Abstract
Isothermal nucleic acid sequence-based amplification (NASBA) was applied to the detection of Chlamydophila pneumoniae 16S rRNA by using the NucliSens basic kit (bioMérieux, Boxtel, The Netherlands). The assay was originally developed as a conventional NASBA assay with electrochemiluminescence detection and was subsequently adapted to a real-time NASBA format by using a molecular beacon. C. pneumoniae RNA prepared from a plasmid construct was used to assess the analytical sensitivity of the assay. The sensitivity of the NASBA assay was 10 molecules of in vitro wild-type C. pneumoniae RNA and 0.1 inclusion-forming unit (IFU) of C. pneumoniae. In spiked respiratory specimens, the sensitivity of the C. pneumoniae NASBA assay varied between 0.1 and 1 IFU/100 μl sample, depending on the type of specimen. Finally, conventional and real-time NASBA were applied to respiratory specimens previously tested by PCR. A 100% concordance between the test results was obtained.
Chlamydophila pneumoniae is a common etiologic agent of respiratory tract infections in humans and is responsible for 3% of all cases of community-acquired pneumonia and 43% of all cases during an isolated miniepidemic (8, 13, 16, 23, 24). It has been associated with asthma (12), chronic obstructive pulmonary disease (21), chronic pharyngitis (9), a wide range of mild to serious extrapulmonary complications (11), and atherosclerosis (5).
In the past, diagnosis of infection by this organism was based on the detection of fourfold rises in antibody titers in the microimmunofluorescence test or an elevated immunoglobulin G (IgG) or IgM titer when only a single serum sample was available. Culture confirmation of the diagnosis of C. pneumoniae infection, followed by the propagation of clinical isolates, has proven difficult to date. Therefore, nucleic acid amplification techniques have been introduced. PCR was shown to be considerably more sensitive than culture for the detection of C. pneumoniae (1, 2).
Real-time nucleic acid sequence-based amplification (NASBA; bioMérieux, Boxtel, The Netherlands) is targeted at RNA. NASBA makes use of the simultaneous enzymatic activities of avian myeloblastosis virus reverse transcriptase, RNase H, and T7 RNA polymerase and molecular beacons (15) under isothermal conditions.
The whole process of amplification and detection runs in a fluorescent reader. The same amplicons can also be used for conventional electrochemiluminescence (ECL) detection (14). The real-time NASBA technique has already been successfully applied for the quantification of human immunodeficiency virus type 1 isolates (7) and for the detection of Mycoplasma pneumoniae (18).
The aim of this study was to develop both a conventional assay and a real-time NASBA assay for the detection of C. pneumoniae in respiratory specimens based on NASBA amplification of a 16S rRNA target sequence by using the NucliSens basic kit (10, 17) and to compare the results of the two assays.
MATERIALS AND METHODS
Bacterial strains
C. pneumoniae (ATCC VR-1355) was grown on HEp-2 cells. Shell vials were inoculated with C. pneumoniae cells. After inoculation, the cell culture plates were centrifuged at 3,500 rpm and 25°C for 60 min and subsequently incubated at 37°C for 1 h. Then, the medium was aspirated and the cell cultures were incubated with fresh medium containing cycloheximide (1 mg/liter). After 3 days, the shell vials were aspirated and fixed with 96% ethanol. The fixed monolayers were rinsed once with phosphate-buffered saline and stained by the fluorescent-antibody technique with C. pneumoniae-specific mouse monoclonal antibodies (Dako A/S, Glostrup, Denmark). Rabbit anti-mouse immunoglobulin labeled with fluorescein isothiocyanate (Dako A/S) was used as a conjugate.
C. pneumoniae (ATCC VR-1355) was quantified by incubation of five replicates of 10-fold dilutions on HEp-2 cells. The numbers of inclusion-forming units (IFUs) were counted 72 h after infection by immunofluorescence microscopy. The titer was expressed in IFU/ml.
