Abstract
Objectives. We sought to determine prevalence rates of vitamin C deficiency and depletion in the United States.
Methods. We used data from the Third National Health and Nutrition Examination Survey to assess intake of dietary, supplemental, and serum vitamin C.
Results. Mean intakes and serum levels of vitamin C were normal; however, vitamin C deficiency and depletion were common (occurring among 5%–17% and 13%–23% of respondents, respectively). Smokers, those who did not use supplements, and non-Hispanic Black males had elevated risks of vitamin C deficiency, while Mexican Americans had lower risks.
Conclusions. Health professionals should recommend consumption of vegetables and fruits rich in vitamin C and should recommend supplementation for individuals at risk of vitamin C deficiency.
Health professionals in the United States generally consider overt vitamin C deficiency, or scurvy, to be a disease of historical significance.1 Despite numerous case studies in the recent medical literature,2–19 scurvy is now presumed to be an uncommon disease in developed nations,20 and patients who present with low-grade inflammation, fatigue, limping, gum bleeding, or swollen extremities may not be screened for vitamin C deficiency.15,17,21,22 Furthermore, because the signs and symptoms of scurvy are similar to those of other conditions (e.g., vascultitis, rheumatic disorders, reduced lung function), patients with vitamin C deficiency initially may be misdiagnosed and prescribed medication without receiving proper therapy.17,23
In the United States, mean vitamin C intakes usually exceed the recommended dietary allowances (RDAs) of 75 and 90 mg per day for women and men, respectively.24 Elevated mean intakes, however, mask the fact that numerous US residents underconsume vitamin C. Data from the US Department of Agriculture’s 1994 to 1996 Continuing Survey of Food Intakes by Individuals showed that 18% of US adults consumed less than 30 mg per day of vitamin C, despite an overall mean intake of 95 mg per day.25 The data from this survey further indicated that 14% of male and 20% of female 13- to 18-year-olds consumed less than 30 mg per day of vitamin C (RDAs are 65 and 75 mg per day for girls and boys, respectively).26
In addition to low dietary intakes, numerous reports have indicated that cigarette smokers are at increased risk of low serum vitamin C owing to the free-radical-quenching role of vitamin C (i.e., the ability to render oxidants harmless),27–29 and the most recent data from the 2000 Behavioral Risk Factor Surveillance System indicate that 23% of US adults smoke cigarettes.30 At the same time, most Americans are not consuming the recommended number of servings of vegetables and fruits or taking vitamin supplements.20,31 Currently, the second leading cause of death in the United States is cancer; as a preventive measure, high vitamin C intakes may reduce the risk of oral, esophageal, stomach, and breast cancers.20 Serum vitamin C levels have been assessed in international studies,32–34 but little is known regarding vitamin C status among American children and adults. The present study was conducted to determine the prevalence of vitamin C deficiency and depletion in the United States.
METHODS
The National Center for Health Statistics conducted the Third National Health and Nutrition Examination Survey (NHANES III) to assess the health status of children and adults in the United States. In this cross-sectional survey, personal household information was collected and health examinations conducted with 30 818 individuals 2 months and older; household interviews were conducted over a 6-year period (1988–1994). Adults 60 years or older, non-Hispanic Blacks, and Mexican Americans were purposively oversampled to produce more precise estimates for these population groups. Detailed descriptions of the plan and operation of the survey, including informed consent, have been reported previously.35
The sample for this study (n = 15 769) included civilian, noninstitutionalized children and adults aged 12 to 74 years. Data regarding demographic characteristics, socioeconomic status, dietary habits, and health history were collected during the household interview. In addition, self-reported race/ethnicity was recorded during the household interview and coded as non-Hispanic White, non-Hispanic Black, or Mexican American.
Quantitative dietary data were collected via 24-hour dietary recalls during the clinic examination, and results were coded with the US Department of Agriculture nutrient database (included with the NHANES III CD-ROM).36 Respondents were queried regarding the supplements they used and how many times they had taken each supplement during the preceding month. As a means of estimating vitamin C intakes from supplements, a monthly total was calculated and then divided by 30 to derive daily supplemental vitamin C intake (as described by Will et al.37). Physical examinations, including venipunctures, were conducted in mobile examination centers approximately 2 to 4 weeks after household interviews. Overall, NHANES III response rates were 86% for the household interview and 78% for the physical examination.35
Participants were asked to fast overnight before arriving in the morning at the mobile examination center for assessment. Serum vitamin C was measured at the Centers for Disease Control and Prevention in Atlanta via isocratic high-performance liquid chromatography with electrochemical detection.38 The coefficient of variation for the vitamin C assay averaged 5.8%.38 (The term vitamin C was defined as comprising all compounds that exhibit the activity of ascorbic acid, including dehydroascorbic acid reduced during analysis.) Serum vitamin C levels, ranging from 0.0 μmol/L to the upper cutoff point of 170 μmol/L, were categorized according to internationally established limits: deficiency (less than 11 μmol/L), depletion (11–28 μmol/L), or normal (more than 28 μmol/L).33,39,40 Several participants (n = 21) were excluded from analyses because their serum vitamin C levels were quite high (more than 170 μmol/L) and of dubious validity.
