Abstract
Objectives. We investigated how, under various conditions, the risk of mother-to-child transmission of HIV through breastfeeding compares with the risk of death from artificial feeding.
Methods. We developed a spreadsheet simulation model to predict HIV-free survival during 7 age intervals from 0 to 24 months for 5 different infant feeding scenarios in resource-poor settings.
Results. Compared with artificial feeding, breastfeeding during the first 6 months by HIV-positive mothers increases HIV-free survival by 32 per 1000 live births. After 6 months, as the age-specific mortality rate and risk of death caused by replacement feeding both decline, replacement feeding appears to be safer.
Conclusions. Under conditions common in countries with high HIV prevalence, replacement feeding by HIV-infected mothers should not be generally encouraged until after the infant is approximately 6 months old.
Each year, HIV infects an estimated 800 000 children, mainly because of transmission from mother to child during pregnancy, delivery, or breastfeeding. Most of these infections could be prevented through the use of antiretroviral drugs taken during pregnancy and delivery and the avoidance of breastfeeding. However, the use of breastmilk substitutes also brings mortality risks that need to be balanced against the risk of HIV transmission. The balance of risks depends on local conditions and should be examined for each situation. For the mother who is HIV-negative or who does not know her status, breastfeeding continues to be recommended.1 For the mother who knows she is infected and for the health worker advising her, the risks associated with different infant feeding strategies need to be understood.
United Nations agencies currently recommend that: “When replacement feeding is acceptable, feasible, affordable, sustainable and safe, avoidance of all breastfeeding by HIV-infected mothers is recommended. Otherwise, exclusive breastfeeding is recommended during the first months of life.”2(p32) They also note that the appropriate time to wean depends on both the individual woman’s situation and the continuing risks of replacement feeding (including malnutrition and infections other than HIV) and must take into account the possible increased risk of HIV transmission with mixed feeding during the transition period between exclusive breastfeeding and complete cessation of breastfeeding.
Health services worldwide are developing and implementing programs to reduce mother-to-child transmission (MTCT) of HIV, including antiretroviral therapy and artificial feeding. These initiatives increase the urgency of efforts to understand the overall mortality risks associated with different infant feeding strategies under different conditions.
A number of studies have been published that use simulation models to answer such questions.3–11 These simulations unanimously conclude that under conditions of high infant mortality and a high risk of death from replacement feeding, breastfeeding is the safer infant feeding strategy, despite the risk of HIV transmission. Although many of the factors affecting the risks of HIV transmission through breastfeeding and infant mortality vary dramatically with age, most of these analyses treat the whole of infancy as a single homogeneous period. The exceptions8,9 lack empirical data on the age-specific risk of death from artificial feeding that are now available.12
We developed a simulation model that takes into account such age-related changes to estimate the effect on HIV-free survival at different ages of a range of infant feeding strategies, including efforts to make breastfeeding safer.
METHODS
Model Structure and Design
The spreadsheet model, derived from an approach originally presented by Hu et al.,4 divides infancy into 6 intervals: 0 to 7 days, 7 days to 2 months, 2 to 4 months, 4 to 6 months, 6 to 9 months, and 9 to 12 months; 12 to 24 months was included as a single interval. The model can simulate any scenario represented by the values of 5 variables, all of which can vary across intervals: the number of live infants at beginning of the interval (N), the MTCT rate during breastfeeding (T), the proportion of women breastfeeding (B), the proportion of uninfected breastfed infants who die during the interval (M), and the relative risk of mortality among nonbreastfed infants (R). For each interval in each scenario, the number of deaths and HIV infections in each of 3 mutually exclusive categories is calculated, as illustrated in the decision tree shown in Figure 1 ▶.
