Abstract
Objectives. We assessed the prevalence of elevated blood lead levels (≥ 10 micrograms of lead per deciliter of blood), risk factors, and previous blood lead testing among children in 2 high-risk Chicago, Ill, communities.
Methods. Through high-intensity targeted screening, blood lead levels were tested and risks were assessed among a representative sample of children aged 1 to 5 years who were at risk for lead exposure.
Results. Of the 539 children who were tested, 27% had elevated blood lead levels, and 61% had never been tested previously. Elevated blood lead levels were associated with chipped exterior house paint.
Conclusions. Most of the children who lived in these communities—where the prevalence for elevated blood lead levels among children was 12 times higher than the national prevalence—were not tested for lead poisoning. Our findings highlight the need for targeted community outreach that includes testing blood lead levels in accordance with the American Academy of Pediatrics’ recommendations.
Lead is an environmental toxicant that affects nearly every system in the body.1 Among children, lead is associated with decreased intelligence, growth and hearing impairment, anemia, and attention and behavioral problems.2 High levels of exposure can cause severe brain damage and death. Young children, especially those who are aged younger than 2 years, are particularly susceptible to lead because their central nervous systems are still developing and because they absorb more lead from their environments than do adults.2 Among children who are aged younger than 6 years, the Centers for Disease Control and Prevention (CDC) defines an elevated blood lead level as greater than or equal to 10 micrograms of lead per deciliter of blood (μg/dL), but there are subtle effects on health at lower levels.3 Deteriorating lead-based paint is the most common high-dose source of lead exposure among young children in the United States.1 In 1973, the Consumer Product Safety Commission established a maximum lead content in paint of 0.5% by weight, and in 1978 that amount was lowered to 0.06%.4 Eighty-nine percent of homes in Chicago were built before 1978.5
A federal strategy to eliminate childhood lead poisoning by 2010 was developed by the CDC, the Department of Housing and Urban Development, the Environmental Protection Agency, and other agencies.6 Two key elements of the strategy are to identify and care for lead-poisoned children and to refine lead poisoning prevention strategies. High-intensity targeted screening is a tool that allows the CDC and its local partners to assess testing levels among children in high-risk communities and to examine the blood lead burden among children in specific locales.
Chicago, Ill, is the third largest city in the United States and has an estimated 308000 children who are aged younger than 6 years.5 It is divided into 77 communities, some of which have many risk factors for childhood lead poisoning, including a high percentage of residents who are poor, who live in old housing, who receive Medicaid assistance, and who are of minority race/ethnicity.7,8 Of the 114126 Chicago children who were tested for lead poisoning in 2001, 11.2% had blood lead levels greater than or equal to 10 μg/dL, and 1.9% had blood lead levels greater than or equal to 20 μg/dL.9
The results of a collaborative blood lead study in 2 Chicago communities by the CDC and the Chicago Department of Public Health (CDPH), which was conducted in partnership with community-based organizations, are presented in this article. The objectives of our study, which was conducted during October and November 2001, were to (1) assess the prevalence of children with elevated blood lead levels who had not been previously tested, (2) obtain an unbiased prevalence estimate of elevated blood lead levels among children aged 1 to 5 years who lived in these 2 Chicago communities, and (3) identify demographic, behavioral, and environmental risk factors for elevated blood lead levels among these children.
METHODS
Study Design
Our study included a population-based cross-sectional blood lead sampling scheme and the administration of personal and environmental risk factor questionnaires. The study population was composed of children aged 1 to 5 years who had lived in their Chicago residence for at least the past 30 days. A population-based cluster survey design was used to select households in 2 high-risk Chicago communities, Austin and Englewood. Because of the large size of these communities (3 and 4 square miles, respectively), a simple random sample was not feasible. Our cluster survey design followed the Expanded Program on Immunization model, but it was improved in accordance with the recommendations of Brogan et al.10
Housing unit information was obtained from 1990 tax assessment data that included information about each property in Chicago, such as the year a unit was built. Samples were selected by (1) dividing each community into clusters, i.e., primary sampling units (Austin = 305 total clusters; Englewood = 308 total clusters), on the basis of a grid system that randomly and equally divided the communities; (2) using the population proportional to estimated size method to randomly select clusters (Austin = 29 random clusters; Englewood=41 random clusters); and (3) selecting a sample of households (Englewood=7 out of 210 total households; Austin = 6 out of 180 total households) within each cluster by randomly selecting 1 household within the cluster that had equal selection probability. A cluster was approximately 4 city blocks.
