Abstract
Objectives. We assessed the association between initiation of highly active antiretroviral treatment (HAART) regimens and sexual risk behaviors among HIVinfected women.
Methods. We analyzed data from 724 women who initiated HAART between January 1996 and January 2001 and who had pre-HAART viral loads at or above 400 copies per milliliter.
Results. Sexually active women were less likely (odds ratio [OR] = 0.79) to report 2 or more partners during a 6-month period after HAART initiation than before HAART initiation. However, the risk for unprotected sex was higher after HAART initiation than before HAART initiation among all sexually active women (both those who reported 2 or more partners [OR = 1.84] and those who reported 1 partner [OR = 1.22]).
Conclusions. Sexual risk behaviors are associated with receipt of therapy but not with therapeutic response, indicating a risk for transmission among female HAART recipients.
Since the introduction of highly active antiretroviral treatment (HAART) in the mid-1990s, decreases in AIDS-related morbidity and mortality in the United States1–2 have coincided with concerns about concomitant increases in sexual risk behaviors both among groups with HIV-1 infection and among the general population.3 Increases in risk behaviors contribute to the transmission of HIV, including drug-resistant HIV strains, and other sexually transmitted diseases (STDs).4–5
Antiretroviral therapy and sexual risk behaviors may be linked through several mechanisms. First, HIV-positive individuals who derive therapeutic benefit from HAART may attain improved quality of life and functional status with the alleviation of physiological, social, and psychological consequences of HIV disease. These gains may be accompanied by increases in sexual risk behaviors among individuals whose illness had previously inhibited those behaviors. Second, individuals may hold unrealistic beliefs about the impact of antiretroviral therapy on disease transmission rates, and thus may perceive the consequences of transmitting HIV as being less serious than in the past. The proven efficacy of HAART in reducing maternal–fetal transmission of HIV may reinforce these beliefs. Individuals who hold such beliefs may be less likely to consistently use condoms or may have a higher number of partners than those who do not. Similarly, individuals at risk for HIV may be less inclined to insist on safe sexual behavior if they perceive the consequences of HIV infection to be somewhat less dire because of the availability of effective antiretroviral therapy.
To help clarify these relationships, we conducted 2 sets of analyses for a national cohort of women with HIV-1 infection. The first analysis examined sexual risk behaviors during the periods preceding and following initiation of HAART regimens, and the second analysis assessed sexual risk behaviors in terms of immunological response to therapy. Behavioral changes in both sets of analyses would imply that attitudes about receiving treatment and the effects of improved immunological parameters were influencing sexual behaviors.
METHODS
Participants
Participants were enrolled in the Women’s Interagency HIV Study (WIHS), a multisite longitudinal cohort study established in 1993 to investigate the natural history of HIV infection among women.6 We collected data from 1168 HIV-positive women who reported receiving HAART at any time from January 1996 through January 2001. In accordance with the International AIDS Society–USA panel and the US Department of Health and Human Services/Henry J. Kaiser Family Foundation7 guidelines, HAART was defined as a regimen consisting of 1 of the following: (1) 2 or more nucleoside reverse transcriptase inhibitors in combination with at least 1 protease inhibitor or 1 non-nucleoside reverse transcriptase inhibitor, (2) 1 nucleoside reverse transcriptase inhibitor in combination with at least 1 protease inhibitor and at least 1 non-nucleoside reverse transcriptase inhibitor, (3) ritonavir and saquinavir in combination with 1 nucleoside reverse transcriptase inhibitor (but no non-nucleoside reverse transcriptase inhibitors), or (4) abacavir and 3 or more nucleoside reverse transcriptase inhibitors (but no protease inhibitors or nonnucleoside reverse transcriptase inhibitors). Per the guidelines, combinations of zidovudine and stavudine with either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor were not considered to be HAART regimens because of the drug–drug antagonism between zidovudine and stavudine. Self-reports of HAART use were obtained for the period since the most recent WIHS semiannual visit; participants’ recognition of drugs was aided by cards with photographs of different drugs.