FIGURE 1—
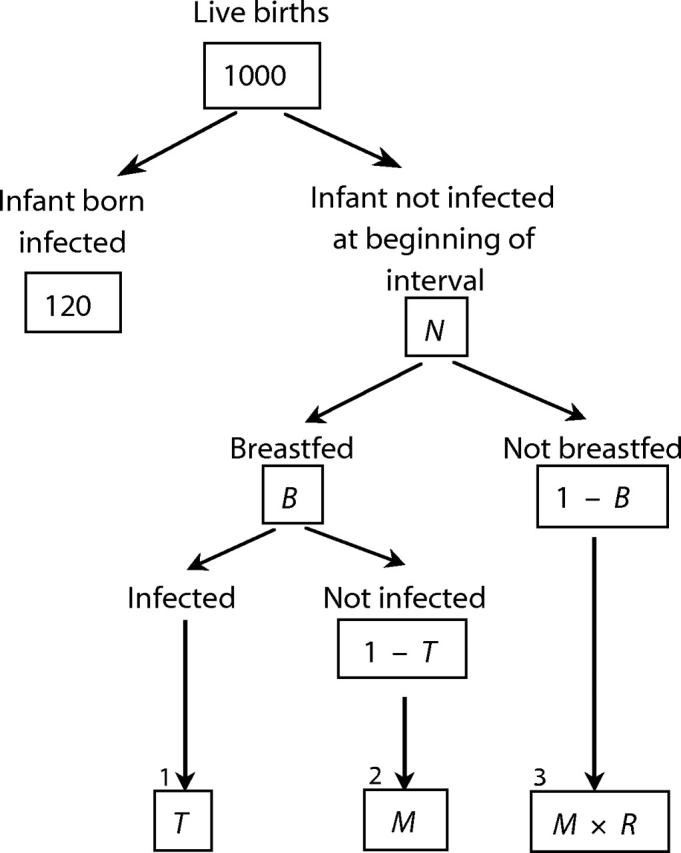
Decision tree with 3 mutually exclusive categories of postnatal infections and deaths: HIV infections, non-AIDS deaths among uninfected breastfed infants, and non-AIDS deaths among uninfected nonbreastfed infants.
Note. The formula for infections or mortality in any category is obtained by multiplying the cells in that path of the tree (1. HIV infections = N × B × T; 2. Non-AIDS deaths among uninfected breastfed infants = N × B × (1 − T) × M; 3. Non-AIDS deaths among uninfected nonbreastfed infants = N × (1 − B) × M × R). The first interval (shown) begins with n = 880 (1000 live births − 120 infants infected during pregnancy and delivery). For each subsequent interval, N is the number of infants surviving HIV-free at the end of the preceding interval.
Model Assumptions
Although the simulation model was designed for whole populations, including uninfected mothers, some of whom become infected while breastfeeding, for the purposes of this analysis, HIV prevalence is set to 1, thus restricting the simulations to mothers who were HIV-positive during pregnancy.
Simulations begin with 1000 live births, the denominator of the infant mortality rate. Current methods of testing for HIV in infants do not permit the timing of transmission to be estimated with precision. The rate of transmission before and during delivery is therefore estimated from the rate of infection observed among infants of nonbreastfeeding women. Because the prevalence of breastfeeding is generally high in poor countries, the transmission rate before and during delivery is estimated mainly from studies in affluent countries. In a large, multicenter study in Europe, transmission among 683 nonbreastfed infants of mothers infected before delivery was 13.6%.13 In a French study of 801 infected nonbreastfeeding mothers, the rate was 19%.14 In a meta-analysis reported by Dunn et al.,15 16.1% of 1567 nonbreastfeeding mothers in 6 studies transmitted the virus to their infants. Evidence suggests that the rate of transmission through breastfeeding is somewhat higher in countries where malnutrition and other infections are more common.16–18 In a 2000 review of then-current knowledge about MTCT in poor countries, transmission before and during delivery was estimated to be 15% to 30%.19 In our analysis, we assumed MTCT during or before delivery was 24%, and perinatal antiretroviral prophylaxis was assumed to reduce this by half, to 12%.20,21 Of 1000 infants born alive, the number entering the first interval HIV-free (N) is therefore 880. All subsequent intervals begin with the number of live, uninfected infants remaining at the end of the previous interval.