An address was randomly selected as a starting point for each data collection team. After visiting the first address, each team went to the next address on the same side of the street, in descending order, and then up the opposite side until its quota of households was met or until the street ended. If the quota was not met when the street ended, the team went to another street on its list, which was mapped in a clockwise direction for safety reasons, so we always knew where the team was going. To account for nonresponse rates, the data collection teams noted the outcome of each household visit (i.e., eligible, ineligible, refused, vacant). A household was recorded as “occupied but the residents not at home” only after the team had visited the household at least 3 times. To increase participation rates, CDPH note cards with a phone number were left at vacant households, and participating families were given a $15 grocery store gift certificate. A household was defined as any unit (i.e., an area that included at least 1 bedroom, 1 bathroom, and a kitchen) where people could live at a given address, and nearly all the addresses visited (mostly houses) had more than 1 household.
A data collection team comprised at least 1 CDPH staff member, 1 CDC staff member or 1 CDC-trained public health graduate student, and 1 community member. Team members were trained in cultural sensitivity, data collection, capillary and venous blood drawing, and personal safety. Approximately 20 teams were used during the study period.
Sample sizes for the 2 communities were calculated to provide a large enough sample so that the margin of error around the prevalence estimate (95% confidence interval [CI]) was ±10%. Sample size calculations assumed an intracluster correlation of 0.23, which implied a design effect of 2.4.
Questionnaire Data
Each team administered 2 questionnaires to a consenting parent or a legal guardian from each eligible household: a child questionnaire to assess risk factors for lead exposure and to obtain information about the eligible children and a household questionnaire to assess the home environment, demographics of the people who lived in the residence, and lead poisoning prevention knowledge (assessed through 5 true/false questions developed by CDPH). It took 15 to 20 minutes to administer the questionnaires.
Health Education
During the interview, the team provided the parent or guardian with educational material about lead poisoning prevention, ways to reduce lead hazards in the home, and childhood immunization. Free blood lead screenings were promoted and were provided by the CDPH to children who were not selected for our study.
Blood Lead Survey
Capillary blood lead samples were collected because it was convenient and because previous studies have shown a high correlation between capillary and venous sampling (the preferred diagnostic method).11–13 CDC technicians with extensive field experience trained team members in the appropriate collection14 of 200-μl samples of capillary blood from each eligible child in the household. A venous blood sample was randomly collected from 10% of the participating children by trained CDPH phlebotomists as a quality-control measure. The CDC laboratory used the Perkin-Elmer Model 4100ZL atomic absorption spectrometer with Zeeman background correction (Perkin-Elmer LAS, Shelton, Conn) to test the capillary and the venous blood samples for lead. When capillary samples did not have a sufficient quantity for measurement (n = 24), CDPH staff collected venous samples within 3 months. These results were used in the analysis when capillary results were not available.
Data Analysis
Data were entered into Epi Info (CDC, Atlanta, Ga), and 10% of the records were completely reentered to assess the accuracy of data entry. Statistical analyses were performed with SAS (SAS Institute Inc, Cary, NC) and SUDAAN (Research Triangle Institute, Research Triangle Park, NC) software. Sampling weights that represented both the number of households in the clusters and the overall number of households in the 2 communities were used to calculate prevalence estimates.
Children were matched with the CDPH childhood lead poisoning prevention database (variables were first name, last name, and date of birth) to determine if they had been previously tested for blood lead. Children were considered previously tested if the 3 variables matched perfectly and if the match occurred before October 27, 2001.
Logistic regression techniques were used to examine risk factors for elevated blood lead levels that were obtained from the household and child questionnaires. Risk factors included child activities and country of birth, length of time at residence, previous renovation activity in the household, parental smoking status, occupation and hobbies, and condition of paint on exterior surfaces of the residence. On the basis of previous studies,15–17 the following were selected as confounding variables: age and gender of child, receipt of public assistance, receipt of public or Section 8 housing, and educational level of the parent or guardian. Age of residence and minority status were not considered confounders because all the homes visited were built before 1978 and nearly all the participants were Black.
A univariate analysis was conducted first to assess each risk factor’s association with elevated blood lead levels; risk factors significantly associated with elevated blood lead levels were evaluated separately in multivariate analyses. During the first multivariate analysis, we assessed each risk factor while the selected confounding variables and interaction terms were controlled. During the second multivariate analysis, we used a forward-selection strategy to add 1 risk factor variable at a time to the most predictive model, which included the a priori confounders, until all risk factors in the model were statistically significant. Only statistically significant risk factors identified in the first multivariate analysis were included in the second multivariate analysis, and interactions between risk factors and confounding variables were assessed during both analyses. The variance inflation factor was used to assess collinearity between variables in the predictive models.