The bacterial strains used to test the specificities of the NASBA primers are presented in Table 1. Mycoplasma strains were cultured in spiroplasma (SP4) medium (25) without thallium acetate and supplemented with amphotericin B (0.5 mg/ml), polymyxin B (500 U/ml), glucose (0.5%), and arginine (0.25%) or urea (0.5%), depending on the nutritional needs of the species. An input of at least 10,000 color-changing units in the extraction was used to test the specificity of the assay. Legionella strains were grown on buffered charcoal-yeast (Oxoid Ltd., Belgium) agar plates at 37°C for 48 to 72 h. An input of 10,000 CFU in the extraction was used to test the specificity of the assay. Other bacteria were cultivated on standard media supporting optimal growth. The clinical isolates were identified by standard methods. An input of 10 μl of a 1/50 dilution of a 0.5 McFarland standard solution in the extraction was used to test the specificity of the assay.
TABLE 1.
Bacterial species and strains
| Strain no. | Strain or source | Species | Origina | ECL countb | Real-time resultc |
|---|---|---|---|---|---|
| 1 | ATCC VR-1355 | Chlamydophila pneumoniae | ATCC | 124562 | 14.30 |
| 2 | Clinical isolate | Chlamydia psittaci | UZA | 250 | 1.51 |
| 3 | Kiq10 | Chlamydia trachomatis | ITG | 247 | 1.23 |
| 4 | ATCC 29085 (PI1428) | Mycoplasma pneumoniae type 1 | ATCC | 285 | 1.31 |
| 5 | ATCC 15492 (MAC) | Mycoplasma pneumoniae type 2 | ATCC | 270 | 1.43 |
| 6 | NC10117 | Mycoplasma fermentans | NTCC | 269 | 1.29 |
| 7 | NC10111 | Mycoplasma hominis | NCTC | 199 | 1.48 |
| 8 | ATCC 33530 (G-37) | Mycoplasma genitalium | ATCC | 245 | 1.51 |
| 9 | NC10112 | Mycoplasma orale | NCTC | 395 | 1.03 |
| 10 | NC10136 | Mycoplasma buccale | NCTC | 296 | 1.33 |
| 11 | NC10113 | Mycoplasma salivarium | NCTC | 221 | 1.44 |
| 12 | NC11702 | Mycoplasma pirum | NCTC | 287 | 1.38 |
| 13 | Clinical isolate | Ureaplasma urealyticum | UZA | 192 | 1.42 |
| 14 | Clinical isolate | Legionella pneumophila | UZA | 170 | 1.38 |
| 15 | Clinical isolate | Moraxella catarrhalis | UZA | 236 | 1.65 |
| 16 | Clinical isolate | Haemophilus influenzae | UZA | 213 | 1.36 |
| 17 | Clinical isolate | Streptococcus pneumoniae | UZA | 240 | 1.49 |
| 18 | Clinical isolate | Streptococcus pyogenes | UZA | 201 | 1.31 |
| 19 | Clinical isolate | Viridans group streptococcus | UZA | 284 | 1.14 |
| 20 | Clinical isolate | Staphylococcus aureus | UZA | 256 | 1.54 |
| 21 | Clinical isolate | Klebsiella pneumoniae | UZA | 265 | 1.12 |
| 22 | Clinical isolate | Escherichia coli | UZA | 278 | 1.28 |
| 23 | Clinical isolate | Neisseria meningitidis | UZA | 230 | 1.14 |
| 24 | Clinical isolate | Pseudomonas aeruginosa | UZA | 199 | 1.35 |
ITG, Instituut voor Tropsiche Geneeskunde, Antwerp, Belgium; ATCC, American Type Culture Collection, Manassas, Va.; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom; UZA, Universitair Ziekenhuis Antwerpen, Edegem, Belgium.
Counts above 750 are positive.
Real-time NASBA assay result; counts above 1.81 are positive.
