after stent-angioplasty that undergo spontaneous intimal regression at late times.10,11 Both PTFE grafts and stented arteries are not distensible, have a proteoglycan rich region near the endothelium with less near the inflammatory foreign material,9,12 and exhibit increased apoptosis near the foreign material and increased cell proliferation near the endothelium.8,12,13 In the PTFE graft neointima undergoing regression in response to high flow, there is a loss of proteoglycans and a relative increase in collagen,9 similar to what is observed in stented arteries undergoing spontaneous intimal regression at late times.14,15 The PTFE graft thus models interesting aspects of clinically observed pathology.
In this report, we describe our investigation of changes in graft neointimal gene expression in response to high shear stress. Observing that several members of the bone morphogenetic protein (BMP) family were upregulated by elevated shear stress, we focused our investigation on the expression of several members of the BMP family and noggin (a BMP antagonist) in the graft neointima and their effects on cultured baboon SMCs.
METHODS
Complete methods and results, including microarray analysis, are presented as an online Appendix.
Baboon model
Male baboons received bilateral aortoiliac PTFE bypass grafts. After 2 months, unilateral femoral arteriovenous fistulas were constructed as described7,8 to increase blood flow and shear stress in the upstream ipsilateral graft and iliac artery. Animals were euthanized at days 1, 4, and 7 (5 each day, 15 total) by using intravenous sodium pentobarbital (160 mg/kg). All procedures were performed in accordance with the guidelines of the University of Washington Institutional Animal Care and Use Committee.
Taqman reverse transcriptase polymerase chain reaction
Total RNA for microarray and Taqman analysis was prepared from the frozen neointima of the graft by using Solution D/phenol/chloroform/isopropanol.16 RNA quantity and quality was characterized spectrophotometrically by using OD 260/280 (1.96 ±0.06), by agarose gel electrophoresis, and analysis of the sample on the Agilent 2100 Bioanalyzer (Palo Alto, Calif). Five micrograms of graft intima total RNA from individual animals was combined to form six pools for each time point and blood flow combination; average yield of total RNA from individual grafts was 11 ±6 μg. The rationale for pooling stems from the fact that these animals were used for microarray analysis of the effects of high blood flow on intimal gene expression (see online Appendix). We did not have enough total RNA from some individual animals, so forming pools of the intimal samples taken at 1, 4, and 7 days allowed us to screen about 32,000 genes with four replicates.
Taqman reverse transcriptase-polymerase chain reaction (RT-PCR) of BMP4 and noggin was performed by using the RNA pools with the GeneAmp 5700 sequence detection system (Applied Biosystems, Foster City, Calif). Primers and probes were designed by using Primer Express 2.0 (Applied Biosystems) to cross multiple exons of BMP4 (forward primer 5′-ACCACGAAGAACATCTGGAGAAC-3′, reverse primer 5′-TGCTGAGGTTAAAGAGGAAACGA-3′, probe 5′-6[6-carboxy-fluorescein]-TCCCAGGGACCAGTGAAAACTCTGCTT-[6-carboxy-tetramethlyl-rhodamine]-3′), and noggin (forward 5′-CCTGGTGGACCTCATCGAA-3′, reverse 5′-CAGCGTCTCGTTCAGATCCTT-3′, probe 5′-6[6-carboxy-fluorescein]-ACCCAGACCCTATCTTTGACCCCAAG-GA-[6-carboxy-tetramethlylrhodamine]- 3′), and were synthesized by Applied Biosystems. The cycling conditions were 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C, and 1 minute at 60° for combined annealing and extension.
Standard curves of dilutions of plasmids containing BMP4 or noggin (I.M.A.G.E. clone 4399276 and 4727725, Research Genetics) were used to calculate the messenger RNA (mRNA) copy number. Ribosomal RNA control reagents (Applied Biosystems) were used as an internal control, and the calculated amount of mRNA was normalized by the amount of 18S present.
In a separate series of assays on the same RNA pools, quantitation of BMPs 2, 4, and 5 was done using Applied Biosystems predesigned Taqman primer and probe sets (PDARs, see ABI P/N 4333458_a.pdf for the complete protocol). We used Stratagene Universal Target total RNA. We synthesized first strand complimentary DNA (cDNA) by using random primers and the Applied Biosystems High Capacity cDNA Archive Kit (P/N 4322171). Two experiments with duplicate PCR reactions, which used 10-ng equivalents of total RNA, were run on the ABI 7900 machine with the standard cycling protocol and increasing the number of cycles to 44. β-Glucuronidase (GUSB) and TATA binding protein (TBP) were used as the internal reference in all of the samples and calibrator. The delta-delta Ct method was used to calculate ratios of each gene in each sample against the calibrator RNA (see ABI P/N 4333458_a.pdf for the complete protocol). Both references gave similar results, and we present the combined average of the GUSB data.