The 1168 women in our analysis represented 60.1% of HIV-positive women who were enrolled in the WIHS and who were alive in 1996. Of the women who were alive in 1996 and did not initiate HAART, 32.9% were not receiving any antiretroviral therapy during the study period, and 67.1% were receiving a form of antiretroviral therapy that did not meet the definition of HAART. To detect significant shifts in therapeutic success as a result of HAART, we further restricted our analyses to 724 participants with viral load levels greater than 400 copies (amount of virus) per milliliter at the time of the study visit and before HAART initiation. This viral load cutoff point represented the minimal threshold for detection of plasma HIV RNA that was available at the beginning of the observation period.
Study Procedures
Participants completed visits at 6-month intervals at study sites in the following areas: Washington, DC, and the surrounding metropolitan area; the San Francisco Bay Area and Los Angeles, Calif; Brooklyn, Bronx, and Manhattan, NY; and Chicago, Ill. Each study visit included an interviewer-administered set of instruments and a physical examination.
At each study visit, participants provided information about the occurrence and the frequency of specific sexual behaviors since the previous study visit. We evaluated 3 separate outcomes related to sexual activities with male partners: (1) any vaginal, oral, or anal sexual activity with 1 or more partners during the past 6 months; (2) among sexually active women, having 2 or more sexual partners during the past 6 months; and (3) among sexually active women who reported vaginal intercourse, consistency of condom use during vaginal sex (always vs not always) in the past six months. We restricted analyses to heterosexual partnerships because of the low proportion of women who reported same-sex relationships.
In addition to information about sexual behavior, a variety of other self-reported variables were included in our analysis: use of crack, cocaine, or heroin; frequency of alcohol use; smoking status; and employment history. Overall quality of life was assessed with scales derived from the Medical Outcomes Study instrument.8 Depressive symptomatology was assessed with the Center for Epidemiologic Studies Depression scale (CES-D), which has been shown to have high test–retest reliability and to be a good predictor of clinical depression.9 A conventional definition of depression (total CES-D score > 16) was used to identify depressed participants. The presence of AIDS-defining clinical conditions was ascertained through self-report. For example, reported conditions that indicated an AIDS diagnosis, in accordance with the Centers for Disease Control and Prevention definition of AIDS (consistent with 1993 CDC clinical surveillance conditions excluding criteria of low CD4 cell counts)10 were recorded.
Participants were asked to report the occurrence of clinical symptoms of AIDS (fever accompanied by a temperature higher than 100 degrees Fahrenheit lasting longer than 1 month, memory problems lasting longer than 2 weeks, numbness lasting longer than 2 weeks, unexpected weight loss of more than 10 pounds, mental confusion, and drenching night sweats) and were then classified into 3 groups by number of symptoms (0, 1, and 2 or more). Finally, participants were asked whether a health care provider had told them in the past 6 months that they had gonorrhea, syphilis, chlamydia, pelvic inflammatory disease, genital herpes, genital warts, or trichomonas.
Lymphocyte subsets were quantified with standard flow cytometric methods at laboratories participating in the National Institutes of Health/National Institute of Allergy and Infectious Diseases Flow Cytometry Quality Assessment Program. CD4 cell counts were assessed at 6-month intervals corresponding with study visits. Participants typically were informed of their CD4 status within 2 to 4 weeks of blood sampling.
Statistical Analysis
To investigate whether the initiation of HAART or the immunological changes that occurred after the initiation of HAART were associated with subsequent changes in sexual behaviors, we conducted 2 sets of analyses for each of our 3 outcomes of interest (sexual activity, multiple partners, condom use consistency). Both of these analyses evaluated behavioral trends only among women who had initiated HAART; comparisons of behavioral trends among women who did not use HAART would have been complicated by selection-by-indication biases resulting from the nonrandomized use of HAART in observational studies.11 Detailed analyses characterizing women in the WIHS who initiated HAART have been published elsewhere.12
In our first analysis, we evaluated the percentages of women who engaged in sexual activity, who reported 2 or more male sexual partners, and who reported inconsistent condom use before and after initiating HAART. Trends in these percentages were statistically evaluated with a repeated-measures logistic model that used generalized estimating equation (GEE) methods to investigate associations between HAART initiation and sexual risk behaviors after adjustment for several variables13 that previous research has linked with the likelihood of engaging in sexual risk behaviors among HIV-positive women,14–16 including frequency of drug or alcohol use, smoking status, presence or absence of AIDS-defining illness, number of AIDS-related clinical symptoms, presence or absence of depression, quality of life score, and participant’s age.
