Abstract
Objectives. We evaluated lifestyle interventions for diabetic persons who live in rural communities.
Methods. We conducted a 12-month randomized clinical trial (n = 152) of “intensive-lifestyle” (modeled after the NIH Diabetes Prevention Program) and “reimbursable-lifestyle” (intensive-lifestyle intervention delivered in the time allotted for Medicare reimbursement for diabetes education related to nutrition and physical activity) interventions with usual care as a control.
Results. Modest weight loss occurred by 6 months among intensive-lifestyle participants and was greater than the weight loss among usual-care participants (2.6 kg vs 0.4 kg, P<.01). At 12 months, a greater proportion of intensive-lifestyle participants had lost 2 kg or more than usual-care participants (49% vs 25%, P<.05). No differences in weight change were observed between reimbursable-lifestyle and usual-care participants. Glycated hemoglobin was reduced among all groups (P<.05) but was not different between groups.
Conclusions. Improvement in both weight and glycemia was attainable by lifestyle interventions designed for persons who had type 2 diabetes and lived in rural communities.
Individuals who live in rural medically underserved communities are an important target population for translational research. Such research evaluates interventions that are designed and implemented for various population settings on the basis of efficacy established during previous randomized controlled trials. In South Carolina, 75% of counties are designated as “medically underserved” by the US Public Health Service,1 and the prevalence of overweight, obesity, and physician-diagnosed diabetes is among the highest in the nation.2 Approximately 30% of the state population is Black, and among Black adults who have type 2 diabetes, glucose control as indicated by glycated hemoglobin (HbA1c) has been shown to be considerably higher than among White adults (10.5% vs 8.4%).3
Previous clinical trials have shown that among persons who have type 2 diabetes, moderate weight loss can improve glycemic control and lipoprotein profile and reduce blood pressure.4–6 Although definitive data on the benefits of long-term weight loss to reduce risk for clinical complications of diabetes are not yet available, the evidence-based nutrition recommendations of the American Diabetes Association emphasize the importance of weight management as a key element of medical nutrition therapy for diabetes. The American Diabetes Association also emphasizes the importance of glycemic control and management of cardiovascular risk factors, regardless of weight status.7
Among urban Black populations, including those who have diabetes, the success of culturally sensitive behavioral weight loss programs has been reported,5,8–12 although some studies reported that, compared with Whites, Blacks lost less weight10 and had an increased tendency to regain weight.11 To date, only a limited number of studies of behavioral programs for persons who have type 2 diabetes have been conducted in rural or semirural communities, where improvements in glycemic control and weight loss varied.13–16 The goal of our study was to develop, implement, and evaluate a 1-year primary care–based lifestyle intervention for weight management that was designed to improve metabolic control among individuals who have type 2 diabetes and live in rural medically underserved communities. The state-of-the art lifestyle intervention program developed for the National Institutes of Health–funded Diabetes Prevention Program (DPP),17 in combination with experiences gained from an 8-week pilot study13 and focus groups, was used to guide the planning and the implementation of our 12-month randomized controlled trial (“intensive-lifestyle” intervention). Because of the limited amount of time for health education that is normally reimbursed by health insurance, we evaluated a second weight management strategy. It was designed to deliver the most salient elements of the intensive-lifestyle intervention within the approximate number of hours that are usually reimbursed by Medicare for 12-month nutritional education among persons who have recently been diagnosed with diabetes (“reimbursable-lifesyle” intervention).
METHODS
Setting
Our 12-month randomized clinical trial—POWER (Pounds Off With Empowerment)—was a collaborative effort between the University of South Carolina and the South Carolina Primary Health Care Association, which represents the federally funded primary health care facilities in the state that provide care to medically underserved communities. Two primary health care centers in rural counties were identified on the basis of large numbers of patient visits for diabetes care.
Both health centers were provided with subcontracts that covered the costs of implementing the study on-site, including funds for hiring study staff and for providing transportation to study participants. Thus, the project was highly visible and was integrated into the daily operations of the health centers.
