Abstract
Objectives. We investigated the relationship between breastfeeding, asthma and atopy, and child body mass index (BMI).
Methods. From a prospective birth cohort (n = 2860) in Perth, Western Australia, 2195 children were followed up to age 6 years. Asthma was defined as doctor-diagnosed asthma and wheeze in the last year, and atopy was determined by skin prick test of 1596 children. Breastfeeding, BMI, asthma, and atopy were regressed allowing for confounders and the propensity score for overweight.
Results. Using fractional polynomials, we found no association between breastfeeding and overweight. Less exclusive breastfeeding was associated with increased asthma and atopy, and BMI increased with asthma.
Conclusions. Less exclusive breastfeeding leads to increases in child asthma and atopy and a higher BMI is a risk factor for asthma.
Asthma is the leading cause of hospitalization among Australian children, and its prevalence is increasing worldwide.1,2 In developed nations, the increase in prevalence of childhood asthma has been paralleled by an increase in childhood obesity.3,4 Overweight and obesity are leading causes of morbidity and a major public health problem,5 as they are associated with numerous risk factors for cardiovascular disease and chronic diseases later in life, including hyperlipidemia, hyperinsulinemia, hypertension, and early atherosclerosis.5–7
There has been considerable debate as to whether formula feeding is a risk factor for childhood obesity. Dewey et al. studied growth patterns for weight, length, head circumference, and indexes of body composition8 and found that infants breastfed for 12 months or longer are leaner than formula-fed infants.9 The impact of breastfeeding on obesity has been investigated in small studies, but no effect was observed.10–12 On the other hand, a protective effect has been reported in a Canadian cross-sectional study of 1320 adolescents born in the late 1960s.13 Another cross-sectional study in Bavaria14 concluded that breastfeeding may help decrease the prevalence of obesity in childhood.
Investigators have speculated that obesity may be a causal factor in the inception of childhood asthma, and a number of studies have shown positive associations between body mass index (BMI; weight in kilograms divided by height in meters squared) or obesity and asthma.15–19 Little is known about the effects of BMI on atopy, but 1 study found positive effects before but not after adjustment for BMI.15 Breastfeeding has been shown to protect against overweight and obesity14,20–22 as well as asthma in childhood,23,24 although the relationship between breastfeeding and childhood asthma remains speculative.25
The aim of this study was to investigate whether the relationship of breastfeeding to asthma was influenced by BMI. The objectives of the report were to determine the interrelationships of (a) breastfeeding with BMI, (b) BMI with asthma and atopy, and (c) breastfeeding with asthma and atopy after adjustment for BMI.
METHODS
Background to the Study
Subjects in the Western Australian Pregnancy Cohort Study were serially recruited from the public antenatal clinic at King Edward Memorial Hospital or from nearby private practice in Perth, Western Australia, between 1989 and 1992.26 The study started as a pregnancy cohort in which 2979 women were enrolled at or before the 18th week of gestation from the antenatal booking clinics at King Edward Memorial Hospital. Approximately 100 women per month were enrolled for 30 months commencing in May 1989 and finishing in November 1991. The criteria for enrollment were gestational age of 16 to 20 weeks, sufficient proficiency in English to understand the implications of participation, an expectation to deliver at King Edward Memorial Hospital, and an intention to remain in Western Australia so that follow-up through childhood would be possible.
By the end of the pregnancy phase, 2888 women remained in the study. Of 2860 live births, 14 children have since died and 244 children have been withdrawn or lost to follow-up (predominantly living outside the metropolitan area, in another state, or overseas). Thus, 2602 of 2860 children (91%) remained available for follow-up at age 6 years.