Paired venous and capillary specimens from 41 children were collected and analyzed. Linear regression analysis showed a close relationship between the test methods, with a slope of 0.92, a correlation of 0.69, and an intercept of 0.71. A bias plot of the paired results showed an equal distribution both above and below the mean, and 93% of the capillary/venous pairs were within 2 standard deviations of the mean.
RESULTS
Of the 4854 households visited (Austin = 2456; Englewood = 2398), 78.5% were ineligible for participation (e.g., no children aged 1–5 years lived at the residence, residence was unoccupied), and the refusal rate was 5.9%. The most common reason for nonparticipation was that the child had had a recent blood lead test. Forty-one children from 25 households were excluded from the analyses because insufficient (n = 36) or no (n = 5) blood was collected. The final sample included 539 children from 366 households.
Household Characteristics
An average of 6 persons (range = 2–18) lived in participating households, and half the housing units in the study were built between 1900 and 1919. Thirty-seven households (10%) had a resident whose occupation possibly involved lead, such as battery recycling or manufacturing, painting or construction, and automobile or radiator repair. Of the 366 households, 222 (61%) had a resident who was a current smoker, 387 (73%) of the families rented the unit in which they lived, 194 (36%) children had a parent or guardian who completed high school, and 165 (31%) had a parent or guardian who completed some high school (Table 1 ▶).
TABLE 1—
Frequency and Weighted Prevalence of Study Population Characteristics by Community and Blood Lead Levels: Chicago, Ill, 2001
| Austin (n = 189)a | Englewood (n = 350)a | ||||
| Total (N = 539) All BLLs | BLLs < 10 μg/dL, % (n = 142) | BLLs ≥ 10 μg/dL, % (n = 47) | BLLs < 10 μg/dL, % (n = 234) | BLLs ≥10 μg/dL, % (n = 116) | |
| Child characteristic | |||||
| Raceb | |||||
| Black | 529 | 96.8 | 98.5 | 98.5 | 97.4 |
| White | 3 | 1.3 | 0.0 | 0.5 | 0.0 |
| Other | 9 | 1.9 | 1.5 | 1.0 | 2.6 |
| Gender | |||||
| Male | 280 | 47.8 | 65.0 | 52.1 | 59.7 |
| Female | 254 | 52.2 | 35.0 | 47.9 | 40.3 |
| Unknown | 5 | ||||
| Age, y | |||||
| 1 | 103 | 11.5 | 21.4 | 18.4 | 15.7 |
| 2 | 120 | 19.4 | 23.6 | 17.2 | 26.3 |
| 3 | 118 | 19.4 | 38.8 | 24.5 | 18.9 |
| 4 | 104 | 32.0 | 7.8 | 19.1 | 25.1 |
| 5 | 94 | 17.7 | 8.4 | 20.8 | 14.0 |
| Asthma | |||||
| Yes | 163 | 30.5 | 30.4 | 30.6 | 32.5 |
| No | 371 | 69.5 | 69.6 | 69.4 | 67.5 |
| Unknown | 5 | ||||
| Any prior blood lead test | |||||
| Yes | 212 | 39.5 | 32.9 | 46.4 | 36.5 |
| No | 327 | 60.5 | 67.1 | 53.6 | 63.5 |
| Household characteristic | |||||
| Receipt of any form of public assistancec | |||||
| Yes | 495 | 82.6 | 92.2 | 94.4 | 93.7 |
| No | 41 | 17.4 | 7.8 | 5.6 | 6.3 |
| Unknown | 4 | ||||
| Caretaker education | |||||
| None | 2 | 0.0 | 0.0 | 0.0 | 1.8 |
| ≤ 8th Grade | 13 | 1.0 | 3.2 | 2.1 | 2.6 |
| Some high school | 165 | 26.5 | 53.8 | 29.7 | 41.3 |
| High school diploma or GED | 194 | 31.8 | 27.6 | 40.6 | 35.9 |
| Some college | 125 | 34.6 | 13.8 | 22.4 | 15.8 |
| College graduate | 25 | 6.1 | 1.6 | 5.2 | 2.6 |