Participant Eligibility and Recruitment
A detailed description of the recruitment process has been published elsewhere.18 Briefly, potentially eligible participants were identified through diabetes registries at each health center. Inclusion criteria included being aged 45 years or older and having had a clinical diagnosis of diabetes. Potential participants also had to have a body mass index (BMI) of 25 kilogram/meter2 (kg/m2) or greater during the previous calendar year, which was confirmed by a brief medical record review. Exclusion criteria included any limitation that would prohibit full participation in the study (e.g., metastatic cancer, multiple or recent [within 6 months] myocardial infarction or stroke, dialysis for end-stage renal disease, severe psychiatric disease or dementia, or inability to walk). An introductory letter cosigned by the health center’s medical director and the study’s principal investigator was sent to eligible potential study participants; this was followed up with a recruitment and eligibility-screening phone call. Those who were both eligible and interested in participating were asked to complete 2 screening visits to further establish eligibility and interest in the study. Eligible participants were required to complete a 3-day “run-in” program designed to confirm both interest in participation and the minimal ability to self-monitor diet and physical activity. This was followed by a third visit to collect baseline study measurements and to assign participants randomly to 1 of 3 study interventions.
Of the 664 potential participants contacted by phone, 143 (21.5%) were randomized into the study, which was similar to the recruitment yield in university-based behavioral trials.19 Another 53 participants attended the initial screening visit on the basis of response to local publication efforts (posters, etc.), and 46 (87%) of these were randomized. Two of the 189 randomized participants were subsequently excluded because of severe congestive heart failure; thus, 187 participants were included in the trial.
Intervention
All participants were given a study goal of achieving and maintaining a 10% weight loss over 12 months on the basis of weight measured at randomization. Participants were randomized into 1 of 3 interventions: intensive-lifestyle intervention, reimbursable-lifestyle intervention, or usual care. The intensive-lifestyle intervention was derived from the lifestyle intervention of the DPP.17 The program focused on moderate weight loss with a goal of 25% of calories from dietary fat and a minimum of 150 minutes of physical activity per week that was similar in intensity to brisk walking. Energy intake goals were added as necessary. The DPP intervention was delivered primarily via individual counseling sessions, and key elements included frequent, sustained contact with a trained interventionist; a structured 16-session core curriculum composed of behavioral strategies for weight loss and physical activity, such as self-monitoring of diet and physical activity; and additional behavioral strategies to assist with achieving weight loss goals that were tailored to individual needs in a culturally appropriate manner.
For our study, we made modifications to the DPP intervention on the basis of an 8-week pilot study13 and findings from 2 focus groups. Modifications included regular use of group sessions, considerable simplification and reduction in the amount of written materials, encouragement of physical activity at low to moderate intensity for individuals who had very sedentary lifestyles, and inclusion of additional regionally/culturally appropriate examples, such as modifications of regularly consumed foods (e.g., substitute turkey neck bone for ham bone to cook greens, low-fat seasoning for grits) and suggestions for physical activity (e.g., identification of safe places to walk in the community, use of chair exercises for individuals who had lower-extremity pain). Self-monitoring tools for diet and physical activity were retained in a very simple format. Information regarding selected aspects of diabetes care (e.g., encouragement to monitor blood glucose at home) was incorporated, although the intervention retained a clear focus on diet and physical activity. Intensive-lifestyle participants met weekly with the study nutritionist for delivery of the first 4 months of the core curriculum (intensive), every other week for the next 2 months (transition), and once a month for the remaining 6 months (maintenance). Nutritionists delivered both the nutritional and the physical activity components of the intervention within these scheduled 1-hour sessions. Sessions were modeled after the NIH-funded Trial of Non-Pharmacologic Interventions in the Elderly20 and were conducted sequentially in a pattern of 3 group sessions and 1 individual session.
The reimbursable-lifestyle intervention was a condensed version of the intensive-lifestyle intervention, in which key elements of the intensive-lifestyle intervention were delivered in 4 1-hour sessions over the course of the 12-month study and included 3 group sessions and 1 individual session. The total time allotted for delivery of this intervention was determined by the approximate number of hours reimbursed annually by Medicare for diabetes education (diet and physical activity) in South Carolina for an individual who was recently diagnosed with diabetes.
Usual care was delivered in 1 individual session by a study nutritionist at the beginning of the 12-month period. Information related to diet and physical activity was derived from materials developed by the American Diabetes Association and the American Dietetic Association.
Outcome Measures
Standardized measurement visits occurred during the randomization visit and during visits scheduled at 3 months, 6 months, and 12 months after randomization. The primary outcome was weight loss; weight was measured to the nearest 0.23 kg (0.5 lb) with a Detecto balance beam scale that had a stadimeter (Cardinal Scale Manufacturing Company, Webb City, Mo). Height was measured to the nearest 0.1 centimeter while the participants stood erect, looking forward, against the stadimeter after taking a full inspiration. BMI was calculated as kg/m2.