Morphometry and immunohistochemistry
Formalin-fixed, paraffin-embedded, sections of tissue obtained from the middle of the PTFE graft were stained with hematoxylin and eosin, and the neointimal area was determined by computer-assisted morphometry. Neointimal cell proliferation and death indices were determined by anti-bromodeoxyuridine staining and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling of fragmented DNA (TUNEL), as previously described.8 For immunohistochemical staining, tissue sections were blocked for 1 hour at 37°C with 20% horse serum and 5% albumin, and then incubated overnight at 4°C with goat antibodies to human BMPR-IA, BMPR-IB, and BMPR–II (Santa Cruz Biotechnology Inc, Santa Cruz, Calif). Sections were then incubated with horse biotinylated secondary antibodies (Vector Labs, Burlingame, Calif) at room temperature for 30 minutes, followed by avidin-biotin-peroxidase (Elite ABC kit; Vector Labs). Normal goat immunoglobulin G (Santa Cruz) was used as a negative control.
Western blotting
Frozen graft neointima from grafts exposed to 4 days of high or normal shear stress were pulverized and extracted with 20 mM Tris, 100 mM sodium chloride, 1 mM EDTA, 0.5% IGEPAL CA-630 (Sigma, St. Louis, Mo), 0.1 mM AEBSF (Calbiochem, San Diego, Calif), pH 8.0. After centrifugation, the concentration of protein in the supernatant was determined (BCA, Pierce, Rockford, Ill), and 30-μg samples of protein were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by Western blotting. Blots for BMP4 were blocked with 20% normal sheep serum plus 5% albumin for 1 hour at 37°C. Blots were then incubated overnight at 4°C with monoclonal anti-BMP4 at 0.5 μg/mL (R & D Systems, Minneapolis, Minn). Protein was visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia, Piscataway, NJ).
In situ hybridization
The BMP4 cDNA clone used for RT-PCR was linearized, transcribed into antisense and sense riboprobes, and labeled with 35S-UTP (Riboprobe Combination System-SP6/T7 RNA Polymerase, Promega, Madison, Wisc) for in situ hybridization on formalin-fixed sections as described.17
Cell proliferation
Baboon aortic and graft neointimal SMCs grown from explants were cultured in Dulbecco’s Modified Eagle Medium with 10% or 20% fetal bovine serum (aortic and neointimal SMCs, respectively). After wiping away endothelial cells, the neointima separates easily and cleanly from the PTFE graft material by dissection. The neointimal SMCs were obtained from the neointimal tissue by outgrowth from about 1-mm2 explants in which >99% of neointimal cells are SMCs (by α-SM actin immunohistochemical staining; <0.05% macrophages by HAM56).8,18 Essentially 100% of the cultured cells are also α-SM actin positive.19 Subconfluent SMCs were changed to serum-free medium for 72 hours before the addition of recombinant BMP2, BMP4, BMP5, noggin, PDGF-BB20 (all from R & D Systems), or serum. After 10 hours, 3H-thymidine (1 μCi/mL) was added. After 28 hours, DNA was harvested and radioactivity was determined as described.21
Cell death
Cell death was determined by detection of cytoplasmic histone/DNA complexes (mono- and oligonucleosomes) by enzyme-linked immunoabsorbent assay (Cell Death Detection kit, Roche Diagnostics, Indianapolis, Ind) following the manufacturer’s instructions.
Statistical analysis
Values reported are the mean ± SEM. Each in vitro experiment was repeated at least three times. Statistical significance of DNA synthesis and cell death assays was determined by using multiple analyses of variance and the Student’s t test (SPSS Inc, Chicago, Ill).
RESULTS
By 2 months, PTFE grafts develop a thick neointima.5 We confirmed and extended previous observations8 that the switch to high flow causes a significant decrease in intimal cross-sectional area by showing that high shear stress increased SMC death and decreased SMC proliferation as early as one day after the switch to high flow (Table I).
Table I.
Hemodynamic, morphologic, and biochemical changes in polytetrafluoroethylene graft intima in response to high blood flow
| Day 1 | Day 4 | Day 7 | |
|---|---|---|---|
| Blood velocity(cm/sec) | |||
| Normal | 18 ± 3 | 21 ± 2 | 24 ± 5 |
| High | 85 ± 9 | 93 ± 11 | 82 ± 6 |
| H/N | 4.82 ± 0.83* | 4.41 ± 1.18* | 3.77 ± 1.02* |
| Shear stress(dyne/cm2) | |||
| Normal | 14 ± 2 | 18 ± 2 | 16 ± 3 |
| High | 66 ± 9 | 74 ± 8 | 64 ± 9 |
| H/N | 4.88 ± 0.74* | 4.01 ± 1.30* | 3.98 ± 1.10* |
| Intimal area(mm2) | |||
| Normal | 3.25 ± 0.28 | 3.98 ± 0.43 | 3.17 ± 0.19 |
| High | 2.67 ± 0.33 | 2.96 ± 0.23 | 2.19 ± 0.13 |
| H/N | 0.82 ± 0.11 | 0.76 ± 0.15† | 0.69 ± 0.08† |
| BrdU index(% positive) | |||
| Normal | 5.69 ± 1.05 | 6.22 ± 0.79 | 5.45 ± 0.37 |
| High | 4.15 ± 0.64 | 4.67 ± 0.51 | 4.52 ± 0.93 |
| H/N | 0.73 ± 0.12† | 0.75 ± 0.23† | 0.83 ± 0.17 |
| TUNEL index(% positive) | |||
| Normal | 2.15 ± 0.84 | 1.96 ± 0.48 | 2.39 ± 0.71 |
| High | 3.66 ± 1.23 | 6.41 ± 0.58 | 9.18 ± 2.01 |
| H/N | 1.70 ± 0.41† | 3.27 ± 1.22* | 3.84 ± 0.51* |
H/N, High to normal ratio; BrdU, bromodeoxyuridine; TUNEL, transferase-mediated dUTP nick-end labeling of fragmented DNA.
