Abstract
Human erythrovirus (parvovirus) B19 (B19) is a common human pathogen. The recent discovery of three genotypes, 1 to 3, raised issues related to the ability of genotype-specific antigens to cross-react with antibodies elicited by other genotypes. This study assessed antibody capture and immunoglobulin G (IgG) cross-reactivity between genotype 1 and genotype 3 recombinant antigens and analyzed the potential gain of adding VP1 protein to VP2 capsid antigen. Plasma samples from genotype 1- or genotype 3-infected populations were blindly tested with blindly prepared reagents. The IgG reactivity was assessed with baculovirus-expressed VP2 or VP1 and VP2 recombinant genotype 1 or genotype 3 proteins in a standardized enzyme immunoassay (EIA). A high degree of agreement (>95%) between EIA results was observed, with Spearman correlation coefficient and kappa reliability coefficient results of ≥0.95 for samples from the United Kingdom and ≥0.77 for samples from Ghanaian children, respectively. Most discrepant results were related to equivocal reactivity. The addition of VP1 to VP2 capsids did not significantly impact antibody detection. These data suggest that the currently available genotype-1-based IgG EIA is suitable to detect antibody to B19 genotype 3 in Ghanaian children. However, samples from the Ghanaian adult population exhibited atypical results in the assay, possibly due to the high levels of nonspecific IgG antibodies found in adults living in this region. Within these limitations, the study demonstrates that genotype 1 and genotype 3 antigens are equally effective in detecting either antibody species.
Human erythrovirus (parvovirus) B19 (called B19 in this report) is a nonenveloped single-stranded DNA virus (9, 32). B19 causes a range of diseases and conditions in humans, including fifth disease of childhood, fetal death, various forms of anemia, and other conditions (reviewed in reference 38).
Three relatively long polypeptides encoded by the virus are two structural proteins (VP1 and VP2) and one nonstructural protein (NS-1) (8, 27). VP1 (781 amino acids [aa]) and VP2 (554 aa) are encoded by the same open reading frame and translated from two differentially spliced messages (25-27). VP1 is identical to VP2 except for a 227-aa N-terminal extension called the VP1 unique region. These two proteins form capsids (diameters, 19 to 25 nm) containing approximately 96% of VP2 and 4% of VP1 (27). In the eukaryotic baculovirus expression system, VP2 protein spontaneously forms virus-like particles that, under electron microscopy, are indistinguishable from the capsid structure of the native virions (4, 17). VP1 protein alone forms these structures very inefficiently, but when cotransfected with VP2- and VP1-containing recombinant baculovirus, chimeric capsids with VP2 and VP1 are produced (4, 17, 37). Up to 41% of VP1 was reported to be possible to incorporate in the capsid (3).
The nature of the immune response to the virus is characterized by the predominance of antibodies against structural proteins. Immunoglobulin G (IgG) antibodies against structural proteins appear relatively early and are generally able to clear the infection and to persist for life (12, 38).
B19 was initially considered to be genetically highly conserved (less than 2% nucleotide variation for the whole or near-whole genome) until genotypes 2 and 3, which differed by >10% from the canonical genotype 1 (15, 16, 22, 23, 31), were described, raising the issue of antigenic differences and antibody detection across genotypes. The cross-reactivity of antibody to genotype 1 was tested with genotype 3 antigen, and no significant difference in the ability of antigens from genotype 1 or 3 to capture antibody, presumably to genotype 1, was found (14). However, the ability of the genotype 1 capsid antigen to capture genotype 3 antibody was not examined because of the rarity of such samples in Europe and the United States (13, 14, 23, 31).
Recently, Ghana, West Africa, was identified as an area where genotype 3 is endemic (6). This provided an opportunity to test antibody reactivity to genotypes 1 and 3 VP2 in individuals exposed to genotype 3. Preliminary published results found a small number of B19 DNA-positive samples containing immune complexes that were undetected by a genotype-1-based commercial assay (Biotrin, Dublin, Ireland) and thus suggesting a decreased reactivity of genotype 3 antibodies with genotype 1 antigen (6). However, because the comparability of an in-house VP1 and VP2 (VP1-2) enzyme immunoassay (EIA) with the commercial kit was unsatisfactory, these observations initiated further investigation (7).
The question of whether there is a benefit in including VP1 antigen together with VP2 in serodiagnostic systems is not fully addressed in the current literature. There are only a few reports suggesting that recombinant VP1 used in VP1-2 capsids in a concentration similar to that of the virion confers either no or slight advantage in the sensitivity of the B19 IgG detection under the EIA format (14, 19, 20, 30). However, these studies either employed relatively small sample numbers or compared EIA systems, which are not standardized with one another, so factors other than the presence of VP1 in the recombinant capsids might have played a role in the higher number of anti-B19-positive samples detected with VP1-2 antigen. Conversely, it has been shown that VP1-2 capsids containing VP1 in a concentration higher than that in the virion elicit better-neutralizing responses, mainly because of the presence of antibodies against the VP1 unique region (3). Whether the increase of VP1 concentration in the VP1-2 capsids would have similar advantage for antibody detection has not been fully addressed.
The present study was designed and conducted to comprehensively assess the respective ability of genotype 1 or 3 VP2 or VP1-2 antigens to detect antibodies in samples from areas where genotype 1 or 3 is endemic and to analyze the potential gain of adding VP1 protein to VP2 capsid.
(These data were presented in part at a May 2005 International Plasma Fractionation Association meeting, Bethesda, Md.)
MATERIALS AND METHODS
Study design and population.
This study included 500 randomly selected samples of plasma in EDTA from the United Kingdom that were collected from 1999 to 2001 and stored at −20°C or below in the Division of Transfusion Medicine, University of Cambridge. Citrated plasma samples from 500 randomly selected, Ghanaian adult blood donors were collected from 2003 to 2004. EDTA plasma samples from 400 Ghanaian children under 7 years of age were randomly selected from approximately 2,000 samples collected between November 2003 and August 2005 at the Pediatric Emergency Unit of the Komfo Anokye Teaching Hospital, Kumasi, Ghana, for cross-matching prior to transfusion as previously described (27a). These children were hospitalized for severe anemia, mostly related to acute malaria (>90%). This study was approved by the Ethics and Publication committee of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Parents or relatives were informed, and informed consents were obtained. Blood samples from Ghana were stored in 0.5-ml aliquots at −20°C or lower until used.
Amplification of VP1 and VP2 regions for expression of genotype 3 proteins.