Secondary outcomes included HbA1c (marker of glycemic control), lipid profile, and blood pressure. Laboratory assays of glycated hemoglobin, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and low-density lipoprotein (LDL) cholesterol were conducted at the Analytic Chemistry Laboratory of the South Carolina Department of Health and Environmental Control with the Boehringer Mannheim (Hitachi 911 Analyzer; Roche Diagnostics, Indianapolis, Ind). These assays were conducted during the randomization and 6-month visits only.
Blood pressure was measured 3 times with a standard mercury sphygmomanometer (appropriate cuff sizes—adult regular, large arm, or thigh—were used); the averages of the second and third readings for systolic and diastolic pressure were included in the statistical analysis.
Quality Control
Research staff participated in a 3-day centralized training and certification process before the start of data collection; recertification took place before the 6-month and 12-month measurement visits. Additionally, the 2 nutritionists received training for the intervention protocols. Following the training, weekly conference calls were held with the on-site nutritionists and the university-based study research nutritionist to ensure continual high-quality delivery of the intervention, with an emphasis on appropriate responses to group or individual needs.
Statistical Analysis
Sample size was determined by the sample size formula for group randomization proposed by Donner.21 The sample size (n = 50 per group) allowed detection at α = 0.05 of a 6% weight loss at the end of the study, with 80% power, for intensive-lifestyle intervention versus usual care and for reimbursable-lifestyle intervention versus usual care.
Intervention effects were first evaluated with paired t tests within each randomization group. Potential differences between intensive-lifestyle intervention and usual care and between reimbursable-lifestyle intervention and usual care were evaluated with linear regression modeling of weight change that accounted for clinical site and for change in use of prescribed diabetes medication (insulin, metformin, and other oral hypoglycemic agents) during the course of the 1-year intervention period. Models did not require adjustment for education, gender, or age because these did not differ significantly between randomization groups at baseline. Additionally, between-group differences were evaluated with random effects and repeated-measures regression models in SAS PROC MIXED (SAS Institute Inc, Cary, NC), with specification of random clinic effects within random subject effects that allowed for missing values from follow-up visits. These results were essentially the same as those from the simpler regression modeling; therefore, the regression model results are presented in the results section. Because of the skewed distribution of plasma triglyceride values, analyses of triglycerides were conducted with a natural log transformation. To eliminate the possibility of health center differences in intervention delivery, we tested the interaction between clinic and randomization group, and no such interactions were detected. Finally, analyses were repeated in the subgroup of study participants (“high attendees”) who attended at least 50% of the core curriculum and transitional sessions for intensive-lifestyle intervention (n = 36) or at least 2 of the 4 sessions for reimbursable-lifestyle intervention (n = 47).
RESULTS
Sample Characteristics
Of the 187 participants, 152 (81%) were retained through the 12-month end-of-study measurement visit. Baseline characteristics of these 152 individuals are shown in Table 1 ▶ according to randomization assignment. Overall, 80% of participants were women, 82% were Black, the average age was 60 years, and the average BMI was 36.7 kg/m2. Forty-eight percent of participants had less than a high school education or equivalent, and the average duration of diabetes was 11 years. None of the measured baseline characteristics differed significantly among intervention groups at baseline (all P > .05).