| Unknown | 15 | ||||
| Home ownership typeb | |||||
| Owner occupied | 138 | 29.6 | 36.6 | 21.9 | 8.8 |
| Rental | 387 | 68.7 | 63.4 | 77.7 | 86.5 |
| Section 8 or public housing | 8 | 1.7 | 0.0 | 0.4 | 4.7 |
| Unknown | 6 | ||||
| Year dwelling was built | (N=366) | ||||
| Before 1900 | 84 | 4.4 | 10.3 | 39.9 | 23.4 |
| 1900–1919 | 181 | 46.9 | 64.5 | 41.6 | 54.2 |
| 1920–1939 | 75 | 41.6 | 20.6 | 11.9 | 22.4 |
| 1940–1959 | 8 | 6.0 | 0.0 | 0.6 | 0.0 |
| 1960–1978 | 14 | 1.1 | 4.6 | 6.0 | 0.0 |
| 1979–present | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Unknown | 4 | ||||
| Potential exposures | |||||
| Parental occupation that involves lead | |||||
| Yes | 50 | 9.5 | 8.3 | 5.3 | 9.7 |
| No | 480 | 90.5 | 91.7 | 94.7 | 90.3 |
| Unknown | 9 | ||||
| Parental hobby that involves lead | |||||
| Yes | 6 | 1.1 | 2.8 | 0.5 | 0.5 |
| No | 524 | 98.9 | 97.2 | 99.5 | 99.5 |
| Unknown | 9 | ||||
| Smoker in the residence | |||||
| Yes | 351 | 59.4 | 82.4 | 60.6 | 79.1 |
| No | 179 | 40.6 | 17.6 | 39.4 | 20.9 |
| Unknown | 9 | ||||
| Home renovation in the past 6 months | |||||
| Yes | 241 | 50.9 | 54.9 | 50.8 | 40.7 |
| No | 278 | 48.7 | 43.9 | 45.7 | 54.2 |
| Not sure | 20 | 0.4 | 1.2 | 3.5 | 5.1 |
| Unknown | 6 | ||||
| Chipped paint on front porch | |||||
| Yes | 78 | 8.6 | 37.1 | 14.5 | 18.9 |
| No | 413 | 91.4 | 62.9 | 85.5 | 81.1 |
| Unknown | 48 | ||||
| Chipped paint on exterior front windows | |||||
| Yes | 123 | 17.5 | 38.9 | 24.7 | 33.4 |
| No | 372 | 82.5 | 61.1 | 75.3 | 66.6 |
| Unknown | 44 | ||||
| Observation of child eating dirt in the past year | |||||
| Yes | 93 | 13.6 | 29.4 | 14.6 | 23.5 |
| No | 438 | 86.4 | 70.6 | 85.4 | 76.5 |
| Unknown | 8 | ||||
| Observation of child eating paint in the past year | |||||
| Yes | 51 | 1.0 | 12.9 | 9.4 | 21.3 |
| No | 477 | 99.0 | 87.1 | 90.6 | 78.7 |
| Unknown | 11 | ||||
| Use of folk remedies/medications | |||||
| Yes | 28 | 0.5 | 2.0 | 6.4 | 9.1 |
| No | 505 | 99.5 | 98.0 | 93.6 | 90.9 |
| Unknown | 6 | ||||
Note. BLL = blood lead level; GED = general equivalency diploma.
a Weighted percentages exclude missing observations.
b Respondents could choose more than 1 category.
c This category includes several questions from the survey that were collapsed (eg, Do you receive Medicaid? food stamps? . . .). Therefore, respondents could choose more than 1 category.
Child Characteristics
Gender was not recorded for 5 children, and the gender could not be determined by the children’s names (CDPH was not able to locate and follow up with these families to determine gender). Among the child participants with known gender, 52% were male, and the average age of the children was 2.9 years. Ninety-nine percent of them were Black, 2% were Hispanic, and less than 1% was White (respondents could choose more than 1 race). One hundred twenty-six (24%) of the children had been told by health-care professionals that they had asthma. Ninety-two percent of the study population reported ever receiving some form of public assistance, the most common of which were Medicaid services (70%), food stamps (60%), public aid (56%), and Women, Infants, and Children (WIC) services (51%).