To see if the study sample represented the potential population for inclusion, records were maintained on all pregnant women attending the antenatal clinics during a 6-month period midway through the study. At these 131 clinic sessions, 1420 women were new attenders, of whom 510 (36%) were more than 20 weeks into gestation, 117 (8%) were ineligible because of language difficulties, 60 (4%) planned to deliver elsewhere, 26 (2%) had psychosocial problems that precluded long-term follow-up, and 707 (50%) met the recruitment criteria. Of this latter group, 633 (90%) agreed to participate.26
At the time of enrollment, data were collected from parents about their general health and socioeconomic situation. Data were collected at the time of the child’s birth and included sex, gestational age, birthweight, and physiological and clinical indicators. Parents completed questionnaires following the first, second, third, and sixth year of the child’s life; shortly after the completion of each questionnaire, the child attended clinical assessments conducted by a child-health nurse. The sixth-year assessment (mean age = 5 years and 11 months) included a questionnaire and examination for clinical measures of asthma and atopy.
At year 1, 2463 questionnaires were received (86% of birth cohort), and 2365 children (83%) attended the clinical assessment. At year 6, questionnaires were received for 2195 children (77%) and physical assessment data were available for 1820 children (64%). Because a number of subjects in the cohort were in another part of Western Australia, in another state, or overseas, they completed the questionnaire but were unable to attend the assessment. Further, some subjects completed the questionnaire but for reasons unknown did not attend the clinical assessment.
Of the 1820 children examined, skin prick test information for atopy was available for 1596 (88%); the remaining children were either uncooperative, unwell, or their parents did not consent.
To assess whether the birth characteristics of the children who participated in the 6-year follow-up differed from those who did not, the mother’s age at conception and the gestational age and birthweight were compared. The mothers in the study were, on average, 3 years older at the time of conception than those who did not participate. Gestational age differed by a mean of 3 days and birthweight differed by a mean of 101 g, with participants being older and heavier, on average, than nonparticipants.
Diary Card
Parents kept a diary card of their child’s feeding history and illnesses throughout the first year. Data from the diary card were transcribed to the questionnaire at the research clinic by a specially trained child health nurse at the year-1 interview (age at completion of questionnaire = 13.9 months ± 1.4 months).
Breastfeeding information was collected on the first-, second-, and third-year questionnaires regarding duration of breastfeeding in months and the age in months at which milk other than breastmilk was introduced. Duration of any breastfeeding was defined as the age at which breastfeeding stopped; it was expressed as a continuous variable (in months). Duration of exclusive breastfeeding was defined as the age at which other milk was introduced; it was expressed as a continuous variable (in months).23,27
Confounding Measures
The potential confounders included in initial analyses for BMI were sex (male/female), gestational age younger than 37 weeks (yes/no), birthweight (in grams), smoking during pregnancy (yes/no), breastfeeding (per month), maternal education (< 16 years/≥ 16 years of education), maternal age at time of infant’s birth (< 20 years ≥ 20 years) and ponderal index at birth (cube root of weight in kilograms divided by crown-to-heel length in meters).28 The final model for child BMI included sex, birthweight, smoking in pregnancy, and breastfeeding per month.
The potential confounders adjusted in analysis for current asthma were sex, gestational age younger than 37 weeks, concurrent parental smoking, exposure to early infections (defined as attendance at child care in the first 3 months of life), skin prick test positivity, and maternal asthma status. A number of potentially important risk factors and confounders, including socioeconomic status, maternal education, maternal age, family income, older siblings at birth, age of introduction of solid food, being of Aboriginal descent, and age of asthma onset, have previously been tested and reported to make no significant contribution to model fit.23 The potential confounders adjusted in analysis for atopy were the same as those considered for current asthma. Interaction terms were assessed between exposures, but none was found. Maternal history of asthma (yes/no) was included in the model as a proxy measure of familial influence.
Asthma and Atopy
To reflect its complex definition and previous research,25 current asthma was defined as asthma ever diagnosed by a doctor and wheeze in the last year.
Atopy was assessed by skin prick testing to 4 common environmental aeroallergens (standardized mite, standardized cat pelt, ryegrass [Lolium perenne], and Mold Mix #4 [Bayer Corporation, Tarrytown, NY]), as described by Dreborg29; tests were conducted when the children attended the 6-year clinical assessment. Atopy was defined by a positive skin prick test if, 10 minutes following the test, the resulting welt to at least 1 of the 4 allergens was more than 2 mm across and larger than that of a control.30 Analyses that included atopy were restricted to 1596 children with skin tests.
