Abstract
Objectives. We evaluated the hepatitis A virus (HAV) control policy (hygienic precautions and passive immunization with immune globulin) for “household contacts” (defined as all people who lived in the same house and who shared the same toilet with the patient, people who took care of an HAV-infected child, and sexual partners of the patient) of acute hepatitis A patients between 1996 and 2000.
Methods. We examined the characteristics and the serological outcomes of household contacts. All susceptible contacts were invited for retesting 6 weeks after they received immune globulin.
Results. Of 1242 contacts of 569 HAV patients, more than 50% (n = 672) were found to be HAV immune. Among the remaining contacts, 161 (28.2%) had a concurrent infection, and 86 of these individuals were symptomatic. The remaining 409 susceptible contacts received immune globulin, with 186 (45%) returning for retesting 6 weeks later (64 [34%] were infected, but only 12 had symptoms).
Conclusions. Immune globulin does not protect all household contacts from HAV infection; however, it attenuates symptoms and effectively reduces further HAV transmission.
Hepatitis A is an acute liver disease caused by the hepatitis A virus (HAV) and transmitted from the feces of an infected person via contaminated food, water, hands, or contaminated items (fomites). Although the disease is rarely symptomatic among children aged younger than 5 years, morbidity and mortality can be high among adults. The prevalence of hepatitis A is strongly associated with economic conditions: in less-developed countries, the disease occurs widely among children; as a result, most adults are immune. In more developed countries, the number of adult symptomatic infections increases. In the Netherlands, as in most Western countries, the seroprevalence of anti-HAV antibodies declined sharply among people born after World War II,1 making a majority of the population susceptible.
In Amsterdam, the Netherlands, the incidence of hepatitis A follows a largely seasonal pattern, peaking in August and September, when children of migrant-worker families (mainly from Turkey and Morocco) return from summer holidays in the country of parental origin.2 Hepatitis A also causes year-round microepidemics among homosexual men,3,4 but sequencing of the viruses suggests that different subgenotypes circulate among different at-risk groups.5
In the Netherlands, a diagnosis of hepatitis A must be reported to the Municipal Health Service (MHS). To prevent secondary cases, persons (“household contacts”) who are cohabitants of each primary patient are identified and are given advice on hygienic precautions and passive immunization with immune globulin if they are found to be susceptible. We evaluated the serological results of testing household contacts for acute hepatitis A (1996–2000) to determine the percentage who were immune at presentation and the predictors for such immunity. We also analyzed the follow-up of susceptible contacts to determine the incidence of symptomatic and asymptomatic HAV infection.
METHODS
We examined all hepatitis A cases reported to the Department of Infectious Diseases of the MHS in Amsterdam between January 1, 1996, and December 31, 2000. After a case was reported, a history was taken to find the most likely route of HAV transmission. We classified patients hierarchically, by the route of transmission, into 5 transmission groups: (1) those infected as a result of homosexual activity during the previous 6 weeks, (2) those infected by a hepatitis A patient living in the immediate environment, (3) those infected while traveling to a highly HAV-endemic country during the previous 6 weeks, (4) primary school students who did not travel and who were infected by an asymptomatic peer at school, and (5) unknown (no likely cause of disease).
Household contacts were all people who lived in the same house and who shared the same toilet with the patient, people who took care of an HAV-infected child, and sexual partners of the patient. All household contacts were offered MHS advice on hygienic precautions, serological testing (total anti-HAV antibodies), and immunization with immune globulin within 14 days of the onset of disease in the patient. The first day symptoms of jaundice appeared in a patient was defined as the first day of disease. Because people born and raised in highly HAV-endemic countries are often immune to the virus, contacts from that type of background were not given immune globulin until the HAV antibody test results were available (usually within 1 day). Children aged ≤ 10 years who tested positive for total anti-HAV also were tested for immunoglobulin M antibodies to ascertain whether they had a recent infection. Contacts aged > 10 years were tested for immunoglobulin M only if they described symptoms indicative of acute hepatitis A.
To detect infections that occurred within 6 weeks of passive immunization, susceptible contacts were invited for retesting. Those who then tested positive for total anti-HAV also were tested for anti-HAV immunoglobulin M to exclude possible false-positive tests caused by immune globulin administration. Only people with positive immunoglobulin M antibody test results were considered to have seroconverted and thus to have acquired a recent hepatitis A infection.
