Abstract
I reviewed ethical and scientific aspects of 6 human pesticide-dosing studies submitted to the Environmental Protection Agency (EPA) for consideration during the pesticide reregistration process. All had serious ethical or scientific deficiencies—or both—including unacceptable informed consent procedures, unmanaged financial conflicts of interest, inadequate statistical power, inappropriate test methods and endpoints, and distorted results.
Given today’s knowledge of the effects of pesticides, there is no assurance that any such study can be completely free of short-term risks, long-term risks, or both. Therefore, there is no basis for allowing pesticide studies to continue or for using them during the pesticide reregistration process. An EPA committee that is free from political and financial conflicts of interest should review this practice.
PESTICIDES ARE DEFINED BY the Environmental Protection Agency (EPA) as “substances used to prevent, destroy, repel or mitigate any pest . . .”1 Their widespread use has both improved crop yields and helped control insects and other pests, which has subsequently led to improvements in health. However, they are inherently toxic and have been linked to a broad range of human health problems, including cancer, damage to the central and peripheral nervous system, and interference with neurodevelopment and the endocrine system.
The federal government regulates pesticide use with 2 major pieces of legislation: the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Federal Food, Drug, and Cosmetic Act (FFDCA). Because of concerns that children may be particularly vulnerable to the effects of pesticides, the US Congress requested the National Academy of Sciences (NAS) to study policy and scientific issues related to pesticides in the diets of infants and children.2 The result led to unanimous Congressional action that amended FIFRA with the Food Quality Protection Act of 1996 (FQPA). Among its provisions, FQPA added a children’s safety factor to existing pesticide tolerances, where tolerance is defined as the maximum concentration of a pesticide residue permitted in food, and a requirement that all pesticides be reregistered.
This move led to experiments that may affect tolerances set during reregistration, including controversial experiments in which human volunteers were given pesticides to determine a “no observable effect level” (NOEL) or a “no observable adverse effect level” (NOAEL). Before FQPA was enacted, tolerances were set by dividing the NOEL by an uncertainty factor that had 2 elements: an inter-species factor of 10 to account for the possibility that humans are more sensitive than the test animal and an intraspecies uncertainty factor of 10 to account for intraspecies variations. With FQPA, the provision of a children’s safety factor added another factor of 10 to the uncertainty factor, which yields a total uncertainty factor of 1000 for children and 100 for the general population. By establishing a NOEL for humans, the inter-species uncertainty factor would become unnecessary and would change the total uncertainty factor to 100 for children and 10 for the general population, with a concomitant effect on tolerances.
The controversies triggered by human testing have resulted in at least 3 major reports. However, close reading of these reports strongly suggests that the authors did not have access to the detailed protocols and reports that described the human studies submitted to the EPA by the pesticide manufacturers and the contract research organizations they employed to conduct the tests. The most recent of these was commissioned by the EPA and was conducted by a committee appointed by the National Research Council of the NAS.3 The committee concluded that intentional-dosing studies among humans can be conducted and can be used for EPA regulatory purposes if stringent conditions are met. Although it could not envision a circumstance in which the deliberate dosing of children would be permissible, the committee failed to recommend prohibiting this practice. Two other groups have examined the ethical aspects of these experiments. In 2000, an EPA subcommittee reported it “in general would not support human experimentation primarily to determine a NOAEL.”4 The terms in general and primarily were seen as loopholes that would permit testing. A minority report stated that the final report was a “distorted and diluted” version of their deliberations that “minimiz[ed] the risks to humans from intentional experimental dosing, and de-emphasiz[ed] the salient issue: that no limited human study will provide information about safe levels of intake of pesticides by humans, especially children.”4(pC1) In another report, Oleskey et al. wrote an emphatic rejection: “NOEL studies inherently violate various ethical guidelines.”5 They recommended that “no results obtained from any NOEL studies can be considered in the formulation of exposure guidelines by EPA.”5
To better understand issues related to human testing, I obtained reports about testing from the EPA under the Freedom of Information Act; 6 reports from 1992 to 1999 were provided by the agency.6–11 A synopsis is shown in Tables 1 ▶ and 2 ▶. I evaluated the reports to determine compliance with the ethical principles contained in whichever version of the Declaration of Helsinki12 was in effect at the time of each study and to evaluate the adequacy of the experimental design and the interpretation of the results. Because all the studies claimed use of the Declaration of Helsinki as the ethical standard, and because none of these studies claimed use of the Common Rule, the latter was not used as an evaluation criterion.