BMI
Height and weight measurements of all children were taken at 6 years of age, and BMI was calculated. Weight was measured to the nearest 500 g with a Wedderburn Digital Chair Scales (Wedderburn, Sydney, Australia), with children wearing only underclothes, and height was measured to the nearest 0.1 cm with a Holtain Stadiometer (Holtain, Birmingham, England). We classified children from the 85th to the 95th percentile of BMI as overweight and children above the 95th percentile as obese; for the purposes of our analysis, all children above the 85th percentile were termed overweight.
Statistical Analysis
Linear and unconditional logistic regression analyses were used with a series of categorical or continuous explanatory covariates. Through the use of standard regression techniques, a “list-wise” deletion was effected, leaving the sample sizes in the models equal to the smallest number of observations on any variable. Thus, although 6-year questionnaires may have been completed for 2195 children, if a smaller number of birth data, confounder data, or data from the physical examination was available, the smaller number was used.
Propensity Score
As a way of examining whether breastfeeding acts through BMI in association with asthma and atopy or, conversely, whether BMI is associated with asthma or atopy only because of its common association with other factors such as sex, birthweight, smoking in pregnancy, and exclusive breastfeeding, we created a propensity score for being overweight (> 85th percentile of BMI). The propensity score is defined as the conditional probability of being overweight given a set of covariates31 for each individual. The score is based on the predicted value from the coefficients for all these variables of being overweight at 6 years of age.32 In the propensity score approach, variables thought to be related to exposure are identified and the probability of being “exposed” (i.e., being above the 85th percentile of BMI) is estimated on the basis of a set of covariates.
The score was used in regression analysis to adjust for the identified risk factors for overweight, thereby removing the adjusted effects of overweight from the final estimations.
Fractional Polynomials
We examined the effects of both BMI and the 2 breastfeeding variables on each other and on asthma and atopy by using fractional polynomials in both linear and logistic regression (Stata; Stata Corp, College Station, Tex).33 This technique allows a flexible curve to be fitted to dose–response data as a combination of 2 fractional polynomial response curves that express the change in odds ratios (ORs), usually in 2 functions of the “risk” variable:
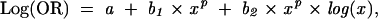 |
(1) |
where a is the constant and b1 and b2 are the regression coefficients in the regression equation and where x is the continuous covariate (e.g., BMI) and p is the power of the polynomial between −2 and 2, with the usual convention of a power of 0 indicating the log transformation. Since these are difficult to interpret from a table, figures were generated to describe the change in odds ratio with change in the level of the risk factor.
All analyses were performed with SPSS-PC (SPSS Inc, Chicago, Ill) and Stata software.
RESULTS
The children’s ages at the time of the 6-year follow-up ranged from 62 to 82 months (mean = 71 months, SD = 2.4 months). The mean BMI was slightly higher for boys than for girls, 48% of mothers had introduced other milk by 4 months, 17% of children had current asthma, and 41% had a positive skin prick test for atopy. Of the mothers in the study, 15% had current asthma (Table 1 ▶). The mean age at which other milk was introduced was 4.5 months (SD = 3.8), whereas the mean age at which breastfeeding stopped was 7.5 months (SD = 7.5). Correlation between the 2 breastfeeding variables was significant at the .01 level.