Blood Lead Results and Follow-up
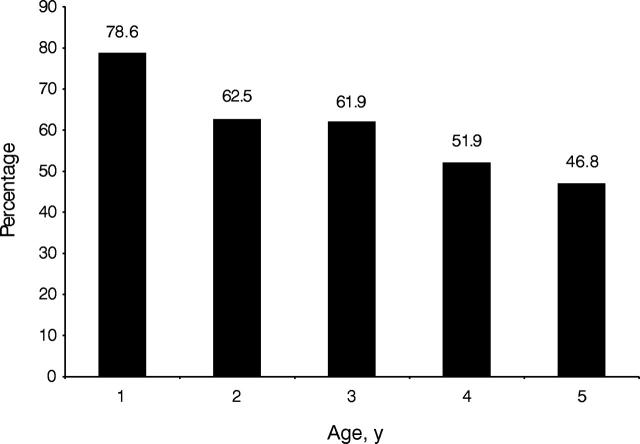
Of the 539 children tested, 327 (61%) never had their blood lead previously tested: 79% were aged 1 year (79%), and the percentages decreased with increasing age (Figure 1 ▶). Fifty-eight percent of the children who received Medicaid services had never had a previous test compared with 67% of the children who did not receive Medicaid services. Among the children who had ever received Medicaid services, 63% of those who had blood lead levels greater than or equal to 10 μg/dL had never had a previous test compared with 55% of those who had blood lead levels less than 10 μg/dL.
FIGURE 1—
Percentage of children identified by a high-intensity targeted screening who had not previously had a blood lead test.
The prevalence of elevated blood lead levels in the 2 communities was 30.0% (unweighted) and 26.6% (weighted); the weighted prevalence was higher in Englewood (33.6%) than in Austin (23.2%). These estimates compare favorably with 2001 CDPH surveillance data in which 30.9% of the children in Englewood and 25.9% of the children in Austin had elevated blood lead levels. The CDPH reported that 44.7% of the children aged younger than 6 years in Englewood and 28.0% of the children in Austin were tested for blood lead during 2001.9 Overall, 163 (30.2%) children in the study had blood lead levels greater than or equal to 10 μg/dL, 62 (11.5%) had blood lead levels greater than or equal to 15 μg/dL, 33 (6.1%) had blood lead levels greater than or equal to 20 μg/dL, and 7 (1.3%) had blood lead levels greater than or equal to 30 μg/dL. The weighted mean blood lead level was 9.0 μg/dL (95% CI= 7.8, 10.2) in Englewood and 8.3 μg/dL (95% CI=7.3, 9.3) in Austin.
The study identified 62 children in 49 households who had confirmed blood lead levels greater than or equal to 15 μg/dL, levels for which the CDPH routinely initiates follow-up home inspections.
Risk Factors
When we controlled selected confounding variables, elevated blood lead levels were associated individually with each of the following: observation of chipped paint on the front porch of the residence (odds ratio [OR]=2.91; 95% CI = 1.37, 6.18), observation of chipped paint on the front exterior windows of the residence (OR = 2.85; 95% CI = 1.42, 5.71), observation by the parent or guardian of the child eating paint during the past year (OR = 3.80; 95% CI = 1.78, 8.12), presence of a smoker in the household (OR = 2.58; 95% CI = 1.43, 4.65), and observation by the parent or guardian of the child eating dirt during the past year (OR=2.08; 95% CI=1.10, 3.93).
During the second multivariate analysis, elevated blood lead levels were associated with each of the following risk factors when selected confounders were controlled: observation by the parent or guardian of the child eating paint during the past year (OR = 4.73; 95% CI = 2.02, 11.10), chipped paint on the front porch of the residence (OR = 2.75; 95% CI = 1.24, 6.10), and presence of a smoker in the house (OR = 2.29; 95% CI = 1.13, 4.65). Together these factors were predictors of elevated blood lead levels after all confounding variables were adjusted (Table 2 ▶). Our ability to assess effect modification between risk factors and confounders was limited because of small numbers in some calculations of the factors of interest. A collinearity assessment did not identify significant correlations between variables in any of the models.
TABLE 2—
Odds ratios for Elevated Blood Lead Levels and Significant Exposure Variables in the Multivariate Model: Chicago, Ill, 2001 (N = 539)a
| Exposure Variable | OR (95% CI) | P |
| Observation of child eating paint in the past year | 4.73 (2.02, 11.10) | .0003 |
| Chipped paint on the front porch | 2.75 (1.24, 6.10) | .0109 |
| Presence of a smoker in the residence | 2.29 (1.13, 4.65) | .0198 |
| Caretaker education < high school diploma or GEDa | 2.92 (1.32, 6.47) | .0200 |
| Child is malea | 2.36 (1.45, 3.83) | .0004 |
| Age, y (continuous)a,b | 0.77 (0.55, 1.07) | .1079 |
| Receipt of public assistancea | 0.67 (0.29, 1.55) | .3406 |
| Receipt of public housing or Section 8 housing a | 1.36 (0.67, 2.77) | .3894 |
Note. OR = odds ratio; CI = confidence interval; GED = general equivalency diploma. aConfounding variables included in the final model were determined to be risk factors on the basis of previous studies and were controlled in the multivariate model.
b The continuous variable is noncategorical and was considered for all ages included in the analysis.
