Abstract
Objectives. We investigated vaccine risk perception among reporters of autism to the Vaccine Adverse Event Reporting System (VAERS).
Methods. We conducted structured interviews with 124 parents who reported autism and related disorders to VAERS from 1990 to 2001 and compared results with those of a published survey of parents in the general population.
Results. Respondents perceived vaccine-preventable diseases as less serious than did other parents. Only 15% of respondents deemed immunization extremely important for children’s health; two thirds had withheld vaccines from their children.
Conclusions. Views of parents who believe vaccines injured their children differ significantly from those of the general population regarding the benefits of immunization. Understanding the factors that shape this perspective can improve communication among vaccine providers, policymakers, and parents/patients.
Despite scientific evidence that vaccination does not cause autism,1–11 many people continue to believe that a causal association exists. Particularly after a case series12 concerning measles–mumps–rubella vaccine (MMR), autism, and gastrointestinal symptoms received wide publicity, the public became concerned about a possible link. Understanding people’s mental models13 of autism, that is, their existing understanding and prior beliefs about vaccines and autism, might help to improve communication about vaccines among vaccine providers, policy makers, and parents/patients. To contribute to this understanding, we identified Vaccine Adverse Event Reporting System (VAERS) reports of autism and related disorders, telephoned those VAERS reporters, and conducted structured interviews to characterize their risk perceptions.
METHODS
VAERS
VAERS was established in 1990. It is jointly managed by the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) and receives more than 14 000 reports annually. Reports are submitted by health care providers, vaccinees, manufacturers, and others. Passive surveillance systems, such as VAERS, are subject to many limitations, including underreporting, incomplete information in many reports, inadequate denominator data, and lack of an unbiased comparison group.14,15 Therefore, it usually is not possible to determine causal associations between vaccines and adverse events from VAERS reports. However, for a follow-up study designed to improve scientific understanding, VAERS can serve as a registry of neurodevelopmental disorders after vaccination.
Subject Selection
To identify adverse event descriptions that suggested autism, we searched VAERS for Coding Symbols for a Thesaurus of Adverse Reaction Terms (standardized words that describe a patient’s symptoms) of autism, schizophrenic reaction, abnormal thinking, and personality disorder. We also searched symptom descriptions for developmental delay, pervasive developmental disorder, and other phrases suggesting autism. We manually reviewed each report to determine whether the event description was consistent with autism.
Survey Instrument
The survey questionnaire addressed demographics, clinical characteristics, comorbidities, family history, and previous experience with vaccines. Gellin, Maibach, and Marcuse conducted a survey among parents to study “knowledge, attitudes, misconceptions, and concerns about vaccine-preventable diseases, vaccines, and immunization policies.”16(p1097) To compare the responses of our respondents with those of the general public, with permission, we incorporated into our survey instrument Gellin et al.’s questions about perceived relative importance of immunization, reasons to immunize, omission of specific vaccines, perceived severity of vaccine-preventable illnesses and the likelihood of acquiring them from nonimmunized individuals, vaccine safety in general, key beliefs about vaccinations, and familiarity with and trustworthiness of various organizations that provide information about immunizations. We adapted the latter items to ask about the familiarity with, and trustworthiness of, organizations that provide information about autism.
To test the clarity of the survey instrument, we conducted pilot interviews with 16 reporters of autism and related disorders. No major problems were identified with the survey, and only minor modifications were made. Therefore, data collected during the pilot study were analyzed with the rest of the data. A contractor with experience in health surveys was hired and trained to conduct interviews and to perform data entry. From January 2001 to May 2002, the investigators and trained medical interviewers conducted structured interviews. As many as 10 attempts were made to reach each reporter: 3 on weekdays, 4 on weeknights, and 3 on weekends. For quality control, FDA personnel monitored a subset of the interviews and the data entered.
Data Entry and Analysis
The data entry program was a Microsoft Windows utility for the entry, validation, and postprocessing of text-based data. The data were summarized with descriptive statistics with the SAS Version 8.02 programming language (SAS Institute Inc, Cary, NC). For open-ended questions such as, “What made you think that ______’s symptoms might be related to a vaccination?” the most frequent answers were summarized by category (newspaper article, family/friends, Web site), and the remainder were independently reviewed by 2 of the authors (A. B. and E. J. W.).