TABLE 1—
Design, Statistical Methods, and Ethical Standards
| Pesticide, Class, Location of Study | Design | Doses Administered (all orally), Number Dosed, Gender | Sample Interval | Analytical Method | Ethical Standard Claimed | Written Informed Consent Available |
| Aldicarb, carbamate, Inveresk Clinical Research, Edinburgh, Scotland | Double-blind, placebo- controlled, single dose | Placebo, 16M, 6F 0.01 mg/kg, 8M 0.025 mg/kg, 8M, 4F 0.05 mg/kg, 8M, 4F 0.075 mg/kg, 4M | (Hours) −16, −3, 0 predose | Mean of 3 predose vs postdose | Declaration of Helsinki, 1989 | Yes |
| 1, 2, 4, 6, 8, 21 postdose | ANOVA (treatment, time, treatment-time interaction) | |||||
| Dichlorvos, organophosphate, Medval Ltd, University of Manchester, UK | Open label, single dose | 70 mg, 6M | (Days) 0, 1, 5 or 6, 7, 14 | Within group: pre vs each time, paired t test within subject: permutation | Declaration of Helsinki, 1989 | No, but protocol states “volunteers completed a consent form.”8 |
| Dichlorvos organophosphate, Medval Ltd, University of Manchester, UK | Single-blind, placebo- controlled, randomized | Placebo, 3M OR 7 mg daily for 21 days, 6M | (Days) 0, 1, 2, 4, 7, 9, 11, 14, 16, 18, 25, 28, 29, or 30 | Group means at each time by repeated measures ANOVA; pre vs post at each time by paired t test; within-subject, permutation | Declaration of Helsinki, 1989 | No, but protocol states “volunteers completed a consent form.”7 |
| Dichlorvos, organophosphate, Medval Ltd, University of Manchester, UK | 2-phase, open label, placebo- controlled | (Days) pre-dose 3 measures in 7 days prior to dose 1 | Between-group, pre vs post dose group means by paired t test within-subject, permutation test | Declaration of Helsinki, 1989 | No, but protocol states “volunteers completed a consent form.”6 | |
| Phase 1: day 1, 35 mg 4M day 8, placebo, 4M, day 14, 35 mg, 6M | Phase 1: 1, 3, 5, 7 or 8 days after dose or placebo | |||||
| Phase 2: 2 week hiatus followed by 21 mg daily up to 15 days, 6M | Phase 2: 3, 5, 8, 10, 12, 15, 17, 19,22, 24, 26, 29, 33, 40, 47, 54 days after initial dose | |||||
| Azinphos methyl organophosphate, Inveresk Research, Tranent, Scotland | Randomized, double-blind, placebo- controlled, repeat dose | Placebo, 4 M 0.25 mg/kg daily for 28 days, 8M | Pre-dose, 8 determinations over 2 weeks; before dose on each day and 4 hours post dose on days 1, 2, 3, 4, 5, 7, 10, 14, 17, 21, 28 | Repeated measures ANOVA (treatment, time, treatment time interaction) pesticide vs placebo, pairwise at each time (error variance from ANOVA) | Declaration of Helsinki, 1996 | Yes |
| Declaration of Helsinki, 1996 | ||||||
| Chlorpyrifos organophosphate, MDS Harris, Lincoln, Nebraska | 2-phase, single dose randomized, double-blind, placebo- controlled | Phase 1: placebo, 6M, 6F 0.5 mg/kg, 6M, 6F 1.0 mg/kg, 6M, 6F | (Hours) Phase 1 and 2: −10, 0, pretreatment and 2, 4, 8, 12, 24, 36, 48, 72, 96, 120, 144, 168 posttreatment | Truncated at 96 h for phase 1 and 48 h for phase 2 and analyzed separately by univariate repeated measures ANOVA and fixed effects modeling | Declaration of Helsinki, 1996, 21 CFR parts 50, 56, 321 [sic] | Yes |
| Phase 2: placebo, 6M, 6F 2.0 mg/kg, 6M, 6F |
Note. ANOVA = analysis of variance; M = males; F = females.
In phase 2 of the 2-phase dichlorvos study, boldface numbers in sample interval column denote days on which subjects were dosed.