Reported rates of transmission through breastfeeding by a mother infected before delivery (T) range from 0.2% to 0.9% per month.15,22–27 However, because of the inability to distinguish early postnatal transmission from that occurring before and during delivery, these rates are based on estimates for transmission after the first 6 weeks to 2 months. De Cock et al.19 estimated that the rate of transmission during the early neonatal period may be several times higher, amounting to a transmission rate over the first 2 months of an additional 5% to 10%. Monthly rates of transmission in the early neonatal period from various studies were 4.5%,25 1.9%,28 2.9%,29 and 1.8%,30 with an arithmetic mean of 2.8%. The value for T used in our analysis for the first 2 months is therefore 2.8% per month. After 2 months, we use the risk of 0.00028 per day reported by Richardson,26 equivalent to 0.85% per month. These risks yield the age period–specific values for T shown in Table 1 ▶. In the absence of effective promotion of exclusive breastfeeding, most breastfeeding mothers begin adding other foods and fluids to the infant diet after the early weeks of life. Exclusive breastfeeding, efforts to prevent and treat breast problems, and prophylactic antiretroviral therapy for the infant or mother during the breastfeeding period are likely to reduce the risk of breastfeeding transmission significantly.28,31–34
TABLE 1—
Actual Values, Critical Values, and Confidence Ratios for Key Variables: Simulation of the Effects of Breastfeeding vs Replacement Feeding on the HIV-Free Survival of Infants of HIV-Positive Mothers
| Age Interval | ||||||||
| Symbol | 0–7 da | 7 d–2 mo | 2–4 mo | 4–6 mo | 6–9 mo | 9–12 mo | 12–24 mo | |
| Actual values | ||||||||
| MTCT rate during breastfeeding | T | 0.0066 | 0.0423 | 0.0169 | 0.0169 | 0.0252 | 0.0252 | 0.0972 |
| Infant mortality rate (deaths per 1000 live births) | M | 0.0293 | 0.0220 | 0.0108 | 0.0101 | 0.0129 | 0.0092 | 0.0356 |
| Additional risk of death from artificial feedingb | R | 1 | 4.2 | 3.6 | 2.5 | 1.7 | 1.4 | 1.8 |
| Critical values | ||||||||
| MTCT rate during breastfeeding | Tcv | 0.0799 | 0.0285 | 0.0153 | 0.00913 | 0.00370 | 0.0295 | |
| Infant mortality rate (deaths per 1000 live births) | Mcv | 0.0130 | 0.00646 | 0.0111 | 0.0348 | 0.0593 | 0.108 | |
| Additional risk of death from artificial feeding | Rcv | 2.69 | 2.54 | 2.66 | 2.93 | 3.73 | 3.63 | |
| Confidence ratiob | 0.53 | 0.59 | 1.11 | 2.74 | 6.71 | 3.20 | ||
Note. MTCT = mother-to-child transmission. The critical value (CV) is the value for which the number of HIV-free survivors is the same regardless of whether the mother breastfeeds, holding all other values constant. If the actual value is higher than the critical value for the relative risk (R) and infant mortality (M) and lower than the critical value for the transmission rate (T), breastfeeding (BF) is safer than replacement feeding (RF).
aCritical values and the confidence ratio are not calculated for the first week, because the assumed value of R is 1.
bTo calculate confidence ratios, the additional risk (R−1) is used rather than the relative risk (R) to permit meaningful comparisons. The confidence ratio is the factor by which the actual value would have to be multiplied or divided to achieve an equal number of HIV-free survivors. Confidence ratios less than 1 favor BF; those greater than 1 favor RF.
This model permits manipulation of the breastfeeding rate (B) to estimate the impact of different infant feeding strategies on transmission and overall risk of infant mortality owing to any cause. To obtain unambiguous estimates of the effects of different infant feeding strategies on HIV-free survival at the individual level, breastfeeding rates were set to simulate perfect compliance (0 or 1).
The underlying infant mortality rate among breastfed infants can be estimated from historical values for infant mortality rate for a given country before the HIV epidemic or, if the HIV prevalence remains low, from current statistics. The average infant mortality rate for the 48 poorest countries was 109 in 1996 and 171 in 1960.35 Mortality rates are known to be higher among younger infants, but the exact relationship depends on the setting. In this study, we used our own unpublished analysis of Demographic and Health Survey data for 31 African countries to derive an infant mortality rate of 91 and the age-specific mortality rates (M) shown in Table 1 ▶, expressed as a proportion of children entering the interval HIV-free.
A number of observational studies have documented increased mortality among nonbreastfed infants.36–43 More recently, an analysis of pooled data from 3 countries has provided age-specific odds ratios for the protective effect of breastfeeding.12 An unpublished analysis of Demographic and Health Survey data from 17 developing countries confirms this pattern of age-specific protection (Shea Rutstein, oral communication, 1999). For the relative risk of death caused by replacement feeding (R), we used odds ratios including deaths from noninfectious causes from the WHO pooled analysis,12 which uses the same age intervals during infancy as does our model, given in Table 1 ▶.
Infants who died during the first week were removed from that analysis “since breastfeeding is unlikely to have had a marked impact on these deaths (which were mainly from perinatal causes and congenital malformations).”12(p451) In our model, we therefore conservatively assumed no protective effect of breastfeeding during the first week.