DISCUSSION
Three fifths (327 of 539) of the children identified by the high-intensity targeted screening as having elevated blood lead levels had not been previously tested for lead, which indicated that children who live in old housing—a known risk factor for elevated blood lead levels—are not being adequately screened. Blood lead testing is the main method for identifying children who have elevated blood lead levels, because children who have elevated blood lead levels of 10 to 30 μg/dL do not show abnormalities on routine medical histories, physical examinations, or other laboratory tests.18 The CDC, the CDPH, and the American Academy of Pediatrics have recommended the development of strategies that target blood lead testing among children who are at the highest risk for lead poisoning.19,20 Nevertheless, this study population, which had many risk factors for lead poisoning, was overlooked by routine health services and public school entrance requirements (among school-aged children).21
Improved approaches are needed to increase testing and follow-up care of the children who most need these services. Increasing door-to-door campaigns (such as high-intensity targeted screening) to reach into communities or providing incentives to parents who bring their children into well-child services may be useful. Educating parents and guardians about the children who are at high risk for lead poisoning and about the importance of having their children tested for blood lead may encourage parents to ask health care providers to test their children. Pediatricians should be reminded of local blood lead–testing policies, and they should be encouraged to review a child’s blood lead–testing history and to test if indicated when children present for any medical care.
A slightly larger percentage of children who were reported to have received Medicaid services had not been previously tested for lead compared with children who were reported to have not received Medicaid services. One explanation for the difference is that some children who received Medicaid services were not tested for lead because they did not have well-child visits. It also is possible that there could have been some misclassification, because parents reported their child’s Medicaid status in this study and we did not validate their responses by matching the child’s name with the Medicaid database. However, this finding indicates that new strategies also are needed to increase testing by Medicaid providers. For example, even when Medicaid-enrolled children receive well-child visits, there may not be laboratory/phlebotomy services onsite, which may result in children not being tested. To improve testing rates among the Medicaid population, the CDPH identifies Medicaid-enrolled children who have not been tested. This is done through a database match between the state Medicaid agency and the CDPH blood lead–testing database. The CDPH provides outreach, including phone calls, letters, and home visits, to the children identified. Efforts are made to ensure that they receive early and periodic screening, diagnosis and treatment services, and help finding a medical home. Additionally, when determining whether or not to test children for lead, health care providers should use risk-assessment questionnaires to assess all children’s risks for lead exposure. Our study found several risk factors that could be used when assessing a child’s risk for an elevated blood lead level. For example, a parent’s or guardian’s observation of the child eating paint or dirt during the past year was predictive of an elevated blood lead level and is consistent with other reports.22,23
The association between children who have elevated blood lead levels and the presence of smokers in their homes is consistent with a recent study that reported an association between environmental tobacco smoke and increased blood lead levels among US children aged 4 to 16 years.24 Although the relationship between smoking and elevated blood lead levels is not understood, our finding reconfirms that children who are at high risk for lead poisoning may be at high risk for multiple environmental exposures that can affect their health, including environmental tobacco smoke. These children also may be at risk for other health conditions, such as asthma. Nearly one fourth of the children in our study were reported to have asthma by their care-givers. While in the home, lead-testing teams can provide health education on asthma and other conditions, make referrals for medical care, and inquire about vaccination status in addition to counseling about lead poisoning prevention. Discussing hazards and health conditions other than lead poisoning while in the home is important because making contact with the family again is often difficult. In our study, returning and meeting with families to collect confirmatory tests for the children who had elevated capillary test results took several weeks. In some cases, the children who had elevated blood lead levels had moved to another address or were not home after repeated visits.