To assess whether report or reporter characteristics changed after the promulgation of news relating to autism and vaccines, we analyzed data by the date that VAERS reports were received. The highly publicized case series about vaccines and autism was published in February 1998.12 In July 1999, the American Academy of Pediatrics (AAP) and the US Public Health Service recommended that manufacturers remove or reduce thimerosal in vaccines.11 Two separate date stratifications—1 with March 1, 1998, as a cutoff and 1 with August 1, 1999, as a cutoff—were performed.
RESULTS
Subject Selection
We identified 351 VAERS reports from July 1, 1990, to July 10, 2001, with Coding Symbols for a Thesaurus of Adverse Reaction Terms or symptom descriptions that suggested autism or a related disorder. One hundred sixty-four originated outside the United States or had no locating information. Of the 187 with contact information available, 36 of the reporters could not be contacted, 26 refused to participate, and 1 stated that her child had never had any autistic symptoms. The study included 124 reporters who agreed to participate.
Characteristics of Respondents, Subjects of Reports, and Vaccines
Respondents.
Most respondents were the child’s biological mother, adoptive mother, or stepmother (91.1%); 30 years of age or older (92.7%); and White (91.9%). The majority had at least some college education (91.1%) and an annual household income of $50 000 or more (62.1%). Compared with respondents in Gellin and colleague’s study,16 the VAERS respondents were older, more educated, more affluent, and more likely to describe themselves as White.
Reports.
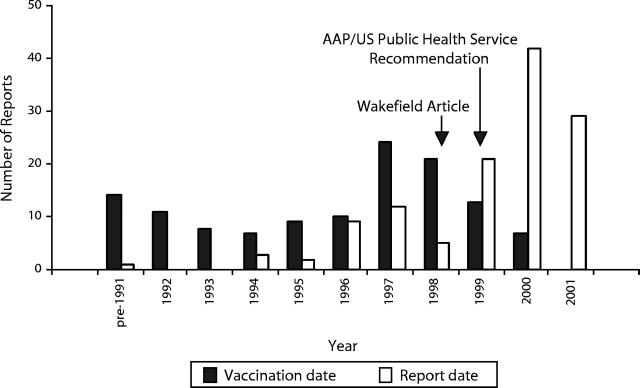
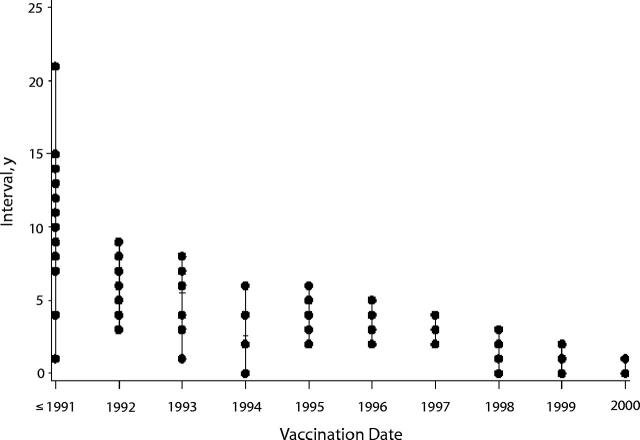
Twenty-seven (21.8%) reports were received from July 1, 1990, to February 28, 1998; 12 (9.7%) were received from March 1, 1998, to July 31, 1999; and 83 (66.9%) were received from August 1, 1999, to July 10, 2001. The date of receipt in VAERS was missing from 2 (2%) reports. Vaccination date for these 124 children peaked in 1997 (Figure 1 ▶), but report date peaked toward the end of our study period (2000–2001). The interval between vaccination date and report date ranged from 11 days to 21 years and tended to decrease over time (Figure 2 ▶).
FIGURE 1—
Vaccination date and report date for 124 VAERS cases of autism and related disorders through July 10, 2001.