Two isolates, one from a Ghanaian blood donor (GenBank accession number AY582124, genotype 3) and another from a Belgian blood donor (GenBank accession number DQ293995, genotype 1), were used to prepare VP2 and VP1-2 antigens. The Ghanaian sample contained 4 × 105 IU/ml of viral DNA. The Expand High FidelityPLUS PCR system (Roche, Lewes, United Kingdom) was used for all of the PCRs described in this study, unless specified otherwise. For the amplification of VP1 region of genotype 3, a nested PCR was performed. The first round was performed in a 50-μl reaction using 10 μl of extracted DNA, 0.6 μM of forward VSTA (5′-CGGGACCAGTTCAGGAGAATCAT-3′) and reverse VOUT (5′-TGAGGACACGGTGGGGAATGTTTA-3′) primers, 10 μl of 5× Expand High FidelityPLUS reaction buffer with MgCl2 (1.5 mM per reaction), an additional 0.5 mM of MgCl2, 200 μM deoxynucleoside triphosphates, and 2.5 U/reaction of enzyme blend. The second round used the same concentrations of reagents but with 5 μl of the first-round reaction and another reverse primer, VEND (5′-CAATGGGTGGACACGGCTTTT-3′). The third round also used the same concentrations of reagents, except 2.5 μl of the second-round reaction was used and primers were substituted with reverse AD2 (5′-ACACGGCTTTTGGCAGTC-3′) and forward AD3 (5′-GGATGGTGTGAGGGGATTG-3′) primers. The 2,569-bp agarose gel-purified amplicon was cloned into pCR-XL-TOPO vector and transformed to One Shot TOP10 chemically competent Escherichia coli cells (Invitrogen, Paisley, United Kingdom) according to the manufacturer's instructions. B19 DNA from positive colonies was sequenced using overlapping primer pairs. The consensus VP1 sequence was derived from seven clones and one of them with an amino acid sequence identical to the consensus was selected for expression. The AD2-AD3 amplicon contained the VP1 region but lacked 13 bp (including 3 bp of the TAA stop codon) and had 236 additional nucleotides upstream of the VP1 ATG start codon. To prepare the VP1 sequence for protein expression, the cloned AD2-AD3 fragment was subjected to PCR with V1ATG (5′-GTGCTTACCTGTCTGGATTACAAAGTTTTGTAGATTATGAGT-3′) and AD2plus (5′-CAATGGGTGCACACGGCTTTTGGCAGTCCAC-3′) primers. The AD2plus primer introduced 10 bp missing upstream of the stop codon, and the V1ATG primer eliminated a 200-bp addition upstream of the translation initiation site of VP1 that was present in the AD2-AD3 product. Thus, the V1ATG-AD2plus product contained the full open reading frame of VP1 without the stop codon (allowing C-terminal expression of V5 and six-His tags available from the commercial vector). PCR was performed using the same reagent concentrations as above but with 5 μl of plasmid DNA. The gel-purified 2,379-bp product was used for cloning into the baculovirus transfer vector pBlueBac4.5/V5-His-TOPO (Invitrogen).
The VP2 coding region was prepared using PCR reagents and concentrations as described above (as for V1ATG-AD2plus PCR), but 5 μl of gel-purified V1ATG-AD2plus product was used with forward primer V2ATG (5′-AGCATGACTTCAGTTAACTCTGCAGAAGC-3′) and reverse primer VSTP (5′-TGTTTACAATGGGTGGACACGGCTTTT-3′). VSTP primer introduced the TAA stop codon back to the VP2 polypeptide. The gel-purified 1,671 bp of expected size were used for cloning into the baculovirus transfer vector pBlueBac4.5/V5-His-TOPO (Invitrogen).
Amplification of VP1 and VP2 regions for expression of genotype 1 proteins.
For expression of the VP2 of genotype 1, B19 DNA from a Belgian strain (see above) was used diluted to 107 IU/ml (34). Nested PCR with 10 μl of sample was performed with the VSTA-VOUT (first round) and V1ATG-VSTP (second round) primer pairs. Reagents and concentrations for both rounds were identical to those for the VSTA-VOUT amplification of genotype 3 (see above). The 2,385-bp agarose gel-purified product was cloned into pCR4-TOPO vector (Invitrogen) and transformed to One Shot TOP10 chemically competent E. coli cells (Invitrogen) according to the manufacturer's instructions. Six clones were sequenced, a consensus sequence was obtained, and one clone translating to the same VP1 amino acid sequence as that of the consensus was used to amplify the VP2 coding region by nested PCR (for reagent concentrations, see above). Five microliters of purified plasmid DNA was used with the V2ATG and VSTP primer pair.
Preparation of recombinant baculovirus and production of proteins.
Respective gel-purified amplicons of VP1 and VP2 were cloned into the baculovirus transfer vector pBlueBac4.5/V5-His-TOPO (Invitrogen) and transformed to One Shot TOP10 chemically competent E. coli cells (Invitrogen) according to the manufacturer's instructions. Baculovirus expression experiments were performed according to the manufacturer's instructions using the Bac-N-Blue expression system (Invitrogen) with Spodoptera frugiperda (Sf9) cells (Invitrogen) grown in SF-900 II medium with l-glutamine (Invitrogen) and the addition of 10 U/ml penicillin, 10 μg/ml streptomycin (Invitrogen), and 2 to 10% heat-inactivated fetal bovine serum (Invitrogen). For the production of capsids containing VP2 and VP1-2 proteins, the cell culture was infected (or coinfected for VP2 and VP1) with the respective baculoviral constructs using a multiplicity of infection of 0.4 to 4. For the production of VP1-2 recombinant proteins with 4% or VP1 protein, the ratio of the multiplicity of infection of VP2 versus VP1 was 10:1 as described previously (3).
Protein purification and analysis.
Protein purification was performed as described previously with some modifications (14). Protein concentration was quantified in duplicate with a bicinchoninic acid protein assay (Perbio Science, Cramlington, United Kingdom) according to the manufacturer's instructions. Confirmatory quantification was performed by scanning Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels using serial dilutions of known concentrations of albumin standard (Perbio). Images were captured using GeneSnap software version 6.03, and an analysis of protein purity and the proportion of VP2 to VP1 was performed with Coomassie-stained SDS-PAGE images by GeneTools software version 3.05 (Syngene, Cambridge, United Kingdom). The identity of the proteins was verified by Western blotting using mouse monoclonal antibodies against VP2 (Chemicon, Chandlers Ford, United Kingdom). Visualization of capsid structures was done on glow-discharged carbon-coated copper grids with 2% phosphotungstic acid and negative staining transmission electron microscopy using a Philips CM 100 (Philips, The Netherlands) electron microscope (Multi-Imaging Centre, Department of Anatomy, University of Cambridge, United Kingdom).
DNA isolation and purification.
DNA was isolated from clinical samples using a High Pure viral nucleic acid kit (Roche) and eluted in 50 μl of elution buffer according to the manufacturer's instructions. Purification of DNA was conducted by excision of amplicons of the expected lengths from the 1 to 1.5% agarose gels and purification by a QIAquick PCR purification kit (QIAGEN Ltd, Crawley, United Kingdom) according to the manufacturer's instructions. Purification of the recombinant plasmids was performed using a QIAprep spin mini prep kit (QIAGEN) according to the manufacturer's instructions.