Details of the protocols for the hemodynamic and morphologic measurements and the BrdU and TUNEL assays are described in supplemental methods. Data are the mean ± SEM (n = 5) of H/N values at 1, 4, and 7 days after the switch to high flow.
P < .01.
P < .05.
BMP and noggin expression
Because microarray results suggested that members of the BMP family are regulated by high blood flow* we performed Taqman RT-PCR analysis to determine message levels for BMP2, 4, and 5. We found that BMP2 message was increased at days 1 and 7, BMP4 message was increased on days 4 to 7, and BMP5 message was decreased on day 4 and increased on day 7 (Table II). In contrast, message levels of noggin, an inhibitor of BMPs, were decreased by high blood flow (Table II).
Table II.
Taqman analysis of changes in expression of bone morphogenetic proteins in the polytetrafluoroethylene graft neointima under high shear stress
|
Expression (H/N) |
|||
|---|---|---|---|
| Gene | Day 1 | Day 4 | Day 7 |
| BMP2 | 1.60 | 1.10 | 1.54 |
| BMP4 | 1.15 | 1.60 | 1.73 |
| BMP5 | 1.00 | 0.72 | 1.32 |
| Noggin | 0.44 | 0.74 | 0.82 |
BMP, Bone morphogenetic protein.
Values are the mean values of the ratios of high to normal (H/N) shear stress samples of assays performed in triplicate on two to five separate occasions.
BMPs inhibit SMC proliferation and increase SMC death
Because BMP2, 4, and 5 were regulated by high blood flow, we investigated the relative activity of each in cultured SMCs. Although each of these BMPs showed a similar inhibitory trend toward PDGF-BB–mediated DNA synthesis by baboon aortic SMCs, only BMP4 had a strong, statistically significant effect (about 60% inhibition) (Fig 1, A). The BMPs alone had no effect on DNA synthesis (data not presented). BMP4 was the most potent, and no additive effects resulted when BMPs were added in combinations of two (each at 30 ng/mL; data not presented); therefore, the effects of BMP4 were further explored by using baboon PTFE graft neointimal SMCs.
Fig 1.
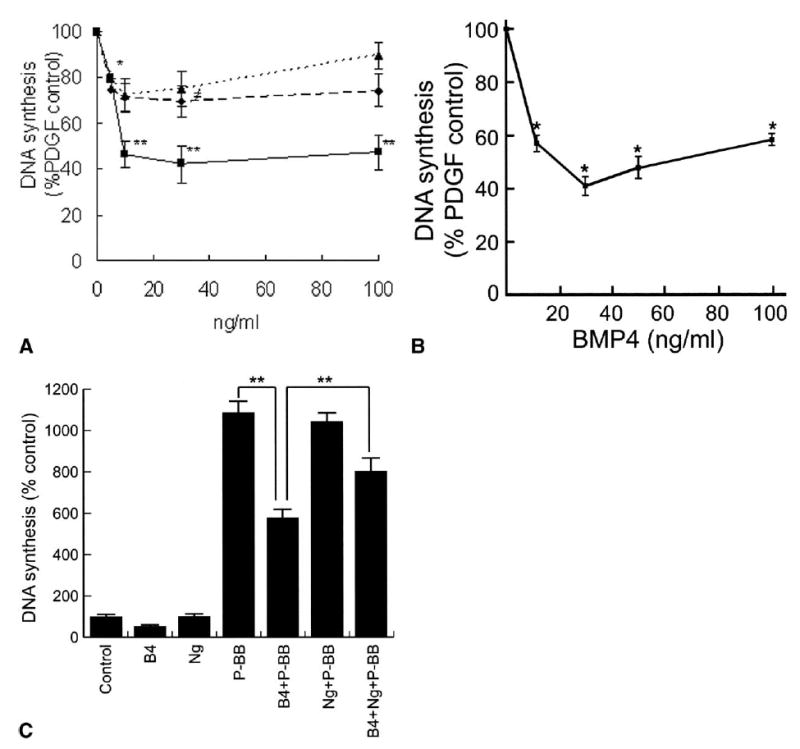
Effect of bone morphogenetic proteins (BMP) on smooth muscle cell (SMC) proliferation. A, Dose-dependant effect of BMP2, 4 (B4) and 5 on DNA synthesis in aortic SMCs treated with 10 ng/mL platelet-derived growth factor-BB (P-BB). Data are presented as the percent of PDGF-BB. Diamonds (dashed line), squares (solid line) and triangles (dotted line) represent BMP2, BMP4, and BMP5, respectively. *P ≤ .05, **P ≤ .01, ‡P = .06. B, Dose-dependant effect of BMP4 on DNA synthesis by polytetrafluoroethylene graft neointimal SMCs treated with 10 ng/mL PDGF-BB. Data are presented as the percent of PDGF-BB. **P ≤ .01. C, Reversal by 300 ng/mL noggin (Ng) of the inhibitory effect of 30 ng/mL BMP4 on the stimulation of SMC proliferation by PDGF-BB (10 ng/mL). Data are presented as the percent of the serum-free medium control. **P ≤ .01.