FIGURE 2—
Interval between vaccination date and report date, by vaccination date.
Note. Each dot represents an interval that occurred at least once for a given year of vaccination. For example, for reports with a vaccination date in 1996, there were 3 reports with a delay of 2 years (reports received in 1998), 4 reports with a delay of 3 years, 2 with a delay of 4 years, and 1 with a delay of 5 years.
Subjects of reports.
The majority of children were male (85.5%). The average age at symptom onset was 19.9 months (SD = 19.16 months), with a median of 15.4 months and 8 missing values, but there was substantial variability (range 2 months to 8 years).
Autism and related disorders.
Three quarters of reporters (75.0%) stated that their child had been diagnosed with autism or autism spectrum disorder, 4.0% reported a diagnosis of mental retardation, and 38.7% stated that their child’s diagnosis was a developmental delay that was not otherwise specified. The diagnoses were not mutually exclusive.
Vaccines.
Almost two thirds of the VAERS reports (81 reports, 65.3%) listed MMR or its component vaccines. MMR or measles–rubella (1 report) was the only vaccine listed on 22 reports (17.7%); on 59 reports (47.6%), it was listed in conjunction with other vaccines, the most common of which were Haemophilus influenzae type B, oral live polio, diphtheria–tetanus–acellular pertussis, and varicella. On the 43 reports (34.7%) that did not list MMR or any of its component vaccines, diphtheria–tetanus–pertussis, diphtheria–tetanus–acellular pertussis, Haemophilus influenzae type B, and oral live polio vaccine were the most commonly reported vaccines. Parent interviews confirmed which vaccines the child had received in relation to the reported symptoms. Reports received on March 1, 1998, or later were somewhat more likely to list MMR (67.0% vs 59.3%) than reports received earlier. Reports received on August 1, 1999, or later were more likely to list hepatitis B (18.1% vs 5.1%), Haemophilus influenzae type B (38.6% vs 28.2%), and diphtheria–tetanus–acellular pertussis (26.5% vs 12.8%) vaccines than reports received earlier. Because manufacturer names and lot numbers were missing from the reports, it was not possible to determine from these VAERS reports how many of the case-patients received thimerosal-containing vaccines that had been distributed to clinics before the request was issued.
Making the Association Between Vaccination and Autism and Related Disorders
In response to the open-ended question, “What made you think that _____’s symptoms might be related to a vaccination?” reporters listed a variety of reasons (Table 1 ▶). The most frequently volunteered reason was the temporal proximity of vaccination and symptom development. Other reasons for making the association included magazine or newspaper articles, health care professionals, Web sites, family or friends, medical and scientific journals, consumer advocacy groups or other parents, a previous vaccine-adverse event in the case-patient or another member of the family, the immunization consent form, and comparison with other children. Responses were not mutually exclusive.
TABLE 1—
Reported Reasons for Making the Association Between Vaccination and Autism and Related Disorders
| Reported Reason for Making the Association | Percentagea of Respondents Who Mentioned the Reason |
| Temporal proximity of vaccination and symptomsb | 31.5% |
| Magazine/newspaper | 24.2% |
| Doctor/nurse | 21.0% |
| Web/Internet | 19.4% |
| Family/friends | 14.5% |
| Medical/scientific journals | 11.3% |
| Consumer advocacy group/other parents | 11.3% |
| Previous experience with vaccine adverse event | 3.2% |
| Immunization consent form | 3.2% |
| Comparison with other children | 1.6% |
aPercentages sum to greater than 100% because the responses are not mutually exclusive.
bFor example, “It happened so quickly after the vaccine.”
The later the reports were received in VAERS, the more likely the respondents were to cite explicitly a temporal association between the children’s symptoms and vaccination. The percentage of reporters who volunteered this reason was 14.8% for reports received before March 1, 1998, and 36.8% for those received later. The percentage of respondents who cited temporal association between the children’s symptoms and vaccination was 17.9% for reports received before August 1, 1999, and 38.6% for those received later.