TABLE 1—
Characteristics of Participants at 6-Year Follow-Up: The Western Australian Pregnancy Cohort Study
| Characteristic | %a | Mean | SD | n |
| Outcomes at 6 y | ||||
| Asthma as diagnosed by a doctor | 31.0 (670/2161) | |||
| Wheeze in last year | 21.6 (471/2179) | |||
| Current asthma (asthma as diagnosed by doctor and wheeze in last year) | 17.3 (373/2161) | |||
| Atopic (positive skin prick test) | 41.4 (660/1596) | |||
| Male sex | 51.8 (1139/2195) | |||
| Gestational age < 37 wk | 10.7 (236/2195) | |||
| Birthweight > 2500 g | 91.7 (2013/2195) | |||
| Birthweight < 2500 g | 8.2 (180/2195) | |||
| Maternal age at birth < 20 y | 7.6 (166/2195) | |||
| Maternal asthma | 15.4 (332/2162) | |||
| Maternal smoking during pregnancy | 36.6 (740/2024) | |||
| Any parental smoking at 6 y | 42.0 (920/2189) | |||
| Never breastfed | 9.9 (204/2062) | |||
| Breastfeeding stopped by 2 mob | 20.2 (416/2062) | |||
| Breastfeeding stopped by 4 mob | 35.6 (734/2062) | |||
| Breastfeeding stopped by 6 mob | 46.1 (950/2062) | |||
| Exclusive breastfeeding stopped by 4 moc | 47.0 (963/2049) | |||
| Body mass indexd at 6 y | ||||
| Total | 15.83 | 1.79 | 2014 | |
| Females | 15.80 | 1.83 | 965 | |
| Males | 15.86 | 1.76 | 1049 | |
an = 2195.
bQuestion asked was “At what age [months] did you stop breastfeeding?”
cQuestion asked was “At what age [months] did you first give your child milk other than breast milk?”
dBody mass index is weight in kilograms divided by height in meters squared.
Breastfeeding and Overweight
In a logistic regression model before adjustment, a shorter duration of any breastfeeding was significantly associated with increased BMI at 6 years (P = .03). Following adjustment for gender, birthweight, and maternal smoking during pregnancy, the effect of longer breastfeeding (per additional month of feeding) did not significantly decrease a child’s overweight: the fractional polynomials for “breastfed in months” gave p = −1 in Equation 1, with the first term (1/breastfed in months) showing an odds ratio of 1.20 (95% confidence interval [CI] = 0.98, 1.46; P = .072) and the second term [log(breastfed in months)/breastfed in months] showing an odds ratio of 1.05 (95% CI = 0.99, 1.12; P = .105). This nonsignificant relationship was not graphed. The other identified risk factors for child overweight were birthweight (OR = 1.91; 95% CI = 1.48, 2.46; P < .0005) and maternal smoking during pregnancy (OR = 1.50; 95% CI = 1.14, 1.96; P = .004). The propensity scores for overweight were generated from the adjusted data.
Current Asthma, Atopy, and Child BMI
Correlations between BMI and asthma were small overall and were significant only in boys (r = 0.111; P < .01). Boys with asthma were significantly more likely than boys without asthma to have a higher BMI (P = .001), although this was not true for girls (P = .22) or children with atopy (P = .44). However, the interaction between sex and BMI became nonsignificant following adjustment for other variables (P = .71). Because the interaction between BMI and sex and its effect on asthma were not significant, combined analyses were done.
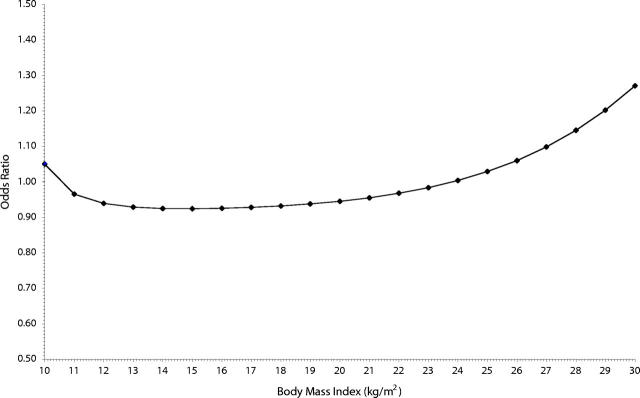
The odds ratio of current asthma within adjusted analyses is shown in Table 2 ▶. The fractional polynomial for BMI (at 6 years) is significant and also gave p = −1 in Equation 1, with the first term (1000/BMI) showing an odds ratio of 1.56 (95% CI = 1.02, 2.38; P = .038) and the second term [1000 × log(BMI)/BMI] showing an odds ratio of 0.77 (95% CI = 0.61, 0.97; P = .028). The resultant association with odds of asthma is graphed in Figure 1 ▶. In other words, the risk for someone whose BMI increases from the healthy to the overweight range (OR = 1.83; 95% CI = 1.34, 2.52; P < .0005) is similar to that of someone whose BMI moves from the overweight to the obese range (OR = 1.86; 95% CI = 1.14, 3.06; P = .014). Atopy was not associated with BMI.
