Abstract
Objectives. This study evaluated the effect of comorbidity at diagnosis on racial differences in survival among men with prostate cancer.
Methods. Clinical and demographic data were abstracted from records of 864 patients diagnosed at 4 Chicago area hospitals between 1986 and 1990. Comorbidity was scored on the basis of clinical information in the Charlson index. Cause-specific relative mortality adjusted for age, stage, differentiation, and treatment was compared across Charlson scores with Cox proportional hazards functions.
Results. Blacks had significantly greater mortality from prostate cancer and other causes (vs Whites, relative risk [95% confidence interval] = 1.84 [1.22, 2.79] and 1.69 [1.33, 2.29], respectively; P < .001). However, differences disappeared as initial comorbidity increased (1.75 [1.33, 2.31] vs 0.90 [0.59, 1.29] for scores = 0 and ≥ 5, respectively).
Conclusions. Absence of a significant preexisting medical diagnosis is associated with a higher risk for excess mortality among Black men diagnosed with prostate cancer.
Black men with prostate cancer have poorer disease-specific and overall survival rates than do their US White counterparts.1 Blacks not only tend to present with more advanced disease but also experience a survival disadvantage within stages.2 Attempts to elucidate the factors responsible for this disparity have focused on hypotheses ranging from genetic factors to health care system failure.3–7 Some investigators have observed a narrowing of the Black–White survival gap with increasing age.8,9 Such a phenomenon could be explained, in part, by race-by-age-group differences in comorbidity. For example, in 1990 the mortality rate from ischemic heart disease among US men aged 45–64 years was higher among Blacks than among Whites (279 vs 237 per 100 000 for Blacks and Whites, respectively).10 However, among men aged 65 years and older, these mortality rate patterns were reversed (1375 vs 1584 per 100 000 for Blacks and Whites, respectively). Hence, it may be that fewer Black men older than 65 years have died from ischemic heart disease than otherwise might have been the case, given that those with more severe disease were removed from the cohort through fatalities at younger ages. Therefore, an improved overall survival among Blacks diagnosed with prostate cancer at older ages could reflect, among other things, the influence of a reduced burden of comorbid conditions.
Comorbidity at the time of diagnosis has been shown to predict both overall survival and cause-specific mortality among White men with localized prostate cancer.11,12 In fact, comorbidity has emerged as an important determinant of interindividual variation in prognosis, and the use of comorbidity to estimate the risks of death from other causes is recommended as a standard part of prostate cancer disease detection and management.13 The role that initial levels of comorbidity play in determining intergroup variation in survival of prostate cancer patients is less well established. Therefore, we performed a retrospective cohort study of the effect of comorbidity on survival outcomes in a biracial cohort of incident cancers diagnosed in the Chicago area. Our objective was to evaluate the prognostic significance of comorbidity at the time of diagnosis in relation to cause-specific mortality in both early- and advanced-stage prostate cancer and in Black and White men. This article focuses on the effect of comorbidity on racial differences in survival. We hypothesized that baseline differences in comorbidity would help explain racial variation in all-cause mortality beyond that caused by differences in age, stage at presentation, histological characteristics, and treatment patterns.
METHODS
Cohort Selection
Our cohort consisted of all cases of adenocarcinoma of the prostate diagnosed among Black and White men at 4 academic medical centers in the Chicago area (2 private university medical centers and 2 Department of Veterans Affairs [VA] medical centers with university affiliations) between January 1, 1986, and December 31, 1990. These hospitals were selected because a substantial proportion (approximately 40%) of the combined cohort consists of Blacks and, according to 1990 US census data, Blacks and Whites admitted to these hospitals form a socioeconomically diverse group.14 We identified from the tumor registry at each hospital 1163 cases (613 university, 550 VA) of adenocarcinoma of the prostate (International Classification of Diseases, Ninth Revision, Clinical Modification code 187.0).15 After obtaining appropriate institutional review board approvals at each of the participating hospitals, we attempted to locate the records of these patients. Of the 1163 patients originally identified, 1007 (87%) had medical records available for detailed review.