TABLE 1—
POWER Participant Characteristics, by Randomization Status, Mean (SD) or %
| Usual Care (n = 56) | Reimbursable-Lifestyle Intervention (n = 47) | Intensive-Lifestyle Intervention (n = 49) | |
| Gender, % | |||
| Women | 79 | 85 | 78 |
| Men | 21 | 15 | 22 |
| Education, % | |||
| < High school | 60.0 | 44.7 | 38.8 |
| Race, % | |||
| Black | 73.2 | 89.4 | 83.7 |
| Non-Hispanic White | 26.8 | 10.6 | 14.3 |
| Other | 0 | 0 | 2.0 |
| Mean age, y | 62.4 (9.5) | 58.9 (7.8) | 59.7 (8.6) |
| Body mass index, kg/m2 | 35.2 (7.5) | 37.5 (6.7) | 37.6 (6.5) |
| Weight, kg | 93.0 (20.3) | 100.0 (19.8) | 99.5 (17.1) |
| Diabetes duration, y | 12.7 (10.6) | 11.6 (10.0) | 8.4 (6.5) |
| Diabetes treatment, % | |||
| Insulin only | 32.1 | 25.5 | 26.5 |
| Oral hypoglycemic only | 57.1 | 53.2 | 46.9 |
| Combination insulin, orals | 8.9 | 17.0 | 24.5 |
| No diabetes medication | 1.8 | 4.3 | 2.04 |
| HbA1c, % | 9.6 (2.9) | 9.7 (3.1) | 10.2 (2.5) |
| Triglyceride, mg/dl | 134.3 (1.8) | 134.3 (1.8) | 125.2 (1.6) |
| Total cholesterol, mg/dl | 217.3 (57.9) | 198.9 (39.6) | 198.6 (47.4) |
| LDL cholesterol, mg/dl | 129.1 (48.6) | 115.1 (37.3) | 119.0 (41.0) |
| HDL cholesterol, mg/dl | 52.4 (16.2) | 51.7 (15.6) | 48.4 (10.4) |
| Systolic blood pressure, mm Hg | 143.2 (17.9) | 136.9 (15.9) | 139.7 (14.6) |
| Diastolic blood pressure, mm Hg | 81.0 (13.1) | 81.2 (8.3) | 83.0 (8.7) |
| Hypertension, % | 80.4 | 78.7 | 73.5 |
Note. POWER = Pounds Off With Empowerment; mm Hg = millimeter mercury; HBA1c = glycated hemoglobin; LDL = low-density lipoprotein; HDL = high-density lipoprotein.
Primary Outcome
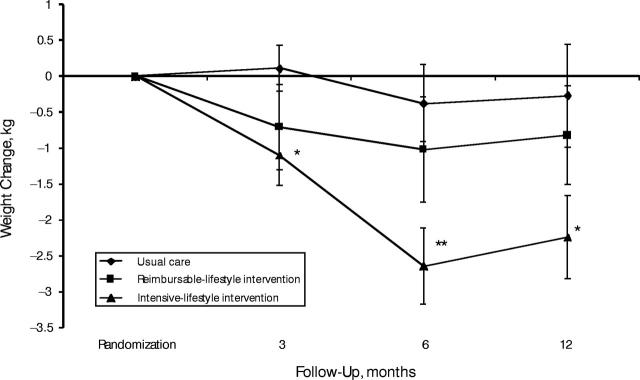
Figure 1 ▶ shows weight change at 3, 6, and 12 months postrandomization for usual care, intensive-lifestyle intervention, and reimbursable-lifestyle intervention. Paired t tests within each group at 6 months showed statistically significant weight loss between randomization and follow-up among intensive-lifestyle participants (paired t test P < .0001) but not among reimbursable-lifestyle or usual-care participants. The regression models showed that weight change at 6 months was significantly greater among intensive-lifestyle participants compared with usual-care participants (P < .01). Although some regain of lost weight was observed at 12 months, weight loss among intensive-lifestyle participants of 2.2 kg was significantly different from baseline (paired t test P < .003). Weight loss among men and women in the intensive-lifestyle group was comparable at 3 and 6 months (P > .05); however, at 12 months, mean weight loss among men was 4.7 kg compared with 1.5 kg among women (P = .02). Weight loss of 2.2 kg at 12 months among the intensive-lifestyle participants compared with 0.3 kg among the usual-care participants (P = .055) did not differ according to gender (P > .05). Weight loss did not differ significantly between reimbursable-lifestyle and usual-care participants at either 6 months or 12 months postrandomization.
FIGURE 1—
Weight change (kg) and standard errors at 3, 6, and 12 months of follow-up, by intervention group.
*Paired t test (P < .05); **paired t test (P < .001).
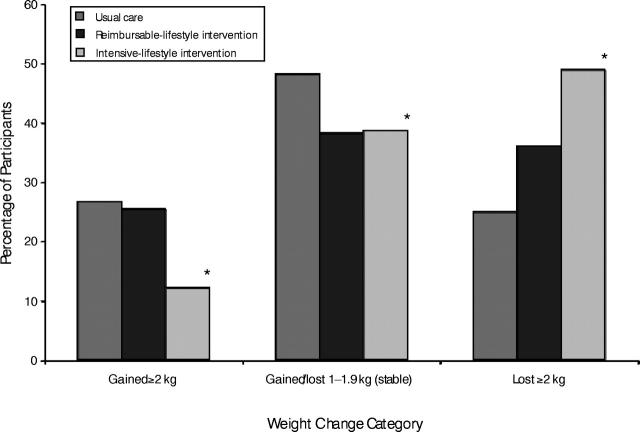
To more fully understand patterns of weight change in this trial, 3 categories were defined arbitrarily as “gained 2 or more kg,” “stable weight (within 2 kg),” and “lost 2 or more kg”; results are shown in Figure 2 ▶ for each group at 12-months postrandomization. Forty-nine percent of the intensive-lifestyle participants lost at least 2 kg compared with 25% of the usual-care participants; conversely, 12% of the intensive-lifestyle participants gained at least 2 kg compared with 27% of the usual-care participants (from χ2 statistic, P < .05). Reimbursable-lifestyle participants did not differ significantly from usual-care participants in these analyses.
