Abstract
Exogenous melatonin is widely used for sleep disorders and has potential value in neuroprotection, cardioprotection and as an antioxidant. Here, a novel method is described for the determination of melatonin and six metabolites in mouse urine by use of LC/MS/MS and GC/MS. LC/MS/MS is used for the measurement of melatonin, N1- acetyl-5-methoxykynuramine (AMK), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and 6-hydroxymelatonin (6-HMEL), while GC/MS is used for the determination of N-[2-(5-methoxy-2-oxo-2,3-dihydro-1H-indol-3-yl)-ethyl]-acetamide (2-OMEL) and cyclic 3-hydroxymelatonin (3-HMEL) with detection limits on column of 0.02 to 0.5 pmol, depending on the metabolite. Following oral administration of melatonin to mice, a 0–24 h urine collection revealed the presence of melatonin (0.2% dose), 6-HMEL (37.1%), and NAS (3.1%) comprising >90% of the total metabolites; AMK and AFMK were also detected at 0.01% each; 2-OMEL was found at 2.2% of the dose, which is >100 times more than the AMK/AFMK pathway, and comprises >5% of the melatonin-related material detected in mouse urine. 3-HMEL was largely found as a sulfate conjugate. These studies establish sensitive assays for determination of six melatonin metabolites in mouse urine and confirm the potential for antioxidant activity of melatonin through the identification in vivo of AMK and AFMK, ring opened metabolites with a high capacity for scavenging reactive oxygen species.
Keywords: antioxidants, melatonin, mouse, urine
Introduction
Exogenous melatonin is widely used in for sleep disturbances associated with jet lag and shift work, for example [1, 2]. In the recent years, the use of exogenous melatonin has been enlarged with the identification of its potential roles in neuroprotection [3], cardioprotection [4], and as an antioxidant [5]. As an antioxidant, protection by melatonin against oxidative damage has been reported in multiple studies using different chemical oxidative stress inducers, such as acetaminophen [6], carbon tetrachloride [7], methotrexate [8], streptozocin [9], amikacin [10], and gentamicin [11]. The efficacy of melatonin against oxidative damage has also been reported using different pathologic animal models, such as hepatic ischemia and reperfusion [12], renal ischemia and reperfusion [13], brain ischemia and reperfusion [14], brain trauma [15], extra-hepatic bile duct ligation [16], and infection with Schistosoma mansoni [17].
Chemical reaction with oxygen free radicals is thought to be one of the mechanisms of the anti-oxidant effects of melatonin [18]. Indeed, melatonin was reported to scavenge the reactive oxygen species, hydroxyl radical (HO·), nitric oxide (NO·), peroxynitrite anion (ONOO−), hypochlorous acid (HOCl), singlet oxygen (1O2), superoxide anion (O2−) and peroxyl radicals (LOO·) [19]. The reaction of melatonin with oxygen free radicals is thought to yield various products of melatonin, including N1-acetyl-5-methoxykynuramine (AMK), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), N-[2-(5-methoxy-2-oxo- 2,3-dihydro-1H-indol-3-yl)-ethyl]-acetamide (2-OMEL) and cyclic 3-hydroxymelatonin (3-HMEL) [18]. AFMK and AMK were first noted as metabolites of melatonin in the central nervous system [20]. It was not until later that AFMK was reported to be a product of the reaction between melatonin and oxygen free radicals [21]. AFMK was identified as reaction product of melatonin with both H2O2 and 1O2 [18, 22]. Formation of AFMK from exogenous melatonin has been reported in serum, retina and brain lateral ventricle after detection by HPLC with fluorescence detection [23]. However, AFMK has never been reported in urine.
2-OMEL was recently identified as a product of the reaction between melatonin and Fenton reagents, as well as with both HOCl and oxoferryl hemoglobin. Analysis of the reaction products by mass spectroscopy (MS), 1H nuclear magnetic resonance (NMR), 13C NMR, correlated spectroscopy (COSY) and heterocorrelated spectroscopy (HETCOR) 2D NMR revealed the formation of a single mono-oxygenated derivative of melatonin under all conditions that was unequivocally identified as 2-OMEL [24]. 2-OMEL had not been reported in vivo and thus it is unknown whether or not this 2-oxidation pathway exists in vivo. 3-HMEL is the product of the reaction of melatonin with HO·, generated in the Fenton reaction and an ultraviolet photolysis system, which was identified using MS, 1H NMR, and COSY 1H NMR, and calculations on the relative thermodynamic stability. Interestingly, 3-HMEL has been reported in the urine of both rats and humans [25].