As with the aortic SMCs, BMP4 dose-dependently attenuated intimal SMC proliferation in response to PDGF-BB (Fig 1, B). In both cases the optimal concentration of BMP4 was 30 ng/mL, with higher doses having less effect in a biphasic manner similar to that observed with BMP2.22 BMP4 also inhibited proliferation of neointimal SMCs mediated by basic fibroblast growth factor (10 ng/mL) and by 1% fetal bovine serum (data not shown). Noggin alone at 300 ng/mL had no effect on intimal SMC proliferation, but reversed the antiproliferative effect of 30 ng/mL BMP4 by 76% ± 6% (Fig 1, C). Similar results with noggin were obtained using aortic SMCs (data not presented).
BMP4 alone increased neointimal SMC death by twofold and had more of an effect in the presence of 10ng/mL PDGF-BB or 0.5% serum (Fig 2, A). Of interest, this effect was only seen after 2 days of treatment (Fig 2, B). Noggin at 300 ng/mL had no effect on intimal SMC death but reversed the death-inducing effect of BMP4 (30 ng/mL) by 65% ± 5% (P < .01, n = 3).
Fig 2.
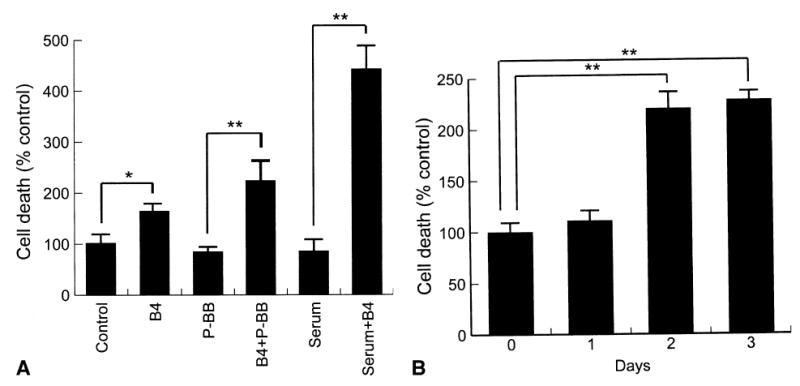
Effects of bone morphogenetic protein 4 (B4) on neointimal smooth muscle cell (SMC) death as determined by enzyme-linked immunoabsorbent assay. A, Effect of 30 ng/mL BMP4 on SMC death after treatment with 10 ng/mL platelet-derived growth factor BB (P-BB) or 0.5% serum for 2 days. *P ≤ .05; **P ≤ .01. B, Time course of SMC death induced by BMP4 (30 ng/mL). Data are presented as percent of the serum-free medium control at each time point.
BMP4 and BMP receptors in vivo
Levels of BMP4 protein in neointimal extracts as determined by Western blotting were found to be increased twofold by high flow (Fig 3), which is consistent with the change in mRNA (Table II). BMP4 mRNA was observed only in endothelial cells by in situ hybridization (Fig 4). Because BMP4 binds to BMPR-IA, -IB, and -II,23,24 we surveyed neointimal expression of these receptors by using immunohistochemistry. Endothelial cells and SMCs in the superficial neointima were positive for BMPR-IA, but SMCs of the deep neointima demonstrated the strongest staining for BMPR-IA, -IB, and –II (Fig 5). Immunostaining for BMPR-IA, -IB, and -II was not appreciably affected by increased flow (data not shown).
Fig 3.
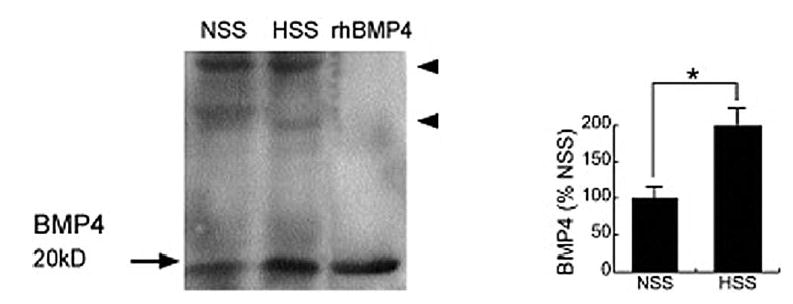
Expression of bone morphogenetic protein 4 in the polytetrafluoroethylene neointima as determined by Western blot analysis of intimal extracts of grafts exposed to high flow for 4 days (left panel.) A graphic display (right panel) of the combined results from three animals is presented as the percent of normal flow values. Recombinant human (rh) BMP4 was used as a positive control. Arrowheads indicate nonspecific bands as determined by results with non-immune mouse immunoglobulin G. HSS, High shear stress; NSS, normal shear stress; *P ≤ .01.