In our second analysis, we assessed whether immunological changes after HAART initiation were associated with subsequent changes in sexual risk behavior. Immunological response to HAART was defined as the amount that CD4 cell counts had increased since the previous visit. These changes were classified into 3 groups: an increase of 33% or more, an increase of 0% to 32%, and no increase. Data from 3 consecutive visits (1-year period) were entered in a model that evaluated changes in sexual behavior. Each of the 3 sexual behavior outcomes of interest was evaluated at the last of these 3 visits, the exposure variables of interest were evaluated at the second of the 3 visits, and immunological response classification was done according to the change from the first and second visits. Because individuals may contribute multiple observations after the initiation of HAART, we used repeated-measures logistic models with GEE methods to determine whether associations between immunological response and sexual risk behaviors remained after adjustment for frequency of drug or alcohol use, smoking status, employment status, presence or absence of AIDS-defining illness, number of AIDS-related clinical symptoms, number of STD diagnoses, presence or absence of depression, quality of life score, and participant’s age. For all sets of analyses, participants who were pregnant or trying to get pregnant were excluded.
RESULTS
Study Population
Of the 724 HIV-infected women who had initiated HAART and whose pre-HAART viral loads were more than 400 copies per milliliter, 372 (52.2%) were Black, 195 (26.9%) were Hispanic, and 151 (20.9%) were White or of other races/ethnicities. The median age at HAART initiation was 38.5 years, and 259 (35.8%) of the women reported having less than a high school education. The majority of the sample (87%) reported engaging in sexual activity at some point during the observation period (for our analysis, we defined sexual activity as heterosexual vaginal sex). At the most recent follow-up visit, 8.4% of the sexually active women reported more than 1 sexual partner, and 22.9% of these women did not consistently use condoms. The lifetime occurrences of several potential confounding variables are summarized in Table 1 ▶.
TABLE 1—
Prevalence of Psychosocial and Behavioral Variables Stratified by Sexual Behavior: HIV-Positive Women, Women’s Interagency HIV Study, 1996–2001
| Sexual Behavior | ||||||||
| Confounders | Sexually Active | Not Sexually Active | ≥ 2 Partners | 1 Partner | Inconsistent Condom Use (1 Partner) | Consistent Condom Use (1 Partner) | Inconsistent Condom Use (≥ 2 Partners) | Consistent Condom Use (≥ 2 Partners) |
| Use crack, cocaine, or heroin, % | 13.6 | 10.2 | 31.1 | 10.2 | 13.5 | 8.5 | 33.8 | 29.6 |
| Smoke, % | 51.0 | 44.8 | 66.5 | 48.0 | 51.0 | 46.6 | 73.1 | 62.2 |
| Consume alcohol, % | 19.8 | 12.7 | 32.1 | 17.4 | 19.6 | 16.2 | 33.8 | 31.1 |
| With depression, % | 45.4 | 47.5 | 58.3 | 42.9 | 41.7 | 43.3 | 55.7 | 60.0 |
| AIDS clinical symptoms, % | 40.3 | 47.8 | 42.5 | 39.9 | 42.5 | 38.5 | 39.0 | 45.2 |
| 0 | 54.1 | 48.7 | 44.9 | 56.0 | 57.0 | 55.6 | 45.8 | 44.8 |
| 1 | 24.2 | 23.4 | 25.5 | 23.9 | 23.8 | 23.9 | 27.2 | 23.9 |
| 2 or more | 21.7 | 27.9 | 29.6 | 20.2 | 19.3 | 20.5 | 26.9 | 31.3 |
| Any sexually transmitted disease, % | 17.2 | 12.5 | 26.2 | 15.4 | 18.0 | 14.3 | 29.4 | 23.9 |
| Quality of life score (median) | 67 | 65 | 61 | 69 | 69 | 69 | 63 | 61 |
| Person-visits | 7423 | 4841 | 3997 | 783 | ||||
We compared the women in our analysis (n = 724) with the women who did not receive HAART and were therefore excluded from our analysis (n = 767). We found no statistically significant differences between these 2 groups in terms of mean age at baseline or reports of alcohol use during the 6 months before baseline. However, the women who had not initiated HAART were more likely than those who had initiated HAART to report crack, cocaine, or heroin use during the 6 months before baseline (34% vs 22%); to be a smoker at baseline (60% vs 50%); and to be Black (59% vs 52%). Women who had not initiated HAART also had a lower mean CD4 cell count at baseline (343 vs 420 cells) and a higher median viral load (27 000 vs 16 000 copies per milliliter; P < .05 for all).