We detected antibodies against HAV with a competitive enzyme immunoassay for total antibodies and an antibody-capture enzyme immunoassay for the detection of immunoglobulin M antibodies (HAVAB and HAVAB-M, Abbott Diagnostic Division, Wiesbaden, Germany). A solid-phase version of both tests was used until April 1998, when that version was replaced by a microparticle version (AXSYM, Abbott Diagnostic Division, Wiesbaden, Germany). These tests have a sensitivity of 99.7% and a specificity of 99%.
The incubation period for hepatitis A is 14 to 50 days. Therefore, we classified contacts whose illness began within 14 days of disease onset in the patient as concurrent primary or coprimary cases. Contacts with disease onset between 15 and 50 days after disease onset in the patient were considered secondary cases.6 To assign these classifications, we extracted the following data for all hepatitis A patients and their contacts from the electronic database of the Department of Infectious Diseases: date of birth, gender, symptoms of disease, date of disease onset, risk factors during the incubation period, country of birth, parents’ country of birth, date of passive immunization, and dates and results of blood tests (total HAV antibodies and immunoglobulin M antibodies). For people aged older than 15 years, country of origin was defined as the country of birth; for people aged 15 years or younger, country of origin was defined as the birth country of their parent(s).
Statistical Analysis
Chi-square tests or Student t tests were used, when appropriate, to compare characteristics between different transmission groups. To calculate risk factors for different outcomes, SPSS (SPSS Inc, Chicago, Ill) was used to obtain univariate and multivariate odds ratios (ORs) and 95% confidence intervals (CIs). In the multivariate modeling, all factors with a P value less than .10 were included.
RESULTS
Reported Patients
Between January 1, 1996, and December 31, 2000, 569 patients with immunoglobulin M–confirmed acute hepatitis A were reported to the MHS in Amsterdam (Table 1 ▶). Of these, 151 (26.5%) had engaged in homosexual activity, 66 (11.6%) had had close contact with a symptomatic patient, 158 (27.8%) had traveled to an HAV-endemic country, 74 (13.0%) were primary school students who had no patients in their immediate environments and who had not traveled, and 120 (21.1%) had no obvious source of infection. Most of the men infected through homosexual encounters were born in Western countries (89%), and most otherwise-infected patients were aged 15 years or younger (71%) and of Moroccan origin (59%).
TABLE 1—
Characteristics of Reported Hepatitis A Patients, by Route of Transmission: Amsterdam, the Netherlands, January 1, 1996 to December 31, 2000
| Characteristics of Patients | Homosexual Transmission | Other Than Homosexual Transmission |
| Total | 151 (26.5%) | 418 (73.5%) |
| Gender | ||
| Female | 216 (51.7%) | |
| Male | 151 (100%) | 202 (48.3%) |
| Mean age, y (range) | ||
| Female | . . . | 14.0 (1–77) |
| Male | 35.1 (19–72) | 15.7 (2–63) |
| Country of origin for persons > 15 ya | ||
| The Netherlands or other Western country | 134 (88.7%) | 107 (85.6%) |
| Morocco | . . . | 4 (3.2%) |
| Turkey | . . . | 1 (0.8%) |
| Other non-Western country | 14 (9.3%) | 10 (8.0%) |
| Unknown | 3 (2.0%) | 3 (2.4%) |
| Country of origin for persons ≤ 15 ya | ||
| The Netherlands or other Western country | 26 (8.9%) | |
| Morocco | . . . | 172 (58.7%) |
| Turkey | . . . | 37 (12.6%) |
| Other non-Western countries | . . . | 22 (7.5%) |
| The Netherlands, parents’ birth country unknown | . . . | 35 (11.9%) |
| Unknown | . . . | 1 (0.3%) |
| Age group, y | ||
| 0–15 | . . . | 298 (71.3%) |
| 16–35 | 92 (60.9%) | 74 (17.7%) |
| > 35 | 59 (39.1%) | 46 (11.0%) |
| No. of household contacts | ||
| None | 88 (58.3%) | 45 (10.8%) |
| 1 | 56 (37.1%) | 38 (9.1%) |
| 2–3 | 6 (4.0%) | 113 (27.0%) |
| > 3 | 1 (0.7%) | 222 (53.1%) |
aFor people aged older than 15 years, country of origin was defined as the country of birth. For people aged 15 years or younger, country of origin was the country of their birth unless that country was the Netherlands, in which case the country of origin was the country of the parents’ birth.