TABLE 2—
Human Testing of Pesticides, Study Objectives, and Additional Design Considerations
| Pesticide | Study Objectives | Variables Measured (including data not specified in study objectives) | Statistical Test Applied to Variable | Findings, Conclusion(s) |
| Aldicarb | Determine general tolerance to various doses | Vital signs, pulmonary function, salivation, pupil size, ECG, clinical signs (nausea, vomiting, sweating, diarrhea, abdominal cramps, slurred speech) | ANCOVA for bolded items at left, none specified for others | 45% reduction in AChE at highest dose, “clinical no effect level . . . is . . . 0.05 mg/kg”11 |
| Determine effect on plasma and RBC AChE | Plasma and RBC AChE | ANCOVA | ||
| Dichlorvos, single dose | Assess effect on RBC AChE | RBC AChE | Paired t test pre vs post dose means | Significant effect at day 5,6, and 14 in group analysis. Five of 6 had significant effect on individual analysis |
| Symptoms reported by volunteers | None specified | NOEL established at 70 mg (approximately 1 mg/kg). | ||
| Dichlorvos, 7 mg daily for 21 days | Assess effect on RBC AChE | RBC AChE | Repeated measures ANOVA | Significant reduction on all dates after 10 days of dosing. |
| Symptoms reported by volunteers | None specified | “Results from this study can unequivocally establish a NOEL at 7 mg dichlorvos per day . . . following repeat administration for 21 days.”6 | ||
| Dichlorvos, 2 phase | Assess effect on RBC AChE after single and multiple doses | RBC AChE | Paired t test pre vs post dose means | 2 of 6 subjects withdrawn because of AChE depressions, 2 more met withdrawal criteria on last day. Significant reductions in AChE from day 5–33 after 15 days of dosing. |
| Symptom form completed by volunteer | None specified | “NOEL established at or close to 21 mg . . . approximately 0.3 mg/kg . . .”5 | ||
| Azinphos methyl | Establish recommended daily intake for chronic dietary exposure | None | None specified | |
| Determine NOEL for plasma and RBC AChE | Plasma and RBC AChE | Repeated measures ANOVA, paired t placebo vs agent at each time | Significant increase in inhibition (paired t) at 4 of 24 time points for plasma AChE and 2 of 24 time points for RBC AchE.a | |
| Conduct risk assessment and obtain information for biological monitoring | Adverse events coded using World Health Organization terminology | None specified | Repeat doses of 0.25 mg/kg were safe | |
| Chlorpyrifos | Determine NOEL for RBC AChE | RBC AChE | ANOVA | NOEL for signs and symptoms was 2.0 mg/kg body weight |
| Signs and symptoms coded using COSTART Adverse Event Dictionary, 5th Edition | None specified |
Note. ANCOVA = analysis of covariance; ANOVA = analysis of variance; NOEL = no observable effect level; RBC = red blood cell; AChE = acetylcholinesterase; ECG = electrocardiogram.
aFor each of the 22 time points, the azinphos methyl group had greater inhibition of plasma AChE than placebo; data not analyzed or commented on by investigators. For RBC AChE, 6 of the 12 values were higher.
RESULTS
Adherence to the Declaration(s) of Helsinki
Study purpose.
Ethical studies begin with a defined statement of purpose. The declaration states that “research . . . must . . . improve diagnostic, therapeutic and prophylactic procedures and the understanding of . . . disease” and “the interests of science and society should never take precedence over the . . . well-being of the subject.”12
Two of the investigations I reviewed were designed to determine the NOEL,9,10 and 3 of them claim to have determined a NOEL.6–8 The sixth investigation was designed to establish “safety and tolerability” of the pesticide tested.11 Because none of these reports have appeared in the scientific literature, they were apparently not intended to advance generalizable scientific knowledge (a MEDLINE search on April 16, 2004, of the name of first author on each of the 6 reports retrieved 35 references; however, none were related to the reports submitted to the EPA).
Protocol review and approval.
The declaration states that protocols must be approved by committees that are “independent of the investigator, the sponsor or any other kind of undue influence.”12 All 6 of the protocols were reviewed and were approved by an ethics committee, usually after minor revisions. However, all of the ethics committees were part of the contract research organization that was paid by the sponsor to perform the study. Potential conflicts of interest were not addressed.
The informed consent process.
Three protocols included an informed consent document. Of these, the aldicarb consent was the least satisfactory. For example, it refers to aldicarb only as “the compound under test.”11 Although the consent states that “I have been given a full explanation of . . . any reasonably foreseeable untoward effects,”11 the nature of the study, the participant’s role, and the risks were not listed.