Baseline Characteristics
Inpatient and outpatient medical records were abstracted on-site by 2 trained reviewers who had no knowledge of the hypotheses under study. The data abstracted from each record included demographics (name, race, date of birth, social security number, and per capita income by zip code), tumor characteristics (stage, tumor differentiation, and Gleason sum16), processes of diagnosis and management (indication for diagnostic evaluation, method of diagnosis, clinical diagnosis date, pathological diagnosis date, metastatic evaluation, and first-course treatments), comorbidities present at the time of diagnosis, and follow-up information (date of last contact, disease recurrence/progression [date and location], vital status, and cancer status). Clinical diagnosis date referred to the date at which prostate cancer was first suspected on the basis of findings from the history, physical exam, and laboratory tests. Pathological diagnosis date was the date on which tissue that led to the cancer diagnosis was obtained. Stage was determined on the basis of review of all of the evidence available in the original patient record, and assignments were made according to American Joint Committee on Cancer TMN (tumor, node, metastasis) staging system.17 A pathologist’s assessment of tumor differentiation was available for all patients. Tumors were classified as either well, moderately, or poorly differentiated, as specified in the pathologist’s report. Corresponding Gleason sums were available in more than 80% of cases. Initial treatment was any treatment directed at the primary tumor received within 4 months of initiation of therapy.18,19 Comorbidity at the time of diagnosis was measured using the index developed by Charlson et al.20 Briefly, this index consists of various qualifying medical conditions that have been weighted according to prospectively derived relative mortality risk estimates. Each condition present is assigned a score; when more than 1 of the conditions is present, the index score for the individual is the sum of the weights for each condition. In our study, qualifying medical conditions detected within 1 year of the patient’s prostate cancer diagnosis were included in the calculation of his comorbidity score. Data from each patient were recorded on a structured data collection form, with each form reviewed by a single physician-reviewer for completeness and coherence. Intra- and interabstractor agreement was monitored for race, clinical diagnosis date, differentiation and TNM stage, treatment date, treatment(s), comorbidities, Charlson score, date of last contact, and vital status. The level of agreement between abstractors was high (κ = 0.45–0.98), on the basis of a 20% random sample of all records reviewed.
Exclusions
We reviewed 1007 records and excluded 90 because the cancers were T1a lesions, which are believed to be clinically insignificant, and excluded 53 because of incomplete data or missing records, leaving 864 records (479 university, 385 VA) for analysis. Whereas Blacks accounted for 38.8% of the analytic cohort, 64% of the cases in Black men were diagnosed at one of the 2 VA hospitals. Of the records not found (n = 156), 42% were of Black cases.