Contacts
Immunity of contacts.
A total of 1715 household contacts were identified; each patient had an average of 3 contacts (range 0–16). Of these 1715 contacts, 473 were excluded because blood samples were not taken or were taken more than 14 days after disease onset in the patient.
The characteristics of the 1242 remaining contacts, 672 (54%) of whom were immune at presentation, are shown in Table 2 ▶. Three hundred fifty nine of the contacts were aged 10 years or younger, and 35 of these were immune.
TABLE 2—
Prevalence of Hepatitis A Antibodies in Blood Samples Taken From Contacts Within 14 Days of Hepatitis A Onset in Patient, by Contact Characteristics: Amsterdam, the Netherlands, January 1, 1996 to December 31, 2000
| Characteristics of Contacts | Total | No. (%) Anti-HAV+ | Univariate OR (95% CI) | Multivariate OR (95% CI) |
| Total susceptible contacts | 1242 | 672 (54.1) | ||
| Age group, y | ||||
| 0–5 | 160 | 5 (3.1)a | 1.00 | 1 |
| 6–10 | 199 | 30 (15.1)a | 5.5 (2.1, 14.5)*** | 5.0 (1.9, −13.4)** |
| 11–15 | 170 | 82 (48.2) | 28.9 (11.2, 73.9)*** | 26.0 (10.0, 67.7)*** |
| > 15 | 713 | 555 (77.8) | 108.9 (43.9, 269.9)*** | 1587.0 (520.3, 4840.5)*** |
| Transmission group index | ||||
| Unknown | 297 | 140 (47.1) | 1 | 1 |
| Travel | 492 | 306 (62.2) | 1.8 (1.4, 2.5)*** | 2.4 (1.5, 3.8)*** |
| Homosexual activity | 51 | 13 (25.5) | 0.4 (0.2, 0.8)** | 0.3 (0.1, 0.9)** |
| School | 232 | 137 (59.1) | 1.6 (1.1, 2.3)** | 2.2 (1.3, 3.7)** |
| Case patient in immediate environment | 170 | 76 (44.7) | 0.9 (0.6, 1.3) | 1.1 (0.6, 2.0) |
| Gender | ||||
| Male | 618 | 304 (49.2) | 1 | 1 |
| Female | 624 | 368 (59.0) | 1.5 (1.2, 1.9)*** | 1.0 (0.7, 1.4) |
| No. of household contacts | ||||
| 1 | 69 | 21 (30.4) | 1 | 1 |
| 2–3 | 223 | 98 (43.9) | 1.8 (1.0, 3.2)* | 0.6 (0.3, 1.6) |
| > 3 | 950 | 553 (58.2) | 3.2 (1.9, 5.4)*** | 2.2 (1.0, 4.9)* |
| Country of originb | ||||
| The Netherlands or other Western country | 310 | 120 (38.7) | 1 | 1 |
| The Netherlands, parents’ birth country unknown Turkey | 53 144 | 8 (15.1) 85 (59.0) | 0.3 (0.1, 0.6)** 2.3 (1.5, 3.4)*** | 30.9 (9.8, 96.8)*** 22.2 (9.7, 50.8)*** |
| Morocco | 647 | 398 (61.5) | 2.5 (1.9, 3.3)*** | 39.8 (19.8, 80.2)*** |
| Other non-Western country | 88 | 61 (69.3) | 3.6 (2.2, 5.9)*** | 14.7 (6.4, 33.6)*** |
Note. HAV = Hepatitis A virus; OR = odds ratio; CI = confidence interval; IgM = immunoglobulin M.
aAll children younger than 10 years were IgM negative.
bFor people older than 15 years, country of origin was defined as the country of birth. For people aged 15 years or younger, country of origin was the country of their birth unless that country was the Netherlands, in which case country of origin was the country of the parents’ birth.