Metabolism of melatonin has been extensively studied. Melatonin is metabolized principally to 6-hydroxymelatonin (6-HMEL) by the cytochromes P450 CYP1A1, CYP1A2, and CYP1B1, and to the minor metabolite N-acetyl-5-hydroxytryptamine (NAS) by CYP2C19 [26, 27]. In the present study, we focused on the pathways of conversion of melatonin to AFMK, AMK, 2-OMEL and 3-HMEL (see Fig. 1), which are thought to arise from nonenzymic reactions with reactive oxygen species. The urinary excretion of the classical metabolites 6-HMEL and NAS were also determined. However, we are unaware of any report of AFMK in urine, of 2-OMEL formation in vivo, and of any published comparison of the different possible antioxidant reactions of melatonin in vivo. In the present study, the urinary concentrations of AMK, AFMK, 6-HMEL and NAS by LC/MS/MS, and of 2-OMEL and 3-HMEL by GC/MS, were determined after administration of melatonin to the mouse. The potential antioxidant products of exogenous melatonin in mouse urine were monitored to confirm the existence of these antioxidant pathways and to evaluate which of the various pathways dominated.
Fig. 1.
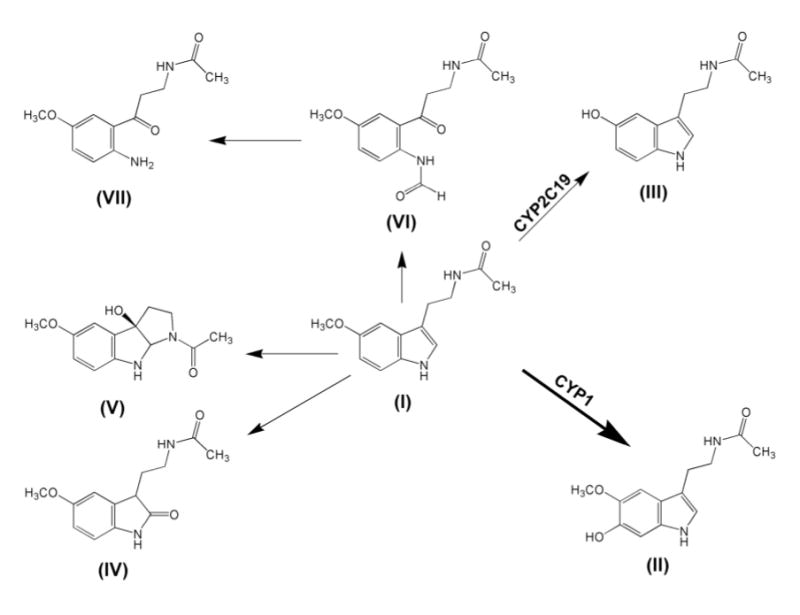
Metabolites derived from melatonin. In humans, melatonin (I) is converted by CYP2C19 to NAS (III) and by CYP1A1, CYP1A2 and CYP1B1 to 6-HMEL (II). Melatonin also undergoes conversion to the ring opened metabolites AFMK (VI), AMK (VII), and to the oxidation products 3-HMEL (V) and 2-OMEL (IV).
Material and methods
Chemicals and reagents
Melatonin, 6-chloromelatonin, 6-hydroxymelatonin, pentafluoropropionic anhydride (PFPA), β-glucuronidase (EC 3.2.1.31) and arylsulfatase (EC 3.1.6.1) were purchased from Sigma-Aldrich (St. Louis, MO). AMK, AFMK, and 3-HMEL were made available by the San Antonio laboratory. 3-HMEL has been described elsewhere [25]. 2-OMEL was made available by the Palermo laboratory and has been described elsewhere [24]. All solvents for LC-MS/MS and GC/MS, and other chemicals were of the highest grade commercially available.
Animals and treatment
Mice (wild-type, 129B6 background) were maintained under a standard 12 h light/12 h dark cycle with water and chow provided ad libitum. Handling was in accordance with animal study protocols approved by the National Cancer Institute Animal Care and Use Committee. Six male mice were used, age 5–6 months. Melatonin (20 mg/kg) was administered by oral gavage and two groups of three mice were immediately housed in separate metabolic chambers (Jencons, Leighton Buzzard, UK). The two pooled urines were collected over a 24 h period following melatonin administration. Urine samples were centrifuged (3000 g, 5 min, 4°C) to discard farragoes and stored at −20°C for further analysis.