Factors That May Have Contributed to the Reported Condition
In response to the question, “How strong or weak a role do you think the following played in _____’s current problem? The choices are very strong, moderate, weak, or no role at all,” 96.0% stated that vaccine ingredients had played a very strong or moderate role. The vast majority of respondents also said that the child’s receiving vaccines at too early an age (95.2%), the child’s receiving too many vaccines at once or over a short period of time (94.4%), thimerosal/mercury in vaccines (86.3%), and the MMR vaccine (78.2%) had played a very strong or moderate role. Fewer than half (41.1%) said that genes, family, or a birth defect had played a moderate or very strong role. Responses were not mutually exclusive. Respondents whose children’s reports were received on March 1, 1998, or later were more likely than earlier reporters to say that the MMR vaccine had played at least a moderate role (81.4% vs 66.7%). Similarly, respondents whose children’s reports were received on August 1, 1999, or later were more likely than earlier reporters to say that thimerosal/mercury in vaccines had played a moderate or very strong role (90.5% vs 77.5%).
Perceived Relative Importance of Immunization
The perceived importance of immunization and other actions that parents can take to keep their children healthy was assessed with a scale of 0 (not at all important) to 10 (extremely important). A small minority of respondents (15.3%) deemed immunization extremely important, and the proportion of extremely important ratings for other actions was substantially higher: ensuring that children eat healthy foods (78.2%), wash their hands regularly (65.3%), and get adequate exercise (67.7%). In contrast, parents in the general population believed that immunization was more important than the other 3.16
Reasons to Immunize
In response to the open-ended question, “Why do/did you have your child(ren) vaccinated?” the VAERS reporters were less likely than parents in the general population to indicate that preventing infections was a reason to immunize their children (56% vs 83%) and more likely to cite government or school requirements (35% vs 8%), doctor recommendations (25% vs 4%), school/government requirements (35% vs 8%), and other reasons (25% vs 4%). Among the VAERS reporters, the latter category included statements such as, “I thought I was doing the right thing” and “We thought we were supposed to.” Responses were not mutually exclusive.
Omitting Vaccines
Respondents were asked, “How many vaccinations has _____ received since you reported these symptoms?” and “How many vaccinations have your other children (or _____’s brothers and sisters) received since you reported these symptoms?” Almost half (46.0%) said that the child about whom a VAERS report had been filed had had no immunizations since the symptoms began (some immunizations, 21.0%; all regularly scheduled immunizations, 16.9%; unsure, 1.6%; and not applicable, because no vaccinations were scheduled, 14.5%). More than one third of the siblings (33.9%) had had all of their regularly scheduled immunizations, but 26.6% had none (some immunizations, 16.1%; unsure, 2.4%; and not applicable, 21.0%). When only some immunizations were given to the case-patients and their siblings, the vaccines most commonly withheld were MMR and diphtheria–tetanus–acellular pertussis. Two thirds of our respondents had omitted at least 1 vaccine in the VAERS report subjects, and almost half had withheld vaccines from their other children compared with only 14% of parents in the Gellin and colleagues study who said that they would opt out of 1 or more vaccines for their children.16
Perceptions of Disease Severity and Likelihood of Infection
Respondents were asked to rate the seriousness of vaccine-preventable diseases by using a scale of 0 (not at all serious) to 10 (extremely serious) and to rate the likelihood that a nonimmunized person would acquire those diseases using a scale of 0 (will definitely not get the disease) to 10 (will definitely get the disease). Spinal meningitis/Haemophilus influenzae and polio were considered to be the most serious vaccine-preventable diseases, with median ratings of 10.0 for both (mean 8.8 and 8.2, respectively). Hepatitis B, whooping cough, and measles were perceived as moderately serious. Influenza and chickenpox were perceived as the least serious, with median severity scores of 4.0 and 3.0 (mean 4.1 and 3.4), respectively. The perceived likelihood that nonimmunized people would acquire the disease ranged from a median of 2.0 (mean 2.3) for polio to 8.0 (mean 7.3) for varicella. The VAERS parents perceived lower severity and lower likelihood of infection for all diseases included in the Gellin and colleagues survey.16
Perceptions of Immunization Safety