FIGURE 2—
Distribution of Pounds Off With Empowerment participants at 12 months, by weight change category.
*Chi-square test for intensive-lifestyle intervention versus usual care (P < .05).
Secondary Outcomes
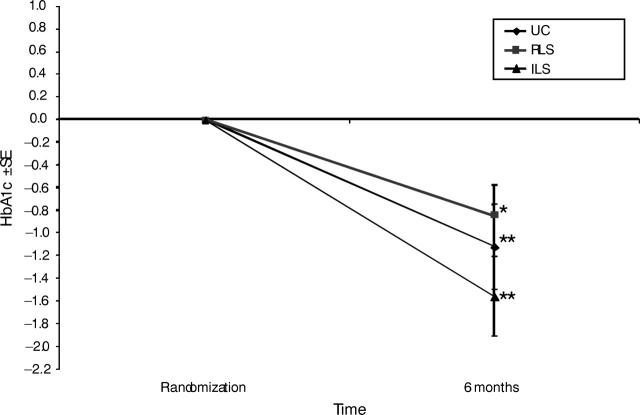
Figure 3 ▶ shows the unexpected decline of HbA1c by 1.1 points among usual-care participants (paired t test P < .01), by 1.6 points among intensive-lifestyle participants (paired t test P < .01), and by 0.8 points among reimbursable-lifestyle participants (paired t test P < .05). On the basis of regression analyses, differences in HbA1c change between the intensive-lifestyle and usual-care participants and between reimbursable-lifestyle and usual-care participants were not statistically significant. This was true before and after consideration of prescribed diabetes medications and weight change. Furthermore, neither diabetes medication regimen nor weight change was predictive of change in HbA1c.
FIGURE 3—
Change in glycated hemoglobin level ±SE at randomization and at 6 months.
*P < .05; **P < .001; paired t test.
Although most of the secondary metabolic outcomes we evaluated improved modestly among all 3 groups (Table 2 ▶), none of the 6-month differences in these measures were significantly different between intensive-lifestyle and usual-care participants or between reimbursable-lifestyle and usual-care participants.
TABLE 2—
Change in Secondary Outcomes Between Randomization and 6 Months
| Usual Care (n = 56) | Reimbursable-Lifestyle Intervention (n =47) | Intensive-Lifestyle Intervention (n =49) | ||||||
| Change | Paired t Test P | Change | Paired t Test P | Versus Usual CareaP | Change | Paired t Test P | Versus Usual CareaP | |
| Body mass index | −0.161 | NS | −0.296 | NS | NS | −0.974 | < .001 | < .01 |
| HbA1c | −1.12 | < .0001 | −0.843 | < .05 | NS | −1.56 | < .0001 | NS |
| Total cholesterol (mg/dl) | −6.32 | NS | −0.03 | NS | NS | −0.09 | NS | NS |
| LDL cholesterol (mg/dl) | −7.07 | NS | −1.44 | NS | NS | −3.37 | NS | NS |
| HDL cholesterol (mg/dl) | −1.12 | NS | 1.58 | NS | NS | 0.73 | NS | NS |
| Triglyceride (mg/dl)b | 0.91 | NS | 0.83 | .0067 | NS | 0.87 | NS | NS |
| Systolic blood pressure (mm Hg) | −9.52 | < .0001 | −4.26 | NS | NS | −3.31 | NS | NS |
| Diastolic blood pressure (mm Hg) | −2.65 | NS | −0.07 | NS | NS | −0.49 | NS | NS |
Note. NS = not significant; HbA1c = glycated hemoglobin; LDL = low-density lipoprotein; HDL = high-density lipoprotein.
a GLM = generalized linear models. Model adjusted for clinic and change in insulin, metformin, and other oral diabetes medication use.
bGeometric mean.