Detection, quantification, and molecular analysis of B19 DNA.
All selected samples from United Kingdom and Ghanaian blood donors and 200 of 400 samples from Ghanaian children were tested for the presence of B19 DNA by quantitative PCR as described previously (6). Viral DNA was isolated singly (Ghanaian children) or in pools of 10 samples (United Kingdom and Ghanaian donors) by the High Pure viral nucleic acid kit (Roche). Positive pools were resolved, and samples reactive in at least one replicate were retested and confirmed by in-house GAPSi-USTO seminested PCR using GAPS/GAPSi and USTO primers in the C-terminal part of the VP1 unique region as described previously (27a). The product of the seminested PCR was digested by DraI restriction endonuclease (New England Biolabs, Hitchin, United Kingdom) to differentiate genotype 1 from genotypes 2 and 3 as described previously (27a). Sequencing was performed as previously described (6). The genotypes of all GAPSi-USTO seminested PCR products were determined by restriction enzyme digestion and subsequent sequencing of the amplicons. GenBank accession numbers of the seminested PCR sequences are DQ234769, DQ234771, DQ234774, DQ234776-DQ234781, DQ234783-DQ234789, and DQ293996-DQ294008. Phylogenetic analysis was performed with PAUP 4.0b10 software using the Kimura 2-parameter model for genetic distances and the neighbor-joining tree inferring method. The reliability of the inferred trees was evaluated with bootstrap analysis of over 100 replicates.
Immunoassays.
IgM and IgG antibodies to B19 were tested using corresponding parvovirus B19 EIAs (Biotrin International, Dublin, Ireland) according to the manufacturer's instructions.
In addition to the commercial Biotrin IgG EIA (also referred to in this report as VP2G1Kit), which employs baculovirus-produced VP2 genotype 1 protein, four different antigens (also referred to in this report as Cam [short for Cambridge] antigens to differentiate between commercial and in-house EIAs) were used to detect IgG antibodies to B19. (i) Cam1 EIA used VP2 genotype 1 (also referred to as VP2G1Cam) protein derived from the Belgian strain (see above). (ii) Cam2 EIA used VP2 genotype 3 (also referred to as VP2G3Cam) protein from the Ghanaian strain (see above). (iii) Cam3 EIA used VP1-2 genotype 3 (also referred to as VP1-2G3Cam) protein from the same Ghanaian strain. (iv) Cam4 EIA used VP1-2 genotype 1 (also referred to as VP1-2G1Cam) produced by MedImmune (3) and, according to the manufacturer, contained approximately 41% of VP1.
Biotrin received coded antigen preparations and used the same standardized method to coat microtiter plates. The same reagents and manufacturing conditions as well as positive and negative controls were used for all four Cam antigen-coated plates. Except those from Ghanaian adults, the same samples were tested simultaneously in duplicate with all five antigens. The four sets of plates coated with the Cam/MedImmune antigens were received and tested under code. Blinding was bilaterally broken after all testing was completed. Five hundred samples from Ghanaian and United Kingdom blood donors were tested with the commercial kit. In addition, 138 Ghanaian adult donors were tested with the four Cam antigens. Four hundred samples from United Kingdom donors and Ghanaian children were tested with all five antigens (the commercial EIA and four “Cam” assays).
Sample dilutions of 1:100 were used. Averaged optical densities (at 450 versus 630 nm) were used to calculate sample-to-cutoff ratios (index, S/CO). The cutoff was set against the International Standard for Anti-Parvovirus B19 plasma (NIBSC code 01/602; Potters Bar, United Kingdom) at a level between 2 and 4 IU. Samples with index values of <0.9 or >1.1 were considered negative or positive, respectively. Samples with index values in the range of 0.9 to 1.1 were considered equivocal. Retested samples whose results were initially negative or equivocal and then equivocal or negative were considered negative. Otherwise, if results were discrepant with the initial test, the retested samples were considered equivocal. The approach recommended by the manufacturer to resolve equivocal results is to repeat testing on another sample collected a few days or two weeks after the initial testing. This could not be done with the retrospective samples used in this study. If final results (negative, positive, or equivocal) of tests comparing antigens differed, the samples were considered discordant; otherwise they were considered concordant.
Statistical analysis.
Statistical analysis was performed using Microsoft Excel (Microsoft Corporation) for Mac (release 1) and NCSS/PASS 2000 (Dawson edition) according to the method of Dawson and Trapp (10). Ninety-five percent confidence intervals (CIs) were calculated by the exact binomial method. Reliability between EIA test results was analyzed using kappa statistics. Correlation between EIA indexes was assessed by the Spearman rank correlation coefficient. Differences between EIA optical density and index values were analyzed by the Wilcoxon signed-rank test for differences in medians after assessing normality. A significance level of <0.05 was used.
RESULTS
Characterization of capture antigens.
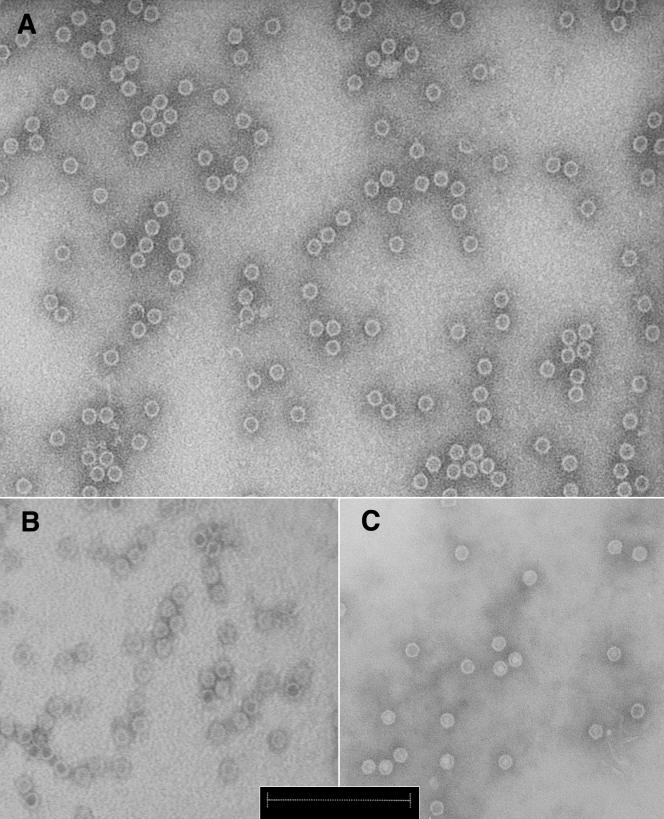
Both VP2-only and VP1-2 proteins formed capsids as shown by electron microscopy (Fig. 1). Purity of the proteins was ≥70%. The genotype 3 VP1-2 protein contained 4 to 10% VP1 according to screening by Coomassie-stained SDS-PAGE (data not shown).