Respiratory specimens
Pools of throat swabs, bronchoalveolar lavage (BAL) specimens, nasopharyngeal aspirates (NPAs), sputum specimens, and bronchial aspirates (BA) that were obtained from the Microbiology Laboratory of the University Hospital of Antwerp and that individually tested negative for C. pneumoniae by PCR (26) were used as the suspension media. Sputum specimens were treated as such. A total of 1,000 μl of a BAL specimen was concentrated by centrifugation at 13,000 rpm for 10 min. A total of 800 μl of the supernatant was removed. The remaining 200 μl was used for DNA extraction with a QiaAmp Blood mini kit (QIAGEN, Hilden, Germany).
Four NPAs previously found to be positive and 100 specimens (18 throat swab, 20 BA, 20 NPA, 20 BAL, 20 sputum, and 2 gargle specimens) previously found to be negative for C. pneumoniae by conventional PCR (26) were also tested.
Nucleic acid extraction
Nucleic acids were extracted as described by Boom et al. (3) by using the NucliSens basic kit extraction module (bioMérieux). Briefly, 100 μl of all protease-treated clinical specimens, protease-treated respiratory specimen pools (20), or aliquots of a bacterial culture was added to a guanidinium thiocyanate (GuSCN) lysis solution, pH 6.2, and mixed vigorously for rapid lysis. Fifty microliters of activated silica was added. The nucleic acid-silica complex was washed twice with GuSCN washing solution, twice with 70% (vol/vol) ethanol, and once with acetone. After the complex was dried at 56°C, nucleic acids were eluted from the silica by using 50 μl elution buffer and were stored at −80°C.
Primer design, biotin capture probe, and molecular beacons
Oligonucleotide primers, the ECL probe, and molecular beacons were derived from the C. pneumoniae 16S rRNA sequence. For real-time NASBA, the sequence of primer P1 was 5′-AATTCTAATACGACTCACTATAGGGAAAGGTCCGAAGATCCCCTTCTTTA-3′, the sequence of primer P2 was 5′-GATGCAAGGTCGCATATGAGGATCTTAGTTCAGATTGAACGCT-3′, the sequence of the ECL capture probe was 5′-biotin-TGTAGTGTAATTAGGCATCTAAT-3′, and the sequence of the molecular beacon was 5′-FAM-CCGATCGTGTAGTGTAATTAGGCATCTAATATCGATCGG-DABCYL-3′, where FAM is 6-carboxyfluorescein and DABCYL is 4-(4-dimethylaminophenylazo)benzoic acid. The generic ECL detection probe was used as provided in the NucliSens basic kit.
Production of wild-type RNA
For the generation of wild-type RNA, cDNA from part of the 16S rRNA from C. pneumoniae strain ATCC VR-1355 was obtained by reverse transcriptase PCR with adapted versions of the NASBA primers, which contained an EcoRI site and a Csp45I site. The cDNA of the 16S rRNA was then inserted into a modified pGEM vector, resulting in plasmid pG3O Cp 16S rRNA.
Plasmids were transformed into Escherichia coli DH5α. Nucleotide sequence analysis did not reveal any mutations in the primer or probe annealing sites. These plasmids were used for large-scale generation of runoff transcripts after linearization with BamHI (Pharmacia Biotech). RNA was generated from the construct with T7 RNA polymerase (Pharmacia Biotech) in vitro, as described by Sambrook et al. (22). Plasmid DNA was removed by treatment with DNase I (Pharmacia Biotech). The RNA was quantitated spectrophotometrically, and the RNA was stored in lysis buffer at −80°C.
NASBA amplification and detection
NASBA reactions were performed with the NucliSens basic kit amplification module (bioMérieux) (10). In negative control reactions, target nucleic acid was replaced by RNase- and DNase-free water. The amplification reactions were incubated in a fluorescence reader, the NucliSens EasyQ analyzer (bioMérieux). The results were calculated with Ascent software (bioMérieux). Afterwards, the same amplification products were diluted 1/10 and were also analyzed by ECL detection with the NucliSens basic kit detection module and the NucliSens reader (bioMérieux). The ECL counts measured were processed and validated by the NucliSens basic kit user software.