*P < .05; **P < .01; ***P < .001.
The included and excluded groups did not differ in gender, number of contacts, or most likely source of infection. However, the median age of the excluded contacts was significantly lower (15 years; range 0–64 years) than that of the included group (20 years; range 0–77 years; P < .001). In addition, the excluded group contained significantly more contacts whose country of origin was “other, non-Western” (P = .03).
In the univariate analysis, contact age, transmission group of patient, contact gender, number of household contacts, and country of origin were significantly associated with immunity at presentation. All of these factors were included in the multivariate analysis. Older age, travel or school transmission groups, 4 or more household contacts, and origin in highly HAV-endemic countries were independently and positively associated with immunity. Contacts of patients in the homosexual transmission group were significantly less likely to be immune at presentation than were contacts in other groups. Finally, significantly more people who originated from HAV-endemic countries than of those who originated from Western countries were immune at presentation.
Coprimary cases: onset of hepatitis A infection in contacts within 14 days of onset in patient.
Of the 570 nonimmune contacts, 161 (28.2%) tested immunoglobulin M positive at their first blood test and were considered to have coprimary infections. Of all coprimary infections, 86 (53%) were symptomatic, and 127 (79%) of these infections were among children aged 10 years or younger (52 [41%] were symptomatic). No asymptomatic infections among contacts older than 10 years were found, because these contacts were not tested for immunoglobulin M antibodies.
Secondary cases: seroconversion among susceptible contacts.
Of the 409 susceptible contacts, 186 (45%) returned for a second blood test 6 weeks later. At that time, 64 (34%) of these 186 contacts were immunoglobulin M positive and had acquired a secondary infection; 12 of the 64 (19%) were symptomatic. Age was not associated with symptomatic hepatitis A infection (data not shown).
Between the 2 groups, 1 that did return and 1 that did not return for retesting, no significant differences were found for age group, mean age, gender, or number of contacts. However, there were differences in the national backgrounds among those who returned (P < .05): 39% were from Western countries, 31% were “children born in the Netherlands, parents’ origin unknown,” 55% were from Morocco, 38% were from Turkey, and 52% were from “other, non-Western” countries. Tertiary cases were not identified or reported to the MHS.
In the univariate analysis, contact age, transmission group of patient, contact gender, number of household contacts, and country of origin were not significantly associated with secondary infection (Table 3 ▶). There also was no association between the time at which immune globulin was given and the likelihood of seroconverting (OR = 0.96; 95% CI = 0.83, 1.04), nor was there an association between the time at which immune globulin was given and the likelihood of contracting symptomatic disease (OR = 0.96; 95% CI = 0.83, 1.12).
TABLE 3—
Seroconversion Among Susceptible Contacts of Patients With Acute Hepatitis A, by Contact Characteristics: Amsterdam, the Netherlands, January 1, 1996 to December 31, 2000
| Characteristics of Contacts | Total | No. (%) Seroconverting | Univariate OR (95% CI) |
| Total susceptible contacts | 186 | 64 (34.4) | |
| Age group, y | |||
| 0–5 | 33 | 11 (33.3) | 1.2 (0.5, 3.1) |
| 6–10 | 59 | 20 (33.9) | 1.3 (0.6, 2.8) |
| 11–15 | 35 | 16 (45.7) | 2.1 (0.9, 5.0) |
| > 15 | 59 | 17 (28.8) | 1.00 |
| Transmission group index | |||
| Travel | 66 | 21 (31.8) | 1.00 |
| Homosexual activity | 17 | 6 (35.3) | 1.2 (0.4, 3.6) |
| Unknown | 55 | 15 (27.3) | 0.8 (0.4, 1.8) |
| School | 31 | 14 (45.2) | 1.8 (0.7, 4.2) |
| Case patient in immediate environment | 17 | 8 (47.1) | 1.9 (0.6, 5.6) |
| Gender | |||
| Male | 103 | 36 (35.0) | 1.1 (0.6, 1.9) |
| Female | 83 | 28 (33.7) | 1.00 |
| No. of household contacts | |||
| 1 | 20 | 6 (30.0) | 1 |
| 2–3 | 31 | 12 (38.7) | 1.5 (0.4, 4.9) |
| > 3 | 135 | 46 (34.1) | 1.2 (0.4, 3.4) |
| Country of origina | |||
| The Netherlands and other Western countries | 60 | 19 (31.7) | 1.00 |
| Country of birth = the Netherlands, parents’ birth country unknown | 8 | 3 (37.5) | 1.3 (0.3, 6.0) |
| Turkey | 17 | 3 (17.6) | 0.5 (0.1, 1.8) |
| Morocco | 90 | 37 (41.4) | 1.5 (0.8, 3.0) |
| Other non-Western countries | 11 | 2 (18.2) | 0.5 (0.9, 2.4) |
Note. OR = odds ratio; CI = confidence interval.