Analyses Restricted to “High Attenders”
Of the 49 individuals in the intensive-lifestyle intervention, 36 (73%) attended at least 50% of the core curriculum and transition sessions, and all 47 individuals in the reimbursable-lifestyle intervention attended at least 2 of the 4 sessions; all usual-care participants were included for comparison. Among the intensive-lifestyle “high attenders,” mean weight loss at 6 and 12 months was 3.1 kg (P < .001 compared with usual-care participants) and 2.7 kg (P < .05 compared with usual-care participants), respectively.
DISCUSSION
The POWER study was conducted as a translational research project designed to evaluate the effectiveness of a state-of-the-art lifestyle intervention for weight management and metabolic control of diabetes. It was applied to older adults, primarily Black, who had physician-diagnosed type 2 diabetes and lived in rural medically underserved communities in South Carolina. Modest weight loss occurred at 6 months and was statistically significantly greater among the intensive-lifestyle participants compared with the usual-care participants. At 12 months, a significantly greater proportion of intensive-lifestyle participants compared with usual-care participants had lost at least 2 kg. No statistically significant weight loss was observed among reimbursable-lifestyle participants. Men were less likely to regain lost weight, although this result should be reviewed with considerable caution because of the small number of men in the intensive-lifestyle group (n = 11). Differences between groups in lipid profile and blood pressure were not statistically significant; however, glycemic control measured by HbA1c improved among all 3 groups.
Weight Loss and Metabolic Status
Weight loss at 6 months among the intensive-lifestyle participants was similar to results from a 6-month lifestyle intervention among older Black adults who had diabetes and lived in an urban setting (average weight loss = 1.3 kg).5 More recently, the Steps to Soulful Living intervention (not limited to individuals who had diabetes) resulted in a 3.7-kg weight loss at 6 months.22 Among the DPP cohort, average weight loss at 6 months postrandomization among the intensive-lifestyle participants was about 7 kg, and at the end of the study, it was 5.6 kg.23 Differences in attained weight loss between POWER and DPP could have been the result of longstanding diabetes (and the attendant diabetes medications, many of which promote weight gain) among POWER participants.24 It also could have been the result of incomplete identification and response to a range of potential barriers to weight loss or weight loss maintenance (see next section, Potential Barriers to Health Action). That the reimbursable-lifestyle participants did not lose weight is of concern because (1) the professional contact time allotted was the maximum normally covered by Medicare in South Carolina for patients recently diagnosed diabetes, and (2) less time is reimbursed for patients who have established diabetes.
The potential relevance of the observed improvement in HbA1c among the intensive-lifestyle participants (1.6%) is evident from controlled clinical trials in which glycemic control was predictive of risk for microvascular complications, including diabetic retinopathy and nephropathy.25,26 Additionally, prospective epidemiologic analyses have estimated that each 1% reduction in HbA1c was associated with a 14% reduction in risk for myocardial infarction and a 21% risk reduction for death related to diabetes.27 Agurs-Collins et al.5 found that HbA1c was reduced from 11.0% to 9.9% among the weight loss intervention group compared with an increase of 10.0% to 11.5% among the control group (P < .05). Change in HbA1c was not statistically attributable to weight loss and was presumed to have improved as a result of improved diabetes self-management that may have occurred as an indirect benefit of the intervention.
Among POWER participants, neither prescribed medication regimen nor weight change was predictive of improved HbA1c. While the POWER trial was ongoing, a “diabetes collaborative” was introduced into both of the clinic sites as part of a federally funded effort to improve chronic disease management among community health center patients. Thus, improvement in day-to-day diabetes management, including consistency in taking prescribed diabetes medication and monitoring blood glucose at home, may have accounted for the improved glycemic control.28 Additionally, individuals who were willing to participate in this 1-year clinical trial may have been inherently motivated to make the needed day-to-day diabetes management changes. Certainly, improvement in HbA1c among usual-care participants was a welcome finding, but it likely precluded identification of a statistically significant difference between usual-care and intensive-lifestyle participants.
With regard to lipid profile and blood pressure, improvements were modest and, like the work of Agurs-Collins et al.,5 were generally nonsignificant. Similarly, a 1-year trial of a culturally sensitive weight management intervention among Black women (n = 529 from 16 churches) demonstrated modest but statistically significant weight loss differences (1.1 kg for intervention vs 0.83 kg for control) but no statistically significant difference between intervention and control for lipids or blood pressure.29 Previous studies that demonstrated statistically significant improvement in these parameters in the context of a weight loss intervention were conducted either among persons who did not have diabetes,30 who had a greater amount of weight loss,31 or both. Thus, given the modest weight loss among POWER participants and the relatively small sample size, it was not surprising that statistically significant improvements in lipid profile or blood pressure were not detected. Wing et al.4 demonstrated a dose–response effect of weight loss on these metabolic outcomes; therefore, it is reasonable to assume that modest weight loss may confer some degree of health benefit.
