Abstract
Recent studies have demonstrated a wide geographic circulation of isolates of Neisseria gonorrhoeae that fail to produce prolyliminopeptidase (PIP). Tests based on the production of this enzyme are important elements of a number of identification systems for gonococci. We documented the appearance, expansion, and contraction of subtypes of 165 PIP-negative gonococci detected in an extended and systematic sample of the 3,926 N. gonorrhoeae isolates collected in Sydney, Australia, from July 2002 to September 2005. Their appearance, peak, and decline followed an “epidemic” curve. At the peak of their prevalence in 2003, PIP-negative gonococci comprised 22% of all isolates. Closely related phenotypes accounted for 162/165 of the PIP-negative gonococci. Algorithms for confirmation of N. gonorrhoeae should take account of the temporal and geographic variability of gonococci by utilizing two or more distinct confirmatory methods.
The tests used to detect and identify Neisseria spp. are directed primarily at the recognition of the pathogenic Neisseria meningitidis and Neisseria gonorrhoeae, and selective agars have been developed for this purpose. However, for public health considerations, as well as those of the individual patient, valid and precise identification of the species of gram-negative diplococci grown on agars selective for the pathogenic Neisseria spp. is needed because a number of species other than N. gonorrhoeae and N. meningitidis may occasionally be isolated (14, 15).
In addition to the considerations of Gram stain and colonial morphology and oxidase and superoxol reactions that establish a provisional identification, additional confirmatory tests should also be used (14, 15). Tests for confirmation of N. gonorrhoeae include those based on carbohydrate utilization patterns, immunologically based coagglutination reactions, and chromogenic substrate tests. An example of the latter is the prolyliminopeptidase (PIP) reaction that relies on demonstration of activity of this enzyme; it is also referred to as hydroxyproline aminopeptidase (HPA or Pro) in some commercial test kits. The Pro reaction performed alone or combined with other tests in commercial applications is considered a useful adjunct to identification. The basis of this assumption is the number of reports showing a high sensitivity of PIP reactions in gonococci examined in assessments of commercial kits used for identification of members of the family Neisseriaceae (3, 4, 7-13, 19-22, 24, 27, 29). However, none of these assessments examined large numbers of strains from diverse sources over time.
Beginning in 2003, a number of gonococci were encountered in Australia where a negative PIP reaction made their confirmation problematic (N.-L. Nguyen, E. A. Limnios, and J. W. Tapsall, Abstr. Natl. Conf. Aust. Soc. Microbiol., abstr. PO5.12, 2004). Around the same period, similar observations were made in New Zealand. Earlier, an increase in PIP-negative gonococci had been reported in the United Kingdom (H. M. Palmer, S. Wu, K. R. Gough, M. N. Hassan, and A. Turner, Abstr. 13th Int. Pathog. Neisseria Conf., abstr. 387, 2002), Denmark (9), and Sweden. Isolates from these countries, examined in a number of collaborative studies, were found to be closely related (9; L. E. M. Unemo, H. H. Palmer, T. Blackmore, G. Herrera, H. Fredlund, E. Limnios, N. Nguyen, and J. Tapsall, Abstr. 16th Int. Soc. Sex. Transm. Dis. Res., abstr. WP-034, 2005). The genetic lesions underlying the PIP-negative gonococci were determined in strains from New Zealand (2). A later point prevalence study within England found a wide variation in the incidence and distribution of PIP-negative gonococci in that country (S. M. Alexander, I. Martin, C. Murphy, H. Patel, M. Perault, K. Fenton, and C. Ison, Abstr. 16th Int. Soc. Sex. Transm. Dis. Res., abstr. WO-101, 2005). The origin and spread of these strains have not been examined further.
We report here the results of an extended study undertaken to examine the prevalence of PIP-negative gonococci in Sydney, Australia, the dynamics of their expansion and spread over time in this defined setting, and the subtypes of the PIP-negative gonococci identified. The implications of these findings in regard to use of this reaction for confirmation of N. gonorrhoeae are discussed.