Respondents 17 years and older were questioned about tobacco use on 2 separate occasions. As part of the household interview, respondents who reported that they had smoked at least 100 cigarettes in their lifetime were asked whether they currently smoked cigarettes. During the private interview conducted in the mobile examination center, all respondents—including those who had not reported any tobacco use in the household interview—were questioned about their use of cigarettes during the past 5 days.
The NHANES III CD-ROM is equipped with the Statistical Export and Tabulation System (SETS), which we used to export data into SPSS 10.0 (SPSS Inc, Chicago, Ill) for data reduction. We conducted all analyses using SPSS and SUDAAN (version 7.5; Research Triangle Institute, Research Triangle Park, NC), which is a statistical program that takes into account the NHANES sampling weights and the survey’s complex design. We used sample weights, based on probability of selection, to adjust for nonresponse; weights were poststratified to the US Bureau of Census 1990 estimates of the total US population.
To improve the normality of the distributions of dietary and serum vitamin C, we log-transformed data before conducting statistical analyses. We assessed data using tabulation to document vitamin C deficiency and depletion. We used odds ratios (ORs) and 95% confidence intervals (CIs) to estimate relative prevalence rates of vitamin C deficiency, with serum vitamin C levels below 11 μmol/L as a cutoff. We used Pearson’s correlation coefficient to assess the relationship between total vitamin C intakes and serum vitamin C. In the case of all tests, we considered 2-tailed P values less than .05 to be statistically significant.
RESULTS
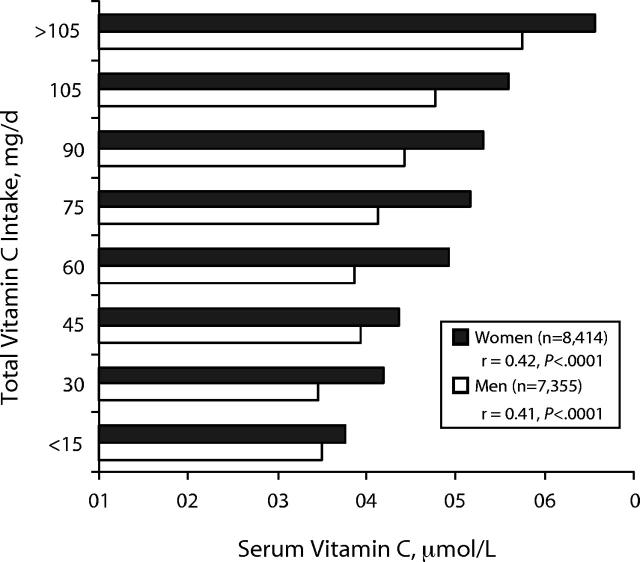
Mean dietary intakes and serum levels of vitamin C were within normal ranges, as indicated in Table 1 ▶. In the case of all age groups, mean vitamin C intakes from diet alone exceeded the RDA, ranging from 110 to 125 mg per day for males and 91 to 107 mg per day for females. Supplemental vitamin C resulted in mean total vitamin C intakes being even higher, ranging from 145 to 210 mg per day for males and 145 to 206 mg per day for females. Mean serum vitamin C levels ranged from 36.3 to 46.0 μmol/L and 42.6 to 55.1 μmol/L for males and females, respectively. As shown in Figure 1 ▶, total vitamin C intakes from diet and supplements were linearly related to serum vitamin C levels among both males (r = 0.41, P < .0001) and females (r = 0.42, P < .0001) across all age groups.