TABLE 2—
Adjusted Odds Ratios of Current Asthma Among 6-Year-Old Children: The Western Australian Pregnancy Cohort Study
| Current Asthma | ||
| Adjusteda OR (95% CI) (n = 1485) | P | |
| BMI (per unit increase) | ||
| 1000/BMI at 6 y | 1.56 (1.02, 2.38) | .038 |
| 1000 × log (BMI at 6 y)/BMI at 6 y | 0.77 (0.61, 0.97) | .028 |
| Exclusive breastfeeding (month other milk introduced) | ||
| log (month other milk introduced) | 0.48 (0.26, 0.91) | .024 |
| [log (month other milk introduced)]2 | 0.79 (0.63, 0.98) | .035 |
| Sex (male/female) | 1.39 (0.96, 2.01) | .083 |
| Gestational age (< 37/≥ 37 wk) | 1.39 (0.75, 2.61) | .299 |
| Parental smoking (yes/no) | 1.42 (0.96, 2.10 | .077 |
| Child care in first 3 months of life (yes/no) | 0.62 (0.25, 1.49) | .286 |
| Maternal asthma (yes/no) | 3.40 (2.33, 4.96) | < .0005 |
| Atopic (positive skin prick test) | 2.35 (1.69, 3.27) | < .0005 |
Note. OR=odds ratio; CI=confidence interval; BMI=body mass index.
aAdjusted for all other variables in the model in addition to the propensity score for overweight (created from predicted values for risk factors for overweight at 6 years: sex, birthweight, smoking in pregnancy, and breastfeeding per month). n is reduced owing to missing data for some variables.
FIGURE 1—
Body mass index (BMI) and risk of asthma in 6-year-old children.
Exclusive Breastfeeding and Risk of Current Asthma and Atopy After Adjustment for Child BMI
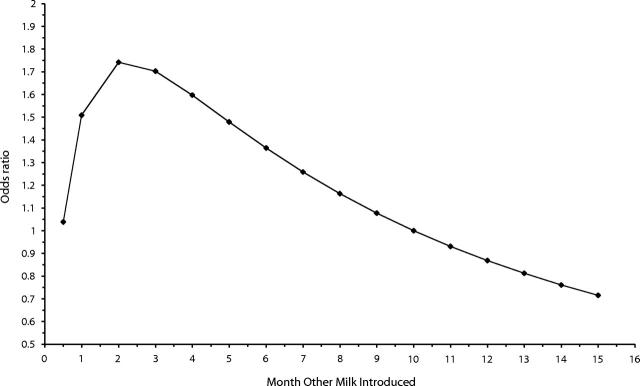
Following adjustment for BMI, the odds of current asthma at age 6 years among children exclusively breastfed per additional month of feeding was significantly decreased (Table 2 ▶). The fractional polynomial for introduction of other milk gave p = 0 in Equation 1 [i.e., log(other milk)], with the 2 terms shown in Table 2 ▶. Figure 2 ▶ demonstrates that the resulting relationship between asthma and the introduction of other milk was not linear. Practically, this means that with each additional month of exclusive breastfeeding, the risk of asthma was reduced by 4% (OR = 0.96; 95% CI = 0.92, 1.00). The effects of breastfeeding on atopy were not significant.
FIGURE 2—
Exclusive breastfeeding (i.e., months until other milk was introduced) and risk of asthma in 6-year-old children.
DISCUSSION
Following adjustment for overweight and its associated risk factors, there was a significant association between BMI and asthma. In addition, the associations between an increased duration of exclusive breastfeeding and asthma and atopy at 6 years were significant. We conclude that BMI and breastfeeding act through different pathways in their association with asthma.