MATERIALS AND METHODS
Gonococcal isolates.
This laboratory is the reference laboratory for N. gonorrhoeae for the State of New South Wales, Australia. It undertakes primary isolation of N. gonorrhoeae from clinical samples from a number of sexual health clinics and hospitals and also receives isolates from a wide network of public and private sector laboratories throughout the state. One principal purpose of this activity is the surveillance of antimicrobial resistance in N. gonorrhoeae, and this surveillance has been conducted continuously since 1981 (1, 25). In order to validate the data obtained from this surveillance, attention has been paid to the requirements for the size of the sample and its representativeness that are currently set by the World Health Organization (28). In these respects, the program is best described as an example of active (i.e., information sought and provided regularly from the primary data collector), comprehensive (i.e., surveillance in the whole population and extensive data capture), and continuous surveillance (28). The sources of referred isolates and the pattern of their referral did not alter over the period of the study. All isolates received are stored in nutrient broth containing 20% glycerol at −70°C. The availability of this comprehensive sample provides a valuable basis for investigating other phenomena related to the epidemiology of gonococci.
In Sydney, Australia, in July 2003, three isolates were received in the reference laboratory; the originating laboratory had provisionally identified the isolates as N. gonorrhoeae but could not confirm the species by a commercial assay kit that included PIP reactions as an integral component of its identification process. Examination in the reference laboratory confirmed the three isolates as N. gonorrhoeae. These three isolates and the next 207 strains consecutively isolated or referred here were also examined for PIP activity in addition to the standard confirmatory tests (see below). A prospective examination of PIP activity in isolated and referred gonococcal isolates was then undertaken to 30 September 2005. Additionally, retrospective analysis of stored isolates was conducted to attempt to determine when these aberrant strains may have appeared and the dynamics of their appearance and spread. This included an assessment of the relatedness of PIP-negative gonococci (see below).
Sampling and analysis.
From 1 July 2002 to 30 September 2005, a systematic but discontinuous sample of consecutively isolated gonococci was taken from the comprehensive, continuous collection of gonococci available. The discontinuous sample was obtained from the gonococci referred and identified the first and third quarters of each year. In each of these quarters, sequential isolates were examined, and results were assessed using the lot quality assurance sampling (LQAS) approach that has been established as relevant for this type of surveillance (28).
The LQAS approach has been adapted for public health use from its original application to monitoring the quality of manufacturing in industry (21). Initially, LQAS was used for assessment of health service delivery (17, 18) and disease prevalence estimates (18). It has the advantages of requiring lower numbers of observations and ease of analysis (21). The disadvantages include a lower sensitivity of some LQAS analyses (21), which may be overcome, as was done here, by use of a larger sample size and sequential sampling (17, 21). Other applications developed include those for surveillance of phenotypic characteristics of bacterial populations, such as antimicrobial resistance (28), and tables have been constructed for estimating requisite sample sizes and for determining significance of LQAS-derived data. An abbreviated table, specific for application to antimicrobial resistance phenotypes in bacterial populations, has also been made available (28). In the study reported here, for each quarter assessed, the number of isolates examined was determined by reference to these established protocols, and this number represented at least a third of all isolates in each quarter studied (for numbers and proportions of isolates examined, see Table 1). When a very low proportion of PIP-negative gonococci was found in the third quarter of 2002, but an increasing number in the first quarter of 2003 (Table 1), enhanced surveillance was undertaken. All 330 isolates originally received in the latter period were tested to ascertain if this was a critical period in the expansion of PIP-negative gonococci (Table 2). The isolates from each of the three months of this first quarter of 2003 were examined separately in regard to the number and subtype of PIP-negative gonococci detected. In this period of January, February, and March 2003, when it was highly likely that a critical increase of PIP-negative isolates had occurred (Table 2 and Fig. 1), Fisher's exact test was used to determine the probability that the association between the PIP-negative gonococci and the time frame of isolation (individual months) was due to chance (5). Calculations were determined at the 5% level of significance using GraphPad Instat version 2.05 for Windows 95 (GraphPad Software, Inc., San Diego, Calif.). In addition to providing reliable estimates of required sample sizes, the tables supplied by the World Health Organization allow assessment of the significance of increasing frequencies of subtypes at the 5% level of significance (28), and these were also applied to the separate data obtained for January, February, and March 2003.
