Abstract
Objectives. We sought to determine the effects of exposure to environmental tobacco smoke (ETS) and childhood cigarette smoking on asthma symptoms among middle school children in North Carolina.
Methods. During 1999–2000, information was collected from a survey completed by the children. Outcomes of asthma symptom reporting were regressed on tobacco smoke exposures.
Results. Children who currently smoked or reported any exposure to ETS were at increased risk of reporting active asthma symptoms. Exposure to ETS and childhood cigarette smoking was responsible for 15% of the asthma cases observed in the study population and $1.34 million in excess medical expenditures.
Conclusions. Even at low levels of exposure, childhood cigarette smoking and ETS are independently associated with asthmatic symptoms.
Asthma is the most common chronic childhood illness. The incidence of asthma among 4- to 15-year-olds increased 74% between 1980 and 1994.1 Similar increases have been reported worldwide with no clear causal link, despite extensive research. Current theories suggest that increased exposure to asthma risk factors may be responsible for a substantial amount of the increase in asthma prevalence rates.2
Some important, established risk factors include tobacco smoke, dust mite and cockroach allergens, pet dander, and household molds.3 Exposure to tobacco smoke is one of the strongest and most consistent risk factors in regard to development and exacerbation of asthma.3 Of the potential sources of tobacco smoke, environmental tobacco smoke (ETS) has become a significant area of research, while cigarette smoking among school-aged children and its association with asthma symptoms have been relatively neglected.
While environmental smoke exposure is a proven significant risk factor,3–6 there is a relative paucity of data corroborating an association between asthma symptom reporting and cigarette smoking among school-aged children. Several studies, mostly conducted among adults, have revealed no association between asthma symptoms and smoking7,8; however, there are notable exceptions.9 In one adult study, active smoking was a dose-dependent risk factor for wheezing symptoms but was not a risk factor for physician-diagnosed asthma.10
Conversely, the data supporting ETS as a risk factor are so strong that in 1992 the US Environmental Protection Agency concluded that ETS is causally associated with additional episodes and increased severity of symptoms among asthmatic children and that it is a risk factor for new cases of asthma in previously symptom-free children.4 Most published studies support this association between parental smoking and childhood asthma or wheezing.5,11,12 Although inconsistent and sometimes crudely measured, a dose–response effect has been reported in numerous studies.13,14
The ability to characterize the etiology of this disease has been limited by the lack of standardized measures designed to assess population levels of asthma and respiratory symptoms. Most survey-based population studies rely on a physician diagnosis of asthma or parental reports of wheezing. While wheezing is often used as a surrogate measure of asthma, the variable between-studies definitions of wheezing make comparisons challenging and imperfect.15 Problems include lack of symptom recognition by parents and children and variations in physicians’ diagnostic criteria.
In response to this issue, the International Study of Asthma and Allergy in Childhood (ISAAC) was developed to assess respiratory and allergy symptoms with a validated questionnaire and a descriptive video according to a standard protocol.16 The predictive validity of the ISAAC survey in terms of clinical outcomes has been demonstrated among both adolescents and adults.17–19 This survey, intended for children, and its paired video demonstrating wheezing attacks have helped bring uniformity to data collection and have been used in studies published in more than 56 countries.20 Yet, to date, few studies employing the ISAAC protocol with US children have been published.
In the present study, we did not rely solely on parental reporting of asthma symptoms, as is the case in much of the literature; instead, we used validated ISAAC data provided by middle school students. Furthermore, in addition to the effects of environmental smoke exposure on asthma symptom reporting, we also estimated the effects of childhood cigarette smoking, an area of study often neglected in the literature. We examined dose–response associations between reported asthma symptoms and both secondary smoke exposure in the home and primary childhood smoking. We used impact measures to estimate attributable cases of active asthma due to tobacco smoke. Finally, building on previous Medicaid cost estimates for asthma services rendered in the state of North Carolina,21 we estimated statewide medical costs of tobacco in the age group under study.