Analyses
Two types of analyses are presented. The first treats each time period independently, comparing the effects of 3 different infant feeding strategies (replacement feeding, breastfeeding, or safer breastfeeding) on HIV-free survival per 1000 infants entering the period HIV-free. In the second analysis, the cumulative 2-year effects of different infant feeding scenarios are compared. These scenarios, chosen to reflect actual policy alternatives, are as follows:
B24: In this default (or “no intervention”) scenario, all HIV-infected mothers breastfeed for 24 months. Other values are given in Table 1 ▶.
B0: All HIV-infected mothers use replacement feeding from birth. Avoidance of breastfeeding reduces B to 0 throughout. All other values remain as in Table 1 ▶.
B6: All HIV-infected mothers breastfeed to 6 months. B is 0 after 6 months, and all other values are as in Table 1 ▶.
SB24: All values are as in scenario B24, but safer breastfeeding interventions (exclusive breastfeeding, prevention and treatment of breast problems, postpartum antiretroviral therapy) reduce T by half throughout.
SB6: All values are as in scenario B6, but safer breastfeeding interventions reduce T by half.
Critical Values
It is important to know how sensitive simulation results are to plausible variations in the key assumptions, especially because such variations cannot be precisely determined. We addressed this problem by calculating critical values for the transmission risk during breastfeeding (T), the relative risk of death caused by artificial feeding (R), and the underlying mortality rate (M) during each age interval. The critical value is the value a variable would have to take to change the policy conclusion when all other variables are held constant. For this analysis, the critical value is defined as the value that would result in an equal number of HIV-free survivors when all HIV-infected mothers breastfeed as when they do not. Expressions for critical values, derived algebraically from the formulas in Figure 1 ▶, are as follows:
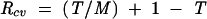 |
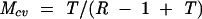 |
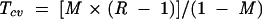 |
where Rcv, Mcv, and Tcv are the critical values of R, M, and T, respectively.
Breastfeeding is favored by values of T smaller than the critical value and by values of R and M larger than the critical value. To avoid confusion in comparing critical values with actual values across time periods for different variables, for each period we calculated a “confidence ratio,” defined here as the ratio of the critical value used in the model to the actual value (for T), or its inverse (for R and M). Confidence ratios are thus standardized such that ratios less than 1 favor breastfeeding and those greater than 1 favor replacement feeding.
RESULTS
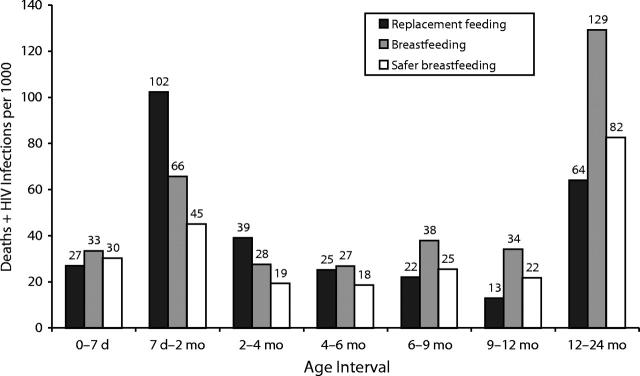
The period-by-period comparison of different infant feeding strategies is presented in Figure 2 ▶, which shows the simulated risk of infection or death from other causes for each interval for each of 3 infant feeding strategies: replacement feeding, breastfeeding, and safer breastfeeding. These risks are calculated per 1000 infants entering the period HIV-free and are therefore independent of the strategy used in the preceding periods. The apparent advantage accorded to replacement feeding in the first week is an artifact of the assumption that there is no protective effect of breastfeeding at this time. During the next 3 periods, safer breastfeeding is safer than replacement feeding, reducing the total of infections and deaths relative to replacement feeding by 56%, 51%, and 27%. Breastfeeding is safer than replacement feeding for the intervals 7 days to 2 months and 2 to 4 months, but for the interval 4 to 6 months, replacement feeding is marginally safer. After 6 months, replacement feeding is safer than both breastfeeding and safer breastfeeding.
FIGURE 2—
Simulated risk of infection or death from other causes in interval for each of 3 infant feeding strategies: replacement feeding (RF), breastfeeding (BF), and safer breastfeeding (SBF), calculated per 1000 infants entering the period HIV-free.