The azinphos methyl study also refers to the pesticide as “the compound under test.” Risks and requirements are listed in an “information document given to me” that states “large increases of acetylcholine in the nervous system can cause increased salivation, sweating, reduced blood pressure, nausea, vomiting and stomach cramps.” It fails to mention weakness, respiratory failure, and death. Although the subject was “free to withdraw from the study at any time without needing to justify my decision,” it goes on to say that if a participant withdraws for nonmedical reasons, “the payment to be made [£1500], if any, shall be at the discretion of the supervising doctor” (emphasis added).9 (Appendix A) The declaration prohibits coercion.
The chlorpyrifos consent is marred by the first sentences in the side effects statement: “Cholinesterase inhibitors are a widely study [sic] class of chemicals. Low doses of these agents have been shown to improve performance on numerous tests of mental function.”9 Several drugs that treat Alzheimer disease are acetylcholinesterase (AChE) inhibitors. However, none are organophosphates. It is misleading to imply participation might improve intellectual function.
Evaluation of risk–benefit considerations.
According to the declaration, human studies “should be based on adequately performed laboratory and animal experimentation and on . . . knowledge of the scientific literature.”12 Investigators must supply this information to enable the institutional review board to make an informed decision. While all 6 studies contained detailed information about the mechanics of their performance, information that justifies the studies and that allows an institutional review board to evaluate the risk–benefit considerations varies substantially. The 3 dichlorvos reports do not include this information. The aldicarb study mentioned exposures that have “given rise to alleged intoxication,” several animal studies, and a previous human study. It concluded with the statement, “In the 17 years of registered use of TEMIK [aldicarb], 193 cases of alleged overexposure have been reported. All . . . resulted from misuse of the product . . .”11 This ignored a report of more than 1000 cases that resulted in 17 hospitalizations and stillbirths by 2 of the 47 pregnant women.13 The azinphos methyl study mentions toxicological studies in animals and several unpublished human studies. The chlorpyrifos study mentioned animal studies without summarizing the data, and 1 published and 1 unpublished human study. There is no indication that institutional review boards requested additional information before approving the studies.
Scientific Considerations
Experimental design and accurate reporting of results.
Because it is unethical to perform studies of poor scientific quality, this area of inquiry bridges the closely related elements of ethics and science. Information about methods, variables measured, statistical tests, and conclusions is shown in Table 2 ▶.
A power analysis to define the proper size of study group(s) is an essential part of the design. If too many participants are enrolled, the excess will be subjected to unnecessary risk. If too few are enrolled, the investigator risks erroneous acceptance of the null hypothesis. Underpowered studies are inconclusive, and all participants in an underpowered study will have been exposed to risk unnecessarily. All of these studies were underpowered.4
All 6 investigations studied young healthy adults, the population least likely to be affected by pesticides. None performed preenrollment pesticide exposure studies, and only 1, the chlorpyrifos study, measured paroxonase levels. Low paroxonase levels increase the sensitivity to some organophosphates, including chlorpyrifos.14 There is no evidence that paroxonase activity was a selection criterion or affected the analysis or interpretation of the results. These data cannot be generalized to children, the focus of FQPA, and probably cannot be generalized to the general population.
The declaration states, “The physician is obliged to preserve the accuracy of the results.”12 Although this generally refers to publication in biomedical literature,15 it also should apply to these reports. In each study, the investigators focused their statistical evaluations on red cell and plasma AChE activity. They then treated this as a biological marker of exposure devoid of clinical relevance. After detecting significant AChE effects, they concluded that they had established a NOEL. However, as shown in Table 2 ▶, few protocols used rigorous methods to collect and evaluate any data other than AChE activity. Thus, it is not clear how they justified the NOEL conclusion.
The aldicarb study has the most appropriate statement: “Due to the multiplicity and investigational nature of these analyses, the observed p-values should be used for descriptive purposes rather than formal hypothesis testing.”11 This minimizes the investigators’ AChE findings. However, they then proceeded to claim that “the NOEL for clinical signs is . . . 0.05 mg/kg”11 because they observed “definite” evidence of toxicity in 1 subject who was given 0.06 mg/kg. This assertion was made in the absence of a prospective strategy for collecting and applying statistical analyses to relevant data.
The most egregious distortion was found in the 2-phase 21-mg/day dichlorvos study in which AChE activity reached the withdrawal criterion in 2 participants on day 12 and 2 more on day 15. Yet, the investigators concluded that a NOEL level was established “at or close to 21 mg dichlorvos following repeated daily oral administration.”6 They do not justify reaching this conclusion.
Adherence to other laws and regulations.