METHODS
Participants were seventh and eighth graders enrolled at participating North Carolina public schools during the 1999–2000 school year, when the North Carolina School Asthma Survey (NCSAS) was conducted. The survey used in this study consisted of the standardized ISAAC questionnaire and video along with additional validated questions on symptoms and risk factors. From this modified questionnaire, we assessed statewide prevalence rates of asthma symptoms and risk factors.22 All participant information was collected via questionnaires completed by the children during a single school day. The returned questionnaires were checked for missing data and correct skip patterns (to determine whether participants who answered no to a question about whether or not they smoked cigarettes correctly skipped the subsequent questions about how many cigarettes they smoked in a day). The STATA software package (Stata Corp, College Station, Tex) was used in conducting all statistical analyses.
Children’s histories of cigarette smoking and exposure to ETS were estimated from their questionnaire responses. Respondents were asked whether they had ever smoked cigarettes and, if so, how many cigarettes they had smoked per day over the past 30 days. They also were asked to report the number of people living in their home who smoked and the average number of days per month they smelled smoke from other people’s cigarettes. No information was available on which members of the household smoked or whether a child’s mother was exposed to smoke while pregnant.
The mutually exclusive outcomes of interest were active physician-diagnosed asthma (defined as a self-reported past history of physician-diagnosed asthma combined with wheezing symptoms in the previous 12 months) and episodes of wheezing (defined as at least one instance of wheezing or whistling in the chest without a past diagnosis of asthma) occurring in the previous 12 months. Children were asked to report whether they had ever been diagnosed by a physician as having asthma. They also were asked to watch a descriptive video depiction of situational wheezing and to indicate whether they had ever experienced any wheezing or whistling in the chest in the past 12 months.
We used multiple logistic regression analyses in examining the data. We calculated adjusted odds ratios (ORs) and 95% confidence intervals from estimated regression coefficients and standard errors. We used Wald χ2 tests in all tests of significance and trend tests.23 The demographic covariates included in the final regression models were indicator variables for gender, race/ethnicity (White, Black non-Hispanic, or other), parental history of asthma (positive in either parent), use of gas stove in the home (more than once per month), respondent history of allergy (any allergy to pets, dust, or pollen), county of residence (rural or urban), and socioeconomic status (free/reduced-price lunch or full-price lunch).
Potentially confounding sources of tobacco smoke exposure were controlled as well. Estimated associations between asthma symptom reporting and childhood cigarette smoking were controlled for level of home ETS exposure. Likewise, associations between asthma symptom reporting and home ETS exposure were controlled for childhood cigarette smoking. Respondents missing data on any of the covariates just described or any of the main exposure or outcome variables were excluded from the analysis. These criteria led to exclusion of 13 052 of the 128 568 completed surveys. Because 47.8% of the respondents did not know the asthma history of either parent, an indicator variable category was added to represent such cases. The final data set, retaining 115 516 respondents, was used in all regression analyses.
In the analysis of the relationship between amount and source of current smoke exposure and symptoms, tobacco smoke exposure was modeled via a set of indicator variables. On the basis of the survey responses, categories were created for number of cigarettes children smoked per day, days per month of ETS exposure, and number of people at home who smoked. We used χ2 tests to conduct trend analyses and examined incremental odds ratios involving the categories just mentioned to evaluate significant dose–response relationships.
The number of children whose asthma symptoms were attributable to tobacco smoke exposure was derived from the formula for attributable cases. The number of attributable cases (Ac) was derived from the number of children exposed to tobacco smoke (Ne), based on the following formula24(p56):
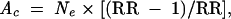 |
where RR is relative risk. Relative risks were calculated from estimated odds ratios according to the formula25(p1691)
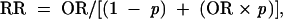 |
where p is the outcome incidence in the unexposed referent group (i.e., children with active diagnosed asthma in North Carolina who were not exposed to tobacco smoke).
We derived estimates of attributable costs due to tobacco smoke exposure by multiplying the number of excess cases of asthma due to tobacco smoke (Ac) by the cost involved in treating each case. We converted the per case annual cost derived from a previous study21 of Medicaid reimbursement data in North Carolina to 2001 dollars using the medical care price index. Since the costs incurred by income-eligible Medicaid children have been shown to be similar to those incurred by commercially insured children,26 we used per case Medicaid costs to represent the per case cost among all children in the state.
RESULTS
A total of 192 248 children were eligible to participate in the survey by virtue of being enrolled in a North Carolina public school during the 1999–2000 school year. Among these eligible children, 128 568 surveys were returned, yielding an overall response rate of 66.8%. The children who completed the survey were representative of the entire seventh- and eighth-grade populations in the state of North Carolina in terms of age, race, gender, socioeconomic status, and county of residence (urban vs rural).27 The children who completed the survey represented 99 of the state’s 100 counties.