Outcomes and Their Ascertainment
Follow-up ended at December 31, 2000, with death from prostate cancer and from other causes serving as the primary outcomes of interest. We used the tumor registries of the participating hospital as our primary source for vital status ascertainment, given that each hospital actively tracked vital status through regular letter and telephone contact with patients and their families. We also conducted multiple searches of the National Death Index and the Veterans Administration’s Beneficiary Identification and Record Locator System through December 31, 2001, for deaths occurring on or before the end of the follow-up date but not recorded in the hospital tumor registry. The sensitivity of Beneficiary Identification and Record Locator System data is comparable to that of National Death Index data.21 Other outcomes of interest included prostate cancer recurrence (date and location), cancer status (presence or absence) as of the date of last contact, and causes of death. Multiple-cause-of-death data were based on death certificate reviews performed by an independent physician-reviewer blinded to the study’s hypotheses. Causes of death were coded according to the International Classification of Diseases.22
Statistical Methods
We used a 2-sample t test for continuous traits and χ2 analysis for categorical traits to compare the baseline demographic and clinical characteristics of Blacks and Whites. Postdiagnosis Kaplan–Meier survival distributions were computed for subgroups of men defined by tumor differentiation (well, moderate, poor), stage (localized or regional [T1b-3any N0-3 M0: tumor confined to prostate gland or extracapsular tumor, with or without regional lymph node involvement] vs distant [T4 NX,0 M0 or T any, N any M1: distant metastases]), race (Black vs White), and combinations thereof, and we used the Mantel–Haenszel statistic to compare distributions within subgroups.22 In our study, localized and regional-stage cases were combined, because they would both be candidates for aggressive primary therapy. The 3 histological subgroups used in our analyses—well, moderately, and poorly differentiated tumors—generally corresponded to Gleason sums of 2–4, 5–6, and 7–10, respectively. Unstaged cases were combined with distant cases, because their respective survival distributions were not significantly different (χ21 = 0.30, P = .58). Charlson comorbidity scores were available for all but 3% of the cases. We used a multiple regression method to impute these values in which the available comorbidity score was regressed on age at diagnosis, race, hospital of origin, stage, tumor differentiation, and presence of other diseases or conditions (coronary artery disease, hypertension, and tobacco and alcohol usage). The primary outcomes for this study were (1) estimates of the effect of comorbidity at the time of prostate cancer diagnosis on overall survival and (2) cause-specific mortality given the patient’s stage of prostate cancer and race after adjustment for the effects of age, tumor differentiation, and first-course treatment. A stratified Cox proportional hazards regression model was used to account for the baseline risk associated with the hospital in which the case originated.23 The regression model included age, race (Black vs non-Black), Charlson comorbidity score, tumor differentiation (well, moderately, or poorly differentiated), stage (localized/regional vs distant), and first-course treatment (surgery, radiation, diethylstilbestrol, castration, or observation). Potential interactions between comorbidity and stage and between comorbidity and race were evaluated by including a comorbidity-by-stage and a comorbidity-by-race term in the regression model. We used a bias-corrected bootstrap approach to compute 95% confidence intervals (95% CIs).24 Briefly, we selected 2500 bootstrap samples and calculated the relative risk for each, generating an estimate of the empirical distribution of the parameter estimator. The 2.5th and 97.5th percentiles of this distribution correspond to the left and right endpoints of the 95% CI. The Cox proportional hazards model used to estimate the survival of Blacks relative to Whites and the Charlson comorbidity score after adjusting for other factors (age, tumor differentiation, treatment) can be written in the following form: λ(t;Z) = λ0(t) exp(β1Black + β2Stage + β3score + β4Black × score + β5Stage × score + other factors), where Z denotes all covariates in the model, Black is an indicator for race (taking the value 1 for Black and 0 for White) and score is the Charlson comorbidity score. Black × score and stage × score are the interaction terms between race and comorbidity and score and comorbidity, respectively. Ninety-five percent confidence CIs for the relative hazards (henceforth referred to as relative risk [RR]) of death of Blacks relative to whites were calculated for each of 6 comorbidity levels, Charlson score = 0, 1, 2, 3, 4 and ≥5.
We considered a number of different regression models before reaching the final model. Time-dependent coefficients and Schoenfeld residuals were used to test the proportional hazards assumption in each of these models.25 No violations of the proportional hazards assumptions were observed (P = .23 to .81). The statistical analyses were performed with the statistical package Stata Release 7.0.26
RESULTS
Baseline Characteristics
Although there were no significant racial differences in age and differentiation in our cohort, Blacks tended to present with distant-stage disease more often than did their White counterparts (41.3% vs 25.1%, P < .001) and to experience a shorter interval between diagnosis and death (4.9 years vs 5.9 years, P < .001) and higher all-cause mortality (67.9% vs 53.1%, P < .001; Table 1 ▶). In addition, Blacks were twice as likely to have localized cancer initially managed with observation (12.1% vs 5.9%, P = .026), half as likely to have regional disease treated with radiation (28.3% vs 58.8%, P < .001), and 22% less likely to undergo surgical or medical castration for distant-stage disease (56.8% vs 72.9% for Black and Whites, respectively, P = .006).