None of the 6 protocols provided evidence of compliance with regulations that govern the administration of chemicals or drugs to human participants. Although the chlorpyrifos study, which was conducted in Nebraska, claims compliance with “21 CFR [Code of Federal Regulations] parts 50, 56 and 321 [sic],”9 it fails to reference an investigative new drug application (an application to the Food and Drug Administration requesting permission to administer a drug, chemical, or test compound to a research participant) made to the Food and Drug Administration (FDA). The chlorpyrifos used in this study and the insecticides used in the other studies appear to be shelf chemicals—chemicals that can be purchased “off the shelf” from a commercial supplier versus chemicals that must be synthesized de novo by a small custom synthesis process. Five of the studies were conducted outside the United States and failed to reference adherence to the regulations of federal FDA-equivalent agencies.
DISCUSSION
I have provided evidence for departures from the ethical standards in effect at the time studies were conducted. The studies reviewed also had inadequate designs, and there were biases in the interpretation of data. The EPA must decide whether or how to use these submissions. This article is designed to inform the discussion and the debate that will occur during that process and to assist any associated rulemaking.
Voluntary informed consent is the core of contemporary ethical guidance, including the Nuremberg Code, the Belmont Report, the Common Rule, and the Declaration of Helsinki. This means that there should be an explanation of the aims, methods, sources of funding, possible conflicts of interest, and anticipated risks and benefits of a study. The study itself must be scientifically acceptable.
Because all 6 studies have been submitted to the EPA and have not been published in any form that is retrievable by MEDLINE, there is little doubt about their real purpose—the production of data that will be used to affect the pesticide regulatory process. This was not revealed to the study participants. Unacceptable deficiencies in the consent documents (e.g., failure to identify the test compound as a pesticide), inclusion of statements that are potentially coercive, misleading statements about the effects, and a failure to identify the source of funding raise serious doubts as to whether the participant’s signatures were a reasonable reflection of informed voluntary consent.
All the studies included evidence of an unmanaged conflict of interest. The institutional review boards and the investigators were all part of the same organization. Conflicts of interest have been central to the critiques of several recent studies that involved human participants. The most notable of these focused on the death of Jesse Gelsinger during a phase-I clinical trial of a gene therapy technique.15 Explicit statements about human subject protection and conflicts of interest are now required by most medical journals,16 and a recent NAS publication urged the development of “distinct mechanisms for the initial focused reviews of scientific and financial conflicts of interest . . . that should precede and inform”15 reviews by institutional review boards.15 These policies should be applied to studies submitted to the EPA.
Ascertaining and weighing risk against potential benefit is perhaps the most difficult yet most important task for institutional review boards. The Declaration of Helsinki states that human studies “should be based on adequately performed laboratory and animal experimentation and on a thorough knowledge of the scientific literature.”12 The reports I reviewed are remarkably devoid of this information. An April 23, 2004, MEDLINE search of the term “pesticide, organophosphates” (restricted to the subtopics poisoning, adverse effects, and toxicity in humans and written in English) yielded 208 articles that were published between 1987 and 1996 and an additional 253 that were published between 1997 and 2004. Although the institutional review boards could have asked for additional data from the investigators, there is no indication that they did so before approving the studies. This may be a manifestation of the conflict of interest or a lack of expertise and experience on the part of the members of the institutional review boards.
Safety monitoring consisted largely of serial measurements of vital signs and standard blood chemistries and the use of adverse-event recording forms. Each of the studies appears to have been performed over a short period of time—the dates on the 3 dichlorvos reports span only 3 weeks—which suggests that the studies may have been designed, approved, and executed as a group. Although inclusion and exclusion criteria were specified, and there were withdrawal criteria for some, there was no evidence of active oversight by the institutional review boards. In the case of the 2-phase dichlorvos study in which AChE activity reached the withdrawal criterion in 4 of the 6 dosed participants, there is no evidence that the investigators or the institutional review board considered halting the study. Post-study monitoring of participants in all 6 studies was limited to the time during which AChE activity was expected to return to normal.
It is important to consider design omissions in addition to the deficiencies I have discussed. In studies of chemicals that act on the central nervous system, it is essential to employ tests that are highly sensitive to small differences in brain function. Neuropsychological and electrophysiological tests do just that, and they have been used widely in studies of mercury exposure and other toxicants17 and to detect minimal brain dysfunction among patients who have cirrhosis of the liver.18 In another relevant study, neuropsychological tests were combined with positron emission tomographic scans of patients who appeared to be clinically normal.19 The researchers found unsuspected deficits in performance on the neuropsychological tests that were correlated with focal reductions in cerebral glucose metabolism. None of the 6 studies I reviewed used tests of this nature. Thus, the conclusions that there were no biologically significant effects are unsupported by rigorous preplanned testing of the type necessary to detect small effects. This may be the most important, and the least appreciated, defect in the design of these studies.