To determine the cutoff for ECL detection, the counts for 100 different individual truly negative samples were measured. In each run, a tube with the reference solution was included. The measured counts were recalculated in reference to the same signal of the reference solution, i.e., 20,000 counts. The average and three times the standard deviation of the 100 measurements were calculated. The cutoff level is expressed relative to the signal of the reference solution, according to the following formula: (three times the standard deviation + average count)/20,000. The results of the NASBA assay were considered positive when the signal was above a cutoff of 0.017 times the signal of the reference solution (or above a cutoff of 2.01 times the signal of the negative control, which corresponds to the average of the negative samples plus three standard deviations).
To determine the cutoff for real-time detection, the signals for the same 100 different individual truly negative samples were measured. The mean was calculated. A sample was considered C. pneumoniae positive when the signal was above the mean of the negative samples plus 20%.
Sensitivity study
The sensitivity of the NASBA assay for C. pneumoniae 16S rRNA was studied by using 10-fold dilutions of suspensions of C. pneumoniae in physiologic water or dilutions of wild-type 16S rRNA generated in vitro in water. The sensitivity was also studied by using 10-fold dilutions of C. pneumoniae added in quadruplicate to protease-treated samples (20) of the respiratory specimen pools.
The intrarun and interrun variations in the conventional and the real-time NASBA assays were estimated by running a dilution series (0.1, 1, 10, and 100 IFU/100 μl) of C. pneumoniae added in duplicate to BAL specimen pools and analyzing five replicates of each nucleic acid extract.
RNA degradation
RNA degradation was monitored by adding a dilution series of C. pneumoniae cells to the BAL specimen pools. The resulting specimens were stored at 4°C or at room temperature for different periods of time after lysis buffer was added to mimic the conditions encountered during transportation of the specimens. Finally, the specimens were stored at −80°C prior to extraction.
RESULTS
Specificity of the C. pneumoniae 16S rRNA NASBA primers
By using the biotin capture probe and the generic ECL detection probe for conventional NASBA and the molecular beacon for real-time NASBA, positive results were obtained with nucleic acid extracts from C. pneumoniae but not with nucleic acid extracts from any of the other organisms listed in Table 1.
Sensitivity of the C. pneumoniae 16S rRNA primers
The sensitivity of the C. pneumoniae NASBA assay was measured by testing dilutions of RNA generated in vitro in quadruplicate and was found to be 10 molecules. When the primers were applied to dilutions of nucleic acids extracted from a titrated culture of C. pneumoniae, they enabled the detection of as little as 0.1 IFU/100 μl of C. pneumoniae.
When conventional and real-time NASBA were applied to clinical specimens spiked with 10-fold dilutions of C. pneumoniae, the sensitivity was 0.1 IFU/100 μl for throat swabs, nasopharyngeal aspirates, and BAL samples. For the bronchial aspirates and sputum specimens, the organism was detected in three of four and two of four samples with an input of 0.1 IFU/100 μl, respectively, and four of four samples of each type with an input of 1 IFU/100 μl. There was no difference in sensitivity between the conventional and the real-time NASBA assays.
The results for all PCR-positive and -negative specimens were confirmed by the conventional and the real-time NASBA assays. The ECL signals for individual C. pneumoniae-negative respiratory specimens ranged from 2 to 495, whereas the signals of the real-time NASBA ranged from 1.00 to 1.68, with one outlier at 1.79. The ECL signals of individual C. pneumoniae-positive specimens ranged from 2,892 to 10,000,001. The ECL counts were greater than 783,483 for all except one of the samples. The ECL signal of the real-time NASBA ranged from 7.18 to 19.93, with one outlier at 24.33.