aFor people aged older than 15 years, country of origin was defined as the country of birth. For people aged 15 years or younger, country of origin was the country of their birth unless that country was the Netherlands, in which case country of origin was the country of the parents’ birth.
DISCUSSION
We analyzed routinely collected data to evaluate the current policy for prevention of secondary transmission of hepatitis A in Amsterdam. The relatively high 50% rate of immunity among contacts may be explained by the high endemicity of HAV in their country of origin. Of the nonimmune contacts, 28% had a coprimary infection, and of the 186 susceptible contacts, 34% developed a secondary infection despite administration of immune globulin within 2 weeks of disease onset in the patient. Only 6% of contacts developed a secondary symptomatic infection.
Patients
In Amsterdam, hepatitis A is observed in 2 main groups: travelers to highly HAV-endemic countries (many of them children from Morocco and Turkey)2 and homosexual men. Molecular sequencing showed that there are 2 main separate groups, and 2 subgenotypes were identified within these 2 groups.7 In 1998, the MHS Amsterdam began an annual vaccination campaign for children aged younger than 16 years who travel to HAVendemic countries (mainly Turkey and Morocco); the campaign has resulted in 50% coverage.8 All other travelers to HAVendemic countries are encouraged to get the vaccination.
The group of homosexual men is more difficult to protect, because transmission occurs year-round and is mainly from anonymous contacts. Because homosexual men comprise 1 of the 2 largest groups of acute hepatitis A infection in Amsterdam, we believe that all homosexual men should be vaccinated against hepatitis A. In the Netherlands in November 2002, a vaccination campaign was started that offered homosexual men free vaccinations against hepatitis B. An effort has been made to offer this group a combined hepatitis A and B vaccine for a reduced price.
The number of reported cases of hepatitis A in Amsterdam has decreased in the past few years, from 200 in 1998 to 50 in 2002.9 It is yet to be determined whether this is an ongoing trend.
Contacts
Immunity of contacts.
Of the 1242 contacts, more than 50% were immune. The average age of contacts excluded because of absent or mistimed blood samples was significantly lower than that of the included group, because parents often object to taking blood samples from children when it is not therapeutically necessary. Because children are more likely than adults to be susceptible to HAV, our rate of immunity may be an overestimation.
We did not test for immunoglobulin M among contacts aged >10 years who were total anti-HAV positive; therefore, we may have misclassified some asymptomatic coprimary-infected contacts aged >10 years as immune, which also may have resulted in overestimation of the immunity rate. However, because the large majority of acute hepatitis A infection among older people is symptomatic,10 we do not believe that not testing for immunoglobulin M in contacts aged >10 years had a major influence on the results. Contacts of patients in the homosexual transmission group had relatively lower immunity at presentation than did contacts of patients in the other transmission groups, probably because most homosexual contacts were from countries with low-HAV endemicity.
Coprimary cases.
Of the susceptible contacts, 28% had seroconverted within 14 days of disease onset in the primary patient and thus were considered to have coprimary infections; half of these contacts had symptoms of hepatitis A. In Athens, Greece, 18.5% of the susceptible contacts of 113 children with hepatitis A were coprimarily infected, and 3.5% of these coprimary cases were symptomatic.11 All blood samples were taken within 7 days of disease onset in the patient, and we hypothesize that the rate of coprimary cases would have been higher than 18.5% if the sampling window had been extended to 14 days. An Italian study of 219 household contacts of 380 hepatitis A patients in Naples found 9 (2.6%) coprimary cases.12 Both the Athens and the Naples studies focused on the contacts of sporadic patients and did not mention the most likely cause of infection among the patients.