Table 1 ▶ summarizes the variables quantifying tobacco smoke exposure among the study population, overall and stratified by race. Of the children taking part in the study, 17.2% reported symptoms of wheezing in the previous 12 months without a diagnosis of asthma, and 9.8% reported active physician-diagnosed asthma.27 Among the children reporting a past diagnosis of asthma, 62.9% reported symptoms in the previous 12 months and thus were categorized as having active diagnosed asthma. Unadjusted prevalence estimates of reported active diagnosed asthma and wheezing in relation to cigarette smoking and ETS exposure are presented in Table 2 ▶. Active physician-diagnosed asthma and wheezing were more frequent among those reporting exposure to tobacco smoke.
TABLE 1—
Prevalence Rates of Cigarette Smoking and Environmental Tobacco Smoke (ETS) Exposure, by Race: Middle School Children in North Carolina
| Overall, No. | (%)a | White (n = 74 403), %b | Black (n = 29 703), %b | Otherc(n = 11 410), %b | |
| Ever smoked | |||||
| No | 79 442 | (68.8) | 69.9 | 68.0 | 63.7* |
| Yes | 36 074 | (31.2) | 30.1 | 32.0 | 36.3 |
| Currently smokesd | |||||
| No | 100 460 | (87.0) | 86.7 | 89.0 | 83.3* |
| Yes | 15 056 | (13.0) | 13.3 | 11.0 | 16.7 |
| No. of cigarettes smoked per daye | |||||
| 0 | 100 460 | (87.0) | 86.7 | 89.0 | 83.3* |
| 1 | 6 494 | (5.6) | 5.2 | 6.3 | 6.5 |
| 2–10 | 6 550 | (5.7) | 6.2 | 3.9 | 6.7 |
| ≥ 11 | 2 012 | (1.7) | 1.9 | 0.8 | 3.5 |
| No. of people smoking at home | |||||
| 0 | 55 124 | (47.7) | 48.3 | 46.3 | 47.4* |
| 1 | 31 529 | (27.3) | 26.2 | 29.9 | 27.7 |
| 2 | 19 874 | (17.2) | 18.2 | 15.6 | 14.9 |
| ≥ 3 | 8 989 | (7.8) | 7.3 | 8.2 | 10.0 |
| Frequency of ETS exposure | |||||
| Never | 20 233 | (17.5) | 15.6 | 20.2 | 23.0* |
| Less than once per month | 21 201 | (18.4) | 19.4 | 15.8 | 17.8 |
| Once per month | 11 582 | (10.0) | 10.4 | 9.2 | 9.4 |
| 2–4 times per month | 15 047 | (13.0) | 13.1 | 12.9 | 13.0 |
| Nearly every day | 47 453 | (41.1) | 41.4 | 41.8 | 36.8 |
aCalculated with a denominator of 115 516 children who completed the survey and were not excluded owing to missing data.
bCalculated with the totals in parentheses as the denominator.
cIncludes Asian/Pacific Islander, Native American/Eskimo, more than one race, and other categories.
dSmoked at least 1 cigarette per day on average over the past 30 days.
eNumber of cigarettes smoked per day during the 30 days before completion of the survey.
*P < .001 (χ2 test of homogeneity with 2 degrees of freedom).
TABLE 2—
Unadjusted Prevalence Rates of Active Diagnosed Asthma and Wheezing in the Past 12 Months, According to Cigarette Smoking Status and Environmental Tobacco Smoke (ETS) Exposure: Middle School Children in North Carolina
| Ever Smoked | Currently Smokes | Exposed to ETS at Homec | ||||||
| Overall, No. | (%)a | Yes (n = 36 074), %b | No (n = 79 442), %b | Yes (n = 15 056), %b | No (n = 100 460), %b | Yes (n = 74 082), %b | No (n = 41 434), %b | |
| Active diagnosed asthma | 11 378 | (9.8) | 11.8 | 8.9* | 13.3 | 9.3* | 11.4 | 7.0* |
| Wheezing in past 12 monthsd | 19 869 | (17.2) | 23.9 | 14.1 | 26.0 | 15.9 | 20.3 | 11.7 |
aCalculated with a denominator of 115 516 children who completed the survey and were not excluded owing to missing data.
bCalculated with the totals in parentheses as the denominator.
cExposure to ETS at least 1 day per month.
dAny wheezing symptoms in past 12 months, without an asthma diagnosis.