FIG. 1.
Electron microscopy of recombinant B19 capsids produced in baculovirus system. (A) VP2 genotype 3 (VP2G3Cam) capsids. (B) VP1-2 genotype 3 (VP1-2G3Cam) capsids, including 4 to 10% of VP1. (C) VP1-2 genotype 1 (VP1-2G1Cam) capsids (MedImmune), including 41% of VP1. Negative staining transmission electron microscopy was performed with 2% phosphotungstic acid. Bar, 200 nm.
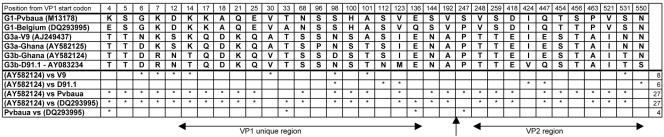
An analysis of differences in deduced amino acid composition between VP1 proteins used in this study and references available from GenBank is presented in Fig. 2. In the VP1 unique and VP2 proteins, three and one amino acid substitutions were found between expressed genotype 1 protein from the Belgian strain and the genotype 1 Pvbaua (M13178) reference, respectively. The expressed genotype 3 strain AY582124 differed from the reference V9 (AJ249437) by seven amino acids in the VP1 unique region and one in VP2. It was closer to the D91.1 (AY083234) strain, differing by three amino acids each in the VP1 unique and VP2 regions. The two expressed VP1 proteins (Belgian and Ghanaian strains) differed by 19 and 8 amino acids in VP1 unique and VP2, respectively.
FIG. 2.
Amino acid differences in VP1-unique/VP2 region between different B19 strains. G1 indicates B19 genotype 1. G3a indicates subcluster A of genotype 3 (V9 prototype), and G3b indicates subcluster B of genotype 3 (D91.1 prototype). Genotypes or subclusters are followed by the respective GenBank accession numbers of the sequences. Stars in the lower half of the figure indicate the positions of differences between pairs of strain sequences shown in the left column. An arrow indicates the boundary between the VP1 unique and VP2 regions. The total number of amino acid differences is shown in the right column.
Molecular epidemiological results.
The prevalence of B19 DNA in adult blood donors from United Kingdom and Ghana was 0.6% (3/500; 95% CI, 0.12 to 1.74). It was 11.5% in the Ghanaian children (23/200; 95% CI, 7.43 to 16.8). Samples from eight (34.8%) children that were positive for B19 DNA were also positive for IgM antibodies against B19. The seminested PCR product DraI restriction endonuclease digestion and subsequent sequencing and phylogenetic analysis indicated genotype 1 in samples from viremic United Kingdom donors and genotype 3 in samples from viremic Ghanaian children and blood donors. As a result, the United Kingdom and the Ghanaian populations were considered exposed to genotype 1 and genotype 3 strains, respectively. The child population had a male-to-female ratio of 1.37 and an average age of 23 months (range, 0.07 to 84 months).
Distribution of indexes (S/CO).
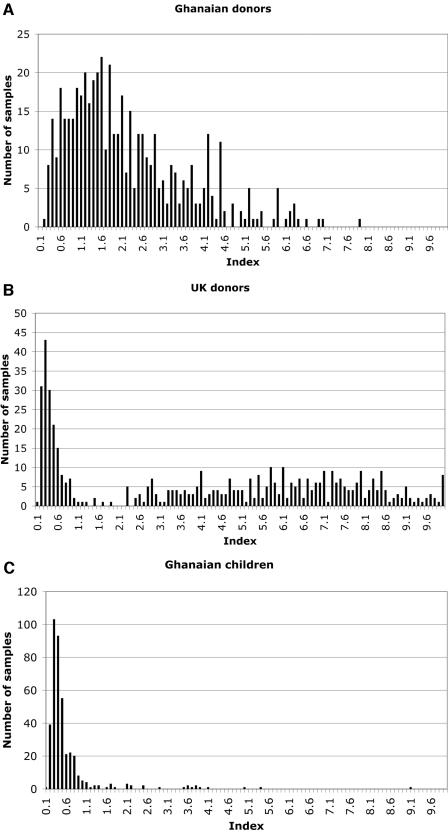
For each lot of experimental plates coated with coded Cam antigens, a cutoff of 2 to 4 IU based on the WHO International Standard was set by the plate manufacturer (Biotrin). The distribution of indexes was initially analyzed with the genotype-1-based Biotrin commercial kit results. As shown in Fig. 3, a bimodal distribution of indexes was clearly seen with samples from both United Kingdom donors and Ghanaian children but not with samples from adult Ghanaian blood donors. As a result, no clear differentiation between positive and negative results using assay index values or optical densities could be achieved in the latter population. Testing with the other four capture antigen preparations in 138 random adult Ghanaian blood donor samples reproduced the phenomenon, i.e., they did not show a bimodal distribution of indexes. As a result, the prevalence of antibodies to B19 in this group of samples could not be determined and the group was no longer included in the comparative evaluation of genotype 1 and genotype 3 antigens.
FIG. 3.
Distribution of VP2G1Kit assay indexes obtained with samples from 500 Ghanaian and United Kingdom blood donors and 400 Ghanaian children. The distribution of indexes reveals a clear separation between negative (index, <0.9) and positive (index, >1.1) in the United Kingdom blood donor and Ghanaian child populations but not in the Ghanaian adult blood donor population. As a result, the prevalence of antibody to B19 could not be determined in the latter population.
Comparison of reactivity of genotype 1 or genotype 3 population with VP2 antigens.
Cross-reactivity between genotype 1 and genotype 3 VP2 proteins was examined using VP2G1Cam and VP2G3Cam EIA tested with samples from areas of genotype 1 (United Kingdom) or genotype 3 (Ghana) prevalence. With the United Kingdom population, 271 samples reacted with both VP2G1Cam and VP2G3Cam antigens (Table 1). Three samples were discrepant in the equivocal category. Overall 99.3% (95% CI, 97.8 to 99.8) agreement between samples tested with VP2G3Cam and VP2G1Cam was observed. The value for the kappa coefficient for agreement between VP2G3Cam and VP2G1Cam was 0.98, while that for the Spearman correlation coefficient for assay indexes was 0.97.
TABLE 1.