Secondary cases.
Compared with other studies, we found a high secondary seroconversion rate (34%) among susceptible contacts despite immune globulin treatment within 1 to 14 days of patient notification. However, of all the 409 susceptible contacts, only 12 (2.9%) secondarily contracted symptomatic hepatitis A, a finding that is in agreement with other studies.12
Symptomatic contacts may have been more likely than nonsymptomatic contacts to return for retesting after 6 weeks, but even after we excluded the symptomatic cases, we still found a seroconversion rate of 30% (52 of 174 cases). In only a few other studies was a second blood test performed to detect secondary asymptomatic infections. In the Naples study,12 12 of the 102 susceptible household contacts (11.8%) who did not receive immune globulin or vaccination seroconverted after 6 weeks (4 of the 12 had symptoms), whereas 2 of the 110 of contacts (1.8%) who did receive active immunization with hepatitis A vaccine seroconverted asymptomatically. The Athens study11 reported no seroconversions among 89 of the 185 susceptible household contacts who returned 4 weeks after administration of immune globulin.
Finally, a review of the Athens and Naples studies and of 4 other studies13 examined the probability of secondary seroconversion among susceptible contacts in the immediate environment of an HAV-infected patient. The reviewers of these studies analyzed the percentage of contacts who were immune at presentation and the percentage of asymptomatic infections. These analyses were based on estimates of HAV immunity and the percentage of symptomatic versus asympotomatic infections if no measurements were provided in the studies; they stratified for age based on the average age distributions among American families. The estimated rate of transmission to susceptible children aged younger than 12 years was 22% (95% CI = 12%, 33%) and to susceptible adults was 15% (CI = 9%, 20%); we found a somewhat higher transmission rate. A possible explanation for this contrast may be the difference in transmission groups: both the Naples and the Athens studies examined family contacts of sporadic patients only, and in the Athens study, all of the patients were children. Even though the most likely route of transmission was not mentioned in these studies, it is doubtful that 28% of the patients were infected by travel, as was the case in our study. Our contacts had often traveled as companions of the patients. Instead of having secondary infections, some of the contacts could have been coprimary patients with long incubation periods (see last Discussion paragraph, “Limitations”); however, we did not find significant differences among the various transmission groups with regard to secondary transmission (Table 3 ▶).
The high seroconversion rate despite immune globulin immunization indicates that although immune globulin prevents or attenuates symptoms, it does not always prevent infection.14 Without immune globulin immunization, the severity of hepatitis A and the percentage of infected people who develop jaundice rises markedly with age, from 0% among children aged 0 to 3 years to 80% among persons aged 16 years and older.10 Of the coprimary infections identified among children aged 10 years and younger in our study, 40% were symptomatic. Although children received immune globulin immunization, 19% of secondary infections were symptomatic, and among contacts aged 16 years and older, 35% were symptomatic, a proportion much lower than the 80% we expected.10 Relatively more secondary than coprimary infections were asymptomatic, and for secondary infections, age was not associated with symptomatic infection. Both of these findings are probably the result of immune globulin vaccinations given to contacts.
When administered within 2 weeks of exposure to HAV, immune globulin reportedly prevents more than 85% of clinical hepatitis A cases.15 Administration of immune globulin in day-care centers has stopped the spread of clinical cases of hepatitis A.16 If administering immune globulin does not prevent seroconversion but does reduce further transmission, a possible explanation for this reduced infectiousness is that immune globulin diminishes HAV excretion. The efficacy of immune globulin is said to be greatest when it is administered early in the incubation period.15 In our study, the interval between disease onset in the patient and administration of immune globulin in the contact was not associated with the seroconversion rate among these contacts. Also, no association was found between this interval and the likelihood of developing symptomatic disease; however, the number of symptomatic cases in our study was small.
Contacts of acute hepatitis A patients in Amsterdam are protected from infection by the administration of immune globulin as soon as possible after the patient has been notified. As a result of recent revisions in the Netherlands national guidelines,17 the hepatitis A vaccine used for preexposure prophylaxis among travelers to HAV-endemic countries is now recommended instead of immune globulin for postexposure prophylaxis. Vaccination may not always be timely enough to prevent clinically overt disease, especially among people who are aged 40 years or older, who are obese, who have a slower immune response to hepatitis A vaccination,18 or who are vaccinated more than 7 days after disease onset in the patient.19 Therefore, vaccination is recommended for all healthy contacts aged younger than 30 years. For contacts aged 30 to 50 years, vaccination is recommended only if administered within 7 days of disease onset in the patient.