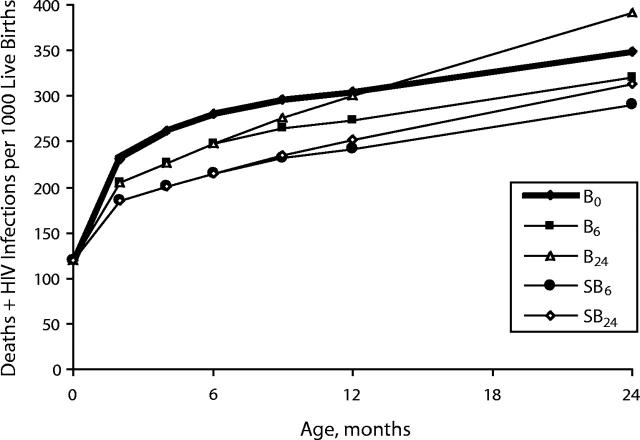
The net effects of different infant feeding scenarios on the cumulative total of infections and deaths are shown in Figure 3 ▶. Under the conditions simulated here, among the cohort of 1000 infants born to HIV-infected mothers who used perinatal antiretroviral prophylaxis, the safest strategy was SB6, which resulted in HIV-free survival to 24 months, exceeding that in the B0 scenario by 65 infants, or 6.5% of the cohort at risk. In comparison with replacement feeding, breastfeeding by HIV-infected mothers during the first 6 months without any intervention to make breastfeeding safer resulted in 68 HIV infections but 100 fewer deaths from other causes, thus increasing net HIV-free survival at 6 months by 32 infants, or 3.2% of the cohort at risk. The most dangerous strategy was continued breastfeeding (B24), which resulted in 391 infants dying or being infected by 24 months. This estimate compares with 291 deaths for the safest strategy (SB6) and 349 for the “no breastfeeding” strategy (B0). Although not shown in Figure 3 ▶, for mothers who breastfeed without any intervention to make breastfeeding safer, beginning replacement feeding at 4 rather than 6 months is marginally safer under the conditions simulated here, reducing the total number of infections plus deaths at 24 months from 320 (in the B6 scenario) to 319.
FIGURE 3—
Cumulative total of deaths and HIV infections per 1000 live births to HIV-infected mothers who used perinatal antiretroviral therapy and different infant feeding strategies: no breastfeeding (B0), breastfeeding to 6 months (B6), breastfeeding to 24 months (B24), safer breastfeeding to 6 months (SB6), and safer breastfeeding to 24 months (SB24).
Critical values are shown in Table 1 ▶, with the estimates used in the model. For the interval 4 to 6 months, the values used for the 3 key assumptions are close to the critical values, and the confidence ratio is close to 1 (1.11). Plausible variations in the actual values therefore could change our conclusions for this interval. However, for the intervals 7 days to 2 months and 2 to 4 months, confidence ratios of 0.53 and 0.59 strongly favor breastfeeding. After 6 months, the critical values favor replacement feeding by a factor of 2.7 or more. Because such large violations of the model assumptions are highly improbable, our confidence in our results is very strong for all intervals but the interval 4 to 6 months. Under conditions of safe breastfeeding, in which transmission risk is reduced by half, all confidence ratios also would be reduced by half.
DISCUSSION
The risk of death from replacement feeding exceeds the risk of MTCT through breastfeeding during the first 4 months of life under the conditions of poverty and poor hygiene simulated here. Exclusive breastfeeding and efforts to prevent and treat breast problems are likely to make breastfeeding even safer. This information should be provided to HIV-positive mothers who live under conditions of poverty and poor hygiene so that they can make informed infant-feeding decisions. Preliminary evidence from current trials suggest that prophylactic antiretroviral drugs given to the infant or mother during the breastfeeding period reduce HIV transmission.34 If this finding is confirmed, breastfeeding may become the safest option under a wider range of conditions.
Randomized trials cannot be used to provide information on the risks of different infant feeding strategies under conditions actually prevailing in resource-poor environments. Although random assignment of feeding strategies may be justified on the basis of genuine uncertainty concerning the balance of risks involved, it is unethical to assign replacement feeding without concurrent improvements in the health environment (hygiene, sanitation, water supply, health care) and economic conditions (free or subsidized supplies), though such improvements would in turn limit the applicability of such trials to real conditions. Therefore, estimates of the risk of death from artificial feeding in our analysis were derived from observational studies. These risks probably underestimate the true risk of artificial feeding in this context, because the mothers in the observational studies were not infected with HIV and therefore must have chosen to feed artificially, not from fear of infecting their infants, but because they considered it a safe, affordable, and feasible alternative to breastfeeding.
However, if mothers stop breastfeeding because of severe infant illness—an example of reverse causality—the risks derived from observation studies may overestimate the true risks. Although we do not know what motivates mothers to choose not to breastfeed their infants, studies in the meta-analysis that generated these estimates avoided reverse causality by assessing breastfeeding status before the onset of the fatal disease or, when such information was not available, 7 days before the infant’s death.