TABLE 1—
Baseline Characteristics, by Race Among Men Diagnosed with Prostate Cancer, Chicago Area, 1986–1990
| All | Blacks | Whites | P | |
| Sample size | 864 | 327 | 537 | |
| Mean age, y (SD) | 69.0 (7.2) | 69.3 (7.8) | 68.8 (7.8) | .307 |
| Mean time from diagnosis to death, y | 5.5 | 4.9 | 5.9 | < .001 |
| Deaths, no. (%) | 507 (58.7) | 222 (67.9) | 285 (53.1) | < .001 |
| Differentiation, no. (%) | ||||
| Well | 235 (27.2) | 80 (24.5) | 155 (28.9) | |
| Moderate | 274 (31.7) | 103 (31.5) | 171 (31.8) | |
| Poor | 355 (41.1) | 144 (44.0) | 211 (39.3) | .276a |
| Stage, no. (%) | ||||
| Localized | 437 (50.6) | 132 (40.3) | 305 (56.8) | |
| Regional | 157 (18.2) | 60 (18.4) | 97 (18.1) | |
| Distant unstagedb | 270 (31.2) | 132 (41.3) | 133 (25.1) | < .001a |
aChi-square P value.
bPresumed to be distant stage by the treating physician but not staged (n = 5).
Comorbidity Scores and Most Common Diagnoses
Initial comorbidity was greater among Blacks relative to Whites (mean Charlson score 2.0 for Blacks vs 1.6 for Whites, P = .001; Table 2 ▶). Further stratification by Charlson score revealed a trend toward overrepresentation of Blacks in each score group, with their proportion relative to Whites generally increasing with increasing score (0.67, 1.08, 1.33, 1.17, and 1.31 for scores of 0, 1, 2, 3 and ≥ 4, respectively). Diabetes mellitus was the most common condition present at the time of prostate cancer diagnosis in our cohort. The disease was one third more prevalent among Blacks (21.7% and 16.0% for Blacks and Whites, respectively, P = .078), with complications (retinopathy, nephropathy, neuropathy) significantly more common among Blacks than among Whites (4.9% and 2.8%, respectively, P = .002). Renal disease, defined as serum creatinine ≥ 3 mg% or a history of renal transplantation, was also significantly more common among Blacks relative to Whites (8.6% vs 5.0%, P = .039), as was cerebrovascular disease with hemiplegia (7.0% vs 4.1%, P < .001). During the follow-up period, 507 (58.7%) men died—215 from prostate cancer and 292 from other causes. Survival varied by race for localized/regional-stage cases (χ2 = 5.59, P < .0181) but not for distant-stage cases (χ2 = 2.10, P < .147).
TABLE 2—
Comorbidity Scores and Most Common Diagnoses, by Race Among Men Diagnosed with Prostate Cancer, Chicago Area, 1986–1990
| Blacks (n = 327) | Whites (n = 537) | P | |
| Mean Charlson comorbidity score (SD) | 2.0 (2.2) | 1.6 (2.0) | .001 |
| Distribution of scores, % | |||
| 0 | 25.7 | 38.4 | |
| 1 | 26.3 | 24.2 | |
| 2 | 18.4 | 13.8 | |
| 3 | 12.2 | 10.4 | |
| ≥ 4 | 17.4 | 13.3 | .003a |
| Most common diagnoses (Charlson score [CS]), % | |||
| Diabetes mellitus, all cases (CS = 1)b | 21.7 | 16.0 | .078 |
| Diabetes mellitus, with microvascular complications (CS = 2)c | 4.9 | 2.8 | .002 |
| History of myocardial infarction (CS = 1)d | 15.0 | 16.6 | .536 |
| Cerebrovascular disease, all cases (CS = 1)e | 17.7 | 12.7 | .083 |
| Cerebrovascular disease, with hemiplegia (CS = 2)f | 7.0 | 4.1 | < .001 |
| Chronic obstructive pulmonary disease (CS = 1)g | 19.6 | 12.1 | .003 |
| Congestive heart failure (CS = 1) | 14.7 | 14.9 | .930 |
| Renal disease (CS = 2)h | 8.6 | 5.0 | .039 |
aChi-square P value.