TABLE 1.
Prolyliminopeptidase reactions of 1,287 Neisseria gonorrhoeae isolates examined in Sydney, Australia, and the auxotype/serovar classes of 165 PIP-negative gonococcia
| Quarter and yrb | No. of gonococci referred per quarter | No. of gonococci (%) tested for PIP | No. (%) of PIP-negative gonococci | No. (%) of PIP-negative gonococci of predominant A/S classes
|
|
|---|---|---|---|---|---|
| NR/Bpyvut | NR/Bpyvt | ||||
| Qtr3 2002 | 375 | 200 (53) | 6 (3) | 4 (2) | 0 (0.0) |
| Qtr1 2003 | 330 | 330 (100) | 44 (13.3) | 17 (5.2) | 26 (7.9) |
| Qtr3 2003 | 251 | 207 (82) | 47 (22.7) | 9 (4.3) | 36 (17.4) |
| Qtr1 2004 | 323 | 111 (34) | 24 (21.6) | 4 (3.6) | 20 (18.0) |
| Qtr3 2004 | 255 | 200 (78) | 31 (15.5) | 6 (3.0) | 24 (12.0) |
| Qtr1 2005 | 322 | 123 (38) | 6 (4.9) | 0 (0.0) | 3 (2.4) |
| Qtr3 2005 | 320 | 116 (36) | 7 (6) | 1 (0.9) | 5 (4.3) |
| Total | 2,176 | 1,287 (59) | 165 (12.8) | 41 (3.2) | 114 (8.9) |
The 1,287 N. gonorrhoeae isolates examined constituted a systematic sample of the 2,176 gonococci available from seven alternate quarters between 1 July 2002 to 30 September 2005.
Qtr, quarter.
TABLE 2.
Expansion in the number and proportion of prolyliminopeptidase-negative N. gonorrhoeae isolates detected in Sydney, Australia, between 1 January 2003 and 31 March 2003
| Mo and yr | No. of N. gonorrhoeae isolates tested | No. (%) of PIP-negative N. gonorrhoeae isolates |
|---|---|---|
| Jan 2003 | 119 | 8a (6.7)b |
| Feb 2003 | 105 | 18a (17.1)b |
| Mar 2003 | 106 | 18 (17) |
| Total | 330 | 44 (13.3) |
These values were significantly different from each other (P value of 0.02 by Fisher's exact test).
These values were significantly different from each other (P < 0.05) (28).
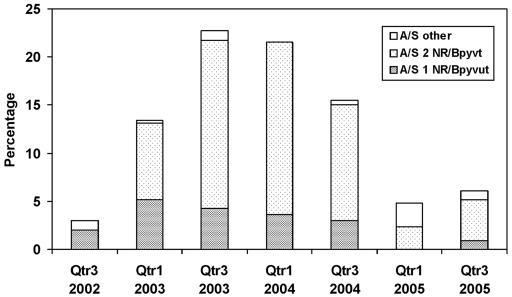
FIG. 1.
Percentage of 165 prolyliminopeptidase-negative gonococci detected in a total of 1,287 gonococci examined in systematic samples of a total of 2,176 isolates over time (seven quarters [Qtr] from 2002 to 2005) and their temporal distribution between the two main auxotype/serovar classes.
Laboratory investigations.