Although most of the excess risk of death from artificial feeding occurs in the intervals 7 days to 2 months and 2 to 4 months, safer breastfeeding offers a survival advantage during the entire first half of infancy. Even without an intervention to make breastfeeding safer, replacement feeding offers little advantage in the interval 4 to 6 months, improving HIV-free survival only by 1 infant per 1000 live births. Other advantages that favor breastfeeding at this point include saving money, delaying fertility, avoiding the social stigma associated with not breastfeeding a young infant, and avoiding the physical and emotional trauma for both infants and mothers caused by early cessation of breastfeeding. Also, for physiological and developmental reasons, appropriate alternative foods for infants younger than 6 months are less available and more expensive than those for older infants. At the public health level, active encouragement of replacement feeding involves logistical challenges and a risk of undermining breastfeeding practices among the majority of mothers, who are HIV-negative or who do not know their status.
Although our analysis appears to suggest that the infant-feeding decision is of little consequence during the first week after birth, this is an artifact of the exclusion of early deaths by the study that provided the relative risk estimates12 and of our assumption, therefore, that there is no increased risk of death from replacement feeding at this time. In reality, although most deaths occurring during the first week may be attributed to congenital defects or perinatal problems, lack of breastfeeding in the first 7 days may contribute to immediate postpartum hypothermia and hypoglycemia and to later deaths from the direct effects of artificial feeding. Also, breastfeeding must be initiated early if at all. Including these effects would increase the estimated deaths from artificial feeding, favoring breastfeeding even more.
It has been suggested that an early transition from breastfeeding (which should be exclusive up to 6 months of age) to replacement feeding among HIV-positive mothers may be the best infant-feeding strategy.19 The optimum timing of this transition depends on the age-related risks associated with available, sustainable replacement-feeding alternatives. Because these risks vary among environments and households, no single recommendation is possible. However, our results suggest that under the conditions simulated here (in which the relative risk of death from artificial feeding is the estimate from the WHO pooled analysis12 and the baseline infant mortality rate is 91 per thousand live births, the average for sub-Saharan Africa), an appropriate time for an HIV-positive mother to switch from breastfeeding to replacement feeding may be approximately 6 months. As economic and environmental conditions worsen, the relative risk of death from replacement feeding increases, shifting the balance of risks to favor a later transition to replacement feeding. Conversely, better conditions would favor an earlier transition to replacement feeding. The critical values and confidence ratios (Table 1 ▶) indicate what conditions favor earlier or later transition. When other variables are held constant, the confidence ratio for the interval 2 to 4 months indicates that mortality would have to be 59% of the current assumed level to make replacement feeding as safe as breastfeeding in this period. This figure corresponds with an infant mortality rate of 54 (91 × 0.59) per 1000 live births, a level that might be found in many communities affected by HIV. Conversely, in the interval 6 to 9 months, breastfeeding would result in better HIV-free survival than replacement feeding only in countries where the infant mortality rate exceeds 249 (91 × 2.74) per 1000 or, under conditions of safer breastfeeding, 124 (91 × 2.74 / 2) per 1000.
Under virtually all conditions in resource-poor communities, a transition from safer breastfeeding to replacement feeding is advised at some point during infancy. Although our model implicitly assumes that this transition is instantaneous and therefore is itself free of risk, the true risk of transmission during the transition may be increased if the risk is higher during mixed feeding28,31 or if breast engorgement and poor drainage from rapid cessation lead to mastitis, also a risk factor for HIV transmission.32,33
Although our simulation included only infected mothers, if some uninfected mothers who do not know their status but who suspect that they are infected also switch to replacement feeding, this switch would increase overall mortality. Therefore, it is important that any policy derived from our model be based on maternal knowledge of HIV status, with appropriate counseling and breastfeeding support for all women.
This model provides new information for public health decisionmakers. Using recent estimates of the age-specific risks of both HIV transmission through breastfeeding and death from artificial feeding, it demonstrates that under conditions common in countries with high HIV prevalence, breastfeeding by the HIV-infected mother has an advantage in terms of HIV-free survival for infants during the first 6 months after birth, despite the risk of MTCT. Additional studies of the risks associated with different infant feeding strategies are urgently needed to refine and confirm this analysis.