Respondents rated the overall safety of vaccines by using a scale of 0 (not at all safe) to 10 (completely safe). The responses ranged from 0 to 9; no respondent stated that vaccines were completely safe. Almost one quarter of respondents (24.2%) said that vaccinations were “not at all safe,” and 78.2% gave a response of 5 or lower. The mean score of 3.4 (median 4.0) was much lower than the average score of 8.2 given by parents in the Gellin and colleagues study.16
Key Beliefs About Vaccination
Respondents were asked to state their agreement (agree or strongly agree), disagreement (disagree or strongly disagree), or neutrality (neither agree nor disagree) in response to 4 belief statements. Most respondents (70.2%) said that children should be immunized only against serious diseases, and 37.9% agreed with the statement “I am more likely to trust vaccines that have been around for a while” (Table 2 ▶). Approximately one third (33.9%) stated that immunization requirements helped to protect their children from acquiring infectious diseases from nonimmunized individuals. A small minority (7.2%) agreed with the statement, “Vaccines are always proven to be very safe before they are approved for use,” compared with 71% in the Gellin and colleagues study.16
TABLE 2—
Key Differences in Beliefs About Vaccination Between VAERS Respondents and the General Population
| % Agree (% Strongly Agree) | ||
| Belief | VAERS (n = 124) | General Population (n = 1600)a |
| Children should be immunized only against diseases that are serious. | 70 (50) | 39 (20) |
| I am more likely to trust vaccines that have been around for a while. | 38 (15) | 88 (64) |
| Vaccines are always proven to be very safe before they are approved for use. | 7 (3) | 71 (33) |
| Government immunization requirements protect my child from getting diseases from nonimmunized children. | 34 (10) | 84 (54) |
aData from Gellin et al.16
Credibility of Key Sources of Information
Respondents were asked to use a scale of 0 (not at all familiar) to 10 (extremely familiar) to indicate their familiarity with various organizations as sources of information about childhood immunizations. For organizations for which the familiarity score was at least 5, respondents were asked to use a scale of 0 (not at all trustworthy) to 10 (extremely trustworthy) to indicate how trustworthy each organization was as a source of such information.
The median familiarity score for the CDC of 7.0 (mean 6.1) was similar to scores for the National Vaccine Information Center, a consumer advocacy group (median 7.0, mean 6.3), and the AAP (median 6.0, mean 5.6). The National Resource Center for Immunization Information, a fictitious organization that Gellin et al. devised as a reference point for the ratings of existing organizations, was as unfamiliar to the VAERS reporters as it was to parents in the general population16; 73% of both groups reported that they were not at all familiar with it. The National Vaccine Information Center was considered a more trustworthy source of immunization information (median trustworthiness score 8.0, mean 7.2) than the CDC (median 5.0, mean 5.0) and the AAP (median 5.0, mean 4.9). In contrast, parents in the general population considered the CDC and AAP to be very trustworthy (mean rating of 8.5 for each).16
Respondents used the same scales to rate the familiarity with, and trustworthiness of, various organizations as sources of information about autism. The Autism Research Institute was a familiar source of information about autism, with a median familiarity score of 8.0 (mean 7.2), as were the Autism Society of America (median 8.0, mean 6.5) and Cure Autism Now (median 7.0, mean 6.0). Families for the Early Treatment of Autism (median 4.0, mean 4.3), the National Alliance for Autism Research (median 2.5, mean 3.7), and Center for the Study of Autism (median 2.0, mean 3.1) were relatively unfamiliar. The Autism Research Institute was considered to be highly trustworthy as a source of information about autism (median 9.0, mean 8.3), as were Autism Society of America (median 8.0, mean 7.6), and Cure Autism Now (median 8.0, 7.4).
DISCUSSION
We set out to describe perceptions of individuals who believe that vaccination caused their children’s autism or related disorder. Three features of these perceptions stand out. First, these parents based their beliefs on their own observations and the temporal association between vaccination and signs and symptoms such as fever, rash, or changed behavior. Media reports reinforced these perceptions. Second, respondents had relatively little trust in the institutions that shape immunization policy. Third, our respondents perceived vaccine-preventable diseases as less serious and less infectious than other parents did and based their decisions on these perceptions. These findings present several challenges and opportunities, which are highlighted in the following discussion.