Fig 4.
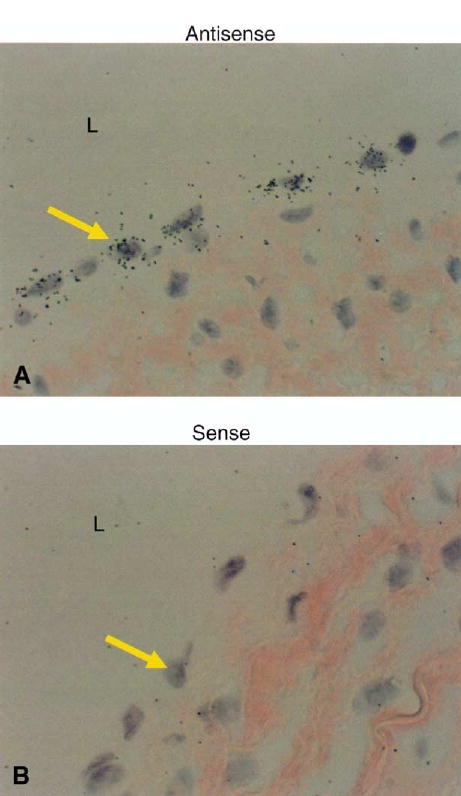
In situ hybridization for bone morphogenetic protein (BMP4) in the polytetrafluoroethylene (PTFE) intima of grafts exposed to high shear stress for 4 days. A, Antisense sequence of BMP4. The black-silver grains indicate a positive signal. B, Sense sequence of BMP4 for negative control. Arrows indicate the endothelial cells. L, Lumen of PTFE. Magnification ×1000.
Fig 5.
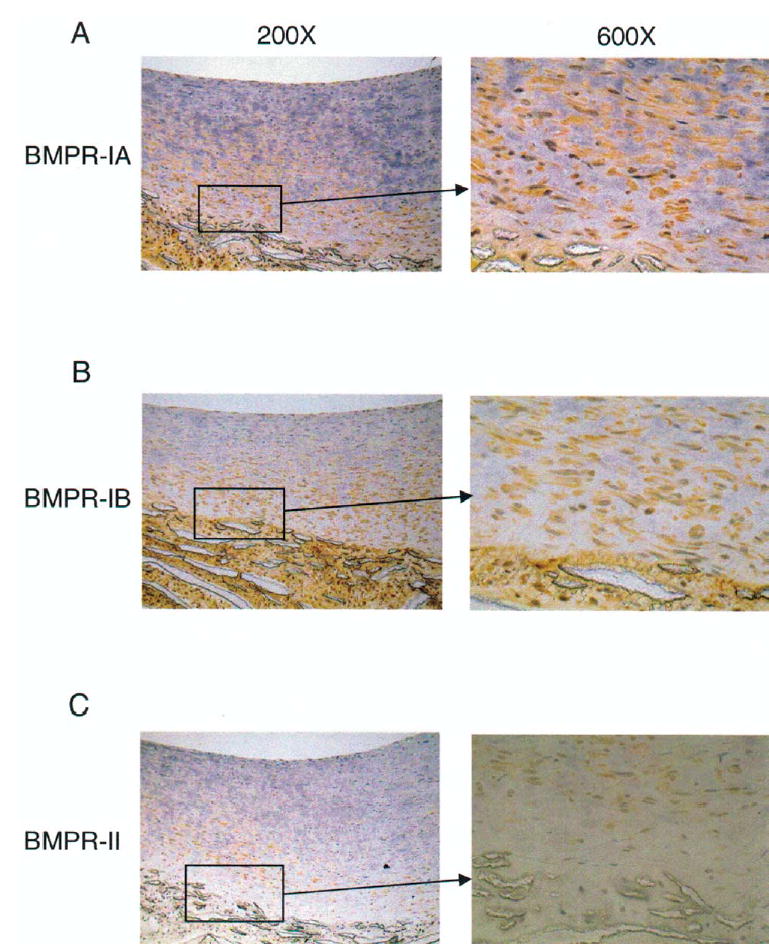
Localization of bone morphogenetic protein receptors (BMPRs). Immunostaining (orange/brown) of BMPR-IA (A), -IB (B), and –II (C) in polytetrafluoroethylene (PTFE) grafts exposed to 4 days of normal shear stress. Location of magnified areas (×1000) are indicated by the boxes. L, Lumen of graft; NI, neointima of graft; MA, matrix of PTFE. Magnification ×200.