Urine sample preparation for analysis
Urine samples were prepared for the detection of both free and potentially conjugated melatonin, AMK, AFMK, 2-OMEL, 3-HMEL, 6-HMEL and NAS. For the unconjugated analytes, 50 μl urine was diluted in 450 μl phosphate buffered saline (PBS), and then extracted with 2 ml ethyl acetate/tert-butyl methyl ether (1:1 v:v). For total (free and conjugated) analytes, 50 μl urine was diluted in 450 μl PBS containing 40 U/ml β- glucuronidase and/or 40 U/ml arylsulfatase, and incubated at 37°C for 18 h with shaking, under a blanket of N2 to prevent oxidation of labile metabolites. The incubation was terminated by the addition of 2 ml ethyl acetate/tert-butyl methyl ether (1:1). 6-CMEL was added as internal standard, which was 100 pmol 6-CMEL for melatonin, AMK, AFMK and 6-HMEL analysis by LC/MS/MS, and 1 nmol for 2-OMEL and 3-HMEL analysis by GC/MS. The samples were extracted by vortexing for 20 sec and then briefly at 4°C. The organic layers were transferred to clean tubes and blown to dryness under N2. The extracted urine samples were reconstituted in 100 μl 70% methanol and 30% H2O containing 0.1% formic acid, and 6 μl was injected to LC/MS/MS for AMK and AFMK analysis. For 2-OMEL and 3-HMEL analysis by GC/MS, the extracted urine samples were reconstituted in 100 μl acetonitrile together with 100 μl PFPA, incubated at 50°C for 20 min, and then blown to dryness under N2. The derivatized samples were reconstituted in 100 μl acetonitrile and 2 μl was injected to GC/MS for 2-OMEL and 3-HMEL determination.
LC/MS/MS method for melatonin, AMK, AFMK, NAS and 6-HMEL
LC/MS/MS analysis was performed using an API 2000 ESI triple quadrupole mass spectrometer (Foster City, CA). A Phenomenex Luna 3 μm C18 50 × 4.6 mm column (Torrance, CA) was used to separate the AMK, AFMK and 6-CMEL. The flow rate through the column at ambient temperature was 0.25 ml/min with 70% methanol and 30% H2O containing 0.1% formic acid. The mass spectrometer was equipped with a turbo ion spray source and run in the positive ion mode. The turbo ion spray temperature was maintained at 350°C and a voltage of 5.0 kV was applied to the sprayer needle. Nitrogen was used as both turbo ion spray and nebulizer gas. The detection and quantification of AMK, AFMK and 6-CMEL were accomplished by multiple reaction monitoring with the transitions m/z 237.0/114.1 for AMK, 265.1/178.3 for AFMK, and 267.0/208.4 for 6-CMEL. Concentrations of melatonin and 6-HMEL were also determined by LC/MS/MS by a method described elsewhere [27], and by monitoring the transitions m/z 232.9/174.0 for melatonin, 249.1/190.0 for 6-HMEL and 219.0/160.1 for NAS. MS/MS conditions were optimized automatically for each analyte and the raw data were processed using Analyst Software.
GC/MS method for 2-OMEL and 3-HMEL
The instrument comprised an Agilent 6890N gas chromatograph and a 5973N mass spectrometry equipped with a 0.25 mm × 30 m, 0.25 μm film thickness HP-5MS (Agilent) capillary column. Helium was used as carrier gas and the constant flow rate was 1 ml/min. The oven temperature was maintained at 90°C for 2 min, then ramped at 15°C/min to 270°C, and held for 10 min. All standards and samples were derivatized with PFPA. The detection and quantification of 2-OMEL and 3-HMEL were accomplished by selected ion monitoring with the ions m/z 376 for 2-OMEL, m/z 540 for 3-HMEL, and m/z 394 for 6-CMEL.
Results
The detection and quantitation of AMK and AFMK were accomplished by multiple reactions monitoring. The retention times were for AMK (m/z 237.0/114.1) and AFMK (m/z 265.1/178.3) were 2.46 and 2.53 min, respectively (Fig 2). The recoveries of AMK and AFMK from urine extracted with ethyl acetate/tert-butyl methyl ether (1:1) were 70–90%. Intra-day and inter-day coefficients of variation were less than 10%. Correlation curves for AMK (0–2 μM) versus peak area ratio (AMK to 6-CMEL) and AFMK (0–2 μM) versus peak area ratio (AFMK to 6-CMEL) were linear (r2=0.996 and 0.999, respectively). The detection limit on column was 0.02 pmol for AMK and 0.04 pmol for AFMK.
Fig. 2.