Several factors strengthen confidence in the validity of our findings. This is a prospective birth cohort study followed from before birth to age 6 years. We determined the relation between infant feeding practice, a strict definition of current asthma at age 6 years (as diagnosed by a doctor and by the presence of wheezing in the last year), and BMI as measured by the child health nurse. Infant feeding data were collected prospectively26 and were confirmed by the child health study nurse by diary card and at interview. Adjustment was made for a wide range of potential confounders, including smoking behavior, maternal asthma history, and child’s atopic status.
We found that the association of concurrent parental smoking and childhood asthma became nonsignificant following adjustment for potential confounders. Maternal smoking in pregnancy and parental smoking at year 1 were tested, but concurrent smoking was of most relevance to current asthma in the children.
The Western Australian Pregnancy Cohort Study had a high response rate. Nevertheless, random nonresponse may have reduced statistical power. Any systematic nonresponse was most likely to be determined by disease status and social class, which may have biased estimated effects in either direction. The addition of social class covariates to our models made little difference to point estimates. Mothers were enrolled in mid-pregnancy, and selection bias was unlikely in relation to key outcomes. Most dropout occurred early in the study and was unlikely to have been associated with the later development of asthma or atopy. The cohort is often viewed as representative of the general Western Australian population.26 Nevertheless, recruitment was mainly through a tertiary obstetric hospital and included a small excess of mothers with preterm babies. However, our models include a covariate reflecting preterm delivery, and confounding owing to at-risk pregnancy should not have distorted our conclusions.
Measurement of outcome data was based on validated methodologies, and the study was powerful enough to detect risk ratios of only moderate size that were likely to be found in the study of a complex disease such as asthma. Standard regression diagnostics showed that our models fitted well.23
The strength of fractional polynomial analyses33 is their flexibility and better representation of adjusted data. Although they may be used in exploratory data analysis, the mathematical expressions for the curves are complex. However, they do allow for retention of the continuous scale of observations (i.e., BMI and exclusive breastfeeding duration in months). Objectivity is greater because arbitrary or data-driven categories are avoided, and a continuous model predicts smoothly changing risk and is more plausible than a cutpoint model.
Breastfeeding and BMI
A number of studies have not found an effect of breastfeeding on obesity before 5 years of age,34,35 with most not finding an effect on later obesity.36,37 One large cross-sectional study14 demonstrated a protective effect of breastfeeding on obesity, although breastfeeding data were collected retrospectively. Retrospective analyses have shown slight protective effects of breastfeeding on obesity,20,21 but the study designs and selected populations differed from those of our study. However, our results agreed with other reports regardless of design, demonstrating an association between duration of breastfeeding and BMI before but not after adjustment.
It is known that breastfed infants are leaner than formula-fed infants9; several mechanisms, behavioral and hormonal, have been suggested to explain this difference. It is not clear whether any of these mechanisms would account for protective influences on the risk of overweight or obesity later in life.
BMI and Asthma
In developed countries, the increased risk of asthma in children has paralleled an epidemic in obesity. An increasing body of epidemiological evidence suggests that there is an association between overweight and asthma.15,38,39 Our study showed that high BMI was a risk factor for asthma following adjustment for most known risk factors. A high BMI was not associated with atopy, in agreement with other studies.15
Reasons for an association between increased body weight and asthma are not clear, but an immunologic mechanism has been suggested.40 In obese individuals, the biological activity of adipose tissue may increase the risk of developing asthma.41 Although it has been suggested that asthma is a risk factor for obesity in children,19 we concluded that obesity may in fact be a risk factor for childhood asthma. From our results, we suggest that early childhood wheezing and asthma can be added to the list of chronic disease linked to obesity.42
Breastfeeding, Obesity, and Asthma
Breastfeeding has been associated with obesity,14,20,21 asthma, and atopy,23,24 but the true effect of breastfeeding may be obscured owing to the use of retrospectively collected data. Using prospectively collected data, we found that breastfeeding protected against stringently defined current asthma at age 6 years following adjustment for sex, gestational age, parental smoking, infections exposure, maternal asthma, concurrent child BMI, an objective measure of atopy, and the propensity score for overweight.