A potentially useful finding for improving targeted screening was the association between elevated blood lead levels and visible chipped exterior paint on the front porch or the windows of older homes. We found that conducting a brief visual inspection of the front exterior of the home was a good predictor of risk for lead exposure among young children who live in older homes. This is consistent with a published study that found children were at greater risk for elevated blood lead levels if they lived in lesser-valued older houses, perhaps because of poorer maintenance and deterioration.25 However, our results differed from a recent study that showed visual assessments of interior items in need of repair or replacement poorly reflected the amount of lead in household dust as measured with dust wipes,26 a technique that we did not use in this study. We found that 46.2% of the children who had elevated blood lead levels had chipped paint on the front porch of their home. If other studies can replicate this finding, it would prove to be a simple, low-cost method for targeting screening efforts and would compliment the use of geographic information system technology for finding children who are at high risk for lead poisoning.27–29
The high prevalence of elevated blood lead levels in these communities (Austin = 23.2%; Englewood = 33.6%) demonstrates the need for state and local surveillance data to accurately describe the number of children who have elevated blood lead levels and to direct prevention efforts. These communities have approximately twice the prevalence of elevated blood lead levels estimated by Chi-cago’s citywide surveillance data (14%)9 and 12 times the current national prevalence estimates (2.2%).30 National data, such as the National Health and Nutrition Examination Survey, are not designed to provide state- and local-level prevalence estimates. Similarly, statewide and even citywide estimates may not accurately reflect elevated blood lead level prevalence in specific communities. One of the advantages of the design used in our study is that we could obtain prevalence estimates that were representative of these communities. Information collected by community studies, such as high-intensity targeted screening projects, can supplement local data, particularly when it validates existing surveillance data. Other urban areas can benefit from high-intensity targeted screening projects; however, because our study required effort and resources above the normal public health outreach activities, future projects may be best suited in communities where there is a need to know the prevalence of elevated blood lead levels or where there is a need to evaluate screening penetration.
Our study had several limitations. First, we were not able to assess possible differences between children who did and did not participate in the study. Second, because we conducted the study in October and November, more families than expected refused testing of their school-aged children because their children had recently been tested in accordance with school entrance requirements. Third, the estimated number of children who had not been previously tested may have been inflated because of the rigorous matching methodology (a perfect match of first name, last name, and date of birth) and because of incomplete reporting of test results to CDPH. Lastly, our project required human and financial resources beyond normal public health outreach activities. The CDC contributed $60000 in addition to staff and laboratory supplies to support the project. The CDPH had several staff work overtime. A separate, more comprehensive cost analysis is planned.
CONCLUSIONS
This community approach found and tested many children who lived in old housing, a known risk factor for elevated blood lead levels, and who had been overlooked by routine health services. Although national policies that restrict the use of lead have successfully reduced lead in children’s environments in the United States, many children still are exposed to lead in their homes and in their communities. Elimination of elevated blood lead levels among young children will require strategic planning and the use of local data to determine which children are at greatest risk and to develop interventions to improve blood lead testing among these children. Elimination of childhood lead poisoning as a public health problem also will require a more intense effort to make the homes of children at risk for elevated blood lead levels lead-safe, ideally before the children develop elevated blood lead levels.
Acknowledgments
Funding for the project was provided by the Centers for Disease Control and Prevention and the Chicago Department of Public Health (grant US7/CCU519879).
We thank the following individuals for their dedication and their hard work in making this project successful: Ed Delisio, Laura Kearns, Kathleen Gallagher, Lana Cohen, Jill Clark, Katherine Berger, Alison Mondul, Kristin Rankin, Jaqueline Reed, Robert Owens, LaTonya Cannon, Valeria Hubbard, Bonnie Dyck, Wendy Blumenthal, Philip Jacobs, Rob Henry, Tim Morta, Charon Gwynn, Jerry Curtis, Blanca Torres, Jamila Rashid, Robert Jones, Courtney Brook Maxwell, Jennifer Hartel, Helen Binns, and Sharunda Buchanan.
Human Participant Protection Institutional review board approval was obtained from both the Centers for Disease Control and Prevention and the Chicago Department of Public Health.
Peer Reviewed
Contributors T.A. Dignam, A. Evens, S. M. Ramirez, and P.A. Meyer developed the study protocol and conducted the study. E. Eduardo provided data analysis. K. L. Caldwell provided laboratory support and expertise. N. Kilpatrick contributed to field work coordination and data entry. G. P. Noonan and M. A. McGeehin contributed to the study concept and staff supervision. W.D. Flanders provided statistical support. All the authors contributed to the writing of the article.
References
- 1.Centers for Disease Control and Prevention (CDC). Preventing Lead Poisoning in Young Children: A Statement by the Centers for Disease Control. Atlanta, Ga: US Department of Health and Human Services, Public Health Service; 1991.
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead. Atlanta, GA: ATSDR; 1999.
- 3.Schwartz J. Low-level leadexposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. [DOI] [PubMed] [Google Scholar]
- 4.Consumer Product Safety Commission. Ban of Lead-Containing Paint and Certain Consumer Products Bearing Lead-Containing Paint; 1997. 42 CFR 1303.1–5.
- 5.US Census Bureau, Population Division. 2000 Population Projections of the United States by City, Age, Sex, and Hispanic Origin. Washington, DC: US Census Bureau; 2001.