Potential Barriers to Health Action
Factors that influenced day-to-day diabetes management among 70 Black women were assessed via a series of 10 focus groups that included both urban- and rural-dwelling women in North Carolina.32 Key themes included spirituality, general life stress and multicaregiving duties, feelings of dietary deprivation, physical and emotional “tiredness,” “worry,” and fear of diabetes complications. These factors also were noted during the POWER trial and were addressed to the extent possible during the intervention. For example, stress related to responsibilities for the care of multiple family members was noted as a common reason for missed intervention sessions. In response, the POWER nutritionist contacted the participant and arranged for make-up sessions via in-person or phone interactions. Attention to highly specific participant needs, including transportation, played an important role in achieving the overall retention rate of 81% and an attendance rate of 73% among intensive-lifestyle participants who were present for at least 50% of the intensive and transitions sessions (i.e., at least 10 sessions).
CONCLUSIONS
Comprehensive intervention approaches that address documented barriers to sustained behavior change for weight management and diabetes self-management for individuals living in rural communities are needed. In particular, the important barrier of payment for professional contact time and for transportation to receive services must be addressed. With POWER, we have documented that modest weight loss and improved glycemic control is attainable by culturally appropriate state-of-the-art lifestyle interventions among Black and White individuals who have type 2 diabetes and live in rural medically underserved communities. We also have shown that the same intervention approach, when delivered in the amount of time normally reimbursed by health insurance (i.e., 4–5 hours over 12 months), was not effective in terms of weight loss; however, some improvement in glycemic control was noted. For persons who have diabetes and live in rural medically underserved communities, future translational research should focus on comprehensive approaches to diabetes management and education and should be designed to elicit greater improvement in metabolic status and health-related quality of life. Likely, focusing on medication compliance, monitoring blood glucose at home, and other aspects of diabetes self-care will be important. Additionally, future research should address the limited numbers of health care providers in rural communities. Use of telemedicine technology, including interactive videoconferencing and Internet support, should be considered. Future studies also should consider health insurance and associated health policy and cost to ensure a means of delivering services that are effective in rural settings.
Acknowledgments
Funding for this project was made possible by the Centers for Disease Control and Prevention in Atlanta, Ga (grant #U48/CCU409664–07).
We thank the leadership of the South Carolina Primary Health Care Association; the Family Health Centers in Orangeburg, SC; and Care South Carolina in Hartsville, SC. We also thank the NIH-funded Diabetes Prevention Program for making the lifestyle intervention materials—from which the POWER interventions were modeled—available to us.
Human Participant Protection The institutional review board of the University of South Carolina approved all study-related activities, and written informed consent was provided by all participants.
Contributors E. J. Mayer-Davis conceived the study, supervised all aspects of the study implementation, and led the writing. A. M. D’Antonio was the lead nutritionist, was instrumental in the delivery of the intervention, and contributed to the analysis and writing. S. M. Smith contributed to the data collection, analysis, and writing. G. Kirkner was the data manager and assisted with the study and analysis. S. Levin Martin was the project manager and directed the daily aspects of the study. D. Parra-Medina assisted with the study design and the recruitment and retention of participants. R. Schultz assisted with the study design and direction of the study.
Peer Reviewed
References
- 1.South Carolina Department of Health and Environmental Control, Diabetes Initiative of South Carolina. Burden of Diabetes in South Carolina. Columbia, SC: South Carolina Department of Health and Environmental Control; 1999.