All isolates were identified as N. gonorrhoeae by combining the provisional tests (14) mentioned earlier, that is, Gram stain, colonial morphology on modified New York City medium, oxidase and superoxol tests (30),a rapid carbohydrate utilization test (26), and coagglutination reactions with Phadebact Monoclonal GC Test reagents (Boule, Huddinge, Sweden). PIP reactions are not used for routine identification of gonococci in this laboratory.
Testing for PIP activity was by conventional assays and commercial methods. All isolates were examined with the enzyme assay method of D'Amato et al. (7) using a 2-h incubation period of the gonococcus with l-proline β-naphthylamide hydrochloride used as the substrate and also by a simpler version of this test in which l-proline-p-nitroanilide was used as the substrate, providing the development of yellow color in a positive result without the need of detector reagents. The API NH (bioMerieux SA, Marcy l'Etoile, France) and the Remel Rapid ID (Remel Inc., Lenexa, KS) panels that include PIP reactions in their protocols were used according to the manufacturers' instructions for PIP determinations with a limited number of isolates.
The extended phenotype of each isolate was derived by determination of the antibiotic susceptibility using the agar plate dilution methods of the Australian Gonococcal Surveillance Programme (25), which uses Sensitest agar (Oxoid, Basingstoke, United Kingdom) supplemented with 8% saponin-lysed horse blood as the test medium, an inoculum of 104 CFU per spot, and auxotype/serovar (A/S) classification. Auxotyping was performed by the method of La Scolea and Young (16), and the serovar was determined by using a panel of 14 monoclonal reagents (Boule, Huddinge, Sweden) and the nomenclature of Bygdeman (6).
RESULTS
From 1 July 2002 until 30 September 2005, 3,926 putative gonococci from New South Wales, Australia, and isolated in or referred to this laboratory, were confirmed as N. gonorrhoeae by conventional procedures. Of these, 2,176 were confirmed in the seven first and third quarters of each year or part of each year in the above period, and of these, 1,287 (33% of all the 3,926 gonococci referred and 59% of the 2,176 received in the seven quarters studied) were examined for PIP activity in the systematic sample (Table 1).
All tests used for PIP activity, whether conventional or commercially derived, gave an identical positive or negative result for each organism tested. Over the entire period, 165 gonococci (12.8% of the 1,287 tested) were PIP negative. However, the proportion of PIP-negative gonococci rose over the first two quarters studied, sustained a peak for the next two periods, and then fell over the remainder of the study. In the third quarter of 2002, 6 (3% of gonococci examined) PIP-negative gonococci were detected, and in the first quarter of 2003, there was a significant rise in the number and proportion of PIP-negative gonococci detected (44 [13.3%]). In the third quarter of 2003, 47 (22.7%) were PIP negative, and in the first quarter of 2004, there were 24 (21.6%) PIP-negative isolates. A decline in the number of PIP-negative gonococci was evident in the third quarter of 2004 when 31 strains represented 15.5% of all isolates, and by the first and third quarters of 2005, 6 (4.9%) and 7 (6.0%) PIP-negative isolates, respectively, were found (Table 1 and Fig. 1). The temporal distribution during the first increase, when 44 PIP-negative gonococci were detected over the 3-month period from 1 January to 31 March 2003, was examined separately (Table 2). In January 2003, 8 of 119 isolates (6.7%) were PIP negative, but in both February and March 2003, 18 of 105 (17.1%) and 18 of 106 (17%) gonococci, respectively, were PIP negative. The increase in PIP-negative phenotypes in February and March 2003 is statistically significantly different (P = 0.02 by Fisher's exact test) from the prevalence recorded in January 2003. Further, the sample sizes examined were sufficient for the detection of a significant increase in the phenotype (P ≤ 0.05) (28).