TABLE 1—
Mean (± SEM) Vitamin C Intakes and Serum Vitamin C Levels
| Gender and Age, y | No. | Population Sizea | Dietary Vitamin C, mg | Total Vitamin C,b mg | Serum Vitamin C, μmol/L |
| Male | |||||
| 12–17 | 975 | 9.59 | 117 ± 5 | 145 ± 7 | 46.0 ± 1.7 |
| 18–24 | 1011 | 11.00 | 125 ± 6 | 179 ± 16 | 36.9 ± 1.1 |
| 25–44 | 2649 | 36.53 | 119 ± 4 | 202 ± 14 | 36.3 ± 1.1 |
| 45–64 | 1765 | 20.39 | 110 ± 4 | 210 ± 16 | 38.0 ± 1.1 |
| 65–74 | 955 | 7.61 | 118 ± 5 | 194 ± 9 | 44.9 ± 1.1 |
| Female | |||||
| 12–17 | 1133 | 9.14 | 101 ± 5 | 145 ± 23 | 50.0 ± 1.7 |
| 18–24 | 1186 | 12.04 | 102 ± 5 | 159 ± 6 | 43.7 ± 1.7 |
| 25–44 | 3212 | 37.66 | 91 ± 2 | 164 ± 5 | 42.6 ± 1.1 |
| 45–64 | 1916 | 22.05 | 97 ± 4 | 206 ± 11 | 47.7 ± 1.1 |
| 65–74 | 967 | 8.95 | 107 ± 3 | 198 ± 10 | 55.1 ± 1.1 |
aIn millions, using weights obtained from NHANES III.
bDietary vitamin C plus supplements.
FIGURE 1—
Mean vitamin C intakes, stratified by serum vitamin C levels.
Table 2 ▶ shows that, overall, 14% of males and 10% of females were vitamin C deficient, as indicated by serum vitamin C values. The percentages of 12- to 17-year-old males and females who were vitamin C deficient were low (5%–6%) relative to other groups. Among all age groups, the percentage of males with vitamin C deficiency was greater than that of females, reaching a peak of 17% among 25- to 64-year-olds. Among females, the greatest prevalence (12%) of vitamin C deficiency was found among 25- to 44-year-olds.
TABLE 2—
Percentages of Vitamin C Deficiency, Depletion, and Normal Serum Values Among US Males and Females
| Serum Vitamin C Value | |||
| Gender and Age, y | <11 μmol/L, % | 11–28 μmol/L, % | >28 μmol/L, % |
| Male | |||
| 12–17 (n = 975) | 6 | 17 | 77 |
| 18–24 (n = 1011) | 13 | 22 | 65 |
| 25–44 (n = 2649) | 17 | 23 | 60 |
| 45–64 (n = 1765) | 17 | 20 | 63 |
| 65–74 (n = 955) | 11 | 15 | 74 |
| Overall | 14 | 20 | 66 |
| Female | |||
| 12–17 (n = 1133) | 5 | 15 | 80 |
| 18–24 (n = 1186) | 11 | 19 | 70 |
| 25–44 (n = 3212) | 12 | 20 | 68 |
| 45–64 (n = 1916) | 10 | 15 | 75 |
| 65–74 (n = 967) | 6 | 13 | 81 |
| Overall | 10 | 17 | 73 |
Across all age groups, proportionately more males and females exhibited vitamin C depletion than vitamin C deficiency. Among males, rates of vitamin C depletion ranged from 15% among 65- to 74-year-olds to 23% among 25- to 44-year-olds. Among females, the lowest rate of vitamin C depletion (13%) was found among 65- to 74-year-olds, and the highest rate (20%) was found among 25- to 44-year-olds.
Table 3 ▶ lists odds ratios related to risk of vitamin C deficiency. As a group, current smokers had the highest risk of vitamin C deficiency. The odds ratio of vitamin C deficiency among smokers was high in the case of both males (OR = 3.6; 95% CI = 3.2, 4.1) and females (OR = 4.2; 95% CI = 3.6, 4.9). Both males (OR = 3.3; 95% CI = 2.8, 4.0) and females (OR = 3.1; 95% CI = 2.6, 3.7) who had not used nutrient supplements in the past month had an increased risk of vitamin C deficiency. Non-Hispanic Black males had a slightly increased risk of vitamin C deficiency (OR = 1.2; 95% CI = 1.1, 1.5) relative to White males, while Mexican American males (OR = 0.83; 95% CI = 0.71, 0.97) and females (OR = 0.80; 95% CI = 0.66, 0.96) both had a lower risk of vitamin C deficiency than White males and females.