Sexual Behavior Before and After HAART Initiation
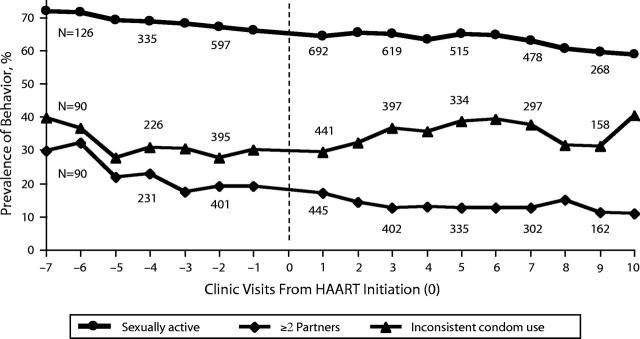
We analyzed data from 7423 person-visits to clinics at study sites (2582 pre-HAART and 4841 post-HAART), with a median of 7 post-HAART follow-up visits per person. Figure 1 ▶ illustrates the occurrence of each sexual behavior reported at study visits before and after HAART initiation. Although this figure depicts somewhat stable trends, after we adjusted for possible confounders we found some change in sexual behavior since the initiation of HAART. The results of the multiple logistic GEE models are summarized in Table 2 ▶. There was a statistically nonsignificant increase in sexual activity reported at post-HAART visits (odds ratio [OR] = 1.12, P = .11). Other covariates, such as drug use (OR = 1.39, P = .035), consuming more than 3 alcoholic drinks per week (OR = 1.67, P < .001), and younger age (measured in 10-year increments; OR = 0.42, P < .001), were associated with increases in sexual activity.
FIGURE 1—
Prevalence of sexual behaviors reported at semiannual study visits before and after HAART initiation: HIV-positive women, Women’s Interagency HIV Study, 1996–2001.
TABLE 2—
Odds of Behavior Change After HAART Initiation Compared With Pre-HAART Time Points: HIV-Positive Women, Women’s Interagency HIV Study, 1996–2001
| Outcome | Model 1a OR (95% CI) | Model 2b OR (95% CI) |
| Sexually active | ||
| No | Reference | Reference |
| Yes | 1.02 (0.89, 1.17) | 1.12 (0.97, 1.30) |
| No. of sexual partners | ||
| 1 | Reference | Reference |
| 2 or more | 0.63 (0.51, 0.77) | 0.79 (0.63, 0.98) |
| Condom use with 1 partner | ||
| Always | Reference | Reference |
| Not always | 1.18 (0.98, 1.43) | 1.22 (1.00, 1.47) |
| Condom use with ≥ 2 partners | ||
| Always | Reference | Reference |
| Not always | 1.61 (1.13, 2.30) | 1.84 (1.26, 2.70) |
Note. OR = odds ratio; CI = confidence interval. OR above 1.0 indicates a greater likelihood of the outcome of interest after initiation of HAART.
aAdjusted for age.
bAdjusted for age; frequency of crack, cocaine, or heroin use; frequency of alcohol use; smoking status; number of clinical symptoms of AIDS; presence or absence of AIDS-defining illness; presence or absence of depression; and quality of life score.
When we restricted the analyses to women who had engaged in vaginal sex with a male partner, we found that participants were less likely (OR = 0.79, P = 0.03) to report having 2 or more partners in a 6-month period at post-HAART visits than at pre-HAART visits, indicating that having 2 or more partners became less common post-HAART. Drug use (OR = 3.05, P < .001), consuming 3 alcoholic drinks per week (OR = 1.41, P = .027), smoking (OR = 1.49, P = .004), and every 10-year increase in age (OR = 0.62, P < .001) were associated with reporting 2 or more sexual partners after the initiation of HAART. Participants who reported inconsistent condom use during vaginal sex were stratified into 2 groups: those who reported having 1 partner and those who reported having 2 or more partners. Participants in both groups were more likely to report inconsistent condom use after HAART initiation than before initiation (1 partner: OR = 1.22, P = .045; ≥ 2 partners: OR = 1.84, P = .002). Drug use was associated with inconsistent condom use in the group that reported having 1 partner (OR = 1.65, P = .008), whereas smoking was associated with inconsistent condom use in the group that reported having 2 or more partners (OR = 1.78, P = .008).