*P < .001 (χ2 test of homogeneity with 1 degree of freedom).
Exposure Variables
After control for the potentially confounding influences of gender, race, allergy, gas stove use, parental asthma history, socioeconomic status, population density, and other sources of tobacco smoke in the logistic regression analysis, our adjusted results showed a significant association between all sources of tobacco smoke exposure and reports of asthma symptoms. Table 3 ▶ presents estimated odds ratios for asthma and wheezing at each level of exposure. Significant increases in the odds of reporting active diagnosed asthma or wheezing in the previous 12 months were seen in terms of both number of cigarettes smoked per day and reported amount of ETS exposure.
TABLE 3—
Relative Odds of Active Diagnosed Asthma and Wheezing in the Past 12 Months for Different Levels and Sources of Exposure to Tobacco Smoke: Middle School Children in North Carolina
| Active Diagnosed Asthma (n = 11 378) | Wheezing in Past 12 Monthsa(n = 19 869) | |||
| OR | 95% CI | OR | 95% CI | |
| Ever smoked | ||||
| No (n = 79 442) | 1.00 | 1.00 | ||
| Yes (n = 36 074) | 1.02 | 0.98, 1.07 | 1.56 | 1.51, 1.61 |
| Currently smokes | ||||
| No (n = 100 460) | 1.00 | 1.00 | ||
| Yes (n = 15 056) | 1.10 | 1.04, 1.16 | 1.47 | 1.41, 1.54 |
| No. of cigarettes smoked per day | ||||
| 0 (n = 100 460) | 1.00 | 1.00 | ||
| 1 (n = 6494) | 1.05 | 0.97, 1.14 | 1.28 | 1.20, 1.36 |
| 2–10 (n = 6550) | 1.10 | 1.02, 1.20 | 1.61 | 1.52, 1.71 |
| ≥ 11 (n = 2012) | 1.24 | 1.09, 1.42 | 1.76 | 1.59, 1.95 |
| P for trend | < .005 | < .001 | ||
| No. of smokers at home | ||||
| 0 (n = 55 124) | 1.00 | 1.00 | ||
| 1 (n = 31 529) | 1.18 | 1.13, 1.24 | 1.12 | 1.08, 1.17 |
| 2 (n = 19 874) | 1.24 | 1.17, 1.31 | 1.27 | 1.21, 1.32 |
| ≥ 3 (n = 8989) | 1.37 | 1.27, 1.48 | 1.31 | 1.24, 1.39 |
| P for trend | < .001 | < .001 | ||
| Frequency of ETS exposure | ||||
| Never (n = 20 233) | 1.0 | 1.00 | ||
| Less than once per month (n = 21 201) | 1.33 | 1.22, 1.44 | 1.27 | 1.20, 1.35 |
| Once per month (n = 11 582) | 1.48 | 1.35, 1.62 | 1.47 | 1.37, 1.57 |
| 2–4 times per month (n = 15 047) | 1.62 | 1.49, 1.76 | 1.61 | 1.52, 1.72 |
| Every day (n = 47 453) | 1.72 | 1.60, 1.84 | 1.86 | 1.76, 1.96 |
| P for trend | < .001 | < .001 | ||
Note. All models are adjusted for gender, race, use of gas stove, parental asthma history, allergy status, socioeconomic status, and other competing sources of tobacco smoke exposure. Numbers in parentheses next to exposure variables indicate the number of respondents with that exposure status. Numbers in parentheses beneath symptoms indicate the number of respondents with that symptom. OR = odds ratio; CI = confidence interval; ETS = environmental tobacco smoke.
aAny wheezing symptoms in past 12 months, without an asthma diagnosis.
In comparison with children who reported never having smoked, children who reported that they currently smoked more than 1 cigarette per day were at a significantly increased risk of reporting active diagnosed asthma and wheezing in the previous 12 months. This increased risk was adjusted for the differing levels of ETS exposure between these groups. The log odds of symptom reports increased significantly as the number of cigarettes smoked per day over the previous 30 days increased.