TABLE 3—
Effects of Smoking, Supplement Use, and Ethnicity on Vitamin C Deficiency
| Serum Vitamin C Value | |||
| <11 μmol/L, % | ≥11 μmol/L, % | Odds Ratio (95% Confidence Interval) | |
| Male | |||
| Smoking status | |||
| Nonsmokers (n = 4429) | 11 | 89 | 1.0 |
| Smokers (n = 2115) | 31 | 69 | 3.6 (3.2, 4.1) |
| Supplement use | |||
| Yes (n = 2119) | 7 | 93 | 1.0 |
| No (n = 5226) | 20 | 80 | 3.3 (2.8, 4.0) |
| Race/ethnicity | |||
| Non-Hispanic White (n = 2613) | 16 | 84 | 1.0 |
| Non-Hispanic Black (n = 2115) | 19 | 81 | 1.2 (1.1, 1.5) |
| Mexican American (n = 2346) | 13 | 87 | 0.83 (0.71, 0.97) |
| Female | |||
| Smoking status | |||
| Nonsmokers (n = 5776) | 7 | 93 | 1.0 |
| Smokers (n = 1686) | 25 | 75 | 4.2 (3.6, 4.9) |
| Supplement use | |||
| Yes (n = 3204) | 5 | 95 | 1.0 |
| No (n = 5204) | 14 | 86 | 3.1 (2.6, 3.7) |
| Race/ethnicity | |||
| Non-Hispanic White (n = 3039) | 11 | 89 | 1.0 |
| Non-Hispanic Black (n = 2605) | 13 | 87 | 1.2 (1.0, 1.4) |
| Mexican American (n = 2379) | 9 | 91 | 0.80 (0.66, 0.96) |
DISCUSSION
These nationwide data indicate that a considerable number of US residents are vitamin C deficient or depleted. Although our findings are contrary to the accepted notion that vitamin C status in the United States is within normal limits, previous work corroborates these data. Dickinson et al.41 assessed NHANES II (in which data were collected from 1976 to 1980) and reported that vitamin C depletion occurred in up to 25% of nonsmoking men and up to 50% of adult male smokers. In smaller, more recent studies, Johnston and colleagues42,43 reported vitamin C depletion in approximately 21% of university students (n = 98) and 30% of outpatients presenting to a local health maintenance organization laboratory (n = 494). Johnston et al.42 further reported that 6.3% of their outpatient sample had plasma ascorbic acid concentrations indicative of vitamin C deficiency.
We found moderately strong correlations (r = 0.41 and r = 0.42 for men and women, respectively) between total vitamin C intakes and serum vitamin C levels, as did Loria et al.40 using NHANES II data (r = 0.54) and Sinha et al.44 (r = 0.56) in a case–control study. These values are higher than that reported by Drewnowski et al.45 for the correlation between serum vitamin C and total vegetable and fruit intakes (r = 0.29). In that study, however, use of supplemental vitamin C was not reported. Measuring supplemental vitamin C is of crucial importance in analyses because total vitamin C intakes and serum vitamin C have an S-shaped relationship. When intakes exceed 70 mg per day, excess vitamin C is excreted in the urine, causing the correlation to flatten off as vitamin C intakes increase.46
Consistent with data reported from NHANES II,40 our data showed that elderly US residents (aged 65 years or older) had a lower prevalence of vitamin C deficiency and depletion than members of other adult age groups. These results differ from those of Bates et al.,47 who found that 33% of their sample of community-dwelling British adults 65 years or older (n = 1310) consumed less than the United Kingdom’s reference nutrient intake48 for vitamin C. Bates et al.47 further reported that 14% of their sample had plasma vitamin C levels below 11 μmol/L, indicative of vitamin C deficiency. In the same study, 40% of elderly individuals residing in nursing homes or residential homes (n = 423) had plasma vitamin C levels below 11 μmol/L, with a mean level of 24.4 μmol/L.47
Seniors are more likely than individuals in other age groups to purchase and use nutrient supplements,49–52 and vitamin C consistently ranks as one of the most frequently purchased supplements.40 Previous research has shown that consumption of vitamin C supplements results in a doubling of total vitamin C intake,44,46 and we showed that individuals who had not used supplements in the previous month had a greatly increased risk of vitamin C deficiency (odds ratios of 3.3 and 3.1 for males and females, respectively). Furthermore, McKay et al.50 noted that supplementation with 250 mg of vitamin C for 8 weeks resulted in a 29% increase in plasma concentrations of vitamin C in a group of community-dwelling, healthy seniors. For many years, physicians, dietitians, and other health professionals have hesitated to discuss supplementation with patients, partly to avoid implying that supplements can substitute for a healthy eating plan; however, this paradigm may be changing. Recently, Fletcher and Fairfield53 recommended that all US adults take a multivitamin every day to reduce their risk of chronic disease, and additional dialogue is needed to determine appropriate levels of supplementation.