All but 1 of the 165 PIP-negative gonococci found here possessed a similar antibiogram (less sensitive to penicillin, MIC 0.125 or 0.25 mg/liter; susceptible to ciprofloxacin and ceftriaxone, MIC ≤ 0.03 mg/liter; susceptible to spectinomycin, MIC ≤ 64 mg/liter; and absence of high-level plasmid-mediated resistance to tetracycline, non-TRNG [25]) and comprised two main phenotypes on the basis of their A/S class. Together, the A/S classes NR/Bpyvut (n = 41 [24.8% of PIP-negative strains]) and NR/Bpyvt (n = 114 [69.1% of PIP-negative strains]) accounted for all but 10 aberrant gonococci. Seven of these ten gonococci had closely related phenotypes, Hyp/Bpyvt (n = 2), Arg/Bpyvt (n = 1), Pro/Bpyvut (n = 1), Pro/Bpyut (n = 2), and NR/Bpyt (n = 1), and the remaining three gonococci were of the less closely related A/S class NR/Bropst. One isolate of the latter A/S class displayed a different antibiogram (penicillin resistant, MIC = 1.0 mg/liter; less sensitive to ciprofloxacin, MIC = 0.5 mg/liter; susceptible to ceftriaxone and spectinomycin; and presence of high-level plasmid-mediated resistance to tetracycline, TRNG [25]). This distribution of gonococcal subtypes with particular phenotypic characteristics was consistent with the findings by previous analyses by other genotypic and phenotypic means (9; Unemo et al., Abstr. 16th Int. Soc. Sex. Transm. Dis. Res.).
DISCUSSION
This extended study of PIP reactions in gonococci isolated from July 2002 to September 2005 in Sydney, Australia, showed a significant increase and then a decrease in the prevalence of PIP-negative gonococci. The time point of rapid expansion of closely related PIP-negative gonococcal phenotypes was identified as February/March 2003 by a systematic retrospective analysis of stored isolates with a reduction in the rate of detection of PIP-negative gonococci not appearing until the first quarter of 2005. The sample sizes used conformed to parameters required for estimating statistically significant changes in antibiotic resistance frequencies in bacterial populations (28).
Together, the A/S phenotypes NR/Bpyvut and NR/Bpyvt accounted for 94% of all PIP-negative gonococci, and most of the remaining PIP-negative strains were also of similar phenotypes, reflecting evolutionary changes of one or two epitopes responsible for the specific serotyping reactions of the gonococcus (6, 23). The strain relatedness was confirmed in genotypic analyses performed elsewhere of a limited number of the isolates as part of an earlier collaborative study (Unemo et al., Abstr. 16th Int. Soc. Sex. Transm. Dis. Res.). This collaborative study also indicated the wide geographic spread of the gonococcal subtypes involved. Another distinct PIP-negative A/S phenotype, NR/Bropst, was present only in low numbers in Sydney, Australia, representing less than 2% of the 165 PIP-negative strains. These combined findings expand existing data on the distribution and prevalence of PIP-negative gonococci. Earlier studies suggested that gonococci lacking PIP activity were rare (3, 4, 7-13, 19-22, 24, 27, 29). However, these examinations were in general confined to low numbers of gonococci from a few locations at a particular time. A recent point prevalence study of larger numbers of gonococci in England found a wide geographic variation in the rate of detection of PIP-negative gonococci with their percentage in different centers ranging between 0 and 21 over a 3-month period in 2004 (Alexander et al., Abstr. 16th Int. Soc. Sex. Transm. Dis. Res.).
Temporal expansion and contraction of gonococcal auxotype/serovar classes are well described, as is the diversity that develops over time in closely related gonococcal subtypes (23). If a particular characteristic is associated with an individual gonococcal subtype, this feature may then become overrepresented in the total gonococcal population as that subtype expands and in turn be reduced in relevance as the overall proportion of that subtype declines. It would appear that in the situation described here that the sensitivity of the PIP test decreased due to the appearance of a subtype lacking this activity and that this situation persisted for approximately 2 years in Sydney, Australia.