The variables assessing children’s exposure to ETS showed a similar positive association with both active diagnosed asthma and wheezing in the previous 12 months. Presence of smokers in the home (relative to no smokers in the home) was a significant risk factor for both measured outcomes, independent of respondent smoking status. The log odds of symptom reports increased significantly as the number of people in the home who smoked increased. A similar dose–response effect was evident in reports of ETS exposure days per month. Relative to children who reported no exposure to ETS, children who reported being exposed to ETS less than 1 day per month were at a significantly elevated risk of reporting asthma symptoms.
Evaluation of Potential Effect Modifiers
We repeated the analyses after stratifying by allergy history. The results yielded similar patterns among respondents with and without such a history, although the odds of asthma symptom reports were slightly elevated in the former. We conducted a similar analysis focusing on parental history of asthma, race, gender, and socioeconomic class. The relationships between reported breathing outcomes and tobacco smoke exposure did not vary significantly when stratified by any of these variables, and the pattern of increasing odds with increased tobacco smoke exposure persisted in all stratified analyses.
Attributable Cases and Cost
The NCSAS data used in this study are representative of the entire cohort of North Carolina seventh- and eighth-grade students, and thus state prevalence estimates can be derived. Combining these statewide estimates with the relative risks from Table 3 ▶ (calculations not shown) allowed estimation of the number of attributable cases of active diagnosed asthma due to the exposures examined (Table 4 ▶). In calculating this estimate, children who were exposed to ETS and who also actively smoked were included only in the active smoking group. Statewide, there are an estimated 2659 cases of asthma attributable to ETS and 198 cases attributable to current childhood cigarette smoking in the age group examined here, representing 15% of overall active asthma cases among the state’s seventh and eighth graders.
TABLE 4—
Excess Cases of Active Physician-Diagnosed Asthma and Excess Costs Among North Carolina Seventh and Eighth Graders Attributable to Various Sources of Tobacco Smoke Exposure
| Exposure | Attributable Excess Cases,a No. (%) | Attributable Excess Annual Costs,b $ |
| Exposure to ETSc | 2659 (14) | 1 252 389 |
| Current cigarette smokingd | 198 (1) | 93 258 |
| Total | 2857 (15) | 1 345 647 |
Note. Per case Medicaid costs represent costs involved in treating a case of physician-diagnosed asthma. ETS = environmental tobacco smoke.
aAttributable cases calculated from estimated odds ratios in Table 3 ▶.
bAttributable costs based on per case annual cost of $471 (2001 dollars).
cExposure to ETS at least 1 day per month.
dCurrent cigarette smokers who were also exposed to ETS at least 1 day per month (89.2% of current smokers also exposed to ETS) were included in current smokers group only.
The annual Medicaid cost of treating a single case of active asthma in North Carolina in the 10- to 14-year age group is estimated to be $471 (in 2001 dollars).21 Since this estimate considers only physician-diagnosed asthma, only costs attributable to excess cases of active physician-diagnosed asthma can be calculated (Table 4 ▶). Approximately $1.34 million is spent each year to provide care associated with these excess cases of active asthma among seventh and eighth graders in North Carolina.
DISCUSSION
In this cross-sectional study of North Carolina schoolchildren, both childhood cigarette smoking and ETS exposure were significantly associated with active physician-diagnosed asthma and wheezing in the previous 12 months. Our results also showed a dose–response effect wherein significantly higher associations were observed at higher levels of tobacco exposure. Dose–response associations were seen across all tobacco exposure categories for both of the breathing outcomes measured, and these associations persisted in all stratified analyses. Our results are consistent with and supplement previous findings indicating that both cigarette smoking and ETS are risk factors for active diagnosed asthma and wheezing.4,5,9,13,14
To our knowledge, no previous studies have described the association between active physician-diagnosed asthma and childhood cigarette smoking in a cross section of US adolescents. In some longitudinal studies following children into adulthood, it has been reported that active smoking is associated, in a dose-dependent fashion, with newly incident adult-onset wheezing and physician-diagnosed asthma.28,29 However, rather than active cigarette smoking, most studies of children tend to concentrate on ETS exposure in the home.