In the United States, individuals who take supplements are least likely to need them,44 and several studies have noted that cigarette smokers are unlikely to purchase supplements.29,54 We showed that cigarette smokers had a high risk of vitamin C deficiency (odds ratios of 3.6 and 4.2 among male and female smokers, respectively). Vitamin C is a strong reducing agent (i.e., an electron donor), both in vivo and in vitro, and the lower level of serum vitamin C reported among smokers probably is caused by higher turnover of vitamin C owing to its antioxidant activity.55,56 The Food and Nutrition Board of the National Academy of Sciences recommends that individuals who smoke consume an additional 35 mg of vitamin C per day (110 and 125 mg per day for adult females and males, respectively).24 In all likelihood, this additional vitamin C is not sufficient to combat the oxidative damage that results from cigarette smoking. High intakes of vitamin C, as achieved by supplementation, may be appropriate for smokers, especially those who do not consume ample servings of vegetables and fruits rich in vitamin C.
Race/ethnicity-specific data regarding vitamin C status are sparse, but our data showed that non-Hispanic Black males had a slightly increased risk of vitamin C deficiency (OR = 1.2). In cross-sectional studies, Koh et al.57 and Loria et al.40 reported that plasma ascorbic acid levels were significantly lower among Black than White residents of the United States, while Ness et al.32 reported that London residents of Caribbean or West African descent had lower levels of plasma vitamin C than did Whites. These lower serum levels seem to be the result of poorer dietary intakes40 rather than any genetic differences in vitamin C absorption or use.
Furthermore, Vitolins et al.52 reported that Black residents of the United States were significantly (P = .001) less likely to use supplements than were Whites or Native Americans. In comparison with non-Hispanic Blacks, we showed that Mexican American males and females had significantly lower risks of vitamin C deficiency (OR = 0.83 and OR = 0.80, respectively). Because it involves common consumption of chiles, tomatoes, and squashes, the traditional Mexican diet is rich in vitamin C and other nutrients.58 However, Mexican Americans are at increased risk of chronic diseases related to hypertension, overweight, and type 2 diabetes, and thus further research is warranted to better understand food availability, eating habits, and disease outcomes in this population.59–61
Internationally, vitamin C deficiency is frequently observed when vegetable and fruit intakes are limited as a result of lack of availability, high prices, and poor storage capacity.33 One would not assume vitamin C deficiency to be common in America, given the variety of US diets; however, Chiplonkar et al.34 reported that the prevalence of vitamin C deficiency among Western Indian adults was quite similar to what we reported here for the United States. Vegetables and fruits serve as primary contributors to total vitamin C intake,10,42,48 and although numerous Americans are meeting the National Cancer Institute’s “5-a-day” goal for vegetables and fruits,62 many of the vegetables and fruits typically consumed are not good sources of vitamin C. The leading vegetables and fruits consumed in the United States—in descending order of consumption—are iceberg lettuce, raw tomatoes, french fries, bananas, and orange juice, representing nearly 30% of all vegetables and fruits consumed by US adults25,31; of these foods, however, only orange juice is a rich source of vitamin C. Broccoli, strawberries, kale, and grapefruit all are rich sources of vitamin C, but, combined, they represent less than 2% of all vegetable and fruit consumption in the United States.31
The amount of vitamin C in any particular food may differ considerably from what is listed in a nutrient database or food label. Vitamin C—the least stable of the vitamins—is readily destroyed by exposure to air,48 and degradation is accelerated further by exposure to heat, alkali, and metals.63 In fact, normal cooking of vegetables and fruits can reduce their vitamin C content by 20% to 40%.10 Johnston and Bowling64 assessed vitamin C oxidation in orange juice and found that the amount of ascorbic acid per 8 fl oz (240 mL) dropped as the expiration date approached, with a decomposition rate of approximately 2% ascorbic acid per day once the container of juice was opened.
One limitation of the present study was that our measure of vitamin C status was based on a single blood sample. Because vitamin C is water soluble and is not stored for a long period of time in body tissues, a single measurement of vitamin C may indicate only an individual’s short-term (1–4 weeks) vitamin C status. Although single 24-hour dietary recalls do not fully describe a given individual’s eating habits, the 1-day recall method applied to a large population is more effective than more complex and expensive methods when the goal is to determine group means.65 In addition, all large-scale nutrition surveys have the potential for underestimating food and nutrient intakes. In the present case, however, the 24-hour dietary recalls were administered via standardized, computerized probes; edited carefully for completeness; and verified to determine the accuracy of extreme values.35 Trained study staff, whose performance was monitored routinely, completed the dietary interviews in private rooms, and nearly all (95%) of the participants provided 24-hour dietary recalls. For these reasons, we believe that our dietary data provide a relatively complete assessment of vitamin C intakes.