The intrarun coefficients of variation for the detection of 0.1, 1, 10, and 100 IFU were 31.9, 28.2, 16.6, and 37.9, respectively, by the conventional NASBA assay and 14.0, 11.7, 11.9, and 9.6, respectively, by the real-time NASBA assay. The interrun coefficients of variation for the same inputs are 56.9, 51.3, 42.6, and 40.0, respectively, by the conventional NASBA assay and 7.8, 6.1, 10.2, and 8.6, respectively, by the real-time NASBA assay.
The hands-on times for the analysis of 12 specimens, including the time for extraction, were 4.5 h for the conventional procedure and 3 h for the real-time procedure.
RNA degradation
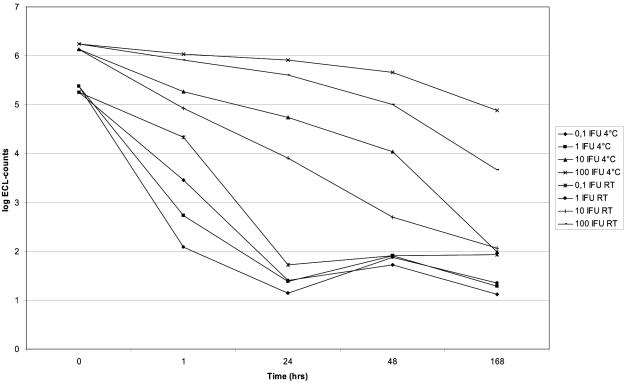
The results of C. pneumoniae RNA degradation at 4°C and at room temperature are presented in Fig. 1. Most C. pneumoniae RNA degradation seemed to occur at room temperature. After incubation for 24 h at both temperatures, inputs of 0.1 and 1 IFU were no longer detectable.
FIG. 1.
C. pneumoniae RNA degradation at 4°C and at room temperature (RT). The input is given in C. pneumoniae IFU; i.e., 0.1, 1, 10, and 100 IFU was added to the BAL pool prior to the extraction. At different time intervals, lysis buffer was added to the aliquots, and the samples were stored at −80°C prior to extraction.
DISCUSSION
The aim of this study was to develop conventional and real-time NASBA assays for the detection of C. pneumoniae based on its 16S rRNA in clinical specimens.
The detection limits of the various C. pneumoniae molecular amplification assays described in the literature are determined to be the lowest number of organisms measured by IFU, by number of cells, or by quantity of DNA, which makes a comparison of the analytical sensitivities of the different assays very difficult. Since the number of rRNA molecules present per C. pneumoniae cell is unknown, the analytical sensitivity of the assay was calculated by using wild-type RNA in vitro, a more objective method for calculation of the sensitivity of the assay. By using the C. pneumoniae 16S rRNA NASBA primers and the ECL probes or the molecular beacon, the detection limit for C. pneumoniae is 10 molecules of wild-type RNA generated in vitro. In our hands the limit of detection of C. pneumoniae in clinical specimens depended on the respiratory specimens tested and ranged from 0.1 and 1 IFU/100 μl sample for both the conventional and the real-time NASBA assay. These results are in line with the NASBA assay results of Coombes and Mahony (6), who found an analytical sensitivity of 100 OmpA molecules and 1 C. pneumoniae IFU. They did not determine the detection level by spiking respiratory specimens with known amounts of C. pneumoniae.
Most degradation of C. pneumoniae RNA seems to occur at room temperature and within 48 h (Fig. 1). Our findings correlate with those of Bruisten et al. (4), who studied the stability of human immunodeficiency virus type 1 RNA in whole blood, plasma, and serum before and after addition of lysis buffer. The authors concluded that the specimens could be kept at 4°C, provided that the transportation time was as short as possible, since most RNA degradation is likely to occur during transportation of the specimens. Furthermore, specimens should be processed on the day of sampling and should be stored at −70°C, thus stabilizing the RNA for at least 6 months, as found previously for NASBA assays for M. pneumoniae (20) and human rhinoviruses (19).