Sexual Behavior and Changes in Immunological Parameters
We examined the 724 participants’ post-HAART initiation visits to assess whether changes in CD4 cell counts were associated with sexual risk behaviors (3128 person-visits were analyzed). The median number of follow-up visits per participant was 4 [interquartile range = 3,6]. Of the 724 women, 14.5% missed 2 or more post-HAART visits. This high percentage was a result of the study design, which required 3 consecutive visits. Missing a study visit was not associated with sexual activity, reporting 2 or more partners, or inconsistent condom use. For 499 women (68.9%), an increase in CD4 cell counts of 33% or higher was observed in at least 1 WIHS visit; for 76 women (10.5%), no increase in CD4 cell count was observed at a post-HAART visit. Immunologic responses were more common: 397 (54.8%) of the WIHS participants had an undetectable level of viral copies (400 or fewer per milliliter) after HAART initiation. Achievement of an undetectable viral load was not associated with subsequent shifts in sexual behavior and was not analyzed further.
The relationships between CD4 cell counts and sexual behaviors are shown in Table 3 ▶. As with the first analysis, model 1 was adjusted for participant’s age, and model 2 was adjusted for frequency of drug or alcohol use, smoking status, employment status, presence or absence of AIDS-defining illness, number of AIDS-related clinical symptoms, presence or absence of STD diagnoses, presence or absence of depression, quality of life score, and participant’s age. Second-order (multiplicative) interaction terms of the confounding factors were examined for all models. Although some were significant, the interaction terms did not change the magnitude or the statistical significance of the primary factors of interest: CD4 cell counts and viral load. Both models indicated that a moderate (0%–32%) increase in CD4 cell count, compared with an increase of 33% or higher, was associated with higher levels of sexual activity (model 1: OR = 1.29, 95% confidence interval [CI] = 1.04, 1.61; model 2: OR = 1.27, 95% CI = 1.02, 1.59). Number of partners and inconsistent condom use were not associated with changes in CD4 cell count. Women who reported drug or alcohol use were more likely than those who did not to be sexually active and to report 2 or more partners. Participants were less likely with increasing age to be sexually active or to report 2 or more partners. Smoking was associated with reporting 2 or more partners and with inconsistent condom use among those who reported 2 or more partners. Reports of any STD diagnoses were associated with inconsistent condom use among women who reported having either 1 partner or 2 or more partners. CD4 cell count at the visit preceding HAART initiation was associated with both sexual activity and inconsistent condom use with 1 partner. Women who had cell counts of 200 or fewer (OR = 0.56, P = .003) and those who had cell counts of 201 to 500 (OR = 0.75, P = .058) were less likely to be sexually active than were women who had CD4 cell counts higher than 500. Finally, depressive symptoms were associated with reports of 2 or more partners.
TABLE 3—
CD4 Cell Counts as Predictors of Sexual Behavior After HAART Initiation: HIV-Positive Women, Women’s Interagency HIV Study, 1996–2001
| Sexual Behavior | ||||||||
| Sexually Active | ≥ 2 Partners | Inconsistent Condom Use With 1 Partner | Inconsistent Condom Use With ≥ 2 Partners | |||||
| CD4 change | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 1a OR (95% CI) | Model 2b OR (95% CI) | Modela OR (95% CI) | Model 2b OR (95% CI) |
| ≥ 33% increase | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 0%–32% increase | 1.29 (1.04, 1.61) | 1.27 (1.02, 1.59) | 0.88 (0.61, 1.28) | 0.96 (0.65, 1.40) | 1.03 (0.78, 1.36) | 1.04 (0.78, 1.38) | 0.95 (0.50, 1.83) | 0.80 (0.45, 1.40) |
| Decrease | 1.05 (0.88, 1.24) | 1.08 (0.90, 1.28) | 1.02 (0.75, 1.40) | 0.99 (0.70, 1.41) | 1.02 (0.81, 1.29) | 1.08 (0.83, 1.39) | 0.81 (0.48, 1.38) | 1.05 (0.51, 2.12) |
Note. OR = odds ratio; CI = confidence interval. OR greater than 1.0 indicates a greater likelihood of the behavior of interest relative to the indicated reference groups.
aAdjusted for age.
bAdjusted for age; frequency of crack, cocaine, or heroin use; frequency of alcohol use; smoking status; number of clinical symptoms of AIDS; presence or absence of AIDS-defining illness; presence or absence of depression; and quality of life score.