DISCUSSION
In this report, we provide evidence that increased BMP expression, including BMP4, may underlie the neointimal atrophy induced by elevated SS in baboon PTFE grafts. Both SMC proliferation and death are altered by day 1 and message levels of BMP2 are increased by day 1. Although BMP4 RNA is not increased on day 1, message levels of the BMP4 inhibitor, noggin,25 are decreased by day 1. Loss of the inhibitor raises the possibility that BMP activity is increased before BMP4 protein changes by day 4. BMP2 and BMP7 have been shown to inhibit SMC or mesangial cell proliferation in vitro (and in vivo for BMP2) mediated by PDGF-BB and 1% serum.22,26–31 Although BMP2, 4, and 7 can inhibit SMC growth, this is not universally observed. Takeda et al27 found that BMPs 2 and 7, but not 4 or 6, inhibited human SMC entry into S phase in response to 1% serum plus PDGF. Nakaoka et al22 found that BMP2, but not BMP7, inhibited entry into S phase by rat SMCs in response to 10% serum, possibly owing to differential binding to BMP receptors.32 These BMPs can have other differential effects. For example, BMP2 inhibits, while BMP4 stimulates, expression of the SMC markers α-SM22 and α-smooth muscle actin.32,33 The observation that BMP4 had similar effects on aortic and graft SMCs is important, because graft SMCs are primarily derived from pericytes and Yang et al34 found opposite effects of BMP4 on the growth of SMCs from different vascular beds.
BMPs are members of the transforming growth factor (TGF)-β superfamily. They regulate cell proliferation, differentiation, migration, and apoptosis during embryonic development.25,35–37 BMPs are dimers that bind two type I (BMPRIA and -IB, or ActR-I) and one type II (BMPR-II or ActR-IIB) serine/threonine kinase receptors.37 Noggin is one of a number of secreted BMP antagonists that acts by binding BMPs and preventing their interaction with BMP receptors.25,38 Binding of BMP to the BMPRs leads to phosphorylation of the type I receptor by the type II receptor. In turn the type I receptor phosphorylates Smads (intracellular mediators), which then move to the nucleus and act in concert with other molecules to regulate transcription.37 Evidence has also accumulated indicating that BMPs can activate members of the mitogen-activated protein kinase (MAPK) pathways. There are reports of both cooperative and antagonistic interactions between the p38 and Smad pathways.39–41
BMPs probably play an important role in vascular disease. Genetic defects of BMP receptors (BMPRs) have been linked to the pathogenesis of pulmonary artery hypertrophy and hypertension in humans.42,43. In addition, BMP2 and BMP4 have been detected in atherosclerotic plaques.44 Although hemodynamic forces, especially shear stress, have been shown to regulate gene expression in arteries and vascular implants45,46 via specific 5′ shear response elements,47–49 there are no known shear response elements in the 5′ promoter region of BMP2 or BMP4. The delayed induction of BMP4 in vivo suggests that intermediate factors control BMP4 expression. Sorescu et al50 reported that increasing levels of laminar shear decreased BMP4 expression in endothelial cells in ≤24 hours. In addition, laminar shear stress increased Smad6 and -7 transcription in ≤3 hours.51 How these in vitro data relate to the in vivo hemodynamic environment is not clear. In contrast to BMP4, the 5′ upstream region of noggin contains a consensus sequence for AP-1 that is required for shear stress-mediated downregulation of vascular cell adhesion molecule-1 in endothelial cells.52,53 Thus, AP-1 may mediate the downregulation of noggin observed after the switch to high shear stress.
The mechanisms by which BMP4 inhibits SMC proliferation and causes cell death are not known. BMP2 increases levels of the cell cycle inhibitor, p21, and antisense-mediated reduction of p21 blocks the inhibitory effect of BMP2 on aortic SMC proliferation.29 Wong et al29 found that 1 day of treatment with BMP2 did not cause SMC death, but Zhang et al54 observed substantial apoptosis in pulmonary SMCs after two days of treatment with either BMP2 or BMP7. These investigators found that BMP2 increased Smad1 phosphorylation by 2 hours and decreased levels of the antiapoptotic protein, Bcl2, by 24 hours.
In contrast to Zhang et al, Dorai et al26 reported that BMP7 inhibited aortic SMC death (in data not shown), which they ascribed to the loss of p53. We also found that longer times were required for the apoptosis inducing effect of BMP4 in the baboon graft intimal SMCs. This suggests a dependence of the process on the synthesis of upstream proapoptotic mediators or slow turnover of antiapoptotic factors. Alternatively, BMP4 may cause SMCs to become dependent on exogenous survival factors.55 In other in vitro cell systems, death caused by BMP4 and BMP2 is mediated by Smad1/5 and p38 MAPK56,57 induction of the transcriptional regulator Msx257,58 and repression of the anti-apoptotic protein Bcl-Xs.57 Of particular interest is the observation that specific cell-cell and cell matrix interactions are required for the effect of BMP4 and Msx2 expression on cell death.58 In this regard, SMC death is only observed in the deep neointima, which has more (and more tightly organized) collagen I and biglycan with less glycosaminoglycans compared with the subendothelial matrix.9 This difference in matrix may alter both the effect and distribution of BMP4. Heparan sulfate proteoglycans such as the glypicans may regulate the distribution and activity of BMP4 and noggin.59,60
CONCLUSION
The counter-regulation of BMP4 and noggin coupled with the growth inhibitory and proapoptotic effects of BMP4 in vitro support a role for BMP4 and noggin in shear stress-mediated neointimal atrophy. These data plus the data linking BMPR mutations to pulmonary hypertension42,43 and showing the increased presence of BMPs in atherosclerotic lesions44 suggest that BMPs play a significant role in vascular pathology. Understanding the function and regulation of BMP ligands, BMPRs, antagonists (eg, noggin) and, in particular, the signaling mechanisms of BMP4 in controlling SMC proliferation and death may lead to therapeutic tools for the prevention or reversal of neointimal hyperplasia.