A notable feature of our analysis of the NCSAS data was a strong and increasing association between number of cigarettes smoked per day by the child and reports of active physician-diagnosed asthma, independent of ETS exposure. At low levels of smoking, this association failed to reach significance; at levels of 2 or more cigarettes per day, however, the association was significant. This association may be explained by the exacerbation of already-prevalent wheezing in a susceptible individual to the point at which it crosses the threshold for diagnosis by a physician, thus converting a child who experiences wheezing to a child with active diagnosed asthma.
Active smoking also may prevent previously diagnosed asthma cases from entering into remission. Alternatively, the presence of this lifestyle risk factor may lead to a diagnostic bias in favor of asthma. In addition, childhood cigarette smoking may, in a small subset of children, actually cause de novo cases of asthma that would otherwise not have occurred. No information was available as to age of diagnosis among the children in our study, but a later diagnosis, after initiation of smoking, would be consistent with this claim.
In our analysis, the associations between asthma symptoms and passive tobacco exposure were stronger than the associations between asthma symptoms and active tobacco exposure. Biologically, the reason may be that those children who are exposed to ETS at home have probably endured more long-term tobacco smoke exposure than those children who actively smoke. This increased exposure over time—especially at younger ages, when children are more susceptible to ETS—may account for these differences. In addition, the “healthy smoker effect,” wherein symptomatic children exposed to ETS self-selectively avoid active exposure, may account for these results.
The dose–response relationship between tobacco smoke exposure and asthmatic symptoms observed in our analysis is consistent with the results of most previous studies.15,30,31 In the NCSAS data, reports of both of the breathing outcomes assessed showed a statistically significant association with tobacco smoke exposure that increased with increasing exposure. These effects were stronger at lower exposure levels, indicating that children at low exposure levels are at the highest incremental risk (classifying children at low levels of exposure as experiencing no exposure will result in underestimates of the overall effects of tobacco smoke).
Importantly, the associations just described achieved significance even at very low levels of exposure. This demonstrates that, in the present study population, smoking more than 1 cigarette per day or being exposed to ETS only 1 day per month was significantly associated with reporting active asthmatic symptoms. The public health implications are manifold. Not only does there appear to be an increased risk for asthmatic symptoms as exposure to tobacco increases, but also there is no safe limit of tobacco smoke exposure in children. Even at low levels of exposure, children may be at increased risk.
A limitation of this study was its dependence on child self-reports for both the predictor and outcome variables. The validity of child reporting has not been as well studied as the validity of parental reporting. Studies do suggest, however, that adolescents are able to understand and validly report health complaints.32 Self-reports of cigarette use in this study were probably slightly inaccurate as a result of underreporting,33 which would tend to underpredict the association with asthma. However, our data correlate well with a 1999 statewide youth tobacco survey estimating that 15% of middle school students smoke cigarettes, and 48.8% live with someone who smokes.34 These statewide estimates are slightly above national youth tobacco survey results, which estimate that 11% of middle school students smoke cigarettes and that 37% live with someone who smokes.35 Furthermore, our statewide estimates of asthma symptoms and ETS exposure are comparable to those seen in other ISAAC studies conducted among US schoolchildren.16,36,37
Previous studies have shown that the association between ETS exposure and asthmatic symptoms diminishes as children grow older, often becoming smaller and less precise in school-aged children.12 Analysis of the NCSAS data demonstrates that significant associations still exist in this cross section of middle school students. While it is probably true that public health measures are most effective if they work to limit ETS exposure among the youngest children—those at greatest risk5,38—our results indicate that these measures must be maintained throughout childhood.
Through a calculation of attributable cases, we estimate that, in the absence of both childhood cigarette smoking and ETS exposure, there would be approximately 15% fewer seventh and eighth graders statewide with active physician-diagnosed asthma. Using Medicaid annual cost estimates, we estimated that $1.34 million excess health care dollars are being spent annually in the state to provide care associated with these excess cases. In similar analyses (calculations not shown), we estimated that more than 23% of cases of wheezing in North Carolina middle school children are attributable to ETS exposure and childhood cigarette smoking. Because of the likelihood that some of these cases of wheezing are undiagnosed cases of asthma, we probably underestimated the true burden of tobacco smoke. While the economic impact of these excess cases of wheezing may be elusive, there are clearly considerable direct and indirect costs for the families involved.