In some gonococcal identification systems, PIP activity is a key reaction. It has not been appreciated until recently that gonococci lacking this characteristic may be commonly encountered and that the frequency of their occurrence may vary over time and place. This suggests that more extensive and continuing evaluation of the distribution of certain crucial features of gonococci should be considered necessary to account for this variability in the sensitivity of confirmatory reactions in gonococci. A continuing appraisal of the frequency of PIP-negative isolates referred to this laboratory will monitor the prevalence of the currently identified phenotypes and the characteristics of any additional PIP-negative phenotypes that may appear. In practical terms, the recommendations that algorithms for confirmation of gonococci should take account of this variability and that two or more distinct confirmation methods should be available within laboratories (14) are endorsed.
REFERENCES
- 1.Australian Gonococcal Surveillance Programme. 1984. Penicillin sensitivity of gonococci in Australia: the development of an Australian gonococcal surveillance programme. Br. J. Vener. Dis. 60:226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackmore, T., G. Herera, S. Shi, P. Bridgewater, L. Wheeler, and J. Byrne. 2005. Characterization of prolyl iminopeptidase-deficient Neisseria gonorrhoeae. J. Clin. Microbiol. 43:4189-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, J. M., and E. B. Mitchell, Jr. 1985. Difficulties in differentiating Neisseria cinerea from Neisseria gonorrhoeae in rapid systems used for identifying pathogenic Neisseria species. J. Clin. Microbiol. 22:731-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. D., and K. R. Thomas. 1985. Rapid enzyme system for the identification of Neisseria spp. J. Clin. Microbiol. 21:857-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budnick, L. D. 1992. Statistics, p. 43-76. In B. J. Cassens (ed.), Preventive medicine and public health, 2nd ed. Harwal Publishing, Philadelphia, Pa.
- 6.Bygdeman, S. M. 1988. Polyclonal and monoclonal antibodies applied to the epidemiology of gonococcal infection, p. 117-166. In H. Young and A. McMillan (ed.), Immunological diagnosis of sexually transmitted diseases. Marcel Dekker, New York, N.Y.
- 7.D'Amato, R. F., L. A. Eriquez, K. A. Tomfohrde, and E. Singerman. 1978. Rapid identification of Neisseria gonorrhoeae and Neisseria meningitidis by enzymatic profiles. J. Clin. Microbiol. 7:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon, J. R., M. Carbello, and M. Pauze. 1988. Evaluation of eight methods for identification of pathogenic Neisseria species: Neisseria-Kwik, RIM-N, Gonobio-Test, Minitek, Gonochek II, GonoGen, Phadebact Monoclonal GC OMNI Test, and Syva MicroTrak test. J. Clin. Microbiol. 26:493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fjeldsoe-Nielsen, H., M. Unemo, H. Fredlund, S. V. Hjorth, L. M. Berthelsen, H. M. Palmer, and A. Friis-Moller. 2005. Phenotypic and genotypic characterization of prolyliminopeptidase-negative Neisseria gonorrhoeae in Denmark. Eur. J. Clin. Microbiol. Infect. Dis. 24:280-283. [DOI] [PubMed] [Google Scholar]
- 10.Hosmer, M. E., M. A. Cohenford, and P. D. Ellner. 1986. Preliminary evaluation of a rapid colorimetric method for identification of pathogenic Neisseria. J. Clin. Microbiol. 24:141-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes, B., M. T. Pezzlo, L. M. de la Maza, and E. M. Peterson. 1987. Rapid identification of pathogenic Neisseria species and Branhamella catarrhalis. J. Clin. Microbiol. 25:2223-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ison, C. A., A. A. Glynn, and S. Bascomb. 1982. Acquisition of new genes by oral Neisseria. J. Clin. Pathol. 35:1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janda, W. M., M. G. Ulanday, M. Bohnhoff, and L. J. LeBeau. 