Comparative distribution of EIA results for United Kingdom blood donor and Ghanaian child samples tested with VP2 genotype 1 and 3 antigens
| VP2G1Cam test result | No. of samples with VP2G3Cam test result
|
No. of samples discordant/total no. of samples | ||
|---|---|---|---|---|
| Positive | Negative | EQa | ||
| United Kingdom donorsb | ||||
| Positive | 271 | 0 | 2 | 2/273 |
| Negative | 0 | 125 | 0 | 0/125 |
| EQ | 0 | 1 | 1 | 1/2 |
| No. of samples discordant/total no. of samples | 0/271 | 1/126 | 2/3 | 3/400 |
| Ghanaian childrenc | ||||
| Positive | 30 | 1 | 2 | 3/33 |
| Negative | 0 | 359 | 2 | 2/361 |
| EQ | 2 | 1 | 3 | 3/6 |
| No. of samples discordant/total no. of samples | 2/32 | 2/361 | 4/7 | 8/400 |
EQ, equivocal.
Agreement of 99.3% (95% CI, 97.8 to 99.8) between samples tested with VP2G3Cam and VP2G1Cam was observed. Kappa and Spearman correlation coefficients were 0.98 and 0.97, respectively.
Agreement of 98.0% (95% CI, 96.1 to 99.1) between samples tested with VP2G3Cam and VP2G1Cam was observed. Kappa and Spearman correlation coefficients were 0.89 and 0.77, respectively.
In the study population of children from Ghana (area of endemicity for genotype 3), a comparison of VP2G1Cam and VP2G3Cam showed 8 of 400 samples to be discordant, with a majority falling into the equivocal category (Table 1). Results from 30 of 400 samples were B19 IgG positive and 359 of 400 were negative by both EIAs. One sample was positive by VP2G1Cam (mean index, 1.35) but negative by VP2G3Cam (mean index, 0.8) antigen. Ninety-eight percent (95% CI, 96.1 to 99.1) agreement between samples tested with VP2G3Cam and VP2G1Cam was observed in Ghanaian children. Kappa and Spearman correlation coefficients were 0.89 and 0.77, respectively.
Comparison of reactivities of G1 and G3 population samples with VP2 and VP1-2 capture antigens of corresponding genotypes.
Reactivities of the samples with VP2 versus VP1-2-based EIA were assessed. Genotype-1-based EIA was used for the analysis of the reactivity in the United Kingdom population, and genotype-3-based EIA was used for the Ghanaian child population. Results are presented in Table 2. For the United Kingdom donor samples, 271 samples were reactive with both VP2 and the VP1-2 assays. Three samples were classified as discordant because of equivocal results. Overall 99.3% (95% CI, 97.8 to 99.8) agreement between samples tested with VP2G1Cam and VP1-2G1Cam was observed. Kappa and Spearman correlation coefficients for this comparison were 0.98 and 0.95, respectively.
TABLE 2.
Comparative distribution of EIA results for United Kingdom blood donor and Ghanaian child samples with VP2 and VP1-2 antigens corresponding to the genotype of prevalent infection
| VP2 test resulta | No. of samples with VP1-2 test result
|
No. of samples discordant/total no. of samples | ||
|---|---|---|---|---|
| Positive | Negative | EQ | ||
| United Kingdom donorsb | ||||
| Positive | 271 | 0 | 2 | 2/273 |
| Negative | 0 | 125 | 0 | 0/125 |
| EQ | 0 | 1 | 1 | 1/2 |
| No. of samples discordant/total no. of samples | 0/271 | 1/126 | 2/3 | 3/400 |
| Ghanaian childrenc | ||||
| Positive | 31 | 0 | 1 | 1/32 |
| Negative | 1 | 353 | 7 | 8/361 |
| EQ | 3 | 1 | 3 | 4/7 |
| No. of samples discordant/total no. of samples | 4/35 | 1/354 | 10/11 | 13/400 |
For samples from United Kingdom donors, test results are for G1Cam; for samples from Ghanaian children, test results are for G3Cam. EQ, equivocal.
Agreement of 99.3% (95% CI, 97.8 to 99.8) between samples tested with VP2G1Cam and VP1-2G1Cam was observed. Kappa and Spearman correlation coefficients were 0.98 and 0.95, respectively.
Agreement of 96.8% (95% CI, 94.5 to 98.3) between samples tested with VP2G3Cam and VP1-2G1Cam was observed. Kappa and Spearman correlation coefficients were 0.83 and 0.80, respectively.
In the Ghanaian children, 13 of 400 samples were discordant between VP2G3Cam and VP1-2G3Cam EIAs (Table 2). By both EIAs, results from 31 of 400 samples were B19 IgG positive and 353 results were negative. One sample was positive by VP1-2G3Cam assay (mean index, 1.27) and negative by VP2G3Cam (mean index, 0.89), and the rest (n = 12) were discordant within the equivocal group. Agreement of 96.8% (95% CI, 94.5 to 98.3) between samples tested with VP2G3Cam and VP1-2G3Cam was observed. For this comparison, the Spearman correlation coefficient was 0.8 and the kappa coefficient was 0.83.
On the basis of these data, the prevalence of B19 antibodies was 67.8% (95% CI, 62.9 to 72.3; 271/400) in the United Kingdom blood donors and 7.75% (95% CI, 5.33 to 10.8; 31/400) in the Ghanaian children.
Comparison of reactivity between the G1 VP2 commercial kit and Cam antigen-based assay.
Since four Cam plates and the commercial kit were prepared with minor manufacturing differences, a comparison between VP2G1Cam and VP2G1Kit (commercial assay) was made (Table 3). Agreement of 97.8% (95% CI, 95.8 to 99.0; 391/400) was found in the United Kingdom population. Only one sample was truly discordant (negative with VP2G1Kit but positive with VP2G1Cam), whereas the rest of the differences were attributable to equivocal results. Spearman and kappa coefficients were 0.94 and 0.95, respectively.
TABLE 3.
Comparative distribution of EIA results for VP2G1 and VP2G1Kit EIA in two study populations
| VP2G1Kit test result | No. of samples with VP2G1Cam test result
|
No. of samples discordant/total no. of samples | ||
|---|---|---|---|---|
| Positive | Negative | EQa | ||
| United Kingdom donorsb | ||||
| Positive | 270 | 0 | 0 | 0/270 |
| Negative | 1 | 121 | 2 | 3/124 |
| EQ | 2 | 4 | 0 | 6/6 |
| No. of samples discordant/total no. of samples | 3/273 | 4/125 | 2/2 | 9/400 |
| Ghanaian childrenc | ||||
| Positive | 27 | 0 | 2 | 2/29 |
| Negative | 5 | 356 | 4 | 9/365 |
| EQ | 1 | 5 | 0 | 6/6 |
| No. of samples discordant/total no. of samples | 5/33 | 5/361 | 6/6 | 17/400 |
EQ, equivocal.
Agreement of 97.8% (95% CI, 95.8 to 99.0) between samples tested with VP2G1Cam and VP2G1Kit was observed. Kappa and Spearman correlation coefficients were 0.95 and 0.94, respectively.
Agreement of 95.8% (95% CI, 93.3 to 97.5) between samples tested with VP2G1Cam and VP2G1Kit was observed. Kappa and Spearman correlation coefficients were 0.75 and 0.63, respectively.