Our data provide robust evidence for an association between breastfeeding, BMI, and early childhood asthma. After account was taken of BMI and its associated risk factors, a protective effect of exclusive breastfeeding against early asthma and atopy was evident. As recently reported, breastfeeding may have an impact on transient cases of asthma (infection related) but not on the incidence of true persistent cases.43 This is why prospective birth cohorts are required to determine the long-term health benefits of breastfeeding.
Our findings support the hypothesis that exclusive breastfeeding protects against the occurrence of asthma and atopy in early childhood following adjustment for BMI. Although additional studies are required to confirm these findings and to understand the mechanisms of breastmilk protection, public health interventions to promote exclusive breastfeeding for at least 6 months may reduce the prevalence and subsequent morbidity of asthma and atopy in early childhood.
Acknowledgments
W. H. Oddy was funded by a National Health and Medical Research Council Public Health Australia Fellowship. The Western Australian Pregnancy Cohort Study is funded by project and program grants from the National Health and Medical Research Council of Australia, Western Australian Health Promotion Foundation, Asthma Foundation of Western Australia, and Glaxo Wellcome.
Acknowledgments are extended to the study investigators and staff responsible for the collection of the data presented here. Sincere thanks are extended to all study families, without whose participation this research could not have been conducted.
Human Participant Protection Informed consent was obtained for follow-up of the children from birth. The ethics committees of Princess Margaret and King Edward Memorial Hospitals approved the protocol for the study.
Contributors W. H. Oddy, J. L. Sherriff, and N. H. de Klerk developed the hypotheses and wrote the main drafts of the article. W. H. Oddy and N. H. de Klerk undertook statistical analyses. G. E. Kendall, P. D. Sly, L. J. Beilin, and K. B. Blake were involved in the design and conduct of the key follow-ups and assisted with the interpretation of the data. L. I. Landau and F. J. Stanley were involved in the initial study design and coordinating the study follow-ups.
Peer Reviewed
References
- 1.Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol. 1999;103:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Robertson CF, Dalton MF, Peat JK, et al. Asthma and other atopic diseases in Australian children: Australian arm of the International Study of Asthma and Allergy in Childhood. Med J Aust. 1998;168:434–438. [PubMed] [Google Scholar]
- 3.Magarey AM, Daniels LA, Boulton TJC. Prevalence of overweight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard worldwide definitions. Med J Aust. 2001;174:561–564. [DOI] [PubMed] [Google Scholar]
- 4.Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 1998. Report No. WHO/NUT/98.1. [PubMed]
- 5.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. [DOI] [PubMed] [Google Scholar]
- 6.Beilin L. Lifestyle and hypertension—an overview. Clin Exp Hypertens. 1999;21:749–762. [DOI] [PubMed] [Google Scholar]
- 7.Berenson GS, Srinivasan SR, Wattigney WA, Harsha DW. Obesity and cardiovascular risk in children. Ann N Y Acad Sci. 1993;699:93–103. [DOI] [PubMed] [Google Scholar]
- 8.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lönnerdal B. Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING study. Pediatrics. 1992;89:1035–1041. [PubMed] [Google Scholar]
- 9.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr. 1993;57:140–145. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson PW, Parkin JM, Pearlson J, Philips PR, Sykes P. Obesity in childhood: a community study in Newcastle upon Tyne. Lancet. 1977;i:350–352. [DOI] [PubMed] [Google Scholar]
- 11.Baranowski T, Bryan GT, Rassin DK, Harrison JA, Henske JC. Ethnicity, infant-feeding practices, and childhood adiposity. J Dev Behav Pediatr. 1990;11:234–239. [PubMed] [Google Scholar]
- 12.Poskitt EM, Cole TJ. Nature, nurture, and childhood overweight. BMJ. 1978;i:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer MS. Do breastfeeding and delayed introduction of solid foods protect against subsequent obesity? J Pediatr. 1981;98:883. [DOI] [PubMed] [Google Scholar]