bOn oral hypoglycemics or insulin.
cRetinopathy, nephropathy, or neuropathy.
dHistory of hospitalization with positive electrocardiogram or cardiac enzymes.
eHistory of transient ischemic attacks or cerebrovascular accident.
fClinical history documented with positive pulmonary function tests or response to bronchodilators.
gDyspnea on exertion or paroxysmal nocturnal dyspnea with response to digoxin, diuretics, or afterload reduction.
hCreatinine >3 mg% or history of renal transplantation.
Relative Risks of Death by Cause
After adjustment for age, stage, differentiation, treatment, and initial comorbidity, Blacks had significantly greater risks of death from prostate cancer (relative risk [RR] = 1.84 [95% CI = 1.22, 2.79], P = .004) and from other causes (RR = 1.69 [95% CI = 1.17, 2.43], P = .005; Table 3 ▶). Determinants of death from prostate cancer included tumor characteristics such as stage and differentiation but not patient characteristics such as age (RR = 1.01 [95% CI = 0.92, 1.11] per 5-year increment, P = .831) or initial comorbidity (RR = 1.04 [95% CI = 0.92, 1.18] per unit change in Charlson score, P = .506). However, age and baseline comorbidity were determinants of death from other causes (RR = 1.24 [95% CI = 1.14, 1.35] and 1.26 [95% CI = 1.14, 1.39] per 5-year increment in age and per unit increase in Charlson score, respectively, P < .001). Stage at diagnosis also correlated with risk of death from other causes (RR = 0.55 [95% CI = 0.35, 0.85] for localized/regional vs distant stage, P = .008). The interaction term was statistically significant.
TABLE 3—
Relative Risk (RR) of Death, by Causea Among Men Diagnosed with Prostate Cancer, Chicago Area, 1986–1990
| Prostate Cancer (n = 215) | Other Causes (n = 292) | Any Cause (n = 507) | ||||
| Characteristic | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P |
| Race (Black vs White) | 1.84 (1.22, 2.79) | .004 | 1.69 (1.17, 2.43) | .005 | 1.75 (1.33, 2.29 | < .001 |
| Age (in 5-year increments) | 1.01 (0.92, 1.11) | .831 | 1.24 (1.14, 1.35) | < .001 | 1.14 (1.07, 1.21 | < .001 |
| Differentiation (vs well) | ||||||
| Moderate | 2.26 (1.36, 3.78) | .002 | 1.09 (0.80, 1.49) | .573 | 1.35 (1.04, 1.74 | .024 |
| Poor | 3.91 (2.40, 6.39) | < .001 | 1.24 (0.90, 1.71) | .181 | 1.83 (1.42, 2.35 | < .001 |
| Stage (localized/regional vs distant) | 0.19 (0.11, 0.31) | < .001 | 0.55 (0.35, 0.85) | .008 | 0.31 (0.23, 0.42 | < .001 |
| Treatment | ||||||
| Surgery | 0.43 (0.24, 0.75) | .003 | 0.62 (0.42, 0.92) | .016 | 0.58 (0.42, 0.78 | .001 |
| Radiation | 1.57 (1.07, 2.30) | .020 | 0.95 (0.67, 1.35) | .795 | 1.17 (0.90, 1.51 | .233 |
| Diethylstilbestrol | 1.79 (1.04, 3.09) | .035 | 1.11 (0.58, 2.15) | .750 | 1.47 (0.97, 2.22 | .070 |
| Castrationb | 1.47 (0.97, 2.23) | .072 | 0.84 (0.55, 1.29) | .425 | 1.13 (0.84, 1.51 | .422 |
| Observation | 0.56 (0.22, 1.43) | .225 | 1.62 (1.02, 2.54) | .041 | 1.37 (0.93, 2.01 | .111 |
| Charlson scorec | 1.04 (0.92, 1.18) | .506 | 1.26 (1.14, 1.39) | < .001 | 1.13 (1.05, 1.22 | .001 |
Note. CI = confidence interval.