DISCUSSION
The HIV-positive women in our study reported higher levels of unprotected vaginal sex post–HAART initiation than pre–HAART initiation. Although women were somewhat less likely to report having 2 or more sexual partners after the initiation of HAART, their overall level of risk may have increased owing to less consistent condom use. Furthermore, the risk for unprotected sex after HAART initiation was somewhat higher among women who reported having 2 or more sexual partners within a 6-month period (OR = 1.84) than among those who reported having only 1 partner (OR = 1.22). These findings suggest that patterns of increasing sexual risk behaviors are occurring among HIV-infected heterosexual women on HARRT. Even though women reported higher levels of unprotected sex following HAART initiation, a concomitant decrease occurred in the likelihood of engaging in sexual activity with multiple partners.
It appears that these changes in sexual risk behaviors occurred independently of improvements in immune function, suggesting that perceptions of HAART’s benefits may be influencing sexual behavior more so than actual improvements in health. We found that sexual activity, number of sexual partners, and condom use consistency were not associated with changes in CD4 cell count classifications occurring after the initiation of HAART. Previous findings from a study of a subgroup of women enrolled in the WIHS also support this interpretation. Thirty percent of the 145 sexually active HIV-infected women enrolled at the Brooklyn site reported either that they believed that they were less infectious as a result of HIV therapy or that they worried less about using condoms after they started HAART. Women who held these beliefs were less likely to report consistent condom use (22.5%) than those who disagreed (50.0%).17
The scope of our study was limited by our lack of specific information regarding psychological factors and beliefs that may have influenced risk behavior. Additionally, at the time of data collection we did not have information on the HIV status of the women’s sexual partners, on whether these partners were regular or casual partners, or on the rate of change to new sexual partners. Changes in risk behavior among women are not necessarily a function of their views about unprotected behavior in the context of HAART. Because men are the primary users of condoms, it may be the male partners’ views or perceptions regarding HIV transmission in the context of HAART that drive changes in sexual risk behaviors. Future research will require delineation of these issues. Furthermore, our sexual risk behavior data are subject to the biases inherent in the collection of any self-reported sensitive behaviors; therefore, the true extent of sexual risk behaviors among our sample may be underestimated. Because we restricted our study sample to women who initiated HAART, we did not have an external reference group; thus, secular changes in behavior may have taken place that we did not appropriately capture.
Finally, although our study protocol required the women in our study to be informed of the results of their virological and immunological tests, it is possible that not all the women were informed early enough to affect subsequent decisions related to sexual behaviors within the next 6 months. Participants at our 6 centers receive HIV care in a number of settings, not all of which are affiliated with WIHS. Those unaffiliated providers monitor participants’ status and provide therapy as they deem appropriate. The WIHS, with participants’ consent, sends WIHS laboratory results to providers. However, these results may arrive after the results of clinical laboratory tests ordered by participants’ private physicians have already been used to guide management. At the time of data collection, WIHS was not structured to measure the provision of testing information to participants.
CONCLUSIONS
Initiation of HAART regimens may be related to continuation of or increases in sexual risk behaviors. It has been estimated that the chance for eradication of an HIV epidemic through widespread antiretroviral therapy use is 85% in the presence of reductions in sexual risk behaviors. In the absence of sexual risk reduction, that chance decreases to 50%, and decreases further in the presence of increased sexual risk behaviors.18 When we reviewed literature on sexual risk behaviors among people who were HIV positive,19 we found only 5 studies that examined the relationship between unprotected sex and factors associated with HIV treatment, clinical status, or medical beliefs among women. One of these studies reported a positive relationship between CD4 cell counts and the risk for unprotected sex,14 and another supported a positive relationship between having no symptoms of HIV infection and engaging in unprotected sex. Our study provides some of the first evidence that primary and secondary prevention efforts targeting sexual risk behaviors among heterosexual HIV-infected women who receive HAART need to be considered. Our study also suggests that these women and their partners should receive services that will promote the knowledge and skills necessary for effective prevention of transmission of HIV. Such an education program may include addressing the impact of HAART on risks for disease transmission at initiation and continuing similar counseling strategies throughout the course of treatment.