Supplementary Material
Acknowledgments
We thank the staff of the Northwest Regional Primate Research Center for their assistance with the animal surgery and care.
Footnotes
Additional material for this article may be found online at www.jvascsurg.org.
See supplemental data for a complete description of the microarray experiment. The complete set of array data is at http://www.cs.washington.edu/homes/ruzzo/papers/ATVB/Flow (user: Hsieh; password: ilikethispaper).
Supported by National Institutes of Health grants HL 30946 and RR00166.
References
- 1.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: New perspectives and therapeutic strategies. Nature Med. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 2.Mudra H, Regar E, Klauss V, Werner F, Henneke KH, Sbarouni E, et al. Serial follow-up after optimized ultrasound-guided deployment of Palmaz-Schatz stents. In-stent neointimal proliferation without significant reference segment response. Circulation. 1997;95:363–70. doi: 10.1161/01.cir.95.2.363. [DOI] [PubMed] [Google Scholar]
- 3.Golden MA, Hanson SR, Kirkman TR, Schneider PA, Clowes AW. Healing of polytetrafluoroethylene arterial grafts is influenced by graft porosity. J Vasc Surg. 1990;11:838–45. doi: 10.1067/mva.1990.18047. [DOI] [PubMed] [Google Scholar]
- 4.Clowes AW, Kirkman TR, Reidy MA. Mechanisms of arterial graft healing. Rapid transmural capillary ingrowth provides a source of intimal endothelium and smooth muscle in porous PTFE prostheses. Am J Pathol. 1986;123:220–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler TR, Kirkman TR, Kraiss LW, Zierler BK, Clowes AW. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991;69:1557–65. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 6.Englesbe MJ, Hawkins S, Hsieh PCH, Davies MG, Daum G, Kenagy RD, et al. Concomitant blockade of PDGF receptors -a and -b induces intimal atrophy in baboon PTFE grafts. J Vasc Surg. 2004;39:440–6. doi: 10.1016/j.jvs.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson EJR, Kohler TR, Vergel SM, Clowes AW. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–9. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- 8.Berceli SA, Davies MG, Kenagy RD, Clowes AW. Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg. 2002;36:1248–55. doi: 10.1067/mva.2002.128295. [DOI] [PubMed] [Google Scholar]
- 9.Kenagy RD, Fischer JW, Lara S, Sandy JD, Clowes AW, Wight TN. Accumulation and loss of extracellular matrix during shear stress-mediated intimal growth and regression in baboon vascular grafts. J Histochem Cytochem. 2005;53:131–40. doi: 10.1369/jhc.4A6493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years. Serial angiographic and angioscopic follow-up. Circulation. 1998;97:2003–6. doi: 10.1161/01.cir.97.20.2003. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda N, Kobayashi Y, Nameki M, Kuriyama N, Kinoshita T, Okuno T, et al. Intimal hyperplasia regression from 6 to 1 2 months after stenting. Am J Cardiol. 2002;89:869–72. doi: 10.1016/s0002-9149(02)02205-1. [DOI] [PubMed] [Google Scholar]
- 12.Inoue S, Koyama H, Miyata T, Shigematsu H. Pathogenetic heterogeneity of in-stent lesion formation in human peripheral arterial disease. J Vasc Surg. 2002;35:672–8. doi: 10.1067/mva.2002.122021. [DOI] [PubMed] [Google Scholar]
- 13.Akimaro KF, Nishibe T, Miyazaki K, Watanabe S, Flores J, Yasuda K. Induction of apoptosis after stent implantation in canine portal vein. Ann Vasc Surg. 2002;16:456–61. doi: 10.1007/s10016-001-0085-9. [DOI] [PubMed] [Google Scholar]
- 14.Finn AV, Gold HK, Tang A, Weber DK, Wight TN, Clermont A, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res. 2002;39:414–25. doi: 10.1159/000064518. [DOI] [PubMed] [Google Scholar]
- 15.Imanaka-Yoshida K, Matsuura R, Isaka N, Nakano T, Sakakura T, Yoshida T. Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angioplasty: clinical significance of early tenascin-C expression. Virchows Arch Int J Pathol. 2001;439:185–90. doi: 10.1007/s004280000390. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Hudkins KL, Giachelli CM, Cui Y, Couser WG, Johnson RJ, Alpers CE. Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol. 1999;10:444–57. doi: 10.1681/ASN.V103444. [DOI] [PubMed] [Google Scholar]
- 18.Golden MA, Au YPT, Kirkman TR, Wilcox JN, Raines EW, Ross R, et al. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991;87:406–14. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden MA, Au YPT, Kenagy RD, Clowes AW. Growth factor gene expression by intimal cells in healing polytetrafluoroethylene grafts. J Vasc Surg. 1990;11:580–5. [PubMed] [Google Scholar]
- 20.Nathe TJ, Deou J, Walsh B, Bourns B, Clowes AW, Daum G. Interleukin-1b inhibits expression of p21(WAF1/CIP1) and p27(KIP1) and enhances proliferation in response to platelet-derived growth factor-BB in smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1293–8. doi: 10.1161/01.atv.0000023428.69244.49. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Daum G, Forough R, Clowes M, Walter U, Clowes AW. Overexpression of human endothelial nitric oxide synthase in rat vascular smooth muscle cells and in balloon-injured carotid artery. Circ Res. 1998;82:862–70. doi: 10.1161/01.res.82.8.862. [DOI] [PubMed] [Google Scholar]
- 22.Nakaoka T, Gonda K, Ogita T, Otawara-Hamamoto Y, Okabe F, Kira Y, et al. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest. 1997;100:2824–32. doi: 10.1172/JCI119830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, et al. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–8. [PubMed] [Google Scholar]