Extending our estimates of excess cases and costs to all children in the state should be a major state public health initiative in the future. The estimates derived for our study population cannot simply be extended to all children, in that many studies have demonstrated that associations between tobacco smoke and asthmatic symptoms vary significantly by age.5,12 However, the association between ETS and asthmatic symptoms is usually stronger among children at younger ages,12 so attributable cases of asthma due to ETS exposure among such children would probably constitute a higher proportion of overall cases than seen in our study population. Similar studies conducted among all children in North Carolina would allow prediction of age-specific excess cases and costs and would help focus public health initiatives where they may yield the most significant improvements.
CONCLUSIONS
From a public health standpoint, given the total morbidity burden of asthma among children, concerted smoking prevention and cessation strategies aimed at parents, women of childbearing age, and children are vital. Not only do sources of ETS have to be reduced, but prevention of cigarette smoking by children must be targeted as well. Health professionals, public health experts, parents, and children must understand that any exposure to tobacco smoke should be considered a risk factor for asthmatic symptoms. Tobacco control efforts that promote maintenance of a smoke-free lifestyle among children of all ages must continue to be implemented and improved.
Acknowledgments
Partial funding of this project was provided by the North Carolina Tobacco Prevention and Control Board.
Human Participant Protection The North Carolina School Asthma Survey was anonymous. A waiver of active consent was obtained from the survey participants. The survey was approved by the University of North Carolina at Chapel Hill’s institutional review board.
Contributors J. J. Sturm and K. Yeatts collaboratively developed the research questions addressed in this article and made most of the revisions. K. Yeatts helped compile the data set from which these data were analyzed. J. J. Sturm analyzed the data. D. Loomis assisted as a valuable advisor in the final stages of the writing of the article.
Peer Reviewed
References
- 1.Mannino DM. Surveillance for asthma—United States. MMWR Morb Mortal Wkly Rep. 1998;47:1–27. [PubMed] [Google Scholar]
- 2.Weiss KB, Gergen PJ, Wagener DK. Breathing better or worse? The changing epidemiology of asthma morbidity and mortality. Annu Rev Public Health. 1993;14:491–513. [DOI] [PubMed] [Google Scholar]
- 3.Skoner D. Controlling asthma: why we must do better. Contemp Pediatr. 2001;18(8):49–62. [Google Scholar]
- 4.Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Washington, DC: US Environmental Protection Agency; 1992. EPA publication 600/6-90/006F.
- 5.Stoddard JJ, Miller T. Impact of parental smoking on the prevalence of wheezing respiratory illness in children. Am J Epidemiol. 1995;141:96–102. [DOI] [PubMed] [Google Scholar]
- 6.Fielding JE, Phenow KJ. Health effects of involuntary smoking. N Engl J Med. 1998;319:1452–1459. [DOI] [PubMed] [Google Scholar]
- 7.Christiani DC, Kern DG. Asthma risk and occupation as a respiratory therapist. Am Rev Respir Dis. 1993;148:671–674. [DOI] [PubMed] [Google Scholar]
- 8.Higgins MW, Keller JB, Metzner HL. Smoking, socioeconomic status, and chronic respiratory disease. Am Rev Respir Dis. 1977;116:403–410. [DOI] [PubMed] [Google Scholar]
- 9.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980;122:567–575. [DOI] [PubMed] [Google Scholar]
- 10.Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Eur Respir J. 2000;15:470–477. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson DM, Horwood LJ. Parental smoking and respiratory illness during early childhood: a six-year longitudinal study. Pediatr Pulmonol. 1985;1:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Gold DR. Environmental tobacco Smoke, indoor allergens, and childhood asthma. Environ Health Perspect. 2000;108(suppl 4):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuspiel D, Rush D, Butler NR, Golding J, Bijur PE, Kurzon M. Parental smoking and post-infancy wheezing in children: a prospective cohort study. Am J Public Health. 1989;79:168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz J, Timonen KL, Pekkanen J. Respiratory effects of environmental tobacco smoke in a panel study of asthmatic and symptomatic children. Am J Respir Crit Care Med. 2000;161:802–806. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham J, O’Connor GT, Dockery DW, Speizer FE. Environmental tobacco smoke, wheezing, and asthma in children in 24 communities. Am J Respir Crit Care Med. 1996;153:218–224. [DOI] [PubMed] [Google Scholar]