Acknowledgments
Data were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group, with centers (principal investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Herminia Palacio); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center, Baltimore, Md (Stephen J. Gange, Alvaro Muñoz). The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute, the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute of Craniofacial and Dental Research (U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-HD-32632 [NICHD], U01-AI-34993, U01-AI-42590, M01-RR00079, and M01-RR00083).
Human Participant Protection Protocol approvals were obtained from the participating sites.
Contributors T. E. Wilson formulated the hypothesis, participated in the planning of the study and the interpretation of the results, and wrote the article. M. E. Gore and S. J. Gange conducted the statistical analyses, contributed to the interpretation of the results, and contributed to the writing of the article. H. Minkoff, M. Cohen, R. Greenblatt, S. Silver, and E. Robison participated in the planning of the article and contributed to the writing.
Peer Reviewed
References
- 1.Centers for Disease Control and Prevention. Update: trends in AIDS incidence, deaths, and prevalence—United States, 1996. MMWR Morb Mortal Wkly Rep. 1997;46:165–173. [PubMed] [Google Scholar]
- 2.Palella F, Delaney K, Moorman A, et al. Declining morbidity and mortality among patients with advanced HIV infection. N Engl J Med. 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 3.Dilley J, Woods W, McFarland W. Are advances in treatment changing views about high-risk sex? N Engl J Med. 1997;337:501–502. [DOI] [PubMed] [Google Scholar]
- 4.Pomerantz R. Primary HIV-1 resistance: a new phase in the epidemic? JAMA. 1999;282:1177–1179. [DOI] [PubMed] [Google Scholar]
- 5.Wainberg M, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279:2000–2002. [DOI] [PubMed] [Google Scholar]
- 6.Barkan S, Melnick S, Preston-Martin S, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 7.Guidelines for the use of antiretroviral agents in HIV-Infected adults and adolescents. Washington, DC: US Dept of Health and Human Services and Henry J. Kaiser Family Foundation. MMWR Morb Mortal Wkly Rep. 1998;47(RR-5):43-82. Also available at: http://aidsinfo.nih.gov//guidelines/default_db2.asp?id=50. Accessed April 29, 2004. [PubMed]
- 8.Bozzette S, Hays R, Berry S, Kanouse D, Wu A. Derivation and properties of a brief health status assessment instrument for use in HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:253–265. [DOI] [PubMed] [Google Scholar]
- 9.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–730. [PubMed] [Google Scholar]
- 11.Ahdieh L, Gange S, Greenblatt R, et al. Selection by indication of potent antiretroviral therapy use in a large cohort of women infected with human immunodeficiency virus. Am J Epidemiol. 2000;152:923–933. [DOI] [PubMed] [Google Scholar]
- 12.Cook J, Cohen M, Grey D, et al. Penetration and predictors of use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002;92:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1994.
- 14.Wilson T, Massad S, Riester K, et al. The sexual, contraceptive, and drug use behaviors of women with HIV and those at high risk for infection: results from the Women’s Interagency HIV Study (WIHS). AIDS. 1999;13:591–598. [DOI] [PubMed] [Google Scholar]
- 15.Wilson T. The Sexual and reproductive behavior of women with HIV. Clin Obstet Gynecol. 2001;44:289–299. [DOI] [PubMed] [Google Scholar]
- 16.Wilson T, Barrón Y, Cohen M, et al. Adherence with antiretroviral therapy and associations with sexual behavior among a national sample of women with HIV. Clin Infect Dis. 2002;34:529–534. [DOI] [PubMed] [Google Scholar]
- 17.Wilson T, Minkoff H. Decreased condom consistency associated with beliefs regarding HIV therapy and disease transmission among women with HIV-infection. J Acquir Immune Defic Syndr Hum Retrovirol. 2001;27:289–291. [DOI] [PubMed] [Google Scholar]
- 18.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2:487–493. [DOI] [PubMed] [Google Scholar]
- 19.Crepaz N, Marks G. Towards an understanding of sexual risk behavior in people living with HIV: a review of social psychological and medical findings. AIDS. 2002;16:135–149. [DOI] [PubMed] [Google Scholar]