In conclusion, conventional and real-time NASBA assays show high degrees of concordance in terms of the sensitivity and the specificity of the test results; but the real-time technology has a clear advantage because of the handling, speed, and number of samples that can easily be analyzed in a single run (4.5 h versus 3 h). Furthermore, fewer intra- and interrun variations are encountered with the real-time NASBA assay. Despite the limited number of C. pneumoniae-positive samples that could be investigated, the NASBA assays described here are promising for the detection of C. pneumoniae in respiratory specimens. However, a large number of clinical specimens from patients with community-acquired pneumonia should be analyzed for further evaluation of our assays.
Acknowledgments
This study was supported by European Commission (Framework V) (grant QLK2-CT-2000-00294).
REFERENCES
- 1.Black, C. M., P. I. Fieds, T. O. Messmer, and B. P. Berdal. 1994. Detection of Chlamydia pneumoniae in clinical specimens by polymerase chain reaction using nested primers. Eur. J. Clin. Microbiol. Infect. Dis. 13:752-756. [DOI] [PubMed] [Google Scholar]
- 2.Boman, J., A. Allard, K. Persson, M. Lundborg, P. Juto, and G. Wadell. 1997. Rapid diagnosis of respiratory Chlamydia pneumoniae infection by nested touchdown polymerase chain reaction compared with culture and antigen detection by EIA. J. Infect. Dis. 175:1523-1526. [DOI] [PubMed] [Google Scholar]
- 3.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruisten, S. M., P. Oudshoorn, P. van Swieten, B. Boeser-Nunnink, P. van Aarle, S. P. Tondreau, and H. T. M. Cuypers. 1997. Stability of HIV-1 RNA in blood during specimen handling and storage prior to amplification by NASBA-QT. J. Virol. Methods 67:199-207. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, L. A., E. R. O'Brien, A. L. Cappuccio, C.-C. Kuo, S.-P. Wang, D. Stewart, D. L. Patton, P. K. Cummings, and J. T. Grayston. 1995. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J. Infect. Dis. 172:585-588. [DOI] [PubMed] [Google Scholar]
- 6.Coombes, B. K., and J. B. Mahony. 2000. Nucleic acid sequence based amplification (NASBA) of Chlamydia pneumoniae major outer membrane protein (OmpA) mRNA with bioluminescent detection. Comb. Chem. High Throughput Screen. 3:315-327. [DOI] [PubMed] [Google Scholar]
- 7.de Baar, M. P., M. W. Van Dooren, E. De Rooij, M. Bakker, B. Van Gemen, J. Goudsmit, and A. De Ronde. 2001. Single rapid real-time monitored isothermal RNA amplification assay for quantification of human immunodeficiency virus type 1 isolates from groups M, N, and O. J. Clin. Microbiol. 39:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekman, M. R., M. Leinonen, H. Syrjälä, E. Linnanmäki, P. Kujala, and P. Saikku. 1993. Evaluation of serological methods in the diagnosis of Chlamydia pneumoniae pneumonia during an epidemic in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 12:756-760. [DOI] [PubMed] [Google Scholar]
- 9.Falck, G., I. Engstrand, A. Gad, J. Gnarpe, H. Gnarpe, and A. Laurila. 1997. Demonstration of Chlamydia pneumoniae in patients with chronic pharyngitis. Scand. J. Infect. Dis. 29:585-589. [DOI] [PubMed] [Google Scholar]
- 10.Fox, J. D., S. Han, Y. Zhang, M. L. Neale, and D. Westmoreland. 2002. Development and evaluation of nucleic acid sequence based amplification (NASBA) for diagnosis of enterovirus infections using the NucliSens basic kit. J. Clin. Virol. 24:117-130. [DOI] [PubMed] [Google Scholar]