1985. Evaluation of the RIM-N, Gonochek II, and Phadebact systems for the identification of pathogenic Neisseria spp. and Branhamella catarrhalis. J. Clin. Microbiol. 21:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janda, W. M., and J. S. Knapp. 2003. Neisseria and Moraxella catarrhalis, p. 585-608. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolkem (ed.), Manual of clinical microbiology, 8th ed., vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 15.Knapp, J. S. 1988. Historical perspectives and identification of Neisseria and related species. Clin. Microbiol. Rev. 1:415-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Scolea, L. J., and F. E. Young. 1974. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl. Microbiol. 28:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemeshow, S., and S. Taber. 1991. Lot quality assurance sampling: single- and double-sampling plans. World Health Stat. Q. 44:115-132. [PubMed] [Google Scholar]
- 18.Myatt, M., H. Limburg, D. Minassian, and D. Katyola. 2003. Field trial of applicability of lot quality assurance sampling survey method for rapid assessment of prevalence of active trachoma. Bull. W. H. O. 81:877-885. [PMC free article] [PubMed] [Google Scholar]
- 19.Perez, J. L., A. Pulido, E. Gomez, G. Sauca, and R. Martin. 1990. Superoxol and aminopeptidase tests for identification of pathogenic Neisseria species and Moraxella (Branhamella) catarrhalis. Eur. J. Clin. Microbiol. Infect. Dis. 9:421-424. [DOI] [PubMed] [Google Scholar]
- 20.Philip, A., and G. C. Garton. 1985. Comparative evaluation of commercial systems for the rapid identification of Neisseria species. J. Clin. Microbiol. 22:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinke, W. A. 1991. Applicability of industrial sampling techniques to epidemiological investigations: examination of an underutilized resource. Am. J. Epidemiol. 134:1222-1232. [DOI] [PubMed] [Google Scholar]
- 22.Robinson, M. J., and T. R. Oberhofer. 1983. Identification of pathogenic Neisseria species with the RapID NH system. J. Clin. Microbiol. 17:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarafian, S. K., and J. S. Knapp. 1989. Molecular epidemiology of gonorrhea. Clin. Microbiol. Rev. 2(Suppl.):S49-S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperry, J. F., M. A. Cohenford, P. Campognone, W. Lawton, and D. O. Chee. 1986. Increased detection of prolylaminopeptidase in Neisseria meningitidis by Identicult-Neisseria. J. Clin. Microbiol. 24:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapsall, J. W., and members of the National Neisseria Network of Australia. 2004. Antimicrobial testing and applications in the pathogenic Neisseria, p 173-186. In J. Merlino (ed.), Antimicrobial susceptibility testing: methods and practices with an Australian perspective. Australian Society for Microbiology, Sydney, Australia.
- 26.Tapsall, J. W., and J. K. Cheng. 1981. Rapid identification of pathogenic species of Neisseria by carbohydrate degradation tests. Importance of glucose in media used for preparation of inocula. Br. J. Vener. Dis. 57:249-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welborn, P. P., C. T. Uyeda, and N. Ellison-Birang. 1984. Evaluation of Gonochek II as a rapid identification system for pathogenic Neisseria species. J. Clin. Microbiol. 20:680-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. 2001. Surveillance standards for antimicrobial resistance. World Health Organization document WHO/CDS/CSR/DRS/2001.5, p. 10. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int/drugresistance/surveillance/en/.
- 29.Yajko, D. M., A. Chu, and W. K. Hadley. 1984. Rapid confirmatory identification of Neisseria gonorrhoeae with lectins and chromogenic substrates. J. Clin. Microbiol. 19:380-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young, H., A. B. Harris, and J. W. Tapsall. 1984. Differentiation of gonococcal and non-gonococcal Neisseriae by the superoxol test. Br. J. Vener. Dis. 60:87-89. [DOI] [PMC free article] [PubMed] [Google Scholar]