Factors Contributing to the Perception of a Link Between Vaccination and Autism
Reporting an adverse event to VAERS implies a temporal association with vaccination; therefore, our observation that a temporal association was the most frequently given reason for making a link between autism and immunization is not surprising. A temporal association is necessary, but not sufficient, to establish causality. Faced with such a serious diagnosis as autism, parents naturally look for explanations in events, such as vaccination, occurring just before the onset of autistic signs and symptoms. Other potential influences are less important in the respondents’ minds. Nevertheless, the media clearly played an influential role.
Approximately three quarters of the VAERS reports were received after the original case series was published,12 and two thirds were received after the AAP/US Public Health Service’s thimerosal recommendation.11 Respondents whose reports were received after the original case series was published were more likely than earlier reporters to cite the MMR vaccine as a contributing factor. One respondent specifically mentioned the Wakefield case series12 as a reason to attribute her child’s symptoms to a vaccine; her VAERS report, which was received 2 years after the article was published, listed MMR as the sole vaccine. Lingam et al. reported that parents who had observed regression in their autistic children—rather than delayed development—were more likely to speculate about the MMR vaccine and other possible causes of autism.17 Because of extensive media coverage, some respondents may have concluded that the reported association was not merely plausible but likely. Similarly, respondents whose reports were received after the AAP/US Public Health Service’s recommendation on thimerosal11 were more likely than earlier reporters to say that thimerosal/mercury had contributed to their children’s symptoms.
Approximately one fifth of the VAERS reporters interviewed said that a Web site had made them think that their children’s symptoms might be related to a vaccination, and many others had searched the Internet for information about autism and related disorders. That the interval between the vaccination date and report date decreased over time demonstrates a secular trend in the perception that autism might be associated with vaccines. Specifically, the longer interval for reports with vaccination dates in the early 1990s suggests that publicity surrounding the original case series and thimerosal recommendation stimulated some parents to submit VAERS reports several years after their children were diagnosed with autism.
These findings illustrate that the media and the Internet influence parental perceptions of vaccine safety and reinforce the need to develop fair and effective ways to communicate with the public about the benefits and risks of vaccines.
Trust of Institutions Promoting Vaccination
VAERS reporters trusted advocacy groups (such as National Vaccine Information Center) and research organizations (such as the Autism Research Institute, Autism Society of America, and Cure Autism Now) more than they trusted the CDC and AAP. Parents may believe that the government health agencies and professional groups have not done enough research into, or made the right decisions regarding, a possible link between vaccination and autism. Our study shows that the views of parents who believe that vaccines harmed their children are very different from those of the general population regarding the dangers of vaccine-preventable illnesses and the potential benefits of immunization.
Perceptions of Severity and Likelihood of Infection and Vaccine Efficacy and Safety
Our findings suggest opportunities for education about infectious diseases and vaccines. Specifically, the risks of immunization should be discussed in the context of the risks of infection. The VAERS parents perceive vaccine-preventable diseases as less serious and less infectious than other parents do. Only 15% of our respondents deemed immunization extremely important, and two thirds had withheld at least 1 vaccine from their children. These findings reinforce recommendations18,19 for vaccine providers and public health officials to provide guidance about immunizations and vaccine-preventable diseases.
Acknowledgments
We thank Elizabeth Begier, Susan S. Ellenberg, and Aparna K. Mohan for critical review of the manuscript. We thank Bruce G. Gellin, Edward W. Maibach, and Edgar K. Marcuse for allowing us to use some of their survey questions. We thank Battelle Center for Public Health Research for assistance with interviews, correspondence, and data entry. We also greatly appreciate the efforts of the VAERS Working Group for their dedication to the maintenance of VAERS. The members of the VAERS Working Group include the following: Elizabeth Begier, Dale R. Burwen, David Davis, Ann W. McMahon, Phil Perucci, Lise Stevens, Frederick Varricchio, and Robert Wise (FDA); Scott Campbell, Robert Chen, Penina Haber, Alena Khromova, Elaine Miller, Gina T. Mootrey, Robert Pless, Vitali Pool, Ali Rashidee, and Michelle Russell (CDC); and Vito Caserta (Health Resources and Services Administration).