In conclusion, our data indicate that a considerable number of children and adults in the United States are vitamin C deficient or depleted. Health professionals should continue to recommend consumption of vegetables and fruits, especially those that are rich in vitamin C. In addition, vitamin C supplementation should be discussed with all patients, but especially those who are at the greatest risk of vitamin C deficiency: cigarette smokers and poor eaters.
Contributors J. S Hampl drafted the article, and C. A. Taylor and C. S. Johnston contributed to critical revisions of the article. J. S Hampl and C. A. Taylor were responsible for acquisition of the data. All of the authors were involved with the study’s conception and design and the analysis of data.
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
References
- 1.Hampl JS, Johnston CS, Mills RA. Scourge of black-leg (scurvy) on the Mormon Trail. Nutrition. 2001;17:416–418. [DOI] [PubMed] [Google Scholar]
- 2.Clark NG, Sheard NF, Kelleher JF. Treatment of iron-deficiency anemia complicated by scurvy and folic acid deficiency. Nutr Rev. 1992;50:134–137. [DOI] [PubMed] [Google Scholar]
- 3.Gómez-Carrasco JA, Lopez-Herce Cid J, de Frutos CB, Ripalda-Crespo MJ, Garcia de Frias JE. Scurvy in adolescence. J Pediatr Gastroenterol Nutr. 1994;19:118–120. [DOI] [PubMed] [Google Scholar]
- 4.McKenna KE, Dawson JF. Scurvy occurring in a teenager. Clin Exp Dermatol. 1993;18:75–77. [DOI] [PubMed] [Google Scholar]
- 5.Case records of the Massachusetts General Hospital: 72-year-old man with exertional dyspnea, fatigue, and extensive ecchymoses and purpuric lesions. N Engl J Med. 1995;333:1695–1702. [DOI] [PubMed] [Google Scholar]
- 6.Gabay C, Voskuyl AE, Cadiot G, Mignon M, Kahn MF. A case of scurvy presenting with cutaneous and articular signs. Clin Rheumatol. 1993;12:278–280. [DOI] [PubMed] [Google Scholar]
- 7.Shelton RM, Ryan MT. Scurvy: a case in a young healthy woman. J Am Acad Dermatol. 1992;27:773–774. [DOI] [PubMed] [Google Scholar]
- 8.Fain O, Mathieu E, Thomas M. Scurvy in patients with cancer. BMJ. 1998;316:1661–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloan B, Kulwin DR, Kersten RC. Scurvy causing bilateral orbital hemorrhage. Arch Ophthalmol. 1999;117:842–843. [DOI] [PubMed] [Google Scholar]
- 10.Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895–906. [DOI] [PubMed] [Google Scholar]
- 11.Levin NA, Greer KE. Scurvy in an unrepentant carnivore. Cutis. 2000;66:39–44. [PubMed] [Google Scholar]
- 12.Mimasaka S, Funayama M, Adachi N, Nata M, Morita M. A fatal case of infantile scurvy. Int J Legal Med. 2000;114:122–124. [DOI] [PubMed] [Google Scholar]
- 13.Oeffinger KC. Scurvy: more than historical relevance. Am Fam Physician. 1993;48:609–613. [PubMed] [Google Scholar]
- 14.Pangan AL, Robinson D. Hemarthrosis as initial presentation of scurvy. J Rheumatol. 2001;28:1923–1925. [PubMed] [Google Scholar]
- 15.Ramar S, Sivaramakrishnan V, Manoharan K. Scurvy—a forgotten disease. Arch Phys Med Rehabil. 1993;74:92–95. [PubMed] [Google Scholar]
- 16.Riepe FG, Eichmann D, Oppermann HC, Schmitt HJ, Tunnessen WW Jr. Special feature: picture of the month. Infantile scurvy. Arch Pediatr Adolesc Med. 2001;155:607–608. [DOI] [PubMed] [Google Scholar]
- 17.Shetty AK, Steele RW, Silas V, Dehne R. A boy with a limp. Lancet. 1998;351:182. [DOI] [PubMed] [Google Scholar]
- 18.Tamura Y, Welch DC, Zic JA, Cooper WO, Stein SM, Hummell DS. Scurvy presenting as painful gait with bruising in a young boy. Arch Pediatr Adolesc Med. 2000;154:732–735. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. [DOI] [PubMed] [Google Scholar]
- 20.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002;287:3116–3126. [DOI] [PubMed] [Google Scholar]
- 21.Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–1868. [DOI] [PubMed] [Google Scholar]
- 22.Johnston CS, Swan PD, Corte C. Substrate utilization and work efficiency during submaximal exercise in vitamin C depleted-repleted adults. Int J Vitam Nutr Res. 1999;69:41–44. [DOI] [PubMed] [Google Scholar]
- 23.Cook DG, Carey IM, Whincup PH, et al. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax. 1997;52:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. [PubMed]