aBased on Cox proportional hazard model adjusted for baseline hazard rate for each hospital.
bOrchiectomy or leuprolide, or flutamide.
cScore range: 0 to 14.
Impact of Initial Comorbidity on Racial Differences in Survival
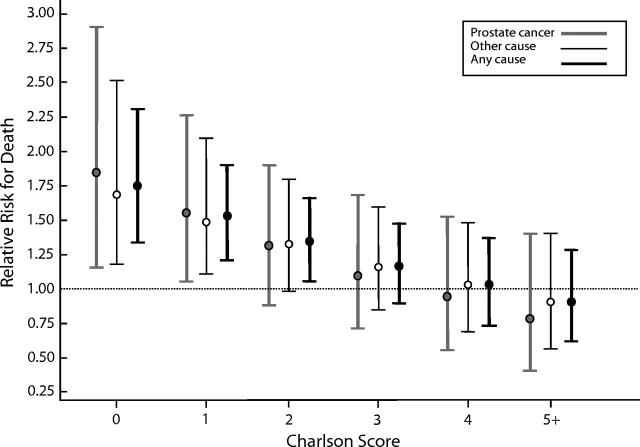
Figure 1 ▶ shows the change in relative risk for death from prostate cancer, other causes, and any cause for Blacks versus Whites by Charlson score adjusted for age, stage, tumor differentiation, and treatment.
FIGURE 1—
Ninety-five percent bootstrap confidence intervals for cause-specific mortality rates of Blacks relative to Whites, by Charlson comorbidity score.
Summary
Comorbidity at the time of diagnosis in our cohort was significantly higher among Black men than among White men. After we controlled for age, tumor characteristics, treatment, and initial comorbidity, Blacks had significantly greater (1.5- to 2-fold) risks of death from prostate cancer and from other causes. Comorbidity as measured with the Charlson index was an independent predictor of death from causes other than prostate cancer (but not of death caused by prostate cancer). After stratification by level of comorbidity at time of diagnosis, significant racial differences in survival eventually disappeared as initial comorbidity increased.
DISCUSSION
Among Black men diagnosed with prostate cancer, the absence of a significant preexisting medical diagnosis was associated with a higher risk of excess mortality from any cause, including prostate cancer. Modification of the Black–White survival gap by comorbidity indicates an interaction between race and comorbidity, and this is supported statistically in our model (coefficient for the race × comorbidity interaction term = −0.132, P = .001). More aggressive tumors among Blacks may render comorbidities less prognostically important because of greater competition from prostate cancer as a cause of death. However, Figure 1 ▶ suggests other, more likely pathways to racial disparities in prostate cancer outcomes. Race may act as a surrogate for social and cultural forces that influence the probability, content, and quality of relationships between health care systems and providers. Indeed, the literature is overflowing with evidence of disparities in the level and quality of care received by US minority populations relative to White populations.27 It is possible that for Black men, the absence of other known major diagnoses around the time their prostate cancer is detected may be attributable to underdiagnosis of those conditions or to underdiagnosis and poorer control of risk factors. Because we did not collect more data pertinent to these factors, we cannot directly test this hypothesis as it relates to racial differences in prostate cancer prognosis in our cohort. However, diagnosis of prostate cancer at an early stage was associated with a significantly lower risk of death from causes other than prostate cancer. To our knowledge, there is no biological reason for this finding. Furthermore, we did not observe an interaction between stage and comorbidity in our cohort (P value = .852). Early-stage diagnosis may correlate with a prior history of care or pattern of early diagnosis and with secondary prevention that favorably affects patient longevity.