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 3.Eberhardt MS, Lackland DT, Wheeler FC, et al. Is race related to glycemic control? An assessment of glycosylated hemoglobin in two South Carolina communities. J Clin Epidemiol. 1994;47:1181–1189. [DOI] [PubMed] [Google Scholar]
- 4.Wing RR, Koeske R, Epstein LH, et al. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147(10)1749–1753. [PubMed] [Google Scholar]
- 5.Agurs-Collins TD, Kumanyika SK, Ten Have TR, et al. A randomized controlled trial of weight reduction and exercise for diabetes management in older African American subjects. Diabetes Care. 1997;20:1503–1511. [DOI] [PubMed] [Google Scholar]
- 6.Kelley DE. Effects of weight loss on glucose homeostasis in NIDDM. Diabetes Reviews. 1995;3:366–377. [Google Scholar]
- 7.American Diabetes Association. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications [position statement]. Diabetes Care. 2002;25 (Suppl 1):S50–S60. [DOI] [PubMed] [Google Scholar]
- 8.Lasco RA, Curry RH, Dickson VJ, et al. Participation rates, weight loss, and blood pressure changes among obese women in a nutrition-exercise program. Public Health Rep. 1989;104:640–646. [PMC free article] [PubMed] [Google Scholar]
- 9.McNabb W, Quinn M, Kerver J, et al. The PATHWAYS church-based weight loss program for urban African American women at risk for diabetes. Diabetes Care. 1997;20:1518–1523. [DOI] [PubMed] [Google Scholar]
- 10.Kumanyika SK, Espeland MA, Bahnson JL, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10(2):96–106. [DOI] [PubMed] [Google Scholar]
- 11.Wing RR, Anglin K. Effectiveness of a behavioral weight control program for blacks and whites with NIDDM. Diabetes Care. 1996;19(5):409–413. [DOI] [PubMed] [Google Scholar]
- 12.McNabb WL, Quinn MT, Rosing L. Weight loss program for inner-city black women with non-insulin-dependent diabetes mellitus: PATHWAYS. J Am Diet Assoc. 1993;93:75–77. [DOI] [PubMed] [Google Scholar]
- 13.Mayer-Davis EJ, D’Antonio A, Martin M, et al. Pilot study of strategies for effective weight management in type 2 diabetes: Pounds Off with Empowerment (POWER). Fam Community Health. 2001;24(2): 27–35. [DOI] [PubMed] [Google Scholar]
- 14.Ridgeway NA, Harvill DR, Harvill LM, et al. Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic. South Med J. 1999;92(7): 667–672. [DOI] [PubMed] [Google Scholar]
- 15.Redhead J, Hussain A, Gedling P, et al. The effectiveness of a primary-care-based diabetes education service. Diabet Med. 1993;10(7):672–675. [DOI] [PubMed] [Google Scholar]
- 16.Schrock LE. Review of cost efficiency and efficacy of delivering a diabetes education program in a southwest rural healthcare facility. Diabetes Educ. 1998;24(4):485–492. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program (DPP) Research Group. Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. Also available at: http://www.bsc.gwu.edu/dpp/index.htmlvdoc. Accessed July 20, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parra-Medina D, D’Antonio A, Smith SM, et al. Successful recruitment and retention strategies for a randomized weight management trial for persons with diabetes living in rural, medically under-served counties of South Carolina: The POWER Study. J Am Diet Assoc. 2004;104(1):70–75. [DOI] [PubMed] [Google Scholar]
- 19.Appel LJ, Espeland MA, Easter L, et al. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med. 2001;161(5):685–693. [DOI] [PubMed] [Google Scholar]
- 20.Donner A. Approaches to sample size estimation in the design of clinical trials—a review. Stat Med. 1984; 3:199–214. [DOI] [PubMed] [Google Scholar]
- 21.Karanja N, Stevens VJ, Hollis JF, et al. Steps to soulful living (steps): a weight loss program for African-American women. Ethn Dis. 2002;12(3):363–371. [PubMed] [Google Scholar]
- 22.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes. JAMA. 2002;287:360–372. [DOI] [PubMed] [Google Scholar]
- 24.Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study Group. Effect of intensive blood-gluccose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 26.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258): 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002; 25(6):1015–1021. [DOI] [PubMed] [Google Scholar]
- 28.Yanek LR, Becker DM, Moy TF, et al. Project Joy: faith-based cardiovascular health promotion for African American women. Public Health Rep. 2001;116 (Suppl 1): 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray G, Bouchard C, James PT, eds. Handbook of Obesity. New York, NY: Marcel Dekker; 1997:855–873.
- 30.Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153(7): 849–858. [PubMed] [Google Scholar]
- 31.Samuel-Hodge CD, Headen SW, Skelly AH, et al. Influences on day-to-day self-management of type 2 diabetes among African-American women: spirituality, the multi-caregiver role, and other social context factors. Diabetes Care. 2000;23(7):928–933. [DOI] [PubMed] [Google Scholar]
- 32.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal Adiposity and Coronary Heart Disease in Women. JAMA. 1998;280:1843–1848. [DOI] [PubMed] [Google Scholar]