Acknowledgments
This work was funded by LINKAGES (Breastfeeding, Lactational Amenorrhea Method (LAM), and Related Complementary Feeding and Maternal Nutrition Program). LINKAGES is supported by the Global Bureau (Global Health/Health, Infectious Diseases and Nutrition), USAID (grant HRN-A-00–97–00007–00). LINKAGES is managed by the Academy for Educational Development (AED). The opinions expressed herein are those of the authors and do not necessarily reflect the views of AED, USAID, or UNICEF.
The authors would like to thank Sandra Huffman, Kristen Marsh, Luann Martin, Lynne Mofenson, and Ellen Piwoz for their useful comments and suggestions.
Human Participant Protection No protocol approval was needed for this study.
Contributors J. Ross developed the original single-period version of the spreadsheet model, modified the multiple-period version to accommodate new data, developed the scenarios for comparison, and ran the simulations. M. Labbok developed the multiple-period version, derived the age-specific baseline infant mortality estimates, and helped design the simulations. Both investigators wrote the article.
Peer Reviewed
References
- 1.WHO/UNICEF/UNFPA/UNAIDS. HIV and Infant Feeding: A Guide for Health Care Managers and Supervisors. Geneva, Switzerland: World Health Organization; 2003.
- 2.WHO. New data on the prevention of mother-to-child transmission of HIV and their policy implications: conclusions and recommendations. Geneva, Switzerland: UNAIDS; January 15, 2001. Available at: http://www.unaids.org. Accessed May 10, 2004.
- 3.Heymann SJ. Modeling the impact of breastfeeding by HIV-infected women on child survival. Am J Public Health. 1990;80:1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu DJ, Heyward WL, Byers RH, et al. HIV infection and breastfeeding: policy implications through a decision analysis model. AIDS. 1992;6:1505–1513. [PubMed] [Google Scholar]
- 5.Kennedy KI, Fortney JA, Bonhomme MG, Potts M, Lamptey P, Carswell W. Do the benefits of breastfeeding outweigh the risks of postnatal transmission of HIV via breastmilk? Trop Doct. 1990;20:25–29. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy KI, Visness CM, Rogan WJ. Breast feeding and AIDS: a health policy analysis. AIDS Public Policy J. 1992;7:18–27. [Google Scholar]
- 7.Del Fante P, Jenniskens F, Lush L, et al. HIV, breast feeding, and under-5 mortality: modelling the impact of policy decisions for or against breastfeeding. J Trop Med Hyg. 1993;96:203–211. [PubMed] [Google Scholar]
- 8.Nagelkerke NJD, Moses S, Embree JE, Jenniskens F, Plummer FA. The duration of breast feeding by HIV-1-infected mothers in developing countries: balancing benefits and risks. J Acquir Immune Defic Syndr. 1995;8:176–181. [PubMed] [Google Scholar]
- 9.Kuhn L, Stein Z. Infant survival, HIV infection, and feeding alternatives in less-developed countries. Am J Public Health. 1997;87:926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walley J, Witter S, Nicoll A. Simplified antiviral prophylaxis with or and without artificial feeding to reduce mother-to-child transmission of HIV in low and middle income countries: modelling positive and negative impact on child survival. Med Sci Monit. 2001;7:1043–1051. [PubMed] [Google Scholar]
- 11.Bertolli J, Hu DJ, Nieburg P, Macalalad A, Simonds RJ. Decision analysis to guide choice of interventions to reduce mother-to-child transmission of HIV. AIDS. 2003;17:2089–2098. [DOI] [PubMed] [Google Scholar]
- 12.WHO Collaborative Team on the Role of Breastfeeding in the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–455. [PubMed] [Google Scholar]
- 13.[No authors listed]. Risk factors for mother-to-child transmission of HIV-1. European Collaborative Study. Lancet. 1992;339:1007–1012. [DOI] [PubMed] [Google Scholar]
- 14.Mayaux M-J, Blanche S, Rouzioux C, et al. Maternal factors associated with perinatal HIV-1 transmission: the French cohort study: 7 years of follow-up observation. The French Pediatric HIV Infection Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:188–194. [PubMed] [Google Scholar]
- 15.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breast feeding. Lancet. 1992;340:585–588. [DOI] [PubMed] [Google Scholar]
- 16.Bobat R, Coovadia H, Coutsoudis A, Moodley D. Determinants of mother-to-child transmission of human immunodeficiency virus type 1 infection in a cohort from Durban, South Africa. Pediatr Infect Dis J. 1996;15:604–610. [DOI] [PubMed] [Google Scholar]