- 25.Taylor CA, Hampl JS, Johnston CS. Low intakes of vegetables and fruits, especially citrus fruits, lead to inadequate vitamin C intakes among adults. Eur J Clin Nutr. 2000;54:573–578. [DOI] [PubMed] [Google Scholar]
- 26.Hampl JS, Taylor CA, Johnston CS. Intakes of vitamin C, vegetables and fruits: which schoolchildren are at risk? J Am Coll Nutr. 1999;18:582–590. [DOI] [PubMed] [Google Scholar]
- 27.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. [DOI] [PubMed] [Google Scholar]
- 28.Weber C, Erl W, Weber K, Weber PC. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation. 1996;93:1488–1492. [DOI] [PubMed] [Google Scholar]
- 29.Hampl JS, Betts NM. Cigarette use during adolescence: effects on nutritional status. Nutr Rev. 1999;57:215–221. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Cigarette smoking among adults aged 18 and older, 2000. Available at: http://www.cdc.gov/tobacco/statehi/html_2002/current_2000.htm. Accessed July 29, 2002.
- 31.Johnston CS, Taylor CA, Hampl JS. More Americans are eating “5 a Day” but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000;130:3063–3067. [DOI] [PubMed] [Google Scholar]
- 32.Ness AR, Cappuccio FP, Atkinson RW, Khaw KT, Cook DG. Plasma vitamin C levels in men and women from different ethnic backgrounds living in England. Int J Epidemiol. 1999;28:450–455. [DOI] [PubMed] [Google Scholar]
- 33.Matilainen T, Vartiainen E, Puska P, et al. Plasma ascorbic acid concentrations in the Republic of Karelia, Russia and in North Karelia, Finland. Eur J Clin Nutr. 1996;50:115–120. [PubMed] [Google Scholar]
- 34.Chiplonkar SA, Agte VV, Mengale SS, Tarwadi KV. Are lifestyle factors good predictors of retinol and vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr. 2002;56:96–104. [DOI] [PubMed] [Google Scholar]
- 35.Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Washington, DC: National Center for Health Statistics; 1994. [PubMed]
- 36.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the Third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65(suppl 4):1203S–1209S. [DOI] [PubMed] [Google Scholar]
- 37.Will JC, Ford ES, Bowman BA. Serum vitamin C concentrations and diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 1999;70:49–52. [DOI] [PubMed] [Google Scholar]
- 38.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atlanta, Ga: Centers for Disease Control and Prevention; 1996.
- 39.Jacob RA. Assessment of human vitamin C status. J Nutr. 1990;120(suppl 11):1480–1485. [DOI] [PubMed] [Google Scholar]
- 40.Loria CM, Whelton PK, Caulfield LE, Szklo M, Klag MJ. Agreement among indicators of vitamin C status. Am J Epidemiol. 1998;147:587–596. [DOI] [PubMed] [Google Scholar]
- 41.Dickinson VA, Block G, Russek-Cohen E. Supplement use, other dietary and demographic variables, and serum vitamin C in NHANES II. J Am Coll Nutr. 1994;13:22–32. [DOI] [PubMed] [Google Scholar]
- 42.Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366–370. [DOI] [PubMed] [Google Scholar]
- 43.Johnston CS, Solomon RE, Corte C. Vitamin C status of a campus population: college students get a C minus. J Am Coll Health. 1998;46:209–213. [DOI] [PubMed] [Google Scholar]
- 44.Sinha R, Frey CM, Kammerer WG, McAdams MJ, Norkus EP, Ziegler RG. Importance of supplemental vitamin C in determining serum ascorbic acid in controls from a cervical cancer case-control study: implications for epidemiological studies. Nutr Cancer. 1994;22:207–217. [DOI] [PubMed] [Google Scholar]
- 45.Drewnowski A, Rock CL, Henderson SA, et al. Serum beta-carotene and vitamin C as biomarkers of vegetable and fruit intakes in a community-based sample of French adults. Am J Clin Nutr. 1997;65:1796–1802. [DOI] [PubMed] [Google Scholar]
- 46.Knutsen SF, Fraser GE, Linsted KD, Beeson WL, Shavlik DJ. Comparing biological measurements of vitamin C, folate, alpha-tocopherol and carotene with 24-hour dietary recall information in non-Hispanic blacks and whites. Ann Epidemiol. 2001;11:406–416. [DOI] [PubMed] [Google Scholar]
- 47.Bates CJ, Prentice A, Cole TJ, et al. Micronutrients: highlights and research challenges from the 1994-5 National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr. 1999;82:7–15. [DOI] [PubMed] [Google Scholar]
- 48.Ministry of Agriculture, Fisheries and Food. Manual of Nutrition. 10th ed. London, England: Her Majesty’s Stationery Office; 1996.