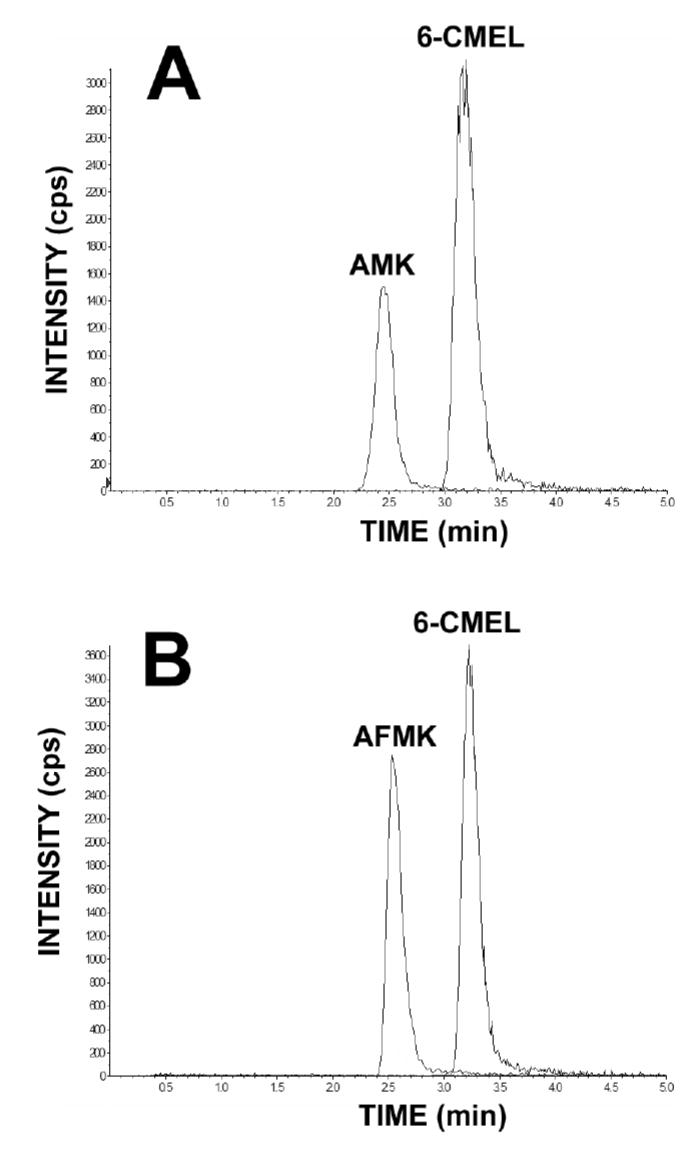
The determination of AMK and AFMK contents by LC/MS/MS. Typical chromatogram of AMK and AFMK eluting at 2.46 and 2.53 min, detected by multiple reaction monitoring. 6-CMEL was used as an internal standard.
The detection and quantitation of 2-OMEL and 3-HMEL were accomplished by selected ion monitoring after derivatization by PFPA (Fig. 3). The retention times for 2-OMEL, 3-HMEL and 6-CMEL (internal standard) were 14.36, 16.13 and 15.09 min, respectively. The recoveries of 2-OMEL and 3-HMEL from urine extracted with ethyl acetate/tert-butyl methyl ether were 60–80%. Intra-day and inter-day coefficients of variation were less than 10%. Correlation curves for 2-OMEL (0–200 μM) versus peak area ratio (2- OMEL to 6-CMEL) and 3-HMEL (0–20 μM) versus peak area ratio (3-HMEL to 6- CMEL) were linear (r2=0.974 and 0.975, respectively). The detection limit on column was 0.25 pmol for 2-OMEL and 0.5 pmol for 3-HMEL.
Fig. 3.
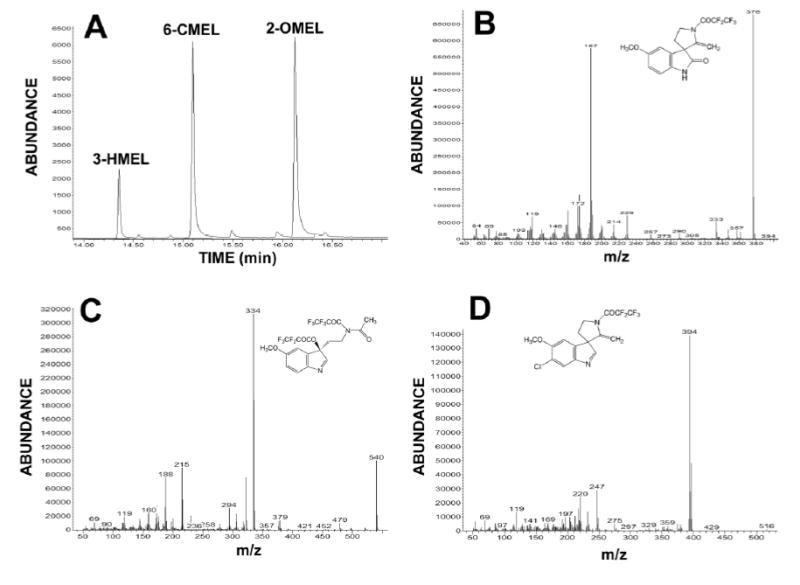
The determination of 2-OMEL and 3-HMEL by GC/MS. Typical chromatogram of 2-OMEL and 3-HMEL eluting at 14.4 and 16.1 min (panel A). 6-CMEL eluting at 15.1 min was used as an internal standard. The fragmentation patterns of the conjugated derivatives 2-OMEL, 3-HMEL and 6-CMEL are shown in panels B, C and D, respectively.