In samples from Ghanaian children, 17 discrepancies were found, with five samples negative by VP2G1Kit but positive by VP2G1Cam and the rest discrepant because of one equivocal result. Agreement of 95.8% (95% CI, 93.3 to 97.5; 383/400) was found comparing these two EIA results. Spearman and kappa coefficients were 0.63 and 0.75, respectively. To assess whether these differences were related to the levels of reactivity, medians of assay index values were compared for samples from the United Kingdom and the Ghanaian child population. For both comparisons, the P value was <0.001, with the VP2G1Kit median index being higher than that of VP2G1Cam in the samples from the United Kingdom population but lower in those from the Ghanaian children. Comparisons of VP2G1Kit with VP2G3Cam and VP1-2G1Cam in the samples from the United Kingdom population and VP2G1Kit with VP2G3Cam in the samples from Ghanaian children were also made, and slightly more discrepancies were found compared to those with the VP2G1Cam (data not shown).
DISCUSSION
This study intended to assess B19 IgG cross-reactivity with genotype 1 and genotype 3 recombinant structural proteins and to evaluate the effect of including VP1 antigen in VP2-only-based assay systems. It confirmed previous findings that, in Kumasi, Ghana, the prevalent genotype is genotype 3 (6, 27a). The genetic distances of two near-full-length sequences of B19 genotype 3 from genotype 1 were estimated to range between 13.7% and 14.2% (31). The previously published VP1 genotype 3 and our additional Ghanaian sequences further supported the proposition that genotype 3 clustered in two subtypes, A and B (6). The subtype A prototype included V9 (AJ249437) and our Ghanaian strain (AY582125). Subtype B included reference strain D91.1 (AY083234) and another Ghanaian strain sequence (AY582124) expressed in this study. Upon phylogenetic analysis (data not shown), separation between these subtypes in the VP1 region was supported by a bootstrap value of 95. The subclustering was retained (although with the lower bootstrap value of 85) when VP1 amino acid sequences were analyzed (data not shown). The majority of amino acid differences were found in the VP1 unique region; only eight were different in the VP2 region (Fig. 2). Although the Parker antigenicity and hydrophobicity/hydrophilicity profile did not identify major antigenic differences (data not shown), the observed amino acid differences might be sufficient to alter the overall antigenicity of the tested capsids.
The reactivity and the cross-genotype cross-reactivity of five B19 structural antigens were evaluated initially with three, but ultimately with two, sample populations from areas where genotype 1 and genotype 3 are endemic. While good separation between IgG-negative and -positive samples was observed for the United Kingdom donor population using all five EIA systems, such separation was not present in the Ghanaian adult blood donor group (Fig. 3). However, in the Ghanaian children, better separation between IgG-negative and -positive samples was observed, allowing the use of the first and third populations for assay comparison. The pattern seen in the adult Ghanaian population was consistently observed, irrespective of the antigen used. This phenomenon was therefore not related to genotype or type of antigen but rather to assay protocol, the samples themselves, or a combination of both.
The data presented here indicate that samples from adult Ghanaians (and likely other sub-Saharan populations) react abnormally under the current enzyme-linked immunosorbent assay, probably due to the hyperimmunized status of adults in this region. This phenomenon has been described for human immunodeficiency virus (HIV) serological testing in Africa. In one study comparing 36 commercial immunoassays for HIV detection, some had higher rates of nonspecific reactions with African samples compared to those with samples of non-African origin (36). A high frequency of false-positive or indeterminate samples was observed in other studies of anti-HIV reactivity in samples of sub-Saharan African origin (11, 18). Similar results showing high or higher false-positive rates in sub-Saharan African countries, including Ghana, were often reported with other viruses, such as hepatitis C virus (HCV) (2, 29, 33, 35). Hypergammaglobulinemia, arising from the multiplicity of intercurrent infections occurring in sub-Saharan Africa, was discussed as a potential cause for the increased anti-HCV false-positive results in samples from this region (29, 35). In one study, mean IgG levels for a population from Tanzania were about two times higher than those of a control U.S. population; however, no clear correlation was observed between anti-HCV EIA false positivity and the level of IgG (33). This is consistent with data observed in the current study. IgG levels for Ghanaian adult blood donors were previously estimated to range from 12 to 30 mg/ml (6, 27a). However, this concentration per se was not accounting for the index distribution since it was not significantly improved by a sample dilution of 1:200 instead of 1:100 for all five EIAs. Interestingly, malaria, a common disease in Ghana, was linked with false-positive anti-HCV results (1) and might play a role in the results obtained here with adult Ghanaian blood donors. It should be kept in mind that cross-reactivity of B19 and simian parvovirus antigens, which show high degrees of similarity with B19 (5, 24) and might be potentially transmitted as zoonosis, might be a reason for an atypical EIA reactivity result in sub-Saharan Africa (5).
The discrepancies observed in the previous study (6, 7) that triggered the present investigation were probably related to the difficulty of identifying truly positive results, as experienced here while investigating a similar population of Ghanaian blood donors. Investigation of the nature of the irregular reactivity of Ghanaian adult plasmas is the subject of a follow-up to the present study.
The assessment of IgG cross-reactivity between VP2 antigens of different genotypes did not reveal any major differences between antigen genotype when utilized to test samples from United Kingdom blood donor or Ghanaian child populations (Table 1). Less then 1% of samples in the United Kingdom were discordant between VP2 genotype 3- and 1-based assays, and a high level of agreement was found, supported by Spearman correlation coefficient and kappa statistics. These data confirm and extend the results of Heegaard et al. (14), who found 100% cross-reactivity in a European population tested for IgG with EIA using VP2 of genotype 1 and 3 but did not have an opportunity to test a population infected by genotype 3. Such a population was provided by Ghanaian child samples that reacted equally well with genotype 1 and genotype 3 VP2 antigens. A high degree of cross-reactivity between genotype 3 and 1 antigens was observed in samples from the population exposed to genotype 1 B19 (99.3%, United Kingdom) and in samples from the population exposed to genotype 3 B19 (98%, Ghana). Spearman correlation and kappa coefficients were higher, however, with genotype 1 than with genotype 3 populations, irrespective of the genotype of the antigen used (data not shown). Results were slightly more discordant when the VP2 genotype 1-based commercial kit was compared to the genotype-3-based VP2 EIA. However these results were not related to the genotype of the antigen, since results were consistent when the genotype 1 VP2 EIA based on the Cam antigen was compared to the genotype 3 VP2 EIA. To further study this apparent discrepancy, VP2G1Cam and VP2G1Kit reactivities were compared with those of United Kingdom and Ghana samples (Table 3). A high level of agreement between these EIAs was seen in results from the United Kingdom population (97.8%) and the Ghanaian children (95.8%). Lower kappa and Spearman correlation coefficients were obtained from analysis of the Ghanaian group versus the United Kingdom group (0.75 and 0.63 versus 0.95 and 0.94, respectively) (Table 3). There were minor, but measurable, differences in the optical density or index values between these groups, suggesting that differences in reactivity are related to the normal range of lot-specific manufacturing variations but not to antigens.