A recent NAS report that concluded that human pesticide testing was permissible contained important qualifiers—the studies should be approached with the “utmost caution and care”3 and were permissible only if there is a “reasonable certainty that participants will experience no adverse effects.”3 In the studies I reviewed, there were no plans for long-term monitoring or for any consideration that there might be delayed or long-term effects. This possibility must be considered because of current knowledge about pesticides.
The discovery that parkinsonism can be caused by the paraquat lookalike MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) led to studies that identified pesticide exposure as a risk factor for the development of parkinsonism.20 These epidemiological studies have been supplemented by the development of animal models of parkinsonism. Animals that were fed rotenone21 or a combination of paraquat and maneb22 exhibited behavioral signs similar to Parkinson’s disease among humans and had neuropathological findings that are typical of the disorder. Two findings are particularly important. First, the combination of maneb and paraquat yielded an effect that was greater than the added effects of separate administration.22 This finding is important to consider in the context of data from the Centers for Disease Control and Prevention that document exposure to multiple pesticides with higher burdens among children.23 Second, early-life exposure to these pesticides sensitized the animals to the effects of a second exposure during adulthood.24 Occupational exposure to organophosphates may cause the development of a peripheral neuropathy or impairments of mood and visual-spatial function.25 None of the 6 studies considered the development of parkinsonism or other neurological conditions as a risk.
Other recent data show that chlorpyrifos dosing at levels that do not alter brain AChE activity affect the calcium-cyclic adenosine monophosphate response element binding protein, a protein important to brain development.26 This may account for reduced birthweights and head circumferences among children who were born to pesticide-exposed mothers.27,28
Neurotoxicological data make it impossible to assure a potential pesticide-dosing study participant that there is no risk for the development of neurological injury.
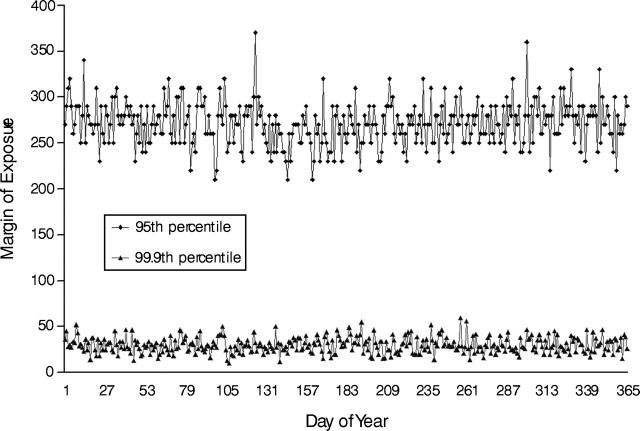
The motivation behind industry-sponsored human-dosing studies is clear: the industries want to abolish, or at least reduce, the interspecies uncertainty factor and thereby allow the EPA to accept higher tolerances.29 The motivation to press for higher tolerances is more apparent after a review of some of the findings from a recent EPA organophosphate risk assessment.30 In the assessment, the EPA estimated composite margins of exposure for all of the organophosphates among children and adults of various ages who live in different parts of the country. The margins of exposure were calculated by dividing a measure of a minimal effect (technically, the point of departure) by the exposure to all organophosphates, which were summed for all routes. Somewhat paradoxically, as the exposure decreases and the margin of exposure increases, the apparent risk decreases. Thus, a high margin of exposure is an indication of low apparent risk, and a low margin of exposure is an indication of higher risk. In the absence of NOEL data for humans, the point of departure used for the margin-of-exposure calculations was based on animal experimentation and was defined as the amount of various organophosphates required to inhibit brain AChE activity by 10%, a level that may affect brain development. In Figure 1 ▶, daily margins of exposure are shown for the most highly exposed children aged 1 to 2 years in the northeast–north-central region of the United States. Margin-of-exposure data are corrected for the tentatively assigned FQPA children’s uncertainty factors, which ranged from 1 to 3. When the current uncertainty factors are used, the target margin of exposure is 100. As shown in Figure 1 ▶, the most highly exposed children—those at the 99.9th percentile—had margins of exposure below this target. Abolition of the interspecies uncertainty factor would lower the target margin of exposure to 10, and the most highly exposed children would then have margins of exposure higher than the target. However, this apparent risk reduction would not change the actual risk or exposure. Dichlorvos accounts for almost all the total organophosphate exposure among the highly exposed group.30 It is undoubtedly no coincidence that dichlorvos was the test substance in 3 of the tests I reviewed. It also is worth noting that if the FQPA children’s safety factor were raised from 3 to 10, and if other uncertainty factors were preserved, then 5% of all children aged 1 to 2 years would have margins of exposure lower than the target, which would be raised to just over 300.