Limitations
In this study, we considered infections in contacts who tested positive for immunoglobulin M antibodies within 14 days of disease onset in the patient to be coprimary cases. It is possible that some of the secondary cases that had longer incubation periods were actually coprimary infections. However, our study design has been used in other studies, so comparison with our results should not to be influenced by this assumption.
CONCLUSIONS
Our study shows that immune globulin does not protect all contacts from HAV infection, although it does attenuate symptoms and reduces further spread of transmission. That no tertiary cases were reported is evidence of reduced infectiousness.
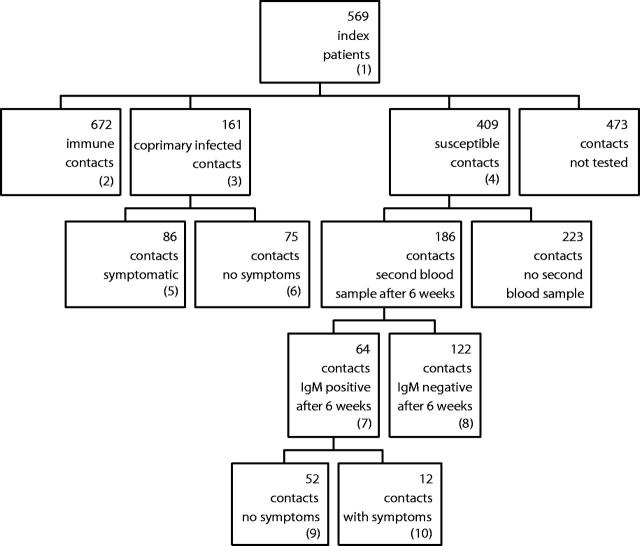
FIGURE 1—
Outcomes of 1715 household contacts of 569 acute hepatitis A patients: Amsterdam, the Netherlands, January 1, 1996 through December 31, 2000.
Note. IgM = immunoglobulin M; HAV = hepatitis A virus.
(1) All reported to Municipal Health Service with jaundice and anti-HAV IgM positive.
(2) No symptoms, anti-HAV total positive; if younger than 10 years, IgM negative.
(3) All IgM positive.
(4) No symptoms and anti-HAV total negative.
(5) With symptoms and IgM positive.
(6) Without any symptoms and IgM positive. Because contacts older than 10 years who did not have symptoms were not tested, everyone in this group was aged 10 years or younger.
(7) Anti-HAV total negative in the first blood-sample, total anti-HAV positive and anti-HAV IgM positive in the sample 6 weeks later.
(8) Anti-HAV total negative in the first blood sample and in the follow-up sample 6 weeks later.
(9) IgM positive but no symptoms of any disease.
(10) IgM positive and symptoms, not always jaundice.
Acknowledgments
The authors thank Mrs. Van der Bij for her advice on statistical methods and Lucy D. Phillips for her editorial review. The authors also thank the public health nurses of the Department of Infectious Diseases for collecting and documenting all data.
Human Participant Protection No protocol approval was needed for this study.
Contributors G. J. B. Sonder wrote the article and analyzed the statistics. J. E. van Steenbergen provided advice on the study design and contributed to the article. L. P. M. J. Bovée collected data, created the database, and reviewed the article. P. G. H. Peerbooms was responsible for the laboratory tests and reviewed the article. R. A. Coutino provided advice on the study design and the article. A. van den Hoek provided advice on the study design, was responsible for the data collection, and contributed to the article.