A similarly high level of agreement between EIA results and index values was found in samples from United Kingdom and Ghanaian populations tested for IgG with VP1-2 or VP2 antigens (Table 2). For genotype 1 antigens in the United Kingdom population, the level of agreement was 99.3%. Only three discrepant results between plates were found in this population, and they were related to equivocal results. When genotype 3 VP1-2 and VP2 IgG reactivities were compared in the Ghanaian child population, the agreement was 96.8%; however, correlation between indexes or the EIA results was slightly lower than that for a similar comparison in the United Kingdom populations. Thirteen samples were discordant, with only one testing IgG positive for VP1-2 and negative for VP2 only. These results are in agreement with the previously reported data on the cross-reactivity between VP2-only and VP1-2 IgG in an EIA system (14, 19, 20, 30). The kappa coefficient reported by others comparing VP1-2 IgG EIA (Medac, Germany) with VP2 EIA (Biotrin, Ireland) in an Italian blood donor population was 0.85 (19). Substantially higher coefficients of agreement found in this study between V1-2 and VP2 EIAs in the United Kingdom population might be accounted for by the higher degree of comparability of VP1-2 and VP2 EIA used in this study. Interestingly, one of the VP1-2 antigens (VP1-2G3Cam) contained only 4 to 10% of VP1 in the capsid structure and the other (VP1-2G1Cam) contained approximately 40% of VP1 and no significant difference in IgG capture with VP2-only EIAs was detected. These data suggest that higher concentrations of VP1 than that found in the natural viral capsid do not significantly improve antibody detectability. It was previously reported that the antigenicity of the capsids containing VP1-2 proteins is different from that of the VP2-only capsids (28). Similarly, there were reports indicating that some samples might react with VP2-only capsids but not with VP2 capsids containing VP1 and vice versa (19-21). Although there might be differences in the antigenicity of VP1-2 and VP2-only capsids, they might be minor and not lead to significant changes in the overall reactivity of the antibody pool comprised of antibodies to multiple epitopes present in the capsid proteins.
Overall, this comparative study indicated that genotype 1 VP2 antigen was as effective as genotype 3 VP2 antigen in detecting IgG to genotype 3 B19 and vice versa. In addition, the inclusion of VP1 in the VP2-based serodiagnostic assays did not significantly improve the sensitivity of detection of IgG in the EIA format. Thus, assays based on VP2-only antigens have sufficient sensitivity to detect antibodies to B19 of genotype 1 and genotype 3 in archetypal populations.
Acknowledgments
We are thankful to E. Addo-Yobo and the staff of the Pediatric Emergency Unit and the staff of the Transfusion Medicine Unit of Komfo Anokye Hospital, Kumasi, Ghana, for assistance in collecting blood samples. We are grateful to MedImmune for providing recombinant genotype 1 VP1-2 protein. We thank I. Thomas and R. Laub for providing the B19 DNA-positive Belgian blood donor sample for genotype 1 protein expression. We are very grateful to J. Skepper, J. Powell, and L. Carter (Multi-Imaging Centre, Department of Anatomy, University of Cambridge, United Kingdom) for assistance with the electron microscopy.
This study was supported in part by a National Blood Service, United Kingdom, PhD grant to A. Parsyan.
REFERENCES
- 1.Aceti, A., G. Taliani, C. de Bac, and A. Sebastiani. 1990. Anti-HCV false positivity in malaria. Lancet 336:1442-1443. [DOI] [PubMed] [Google Scholar]
- 2.Ampofo, W., N. Nii-Trebi, J. Ansah, K. Abe, H. Naito, S. Aidoo, V. Nuvor, J. Brandful, N. Yamamoto, D. Ofori-Adjei, and K. Ishikawa. 2002. Prevalence of blood-borne infectious diseases in blood donors in Ghana. J. Clin. Microbiol. 40:3523-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal, G. P., J. A. Hatfield, F. E. Dunn, A. A. Kramer, F. Brady, C. H. Riggin, M. S. Collett, K. Yoshimoto, S. Kajigaya, and N. S. Young. 1993. Candidate recombinant vaccine for human B19 parvovirus. J. Infect. Dis. 167:1034-1044. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. S., J. W. Van Lent, J. M. Vlak, and W. J. Spaan. 1991. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J. Virol. 65:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, K. E., Z. Liu, G. Gallinella, S. Wong, I. P. Mills, and M. G. O'Sullivan. 2004. Simian parvovirus infection: a potential zoonosis. J. Infect. Dis. 190:1900-1907. [DOI] [PubMed] [Google Scholar]
- 6.Candotti, D., N. Etiz, A. Parsyan, and J. P. Allain. 2004. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J. Virol. 78:12169-12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran, A., S. Doyle, J. P. Allain, D. Candotti, and A. Parsyan. 2005. Evidence of serological cross-reactivity between genotype 1 and genotype 3 erythrovirus infections. J. Virol. 79:5238-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotmore, S. F., V. C. McKie, L. J. Anderson, C. R. Astell, and P. Tattersall. 1986. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J. Virol. 60:548-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotmore, S. F., and P. Tattersall. 1984. Characterization and molecular cloning of a human parvovirus genome. Science 226:1161-1165. [DOI] [PubMed] [Google Scholar]
- 10.Dawson, B., and R. G. Trapp. 2001. Basic and clinical biostatistics, 3rd ed. McGraw-Hill, New York, N.Y.