We also observed a trend of decreasing excess all-cause and cause-specific mortality among Blacks as baseline comorbidity scores increased. In fact, this inverse association seemed to follow a dose–response relation. However, a “crossover” effect characterized by progressively better survival among Blacks relative to Whites as comorbidity increases seems unlikely.
Limitations
Because our cohort was limited to the Chicago metropolitan area, our results may not be generalizable to other settings. Also, our case patients were diagnosed between 1986 and 1990. Prostate-specific antigen testing was just being introduced during this period, and its use was not yet widespread. Therefore, more men had advanced-stage cancer at diagnosis, especially early on, and their care may have differed from current management in clinically important ways. As mentioned, 26% of the patients originally identified could not be included in the analysis. However, it seems unlikely that significant biases were introduced as a result of the exclusions, for several reasons. First, the excluded and analyzed groups each contained a comparable proportion of Blacks (42.0% vs 38.8% for excluded and analyzed cases, respectively). Second, after exclusion of the 90 incidental prostatic adenocarcinomas from the 1163 cases originally identified, 1073 men had lesions deemed clinically significant. Therefore, 80.5% of the clinically significant carcinomas were included in the analysis. Nevertheless, sample sizes for groups with the highest scores were relatively small. Larger sample sizes would have improved precision in estimating the effect of comorbidity on the Black–White survival gap.
CONCLUSIONS
The causes of prostate cancer may continue to elude investigators for some time. Until these causes are determined, a sustained focus on equalizing outcomes of the disease among groups—to the extent possible given our limited understanding of the causative exposures—will continue to be necessary.28 We evaluated the hypothesis that comorbidity at the time of diagnosis would help explain some of the racial disparity in all-cause mortality beyond that explained by differences in demographic, tumor, and treatment characteristics. Comorbidity did account for some of the increased mortality from other causes among Blacks relative to Whites. However, our data further indicated that the absence of a significant preexisting medical diagnosis was in itself a risk factor for excess mortality among Black men diagnosed with prostate cancer. This association probably reflects the action of cultural and social forces (rather than a racial effect per se) that lead to underdetection or delayed detection of prognostically important comorbidities or risk factors thereof.
Acknowledgments
This study was supported by research grants from the Robert Wood Johnson Foundation (MMFDP 033363) and the Department of Veterans Affairs (RCD 97-317).
Human Participant Protection The research protocol was approved by the human subjects committee at each of the involved institutions.
Contributors V. L. Freeman, R. Durazo-Arvizu, L. C. Keys, M. P. Johnson, K. Schafernak, and V. K. Patel contributed to the design. V. L. Freeman, R. Durazo-Arvizu, and V. K. Patel contributed to the analysis. V. L. Freeman, R. Durazo-Arvizu, L. C. Keys, and K. Schafernak contributed to the interpretation of the data. Finally, V. L. Freeman, R. Durazo-Arvizu, L. C. Keys, M. P. Johnson, K. Schafernak, and V. K. Patel contributed to drafting the article
Peer Reviewed
References
- 1.Stanford JL, Stephenson RA, Coyle LM, et al. Prostate Cancer Trends 1973–1995. SEER Program. National Institutes of Health Publication 99-4543. Bethesda, Md: National Cancer Institute; 1999.