- 16.Maier WC, Arrighi HM, Morray B, Llewellyn C, Redding GJ. Indoor risk factors for asthma and wheezing among Seattle school children. Environ Health Perspect. 1997;105:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burney PG, Laitinen LA, Perdrizet S, et al. Validity and repeatability of the IUALTD bronchial symptoms questionnaire: an international comparison. Eur Respir J. 1989;2:940–945. [PubMed] [Google Scholar]
- 18.Riedler J, Reade T, Dalton M, Holst D, Robertson C. Hypertonic saline challenge in an epidemiologic survey of asthma in children. Am J Respir Crit Care Med. 1994;150:1632–1639. [DOI] [PubMed] [Google Scholar]
- 19.Clarke JR. Validation of asthma questionnaires with respiratory physician assessment. Am J Crit Care Med. 1995;151:A568. [Google Scholar]
- 20.Sears MR. Epidemiology of childhood asthma. Lancet. 1997;350:1015–1020. [DOI] [PubMed] [Google Scholar]
- 21.Buescher P, Jones-Vessey K. Childhood Asthma in North Carolina. Raleigh, NC: State Center for Health Statistics; 1999.
- 22.Yeatts K, Shy C. Statewide asthma surveillance. J Asthma. 2000;37:425–434. [DOI] [PubMed] [Google Scholar]
- 23.Kleinbaum D, Kupper L. Applied Regression and Other Multivariable Methods. Pacific Grove, Calif: Duxbury Press; 1998.
- 24.Rothman K, Greenland S. Modern Epidemiology. 2nd ed. New York, NY: Lippincott; 1998.
- 25.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1692. [DOI] [PubMed] [Google Scholar]
- 26.Ray GT, Lieu T, Weinick RM, Cohen JW, Fireman B, Newacheck P. Comparing the medical expenses of children with Medicaid and commercial insurance in an HMO. Am J Managed Care. 2000;6:753–760. [PubMed] [Google Scholar]
- 27.Yeatts K, Shy C. Prevalence and consequences of asthma and wheezing in African-American and white adolescents. J Adolesc Health. 2001;29:314–319. [DOI] [PubMed] [Google Scholar]
- 28.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams L, Lonsdale D, Robinson M, Rawbone R, Guz A. Respiratory impairment induced by smoking in children in secondary schools. BMJ. 1984;288:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasselblad V, Humble CG, Graham MG, Anderson HS. Indoor environmental determinants of lung infection in children. Am Rev Respir Dis. 1981;123:479–485. [DOI] [PubMed] [Google Scholar]
- 31.Ware JH, Dockery DW, Spiro A, Speizer FE, Ferris BG Jr. Passive smoking, gas cooking, and respiratory health of children living in six cities. Am Rev Respir Dis. 1984;129:366–374.6703495 [Google Scholar]
- 32.Haugland S, Wold B. Subjective health complaints in adolescence—reliability and validity of survey methods. J Adolesc. 2001;24:611–624. [DOI] [PubMed] [Google Scholar]
- 33.Barrueco M, Cordovilla R, Hernandez-Mezquita MA, et al. The truthfulness of the answers of children, adolescents and young people to surveys on tobacco consumption conducted in schools. Med Clin. 1999;112:251–254. [PubMed] [Google Scholar]
- 34.North Carolina Youth Tobacco Survey Results. Raleigh, NC: North Carolina Dept of Health and Human Services; 1999.
- 35.Youth Tobacco Surveillance–United States, 2000. Washington, DC: US Dept of Health and Human Services; 2000.
- 36.Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M. Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann Allergy Asthma Immunol. 2001;86:177–184. [DOI] [PubMed] [Google Scholar]
- 37.Hu F, Persky V, Flay B, Zelli A, Cooksey J, Richardson J. Prevalence of asthma and wheezing in public schoolchildren: association with maternal smoking during pregnancy. Ann Allergy Asthma Immunol. 1997;79:80–84. [DOI] [PubMed] [Google Scholar]
- 38.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to ETS exposure in children in the United States. Arch Pediatr Adolesc Med. 2001;155:36–41. [DOI] [PubMed] [Google Scholar]