- 24.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, et al. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–25. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- 25.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–50. [PubMed] [Google Scholar]
- 26.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and 20 stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Takeda M, Otsuka F, Nakamura K, Inagaki K, Suzuki J, Miura D, et al. Characterization of the bone morphogenetic protein (BMP) system in human pulmonary arterial smooth muscle cells isolated from a sporadic case of primary pulmonary hypertension: roles of BMP type IB receptor (activin receptor-like kinase-6) in the mitotic action. Endocrinology. 2004;145:4344–54. doi: 10.1210/en.2004-0234. [DOI] [PubMed] [Google Scholar]
- 28.Emmanuele L, Ortmann J, Doerflinger T, Traupe T, Barton M. Lovastatin stimulates human vascular smooth muscle cell expression of bone morphogenetic protein-2, a potent inhibitor of low-density lipoprotein-stimulated cell growth. Biochem Biophys Res Commun. 2003;302:67–72. doi: 10.1016/s0006-291x(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 29.Wong GA, Tang V, El Sabeawy F, Weiss RH. BMP-2 inhibits proliferation of human aortic smooth muscle cells via p21Cip1/Waf1. Am J Physiol Endocrinol Metab. 2003;284:E972–79. doi: 10.1152/ajpendo.00385.2002. [DOI] [PubMed] [Google Scholar]
- 30.Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36:120–5. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh CG, Kim YS, Simon M, Wozney J, Harris S, Ghosh-Choudhury N, et al. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J Biol Chem. 1999;274:10897–902. doi: 10.1074/jbc.274.16.10897. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Physiol Lung Cell Mol Physiol. 2005;288:L370–8. doi: 10.1152/ajplung.00242.2004. [DOI] [PubMed] [Google Scholar]
- 33.King KE, Iyemere VP, Weissberg PL, Shanahan CM. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem. 2003;278:11661–9. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery T, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–63. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 35.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 36.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman LB, Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 39.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–7. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 40.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647–52. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 41.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 42.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet. 2000;26:81–4. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 43.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, et al. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–9. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 44.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 45.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dekker RJ, Van Soest S, Fontijn RD, Salamanca S, De Groot PG, VanBavel E, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–98. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 47.Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA, Jr, Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A chain promoter. Arterioscler Thromb Vasc Biol. 1997;17:2280–86. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- 48.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor kappa B interacts functionally with the platelet- derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–75. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA. 1993;90:4591–5. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biologic Chem. 2003;278:31128–35. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 51.Topper JN, Cai JX, Qiu YB, Anderson KR, Xu YY, Deeds JD, et al. Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA. 1997;94:9314–9. doi: 10.1073/pnas.94.17.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korenaga R, Ando J, Kosaki K, Isshiki M, Takada Y, Kamiya A. Negative transcriptional regulation of the VCAM-1 gene by fluid shear stress in murine endothelial cells. Am J Physiol Cell Physiol. 1997;273:C1506–15. doi: 10.1152/ajpcell.1997.273.5.c1506. [DOI] [PubMed] [Google Scholar]
- 53.Shyy JY, Lin MC, Han J, Lu Y, Petrime M, Chien S. The cis-acting phorbol ester “12-O-tetradecanoylphorbol 13-acetate”-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc Natl Acad Sci U S A. 1995;92:8069–73. doi: 10.1073/pnas.92.17.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L740–54. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 55.Song Q, Mehler MF, Kessler JA. Bone morphogenetic proteins induce apoptosis and growth factor dependence of cultured sympathoadrenal progenitor cells. Dev Biol. 1998;196:119–27. doi: 10.1006/dbio.1998.8847. [DOI] [PubMed] [Google Scholar]
- 56.Hallahan AR, Pritchard JI, Chandraratna RAS, Ellenbogen RG, Geyer JR, Overland RP, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nature Med. 2003;9:1033–8. doi: 10.1038/nm904. [DOI] [PubMed] [Google Scholar]
- 57.Kiyono M, Shibuya M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol. 2003;23:4627–36. doi: 10.1128/MCB.23.13.4627-4636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol. 1997;186:127–38. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- 59.Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277:2089–96. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- 60.Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S. Glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development. Dev Biol. 2000;225:179–87. doi: 10.1006/dbio.2000.9831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