- 17.Semba RD, Miotti PG, Chiphangwi JD, et al. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clin Infect Dis. 1995;21:966–972. [DOI] [PubMed] [Google Scholar]
- 18.Landers D. Nutrition in pediatric HIV infection: setting the research agenda. Nutrition and immune function, II: maternal factors influencing transmission. J Nutr. 1996;126(suppl 10):2637S–2640S. [DOI] [PubMed] [Google Scholar]
- 19.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. [DOI] [PubMed] [Google Scholar]
- 20.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 1999;353:773–780. [DOI] [PubMed] [Google Scholar]
- 22.Leroy V, Newell M-L, Dabis F, et al. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Lancet. 1998;352:597–600. [DOI] [PubMed] [Google Scholar]
- 23.Miotti P, Taha TET, Kumwenda NI, et al. HIV transmission through breast feeding: a study in Malawi. JAMA. 1999;282:744–749. [DOI] [PubMed] [Google Scholar]
- 24.Ekpini ER, Wiktor SZ, Satten GA, et al. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire. Lancet. 1997;349:1054–1059. [DOI] [PubMed] [Google Scholar]
- 25.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breast feeding and formula feeding on transmission of HIV-1: a randomised clinical trial. JAMA. 2000;283:1167–1174. [DOI] [PubMed] [Google Scholar]
- 26.Richardson BA, John-Stewart GC, Hughes JP, et al. Breast milk infectivity in human immunodeficiency virus type 1-infected mothers. J Infect Dis. 2003;187:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawzi W, Msamanga G, Spiegelman D, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–338. [DOI] [PubMed] [Google Scholar]
- 28.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai W-Y, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. [DOI] [PubMed] [Google Scholar]
- 29.Moodley D, Moodley J, Coovadia H, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. [DOI] [PubMed] [Google Scholar]
- 30.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. [DOI] [PubMed] [Google Scholar]
- 31.Smith MM, Kuhn L. Exclusive breastfeeding: does it have the potential to reduce breastfeeding transmission of HIV-1? Nutr Rev. 2000;58:333–340. [DOI] [PubMed] [Google Scholar]
- 32.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–162. [DOI] [PubMed] [Google Scholar]
- 33.Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. [DOI] [PubMed] [Google Scholar]
- 34.Vyankandondera J, Luchters S, Hassink E, et al. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA study). Program and abstracts of the 2nd IAS Conference on HIV Pathogenesis and Treatment; 13–16July2003; Paris, France. Abstract LB7
- 35.UNICEF. State of the World’s Children, 1998. Oxford, England: Oxford University Press; 1998.
- 36.Victora CG, Smith PG, Vaughan JP, et al. Evidence for protection by breastfeeding against infant deaths from infectious diseases in Brazil. Lancet. 1987;2(8554):319–322. [DOI] [PubMed] [Google Scholar]
- 37.Victora CG, Smith PG, Vaughan JP, et al. Infant feeding and deaths due to diarrhea: a case-control study. Am J Epidemiol. 1989;129:1032–1041. [DOI] [PubMed] [Google Scholar]
- 38.Victora CG, Smith PS, Barros F, Vaughan JP, Fuchs SC. Risk factors for deaths due to respiratory infections among Brazilian infants. Int J Epidemiol. 1989;18:918–925. [DOI] [PubMed] [Google Scholar]
- 39.Victora CG, Huttly SR, Fuchs SC, et al. Deaths due to dysentery, acute and persistent diarrhoea among Brazilian infants. Acta Paediatr Suppl. 1992;381:7–11. [DOI] [PubMed] [Google Scholar]
- 40.Yoon PW, Black RE, Moulton LH, Becker S. Effect of not breast feeding on the risk of diarrhea and respiratory mortality in children under 2 years of age in Metro Cebu, The Philippines. Am J Epidemiol. 1996;143:1142–1148. [DOI] [PubMed] [Google Scholar]
- 41.Sachdev HPS, Kumar S, Singh KK, Puri RK. Does breast feeding influence mortality in children hospitalized with diarrhoea? J Trop Pediatr. 1991;37:275–279. [DOI] [PubMed] [Google Scholar]
- 42.Habicht J-P, DaVanzo J, Butz WP. Does breast feeding really save lives, or are apparent benefits due to biases? Am J Epidemiol. 1986;123:279–290. [DOI] [PubMed] [Google Scholar]
- 43.Habicht J-P, DaVanzo J, Butz W. Mother’s milk and sewage: their interactive effects on infant mortality. Pediatrics. 1988;81:456–461. [PubMed] [Google Scholar]