FIGURE 1—
Daily margins of exposure at the 99.9th and 95th percentiles of exposure to organophosphates among children aged 1 to 2 years who lived in the northeast–north-central region of the United States.29,34
CONCLUSIONS
Two of the 3 committees that evaluated the ethics of human pesticide-dosing studies have concluded that human pesticide-dosing studies pose serious ethical concerns, and the third committee set conditions that would apparently protect study participants. However, my empirical examination of 6 studies submitted to the EPA shows that these protections were not achieved and are probably not achievable. Hence, these tests should not be conducted, and the EPA should rely on other data during the pesticide reregistration process.
Human-dosing studies have failed to meet widely accepted ethical standards for the conduct of research. The studies I reviewed are all flawed by ethical lapses and poor design, particularly with regard to low statistical power, inadequate test methods, and endpoints that fail to detect small effects on the central nervous system. Therefore, the EPA should not rely on these data during the pesticide reregistration process. In particular, these data should not form the basis for the abolition or the alteration of the interspecies uncertainty factor, a decision that would benefit the pesticide industry financially. To accept these studies would open the door to other poorly conducted studies and would violate the principle that those who engage in unethical activity should not reap rewards.31
The EPA should promulgate rules that allow it to convene an in-house ethics review panel that is free of financial conflicts of interest and political influence32,33,34 and that is charged with the task of deciding the fate of these and similar studies.
Acknowledgments
I am grateful to James P. Donnelly of the Roswell Park Cancer Institute in Buffalo, NY, for his advice about the statistical power of the studies I reviewed.
Human Participant Protection No human participants were involved in this study.
Peer Reviewed
References
- 1.US Environmental Protection Agency. Terms of environment Web site. Available at: http://www.epa.gov/OCEPAterms. Accessed October 6, 2004.
- 2.Committee on Pesticide Residues in the Diets of Infants and Children. Pesticides in the Diets of Infants and Children. Washington, DC: National Academy Press; 1993.
- 3.Committee on the Use of Third Party Toxicity Research with Human Research Participants, National Research Council, National Academy of Sciences. Intentional Human Dosing Studies for EPA Regulatory Purposes: Scientific and Ethical Issues. Washington, DC: National Academy Press; 2004. [PubMed]
- 4.Scientific Advisory Board and FIFRA Scientific Advisory Panel. Comments on the use of data from the testing of human subjects. EPA-SAB-EC-00-017. Washington, DC: US Environmental Protection Agency; 2000.
- 5.Oleskey C, Fleischman A, Gold-man LR, et al. Pesticide testing in humans: ethics and public policy. Environ Health Perspect. 2004;112:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gledhill AJ. Dichlorvos: a study to investigate erythrocyte cholinesterase inhibition following oral administration to healthy male volunteers. Alderley Park MacClesfield, Cheshire, UK: Central Toxicology Laboratory; February 3, 1997. EPA MIRD number 443179-01.
- 7.Gledhill AJ. Dichlorvos: a single blind, placebo controlled, randomized study to investigate the effects of multiple oral dosing on erythrocyte cholinesterase inhibition in healthy male volunteers. Alderley Park Mac-Clesfield, Cheshire, UK: Central Toxicology Laboratory; March 24, 1997. EPA MIRD number 442488-01.
- 8.Gledhill AJ. Dichlorvos: a study to investigate the effect of a single oral dose on erythrocyte cholinesterase inhibition in healthy male volunteers. Alderley Park MacClesfield, Cheshire, UK: Central Toxicology Laboratory; March 25, 1997. EPA MIRD number 442488-02.
- 9.Kisicki JC, Seip CW, Combs ML. A rising dose toxicology study to determine the no-observable-effect levels (NOEL) for erythrocyte acetyl-cholinesterase (AChE) inhibition and cholinergic signs and symptoms of chlorpyrifos at three dose levels. Lincoln, Neb: MDS Harris; April 19, 1999. Project 21438.