- 2.Merrill RM, Brawley OW. Prostate cancer incidence and mortality rates among whites and black men. Epidemiology. 1997;8:126–131. [DOI] [PubMed] [Google Scholar]
- 3.Makridakis N, Ross RK, Pike MC, et al. Association of mis-sense substitution in SDR5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet. 1999;354:975–978. [DOI] [PubMed] [Google Scholar]
- 4.Jepson C, Kessler LG, Portnoy B, Gibbs T. Black-white differences in cancer prevention knowledge and behavior. Am J Public Health. 1991;81:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers RE, Wolf TA, Balshem AM, Ross EA, Chodak GW. Receptivity of African-American men to prostate cancer screening. Urology. 1994;43:480–487. [DOI] [PubMed] [Google Scholar]
- 6.Harlan L, Brawley O, Pommerenke F, Wall P, Kramer B. Geographic, age and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13:93–100. [DOI] [PubMed] [Google Scholar]
- 7.Blendon RJ, Aiken LH, Freeman HE, Corey CR. Access to medical care for black and white Americans: a matter of cost concern. JAMA. 1989;261:278–281. [PubMed] [Google Scholar]
- 8.Pienta KJ, Demers R, Hoff M, Kau TY, Montie JE, Severson RK. Effect of age and race on the survival of men with prostate cancer in the metropolitan Detroit tri-county area, 1973–1987. Urology. 1995;45:93–101. [DOI] [PubMed] [Google Scholar]
- 9.Powell IJ, Schwartz K, Hussain M. Removal of the financial barrier to health care: does it impact on prostate cancer at presentation and survival? A comparative study between black and white men in a Veteran’s Affairs system. Urology. 1995;46:825–830. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Chronic Disease Prevention and Health Promotion. Chronic Disease in Minority Populations. Atlanta, Ga: Centers for Disease Control and Prevention; 1992.
- 11.Albertsen PC, Fryeback DG, Storer BE, Kolon TF, Fine J. Long-term survival among men with conservatively treated prostate cancer. JAMA. 1995;274:626–631. [PubMed] [Google Scholar]
- 12.Sweat SD, Bergstralh EJ, Slezak J, Blute ML, Zincke H. Competing risk analysis after radical prostatectomy for clinically nonmetastatic prostate adenocarcinoma according to clinical Gleason score and patient age. J Urol. 2002;168:525–529. [PubMed] [Google Scholar]
- 13.American Cancer Society and the National Comprehensive Cancer Network. Prostate Cancer Treatment Guidelines for Patients (Version III, October 2002). Available at: http://www.nccn.org. Accessed March 21, 2004.
- 14.US Census Bureau. 1990 Census of Population—Social and Economic Characteristics. Washington, DC: US Dept of Commerce; 1993. Publication CP-2.
- 15.International Classification of Diseases, 9th Revision, Clinical Modification. Geneva, Switzerland: World Health Organization; 1980.
- 16.Gleason DF. Histologic grading of and staging of prostatic carcinoma. In: Tannenbaum M, ed. Urologic Pathology: The Prostate. Philadelphia, Pa: Lea & Febiger; 1977: 171–197.
- 17.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 5th ed. Philadelphia, Pa: Lippincott-Raven; 1997.
- 18.Schmidt JD, Mettlin CJ, Natarajan N, Peace BB, Beart RS Jr, Winchester DP. Trends in patterns of care for prostatic cancer 1974–1983: results of surveys by the American College of Surgeons. J Urol. 1987;136:416–421. [DOI] [PubMed] [Google Scholar]
- 19.Natarajan N, Murphy GP, Mettlin C. Prostate cancer in blacks: an update from the American College of Surgeon’s pattern of care studies. J Surg Oncol. 1989; 40:232–236. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Alex KL, MacKenzi CR. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Fisher SG, Weber L, Golber J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995;141:242–250. [DOI] [PubMed] [Google Scholar]
- 22.International Classification of Diseases, 9th Revision. Geneva, Switzerland: World Health Organization; 1980.
- 23.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 1980.
- 24.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- 25.Therneau TM, Grambsch PM. Modeling Survival Data. Extending the Cox Model. New York, NY: Springer; 2000.
- 26.Stata Statistical Software: Release 7.0 [computer program]. College Station, Tex: Stata Corp; 2001.
- 27.Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. [PubMed]
- 28.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health Care System for the 21st Century. Washington, DC: National Academy Press; 2001.