Peer Reviewed
References
- 1.Termorshuizen F, Dorigo-Zetsma JW, De Melker HE, van den Hof S, Conyn-van Spaendonck MAE. The prevalence of antibodies to hepatitis A virus and its determinants in the Netherlands: a population-based survey. Epidemiol Infect. 2000;124:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorkom J van, Leentvaar-Kuijpers A, Kool JL, Coutinho RA. [Association between the yearly hepatitis A epidemic and travel behaviour of children of immigrants in the four major cities of the Netherlands.] Ned Tijdschr Geneesk. 1998;34:1919–1923. [PubMed] [Google Scholar]
- 3.Coutinho RA, Albrecht-van Lent P, Lelie N, Nagelkerke N, Kuipers H, Rijsdijk T. Prevalence and incidence of hepatitis A among male homosexuals. BMJ. 1983;287:1743–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leentvaar-Kuijpers A, Kool JL, Veugelers PJ, Coutinho RA, van Griensven GJ. [An outbreak of hepatitis A among homosexual men in Amsterdam, 1991–1993.] Int J Epidemiol. 1995;24:218–22. [DOI] [PubMed] [Google Scholar]
- 5.Bruisten SM, van Steenbergen JE, Pijl AS, et al. Molecular epidemiology of hepatitis A virus in Amsterdam, Netherlands. J Med Virol. 2001;63:88–95. [PubMed] [Google Scholar]
- 6.Ascertainment of secondary cases of hepatitis A—Kansas, 1996–1997 [editorial]. MMWR Morb Mortal Wkly Rep. 1999;48:608–610, 619. [PubMed] [Google Scholar]
- 7.van Steenbergen JE, Tjon G, van den Hoek JAR, Koek A, Coutinho RA, Bruisten SM. Two years prospective collection of molecular and epidemiological data shows limited spread of hepatitis A outside risk groups in Amsterdam 2000–2002. J Infect Dis. 2004;189:471–482. [DOI] [PubMed] [Google Scholar]
- 8.Dijkshoorn H, Schilthuis HJ, van den Hoek JAR, Verhoeff AP. [Travel advice on the prevention of infectious diseases insufficiently obtained by indigenous and non-native inhabitants of Amsterdam, the Netherlands.] Ned Tijdschr Geneeskd. 2003;147:658–662. [PubMed] [Google Scholar]
- 9.Bovée LPMJ, van den Hoek JAR. Annual Report 2002, Department of Infectious Diseases, GG&GD Amsterdam. Amsterdam, the Netherlands: GG&GD Amsterdam; 2003:15.
- 10.Gingrich GA, Hadler SC, Elder HA, Ash KO. Serologic investigation of an outbreak of hepatitis A in a rural day-care center. Am J Public Health. 1983;73:1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rameliotou A, Papachristopoulos A, Alexiou D, Papaevangelou V, Stergiou G, Papaevangelou G. Intrafamilial clustering of hepatitis A. Infection. 1994;22:36–38. [DOI] [PubMed] [Google Scholar]
- 12.Sagliocca L, Amoroso P, Stroffolini T, et al. Efficacy of hepatitis A vaccine in prevention of secondary hepatitis A infection: a randomized trial. Lancet. 1999;353:1136–1139. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhoff AS, Jacobs RJ. Transmission of hepatitis A through household contact. J Viral Hepatitis. 2001;8:454–458. [DOI] [PubMed] [Google Scholar]
- 14.Mutton KJ, Gust ID. Public health aspects of hepatitis A. In: Gerety RJ, ed. Hepatitis A. Orlando, Fla: Academic Press; 1984:152.
- 15.Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48(RR–12):1–37. [PubMed] [Google Scholar]
- 16.Hadler SC, Erben JJ, Matthews D, Starko K, Francis DP, Maynard JE. Effect of immunoglobulin on hepatitis A in day-care centers. JAMA. 1983;248:48–53. [PubMed] [Google Scholar]
- 17.LCI. Hepatitis A. In: van Steenbergen JE, Timen A, eds. LCI Guidelines Communicable Disease Control. Utrecht, The Netherlands: Landelijke Coördinatie van Infectieziekten Nederland (LCI); 2003. In press.
- 18.Reuman PD, Kubilis P, Hurni W, Brown L, Nalin D. The effect of age and weight on the response to formalin-inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine. 1997;15:1157–1161. [DOI] [PubMed] [Google Scholar]
- 19.Conaty S. Continued role for HNIG in hepatitis A post-exposure prophylaxis [letter]. Comm Dis Public Health. 2001;4:319. [PubMed] [Google Scholar]