Following melatonin (20 mg/kg p.o.) administration to male 129B6 mice, 0–24 h urines were analyzed for melatonin, AMK, AFMK, 2-OMEL, 3-HMEL, 6-HMEL and NAS. The total recovery of melatonin and these six metabolites was 43.1% of the administered dose. Unchanged melatonin (0.2% dose), 6-HMEL (37.1%), and NAS (3.1%) comprised >90% of the melatonin-related materials detected (Fig. 4). All four products of melatonin antioxidant activity were also detected (Fig. 4), and total (free and conjugated) amount of each product was determined. The percent of dose recovered as AMK and AFMK was the same, at 0.01% each. To our knowledge, this is the first report of the detection of AFMK in the urine. Additionally, the percent dose recovered as 3- OHMEL was 0.5%, which was ~25 times greater than AMK/AFMK pathway. Finally, the percent dose recovered as 2-OMEL was 2.2%, which is >100 times more than the AMK/AFMK pathway, and comprises >5% of the melatonin-related material detected in urine.
Fig. 4.
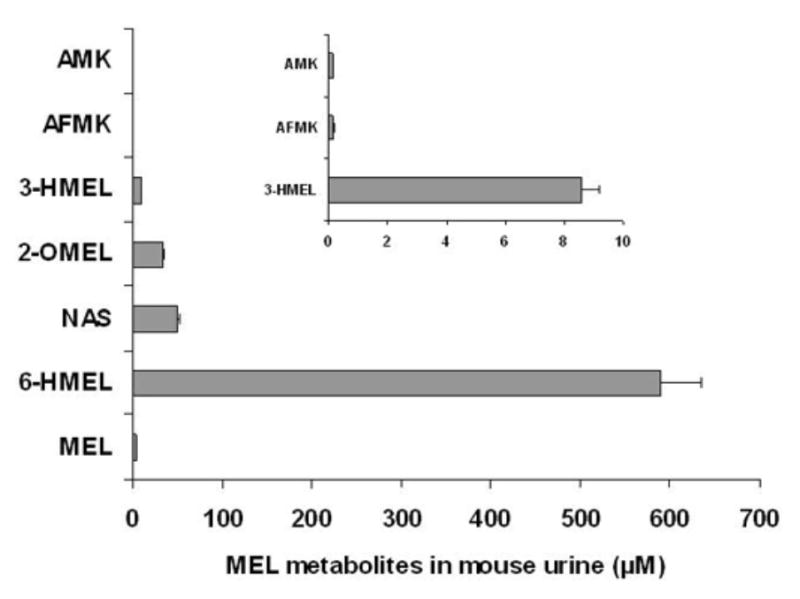
Quantitation of the concentrations of melatonin (MEL) and six metabolites in mouse urine by GC/MS and LC/MS/MS analysis. The inset is an expanded scale showing AMK, AFMK and 3-HMEL. The results represent the mean ± SD (n=6).
To identify potential Phase II (sulfate or glucuronide) conjugation, urine samples were incubated with 40 U/ml β-glucuronidase and/or 40 U/ml arylsulfatase (Fig. 5). After incubation with β-glucuronidase, 3-HMEL increased 75%, when compared with incubation with buffer alone. However, after incubation with arylsulfatase, 3-HMEL increased about 420% when compared with incubation with buffer alone. For the total 3- HMEL (free and conjugated), the urine sample was incubated both with β-glucuronidase and arylsulfatase, which elicited a 5-fold increase in 3-HMEL concentration. Sulfate or glucuronide conjugation for AMK, AFMK and 2-OMEL was not noted.
Fig. 5.