- 49.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. Vital Health Stat 11. 1999;No. 244. [PubMed]
- 50.McKay DL, Perrone G, Rasmussen H, et al. The effects of a multivitamin/mineral supplement on micronutrient status, antioxidant capacity and cytokine production in healthy older adults consuming a fortified diet. J Am Coll Nutr. 2000;19:613–621. [DOI] [PubMed] [Google Scholar]
- 51.Greger JL. Dietary supplement use: consumer Characteristics and interests. J Nutr. 2001;131(suppl 4):1339S–1343S. [DOI] [PubMed] [Google Scholar]
- 52.Vitolins MZ, Quandt SA, Case LD, Bell RA, Arcury TA, McDonald J. Vitamin and mineral supplement use by older rural adults. J Gerontol Med Sci. 2000;55:M613–M617. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults: clinical applications. JAMA. 2002;287:3127–3129. [DOI] [PubMed] [Google Scholar]
- 54.Kirk SF, Cade JE, Barrett JH, Conner M. Diet and lifestyle characteristics associated with dietary supplement use in women. Public Health Nutr. 1999;2:69–73. [DOI] [PubMed] [Google Scholar]
- 55.Faruque MO, Khan MR, Rahman MM, Ahmed F. Relationship between smoking and antioxidant nutrient status. Br J Nutr. 1995;73:625–632. [DOI] [PubMed] [Google Scholar]
- 56.Hininger I, Chopra M, Thurnham DI, et al. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr. 1997;51:601–606. [DOI] [PubMed] [Google Scholar]
- 57.Koh ET, Chi MS, Lowenstein FW. Comparison of selected blood components by race, sex, and age. Am J Clin Nutr. 1980;33:1828–1835. [DOI] [PubMed] [Google Scholar]
- 58.Algert SJ, Brzezinski E, Ellison TH. Mexican American Food Practices, Customs, and Holidays. 2nd ed. Chicago, Ill: American Dietetic Association; 1998.
- 59.Aguilar-Salinas CA, Vazquez-Chavez C, Gamboa-Marrufo R, et al. Obesity, diabetes, hypertension, and tobacco consumption in an urban adult Mexican population. Arch Med Res. 2001;32:446–453. [DOI] [PubMed] [Google Scholar]
- 60.Hampl JS, Sass S. Focus groups indicate that vegetable and fruit consumption by food stamp-eligible Hispanics is affected by children and unfamiliarity with non-traditional foods. J Am Diet Assoc. 2001;101:685–687. [DOI] [PubMed] [Google Scholar]
- 61.Elder JP, Candelaria JI, Woodruff SI, Criqui MH, Talavera GA, Rupp JW. Results of language for health: cardiovascular disease nutrition education for Latino English-as-a-second-language students. Health Educ Behav. 2000;27:50–63. [DOI] [PubMed] [Google Scholar]
- 62.Heimendinger J, Chapelsky D. The National 5 a Day for Better Health Program. Adv Exp Med Biol. 1996;401:199–206. [DOI] [PubMed] [Google Scholar]
- 63.Williams PG. Vitamin retention in Cook/chill and cook/hot-hold hospital food-services. J Am Diet Assoc. 1996;96:490–498. [DOI] [PubMed] [Google Scholar]
- 64.Johnston CS, Bowling DL. Stability of ascorbic acid in commercially available orange juices. J Am Diet Assoc. 2001;101:525–529. [DOI] [PubMed] [Google Scholar]
- 65.Margetts BM, Nelson M. Design Concepts in Nutritional Epidemiology. New York, NY: Oxford University Press Inc; 1991.