- 11.Grayston, J. T., L. A. Campbell, C.-C. Kuo, C. H. Mordhorst, P. Saikku, D. H. Thom, and S.-P. Wang. 1990. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 161:618-625. [DOI] [PubMed] [Google Scholar]
- 12.Hahn, D. L., and R. Golubjatnikov. 1994. Asthma and chlamydial infection: a case series. J. Fam. Pract. 38:589-595. [PubMed] [Google Scholar]
- 13.Kauppinen, M. T., E. Herva, P. Kujala, M. Leinonen, P. Saikku, and H. Syrjälä. 1995. The etiology of community acquired pneumonia among hospitalised patients during a Chlamydia pneumoniae epidemic in Finland. J. Infect. Dis. 172:1330-1335. [DOI] [PubMed] [Google Scholar]
- 14.Kenten, J. H., S. Gudibande, J. Link, J. J. Willey, B. Curfman, E. O. Major, and R. J. Massey. 1992. Improved electrochemiluminescent labels for DNA probe assays for HIV-1 PCR products. Clin. Chem. 38:873-879. [PubMed] [Google Scholar]
- 15.Leone, G., H. van Schijndel, B. van Gemen, F. Russel Kramer, and C. D. Schoen. 1998. Molecular beacon probes combined with amplification by NASBA enable homogeneous, real-time detection of RNA. Nucleic Acids Res. 26:2150-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman, D., F. Schlaeffer, I. Boldur, D. Lieberman, S. Horowitz, M. G. Friedman, M. Leinonen, O. Horovitz, E. Manor, and A. Porath. 1996. Multiple pathogens in adult patients admitted with community acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 51:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loens, K., M. Ieven, D. Ursi, H. Foolen, P. Sillekens, and H. Goossens. 2003. Application of NucliSens basic kit for the detection of Mycoplasma pneumoniae in respiratory specimens. J. Microbiol. Methods 54:127-130. [DOI] [PubMed] [Google Scholar]
- 18.Loens, K., M. Ieven, D. Ursi, T. Beck, M. Overdijk, P. Sillekens, and H. Goossens. 2003. Detection of Mycoplasma pneumoniae by real-time nucleic acid sequence-based amplification. J. Clin. Microbiol. 41:4448-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loens, K., M. Ieven, S. Pattyn, P. Sillekens, and H. Goossens. Sensitivity of detection of rhinoviruses in spiked clinical samples by nucleic acid sequence-based amplification in the presence of an internal control. J. Microbiol. Methods, in press. [DOI] [PubMed]
- 20.Loens, K., D. Ursi, M. Ieven, P. van Aarle, P. Sillekens, P. Oudshoorn, and H. Goossens. 2002. Detection of Mycoplasma pneumoniae by nucleic acid sequence-based amplification in spiked clinical samples. J. Clin. Microbiol. 40:1339-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogulkoc, N., S. Karakurt, B. Isalska, U. Bayindir, T. Celikel, and V. Korten. 1999. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am. J. Respir. Crit. Care Med. 160:349-353. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Steinhoff, D., H. Lode, G. Ruckdeschel, B. Heidrich, A. Rolfs, F. J. Fehrenbach, H. Mauch, G. Höffken, and J. Wagner. 1996. Chlamydia pneumoniae as a cause of community-acquired pneumonia in hospitalised patients in Berlin. Clin. Infect. Dis. 22:958-964. [DOI] [PubMed] [Google Scholar]
- 24.Sundelhöf, B., J. Gnarpe, H. Gnarpe, L. Grillner, and S. Darougar. 1993. Chlamydia pneumoniae in Swedish patients. Scand. J. Infect. Dis. 25:429-433. [DOI] [PubMed] [Google Scholar]
- 25.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered Mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 26.Ursi, D., M. Ieven, H. P. Van Bever, and H. Goossens. 1998. Construction of an internal control for the detection of Chlamydia pneumoniae by PCR. Mol. Cell. Probes 12:235-238. [DOI] [PubMed] [Google Scholar]