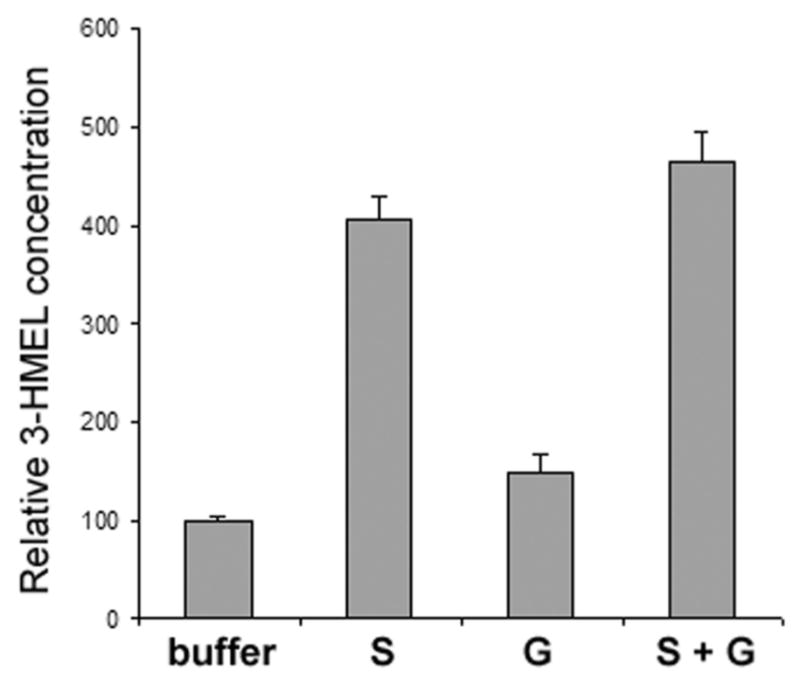
Determination of the conjugated metabolites of 3-HMEL in mouse urine by enzyme treatment. The relative concentrations of 3-HMEL were determined after treatment with arylsulfatase (S) and β-glucuronidase (G) and a mixture of S and G. The results represent the mean ± SD (n=6).
Discussion
When melatonin is administered orally to mice, it is not only 6-hydroxylated to 6-HMEL and O-demethylated to NAS, but also undergoes additional oxidations to yield 2-OMEL and 3-HMEL, together with some oxidative ring opening to yield AMK and AFMK. The total recovery of melatonin and these six metabolites in the urine was 43.1% of the administered dose. Melatonin and its metabolites in the feces were not monitored in this study. The urinary excretion of only 0.2% unchanged melatonin agrees well with previous work. For example, oral administration to dogs of 10–80 mg/kg melatonin resulted in the urinary excretion of 0.25% unchanged melatonin [28]. The excretion of 37.1% of the dose as 6-HMEL (free and conjugated) is also acceptable. Studies in humans given 3 mg melatonin p.o. yielded urinary concentrations of 6-HMEL (based upon the sulfate conjugate) that were ~600-times greater that the melatonin concentrations [29]. In the present study, this ratio was a little lower. However, the mg/kg dose given to mice was 500-times greater than the human dose. Moreover, humans appear to excrete relatively little unchanged melatonin (0.01%), even after doses as large as 100 mg [30]. Regarding NAS, this has been reported in humans to be a significant metabolite of endogenous melatonin, representing ~15% of the urinary melatonin metabolites [26]. In this study, the excretion of NAS in the mouse comprised 7.2% of the administered melatonin dose.
A number of novel findings are reported here. Firstly, AMK and AFMK were found excreted in mouse urine after melatonin administration. Taken together, these ring-opened compounds represent ~0.04% of the melatonin-related materials detected. It is possible that they have escaped detection hitherto because their concentration in urine is very low (0.15 μM), even after a large exogenous dose. However, it was possible to detect and quantitate these compounds by LC/MS/MS with multiple reaction monitoring. These compounds have also been reported to possess potent antioxidant activity [31, 32], and may thus be labile to further biological oxidations by reactive oxygen species. This alone might explain their low abundance in the urine. However, it should be noted that different exposure pathways may also affect melatonin metabolism. By intravenous injection of [14C]melatonin, 15% of urinary radioactivity was attributed to AMK, and by intracisternal injection, 35% of urinary radioactivity was attributed to AMK [20]. The formation of 3-HMEL in vivo and its excretion in the urine of humans and rats has been documented, and proposed as a biomarker of endogenous HO· generation [25]. In the present study, it was found excreted in mouse urine after administration of melatonin, where it comprises 0.5% dose administered. This compound is presumably formed by the reaction of melatonin with hydroxyl radicals, as proposed [25], rather than by a P450-mediated reaction, as is the case for melatonin 6-hydroxylation [27]. Finally, the excretion of 2-OMEL represented 2.2% of the administered dose. The available evidence suggests that this compound is formed from melatonin by the reaction either with various oxygen free radical generating systems or other oxidant species in vitro [24]. To our knowledge, it has never been reported to occur in vivo,.