- 6.President’s Task Force on Environmental Health Risks and Safety Risks to Children. Eliminating Childhood Lead Poisoning: A Federal Strategy Targeting Lead Paint Hazards. 2000. Prepared by ICF Consulting.
- 7.US General Accounting Office. Medicaid: Elevated Blood Lead Levels in Children. Washington, DC: US General Accounting Office; 1998. GAO Pub. No. GAO/HEHS-98-78.
- 8.Binns HJ, Kim D, Campbell C. Targeted screening for elevated blood lead levels: populations at high risk. Pediatrics. 2001;108:1364–1366. [DOI] [PubMed] [Google Scholar]
- 9.Chicago Dept of Public Health, Childhood Lead Poisoning Prevention. Unpublished 2001 blood lead surveillance data.
- 10.Brogan D, Flagg EW, Deming M, Waldman R. Increasing the accuracy of the expanded programme on immunization’s cluster survey design. Ann Epidemiol. 1994;4:302–311. [DOI] [PubMed] [Google Scholar]
- 11.Parsons PJ, Reilly AA, Esernio-Jenssen D. Screening children exposed to lead: an assessment of the capillary blood lead fingerstick test. Clin Chem. 1997;43:302–311. [PubMed] [Google Scholar]
- 12.Schonfeld DJ, Cullen MR, Rainey PM, Berg AT, Brown DR, Hogan JC Jr. Screening for lead poisoning in an urban pediatric clinic using samples obtained by fingerstick. Pediatrics. 1994;94:174–179. [PubMed] [Google Scholar]
- 13.Schlenker TL, Fritz CJ, Mark D, et al. Screening for pediatric lead poisoning. Comparability of simultaneously drawn capillary and venous blood samples. JAMA. 1994;271:1346–1348. [DOI] [PubMed] [Google Scholar]
- 14.Analytical Procedures for the Determination of Lead in Blood and Urine; Approved Guideline. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2001:13. NCCLS document C40-A (ISBN 1-56238-437-6).
- 15.Pirkle JL, Brody DJ, Gunter EW, et al. The decline in blood lead levels. JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- 16.Brody DJ, Pirkle JL, Kramer RA, et al. Blood lead levels in the US population. JAMA. 1994;272:277–283. [DOI] [PubMed] [Google Scholar]
- 17.CDC. Update: blood lead levels—United States, 1991–1994. Morb Mortal Wkly Rep. 1997;46:141–146. [PubMed] [Google Scholar]
- 18.CDC. Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Atlanta, Ga: Dept of Health and Human Services (DHHS); 2002.
- 19.CDC. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, Ga: DHHS; 1997.
- 20.American Academy of Pediatrics, Committee on Environmental Health. Screening for elevated blood lead levels. Pediatrics. 1998;101:1072–1078. [PubMed] [Google Scholar]
- 21.Ill School Code [105 ILCS 5/27-8.1] §27-8.1 (2004) and Ill Lead Poisoning Prevention Act [410 ILCS 45/6.2] §6.2 (1994).
- 22.ATSDR. Summary Report for the ATSDR Soil-Pica Workshop. Atlanta, Ga: ATSDR, Division of Health Assessment and Consultation; 2001.
- 23.Mielke HW, Reagan PL. Soil is an important pathway of human lead exposure. Environ Health Perspect. 1998;106(Suppl 1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in US children. Epidemiology. 2003;14:719–727. [DOI] [PubMed] [Google Scholar]
- 25.Kim DY, Staley F, Curtis G, Buchanan S. Relation between housing age, housing value, and childhood blood lead levels in children in Jefferson County, KY. Am J Public Health. 2002;92:769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallicchio L, Sexton M, Werner ML. A comparison of household lead exposure assessment methods in an old, urban community. Environ Res. 2002;89:50–57. [DOI] [PubMed] [Google Scholar]
- 27.Reissman DB, Staley F, Curtis GB, Kaufmann RB. Use of geographic information system technology to aid health department decision-making about childhood lead poisoning prevention activities. Environ Health Perspect. 2001;109:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda ML, Dolinoy DC, Overstreet MA. Mapping for prevention: GIS models for directing childhood lead poisoning prevention programs. Environ Health Perspect. 2002;110:947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts JR, Hulsey TC, Curtis GB, Reigart JR. Using geographic information systems to assess risk for elevated blood lead levels in children. Pub Health Rep. 2003;118:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D. Surveillance for elevated blood lead levels among children—United States, 1997–2001. Morb Mortal Wkly Rep. 2003;52(SS10):1–2. [PubMed] [Google Scholar]