- 10.McFarlane P, Freestone S. A randomized double blind placebo controlled study with azinphos-methyl to determine the no effect level on plasma and RBC cholinesterase activity after repeat doses. Edinburgh, Scotland: Inveresk Clinical Research Ltd.; April 15, 1999.
- 11.Wyld PJ, Watson CE, Nimmo WS, Watson M. A safety and tolerability study of aldicarb at various dose levels in healthy male and female volunteers. EPA MIRD Number 423730-01. Edinburgh, Scotland: Inveresk Clinical Research Ltd.; March 11, 1992.
- 12.World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects (amended up to and including 52nd WMA General Assembly, Edinburgh, Scotland, October 2000 with note of clarification on paragraph 29 added, Washington, DC 2002: World Medical Association; 2002.
- 13.Goldman LR, Smith DF, Neutra RR, et al. Pesticide food poisoning from contaminated watermelons in California, 1985. Arch Environ Health. 1990;45: 229–236. [DOI] [PubMed] [Google Scholar]
- 14.Furlong CE, Li WF, Richter RJ, et al. Genetic and temporal determinants of pesticide sensitivity: role of paraoxonase (PON1). Neurotoxicology. 2000;21: 91–100. [PubMed] [Google Scholar]
- 15.Committee on Assessing the System for Protecting Human Research Participants, Institute of Medicine. Responsible Research, a Systems Approach to Protecting Research Participants. Washington, DC: National Academy Press; 2002.
- 16.International Committee of Medical Journal Editors. Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication. Available at: http://www.icmje.org. Accessed April 23, 2004. [PubMed]
- 17.Amler RW, Rice DC, Johnson BL. Assessment of mercury neurotoxicity through psychometric and neurobehavioral testing: session summary. Neurotoxicology. 1996;17:237–239. [PubMed] [Google Scholar]
- 18.Weissenborn K, Scholz M, Hinrichs H, Wiltfang J, Schmidt FW, Kunkel H. Neurophysiological assessment of early hepatic encephalopathy. Electroencephalogr Clin Neurophysiol. 1990;75: 289–295. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood AH, Weissenborn K, Bokemeyer M, Tietge U, Burchert W. Correlations between cerebral glucose metabolism and neuropsychological test performance in non-alcoholic cirrhotics. Metab Brain Dis. 2002;17:259–270. [DOI] [PubMed] [Google Scholar]
- 20.Lockwood AH. Pesticides and parkinsonism: is there an etiological link? Curr Opin Neurol. 2000;13: 687–690. [DOI] [PubMed] [Google Scholar]
- 21.Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20: 9207–9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Science. Second National Report on Human Exposure to Environmental Chemicals. Atlanta, Ga: US Department of Health and Human Services; 2003.
- 24.Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology. 2002;23:621–633. [DOI] [PubMed] [Google Scholar]
- 25.Steenland K, Jenkins B, Ames RG, O’Malley M, Chrislip D, Russo J. Chronic neurological sequelae to organophosphate pesticide poisoning. Am J Public Health. 1994;84:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–185. [DOI] [PubMed] [Google Scholar]
- 27.Whyatt RM, Rauh V, Barr DB, et al. Prenatal pesticide exposures, birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkowitz TS, Wetmur JGB-DE, Obel J, et al. In utero pesticide exposure, maternal paraoxonase activity and head circumference. Environ Health Perspect. 2004;112:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gemert M, Dourson M, Moretto A, Watson M. Use of human data for the derivation of a reference dose for chlorpyrifos. Regul Toxicol Pharmacol. 2001;33:110–116. [DOI] [PubMed] [Google Scholar]
- 30.Office of Pesticide Programs USEPA. Revised Organophosphate Cumulative Risk Assessment. Available at http://www.epa.gov/pesticides/cumulative/rra-op/. Accessed June 10, 2002.
- 31.Caplan AL. Am I My Brother’s Keeper? The Ethical Frontiers of Biomedicine. Bloomington, Ind: University of Indiana Press; 1997.
- 32.Blackburn E. Bioethics and the political distortion of biomedical science. New Engl J Med. 2004;350:1379–1380. [DOI] [PubMed] [Google Scholar]
- 33.Michaels D, Bingham E, Boden L, et al. Advice without dissent. Science. 2002;298:703. [DOI] [PubMed] [Google Scholar]
- 34.Lockwood AH. Organophosphate pesticides and public policy. Curr Opin Neurol. 2002;15:725–729. [DOI] [PubMed] [Google Scholar]