One of the principal aims of this study was to compare, in an in vivo setting, the various non-enzymic oxidation products that occur when melatonin acts as an antioxidant with various reactive oxygen species. It is possible that the ring-opened metabolites AMK and AFMK are formed in abundance but do not survive to be excreted into the urine, due to their intrinsic antioxidant activity. Over half the administered melatonin dose is unaccounted for and so the 0.01% dose of each excreted in urine may represent a major underestimate of their formation. The excretion of both 2- and 3-oxidized melatonin derivatives demonstrates that these are formed in vivo, almost certainly by non-enzymic processes and are the dominant urinary products of melatonin’s antioxidant activity. Taken together, AMK, AFMK, 2-OMEL and 3-HMEL represent a cluster of biomarkers of in vivo exposure to reactive oxygen species. Their evaluation in various clinical circumstances, such as chronic inflammatory diseases, would be of great interest.
Historically, the use of exogenous melatonin has been restricted to the treatment of sleep disorders associated with jet lag, shift work, etc. [1, 2]. It is becoming increasingly recognized that melatonin may have utility as an anti-inflammatory agent [33] and as an antioxidant [34]. The evidence that reported here confirms that orally administered melatonin does indeed act as an in vivo antioxidant and that biomarkers of this activity are readily discernible in the urine.
Acknowledgments
Supported by the Intramural Research Program of the National Cancer Institute. JRI is grateful to U.S. Smokeless Tobacco Company for a grant for collaborative research. The University of Palermo (RS ex 60 %) support is also gratefully acknowledged.
References
- 1.SKENE DJ, LOCKLEY SW, ARENDT J. Use of melatonin in the treatment of phase shift and sleep disorders. Adv Exp Med Biol. 1999;467:79–84. doi: 10.1007/978-1-4615-4709-9_10. [DOI] [PubMed] [Google Scholar]
- 2.SHARKEY KM, EASTMAN CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol. 2002;282:R454–463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.THOMAS B, MOHANAKUMAR KP. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J Pineal Res. 2004;36:25–32. doi: 10.1046/j.1600-079x.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 4.CHEN Z, CHUA CC, GAO J, et al. Protective effect of melatonin on myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1618–1624. doi: 10.1152/ajpheart.00874.2002. [DOI] [PubMed] [Google Scholar]
- 5.REITER RJ, TAN DX, OSUNA C, et al. Actions of melatonin in the reduction of oxidative stress. A review J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 6.SENER G, SEHIRLI AO, AYANOGLU-DULGER G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res. 2003;35:61–68. doi: 10.1034/j.1600-079x.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 7.OGETURK M, KUS I, KAVAKLI A, et al. Effects of melatonin on carbon tetrachloride-induced changes in rat serum. J Physiol Biochem. 2004;60:205–210. doi: 10.1007/BF03167030. [DOI] [PubMed] [Google Scholar]
- 8.JAHOVIC N, SENER G, CEVIK H, et al. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct. 2004;22:169–178. doi: 10.1002/cbf.1071. [DOI] [PubMed] [Google Scholar]
- 9.BAYDAS G, CANATAN H, TURKOGLU A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J Pineal Res. 2002;32:225–230. doi: 10.1034/j.1600-079x.2002.01856.x. [DOI] [PubMed] [Google Scholar]
- 10.PARLAKPINAR H, OZER MK, SAHNA E, et al. Amikacin-induced acute renal injury in rats: protective role of melatonin. J Pineal Res. 2003;35:85–90. doi: 10.1034/j.1600-079x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 11.SHIFOW AA, KUMAR KV, NAIDU MU, et al. Melatonin, a pineal hormone with antioxidant property, protects against gentamicin-induced nephrotoxicity in rats. Nephron. 2000;85:167–174. doi: 10.1159/000045650. [DOI] [PubMed] [Google Scholar]
- 12.SEWERYNEK E, REITER RJ, MELCHIORRI D, et al. Oxidative damage in the liver induced by ischemia-reperfusion: protection by melatonin. Hepatogastroenterology. 1996;43:898–905. [PubMed] [Google Scholar]
- 13.KUNDUZOVA OR, ESCOURROU G, SEGUELAS MH, et al. Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J. 2003;17:872–874. doi: 10.1096/fj.02-0504fje. [DOI] [PubMed] [Google Scholar]