- 14.von Kries R, Koletzko B, Sauerwald T, et al. Breastfeeding and obesity: cross sectional study. BMJ. 1999;319:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Kries R, Hermann M, Grunert VP, von Mutius E. Is obesity a risk factor for childhood asthma? Allergy. 2001;56:318–322. [DOI] [PubMed] [Google Scholar]
- 17.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luder E, Melnik TA, DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. J Pediatr. 1998;132:699–703. [DOI] [PubMed] [Google Scholar]
- 19.Gennuso J, Epstein LH, Paluch RA, Cerny F. The relationship between asthma and obesity in urban minority children and adolescents. Arch Pediatr Adolesc Med. 1998;152:1197–1200. [DOI] [PubMed] [Google Scholar]
- 20.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young children. JAMA. 2001;285:2453–2460. [DOI] [PubMed] [Google Scholar]
- 21.Gillman MW, Rifas-Shiman SL, Camargo CA, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461–2467. [DOI] [PubMed] [Google Scholar]
- 22.Butte NF. The role of breastfeeding in obesity. Pediatr Clin North Am. 2001;48:189–198. [DOI] [PubMed] [Google Scholar]
- 23.Oddy WH, Holt PG, Sly PD, et al. Association between breastfeeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ. 1999;319:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. 1995;346:1065–1069. [DOI] [PubMed] [Google Scholar]
- 25.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001;56:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–891. [DOI] [PubMed] [Google Scholar]
- 27.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. [DOI] [PubMed] [Google Scholar]
- 28.Karim E. Environmental factors influencing birthweight. In: Ulijaszek SJ, Johnston FE, Preece MA, eds. The Cambridge Encyclopedia of Human Growth and Development. Cambridge, England: Cambridge University Press; 1998:297–299.
- 29.Dreborg S. Skin test in diagnosis of food allergy. Allergy Proc. 1991;12:251–254. [DOI] [PubMed] [Google Scholar]
- 30.Haby MM, Peat JK, Marks GB, Woolcock AJ, Leeder SR. Asthma in preschool children: prevalence and risk factors. Thorax. 2001;56:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum PR, Rubin DB. The central role for the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 32.Drake C, Fisher L. Prognostic models and the propensity score. Int J Epidemiol. 1995;24:183–187. [DOI] [PubMed] [Google Scholar]
- 33.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. [DOI] [PubMed] [Google Scholar]
- 34.Butte NF, Wong WW, Hopkinson JM, Smith EO, Ellis KJ. Infant feeding mode affects early growth and body composition. Pediatrics. 2000;106:1355–1366. [DOI] [PubMed] [Google Scholar]
- 35.Michaelson KF, Larsen PS, Samuelson G. The Copenhagen Cohort Study on Infant Nutrition and Growth: breast-milk intake, human milk macronutrient content, and influencing factors. Am J Clin Nutr. 1994;59:600–611. [DOI] [PubMed] [Google Scholar]
- 36.O’Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM. Prediction of obesity in children at 5 years: a cohort study. J Paediatr Child Health. 1997;33:311–316. [DOI] [PubMed] [Google Scholar]
- 37.Wadsworth M, Marshall S, Hardy R, Paul A. Breastfeeding and obesity: relation may be accounted for by social factors [letter]. BMJ. 1999;319:1576. [PMC free article] [PubMed] [Google Scholar]
- 38.Shaheen SO. Obesity and asthma: cause for concern? Clin Exp Allergy. 1999;29:291–293. [DOI] [PubMed] [Google Scholar]
- 39.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthma-like symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–1349. [DOI] [PubMed] [Google Scholar]
- 40.Varner AE. An immunologic mechanism for the association between obesity and asthma. Arch Intern Med. 2000;160:2395–2396. [DOI] [PubMed] [Google Scholar]
- 41.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax. 2001;56:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. [DOI] [PubMed] [Google Scholar]
- 43.Infante-Rivard C, Amre D, Gautrin D, Malo JL. Family size, day-care attendance, and breastfeeding in relation to the incidence of childhood asthma. Am J Epidemiol. 2001;153:653–658. [DOI] [PubMed] [Google Scholar]