Human Participant Protection The FDA’s committee for research involving human subjects reviewed and approved the questionnaire, consent form, follow-up letters, and all procedures regarding the interviews, correspondence, data entry process, and file storage.
Contributors M. Braun conceived the study and supervised its implementation. E. J. Woo and S. V. Shadomy conducted interviews. E. J. Woo synthesized analyses and, along with R. Ball, led the writing. A. Bostrom, L. K. Ball, and G. Evans interpreted the findings with regard to risk perception theory. All authors helped to conceptualize study design, develop the survey instrument, interpret findings, and review drafts of the article.
Peer Reviewed
References
- 1.Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763–1766. [DOI] [PubMed] [Google Scholar]
- 2.Madsen KM, Lauritsen MB, Pedersen CB, et al. Thimerosal and the occurrence of autism: negative ecological evidence from Danish population-based data. Pediatrics. 2003;112(3 Pt 1):604–606. [DOI] [PubMed] [Google Scholar]
- 3.Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347(19):1477–1482. [DOI] [PubMed] [Google Scholar]
- 4.Makela A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics. 2002;110(5):957–963. [DOI] [PubMed] [Google Scholar]
- 5.Taylor B, Miller E, Lingam R, Andrews N, Simmons A, Stowe J. Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: a population study. BMJ. 2002;324(7334):393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics. 2001;108(4):E58. [DOI] [PubMed] [Google Scholar]
- 7.Immunization Safety Review Committee, Institute of Medicine. Measles-Mumps-Rubella Vaccine and Autism. Washington, DC: National Academy Press; 2001.
- 8.Kaye JA, del Mar Melero-Montes M, Jick H. Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: a time trend analysis. BMJ. 2001;322(7284):460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akobeng AK, Thomas AG. Inflammatory bowel disease, autism, and the measles, mumps, and rubella vaccine. J Pediatr Gastroenterol Nutr. 1999;28(3):351–352. [DOI] [PubMed] [Google Scholar]
- 10.Taylor B, Miller E, Farrington CP, et al. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet. 1999;353(9169):2026–2029. [DOI] [PubMed] [Google Scholar]
- 11.Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the US Public Health Service. MMWR Morb Mortal Wkly Rep 1999;48(26):563–565. [PubMed] [Google Scholar]
- 12.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–641. [DOI] [PubMed] [Google Scholar]
- 13.Bostrom A, Atman CJ, Fischhoff B, Morgan MG. Evaluating risk communications: completing and correcting mental models of hazardous processes, Part II. Risk Anal. 1994;14(5):789–798. [DOI] [PubMed] [Google Scholar]
- 14.Chen RT, Rastogi SC, Mullen JR, et al. The Vaccine Adverse Event Reporting System (VAERS). Vaccine. 1994;12(6):542–550. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg SS, Braun MM. Monitoring the safety of vaccines: assessing the risks. Drug Saf. 2002;25(3):145–152. [DOI] [PubMed] [Google Scholar]
- 16.Gellin BG, Maibach EW, Marcuse EK. Do parents understand immunizations? A national telephone survey. Pediatrics. 2000;106(5):1097–1102. [DOI] [PubMed] [Google Scholar]
- 17.Lingam R, Simmons A, Andrews N, Miller E, Stowe J, Taylor B. Prevalence of autism and parentally reported triggers in a north east London population. Arch Dis Child. 2003;88(8):666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR Recomm Rep. 8February2002;51(RR-2):1–35. [PubMed] [Google Scholar]
- 19.Pickering LK, ed. American Academy of Pediatrics, Committee on Infectious Diseases. 2000 Red Book: Report of the Committee on Infectious Diseases. 25th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2000.