- 14.WAKATSUKI A, OKATANI Y, SHINOHARA K, et al. Melatonin protects against ischemia/reperfusion-induced oxidative damage to mitochondria in fetal rat brain. J Pineal Res. 2001;31:167–172. doi: 10.1034/j.1600-079x.2001.310211.x. [DOI] [PubMed] [Google Scholar]
- 15.OZDEMIR D, TUGYAN K, UYSAL N, et al. Protective effect of melatonin against head trauma-induced hippocampal damage and spatial memory deficits in immature rats. Neurosci Lett. 2005;385:234–239. doi: 10.1016/j.neulet.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 16.LOPEZ PM, FINANA IT, DE AGUEDA MC, et al. Protective effect of melatonin against oxidative stress induced by ligature of extra-hepatic biliary duct in rats: comparison with the effect of S-adenosyl-L-methionine. J Pineal Res. 2000;28:143–149. doi: 10.1034/j.1600-079x.2001.280303.x. [DOI] [PubMed] [Google Scholar]
- 17.EL-SOKKARY GH, OMAR HM, HASSANEIN AF, et al. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic Biol Med. 2002;32:319–332. doi: 10.1016/s0891-5849(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 18.TAN DX, MANCHESTER LC, REITER RJ, et al. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic Biol Med. 2000;29:1177–1185. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 19.REITER RJ, TAN DX, MANCHESTER LC, et al. Melatonin: detoxification of oxygen and nitrogen-based toxic reactants. Adv Exp Med Biol. 2003;527:539–548. doi: 10.1007/978-1-4615-0135-0_62. [DOI] [PubMed] [Google Scholar]
- 20.HIRATA F, HAYAISHI O, TOKUYAMA T, et al. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974;249:1311–1313. [PubMed] [Google Scholar]
- 21.HARDELAND R, BALZER I, POEGGELER B, et al. On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J Pineal Res. 1995;18:104–111. doi: 10.1111/j.1600-079x.1995.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 22.DE ALMEIDA EA, MARTINEZ GR, KLITZKE CF, et al. Oxidation of melatonin by singlet molecular oxygen (O2(1deltag)) produces N1-acetyl-N2-formyl-5-methoxykynurenine. J Pineal Res. 2003;35:131–137. doi: 10.1034/j.1600-079x.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 23.ROZOV SV, FILATOVA EV, ORLOV AA, et al. N1-acetyl-N2-formyl-5-methoxykynuramine is a product of melatonin oxidation in rats. J Pineal Res. 2003;35:245–250. doi: 10.1034/j.1600-079x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.AGOZZINO P, AVELLONE G, BONGIORNO D, et al. Melatonin: structural characterization of its non-enzymatic mono-oxygenate metabolite. J Pineal Res. 2003;35:269–275. doi: 10.1034/j.1600-079x.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 25.TAN DX, MANCHESTER LC, REITER RJ, et al. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- 26.YOUNG IM, LEONE RM, FRANCIS P, et al. Melatonin is metabolized to Nacetyl serotonin and 6-hydroxymelatonin in man. J Clin Endocrinol Metab. 1985;60:114–119. doi: 10.1210/jcem-60-1-114. [DOI] [PubMed] [Google Scholar]
- 27.MA X, IDLE JR, KRAUSZ KW, et al. Metabolism of melatonin by human cytochromes P450. Drug Metab Dispos. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- 28.SAAF J, WETTERBERG L, BACKSTROM M, et al. Melatonin administration to dogs. J Neural Transm. 1980;49:281–285. doi: 10.1007/BF01252131. [DOI] [PubMed] [Google Scholar]
- 29.KOVACS J, BRODNER W, KIRCHLECHNER V, et al. Measurement of urinary melatonin: a useful tool for monitoring serum melatonin after its oral administration. J Clin Endocrinol Metab. 2000;85:666–670. doi: 10.1210/jcem.85.2.6349. [DOI] [PubMed] [Google Scholar]
- 30.VAKKURI O, LEPPALUOTO J, KAUPPILA A. Oral administration and distribution of melatonin in human serum, saliva and urine. Life Sci. 1985;37:489–495. doi: 10.1016/0024-3205(85)90412-6. [DOI] [PubMed] [Google Scholar]
- 31.SILVA SO, RODRIGUES MR, CARVALHO SR, et al. Oxidation of melatonin and its catabolites, N1-acetyl-N2 -formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine, by activated leukocytes. J Pineal Res. 2004;37:171–175. doi: 10.1111/j.1600-079X.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 32.RESSMEYER AR, MAYO JC, ZELOSKO V, et al. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–213. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- 33.MAYO JC, SAINZ RM, TAN DX, et al. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165:139–149. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 34.HARDELAND R, PANDI-PERUMAL SR. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr Metab (Lond) 2005;2:22. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