- 11.Delaporte, E., M. Peeters, F. Simon, A. Dupont, D. Schrijvers, D. Kerouedan, R. Josse, M. Merlin, A. Trebucq, M. Collet, et al. 1989. Interpretation of antibodies reacting solely with human retroviral core proteins in western equatorial Africa. AIDS 3:179-182. [DOI] [PubMed] [Google Scholar]
- 12.Erdman, D. D., M. J. Usher, C. Tsou, E. O. Caul, G. W. Gary, S. Kajigaya, N. S. Young, and L. J. Anderson. 1991. Human parvovirus B19 specific IgG, IgA, and IgM antibodies and DNA in serum specimens from persons with erythema infectiosum. J. Med. Virol. 35:110-115. [DOI] [PubMed] [Google Scholar]
- 13.Heegaard, E. D., I. Panum Jensen, and J. Christensen. 2001. Novel PCR assay for differential detection and screening of erythrovirus B19 and erythrovirus V9. J. Med. Virol. 65:362-367. [DOI] [PubMed] [Google Scholar]
- 14.Heegaard, E. D., K. Qvortrup, and J. Christensen. 2002. Baculovirus expression of erythrovirus V9 capsids and screening by ELISA: serologic cross-reactivity with erythrovirus B19. J. Med. Virol. 66:246-252. [DOI] [PubMed] [Google Scholar]
- 15.Hicks, K. E., R. C. Cubel, B. J. Cohen, and J. P. Clewley. 1996. Sequence analysis of a parvovirus B19 isolate and baculovirus expression of the non-structural protein. Arch. Virol. 141:1319-1327. [DOI] [PubMed] [Google Scholar]
- 16.Hokynar, K., M. Soderlund-Venermo, M. Pesonen, A. Ranki, O. Kiviluoto, E. K. Partio, and K. Hedman. 2002. A new parvovirus genotype persistent in human skin. Virology 302:224-228. [DOI] [PubMed] [Google Scholar]
- 17.Kajigaya, S., H. Fujii, A. Field, S. Anderson, S. Rosenfeld, L. J. Anderson, T. Shimada, and N. S. Young. 1991. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc. Natl. Acad. Sci. USA 88:4646-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhls, T. L., P. G. Nishanian, J. D. Cherry, J. P. Shen, C. G. Neumann, E. R. Stiehm, R. B. Ettenger, N. O. Bwibo, and D. Koech. 1988. Analysis of false positive HIV-1 serologic testing in Kenya. Diagn. Microbiol. Infect. Dis. 9:179-185. [DOI] [PubMed] [Google Scholar]
- 19.Manaresi, E., G. Gallinella, A. M. Morselli Labate, P. Zucchelli, D. Zaccarelli, S. Ambretti, S. Delbarba, M. Zerbini, and M. Musiani. 2004. Seroprevalence of IgG against conformational and linear capsid antigens of parvovirus B19 in Italian blood donors. Epidemiol. Infect. 132:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manaresi, E., G. Gallinella, S. Venturoli, M. Zerbini, and M. Musiani. 2004. Detection of parvovirus B19 IgG: choice of antigens and serological tests. J. Clin. Virol. 29:51-53. [DOI] [PubMed] [Google Scholar]
- 21.Manaresi, E., E. Zuffi, G. Gallinella, G. Gentilomi, M. Zerbini, and M. Musiani. 2001. Differential IgM response to conformational and linear epitopes of parvovirus B19 VP1 and VP2 structural proteins. J. Med. Virol. 64:67-73. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen, Q. T., C. Sifer, V. Schneider, F. Bernaudin, V. Auguste, and A. Garbarg-Chenon. 1998. Detection of an erythrovirus sequence distinct from B19 in a child with acute anaemia. Lancet 352:1524. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen, Q. T., S. Wong, E. D. Heegaard, and K. E. Brown. 2002. Identification and characterization of a second novel human erythrovirus variant, A6. Virology 301:374-380. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan, M. G., D. C. Anderson, J. D. Fikes, F. T. Bain, C. S. Carlson, S. W. Green, N. S. Young, and K. E. Brown. 1994. Identification of a novel simian parvovirus in cynomolgus monkeys with severe anemia. A paradigm of human B19 parvovirus infection. J. Clin. Invest. 93:1571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozawa, K., J. Ayub, Y. S. Hao, G. Kurtzman, T. Shimada, and N. Young. 1987. Novel transcription map for the B19 (human) pathogenic parvovirus. J. Virol. 61:2395-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozawa, K., J. Ayub, and N. Young. 1988. Functional mapping of the genome of the B19 (human) parvovirus by in vitro translation after negative hybrid selection. J. Virol. 62:2508-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozawa, K., and N. Young. 1987. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J. Virol. 61:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Parsyan, A., et al. Effects of transfusion on human erythrovirus B19 susceptible or infected pediatric recipients in a genotype 3 endemic area. Transfusion, in press. [DOI] [PubMed]
- 28.Rosenfeld, S. J., N. S. Young, D. Alling, J. Ayub, and C. Saxinger. 1994. Subunit interaction in B19 parvovirus empty capsids. Arch. Virol. 136:9-18. [DOI] [PubMed] [Google Scholar]
- 29.Rouet, F., M. L. Chaix, A. Inwoley, P. Msellati, I. Viho, P. Combe, V. Leroy, F. Dabis, and C. Rouzioux. 2004. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Cote d'Ivoire: the ANRS 1236 study. J. Med. Virol. 74:34-40. [DOI] [PubMed] [Google Scholar]
- 30.Salimans, M. M., M. J. van Bussel, C. S. Brown, and W. J. Spaan. 1992. Recombinant parvovirus B19 capsids as a new substrate for detection of B19-specific IgG and IgM antibodies by an enzyme-linked immunosorbent assay. J. Virol. Methods 39:247-258. [DOI] [PubMed] [Google Scholar]
- 31.Servant, A., S. Laperche, F. Lallemand, V. Marinho, G. De Saint Maur, J. F. Meritet, and A. Garbarg-Chenon. 2002. Genetic diversity within human erythroviruses: identification of three genotypes. J. Virol. 76:9124-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summers, J., S. E. Jones, and M. J. Anderson. 1983. Characterization of the genome of the agent of erythrocyte aplasia permits its classification as a human parvovirus. J. Gen. Virol. 64:2527-2532. [DOI] [PubMed] [Google Scholar]
- 33.Tess, B. H., A. Levin, G. Brubaker, J. Shao, J. E. Drummond, H. J. Alter, and T. R. O'Brien. 2000. Seroprevalence of hepatitis C virus in the general population of northwest Tanzania. Am. J. Trop. Med. Hyg. 62:138-141. [DOI] [PubMed] [Google Scholar]
- 34.Thomas, I., M. Di Giambattista, C. Gerard, E. Mathys, V. Hougardy, B. Latour, T. Branckaert, and R. Laub. 2003. Prevalence of human erythrovirus B19 DNA in healthy Belgian blood donors and correlation with specific antibodies against structural and non-structural viral proteins. Vox Sang. 84:300-307. [DOI] [PubMed] [Google Scholar]
- 35.Trepo, C., F. Zoulim, C. Alonso, M. A. Petit, C. Pichoud, and L. Vitvitski. 1993. Diagnostic markers of viral hepatitis B and C. Gut 34:S20-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Kerckhoven, I., G. Vercauteren, P. Piot, and G. van der Groen. 1991. Comparative evaluation of 36 commercial assays for detecting antibodies to HIV. Bull. W. H. O. 69:753-760. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong, S., M. Momoeda, A. Field, S. Kajigaya, and N. S. Young. 1994. Formation of empty B19 parvovirus capsids by the truncated minor capsid protein. J. Virol. 68:4690-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young, N. S., and K. E. Brown. 2004. Parvovirus B19. N. Engl. J. Med. 350:586-597. [DOI] [PubMed] [Google Scholar]