Abstract
Most experience in the comparison of diagnostic tools for canine leishmaniasis comes from cross-sectional surveys of dogs of different ages and breeds and in cases with unknown onset and duration of leishmaniasis. A longitudinal study was performed on 43 beagle dogs exposed to three transmission seasons (2002 to 2004) of Mediterranean leishmaniasis and examined periodically over 32 months through bone marrow microscopy and nested PCR (n-PCR), lymph node culture, serology (immunofluorescent-antibody test), and evaluation of clinical parameters. Starting from January 2003, the highest rate of positives was detected by n-PCR at all assessments (from 23.3% to 97.3%). Sensitivities of serologic and parasitological techniques were lower but increased with time, from 15.8% to 75.0 to 77.8%. Some dogs that tested positive by n-PCR but negative by other tests (“subpatent infection”) remained so until the end of the study or converted to negative in subsequent assessments, whereas all dogs with positive serology and/or microscopy/culture (“asymptomatic patent infection”) exhibited progressive leishmaniasis; 68% of them developed clinical disease (“symptomatic patent infection”) during the study, at 7 (range, 3 to 14) months after being positive to all tests. Postexposure infection incidences were high and were significantly different between 2002 and 2003 exposures (39.5% and 91.7%, respectively). The time course of infection was highly variable in each dog, with three patterns being identified: (i) rapid establishment of a patent condition (0 to 2 months from detection of infection); (ii) a prolonged subpatent condition (4 to 22 months) before progression; and (iii) a transient subpatent condition followed by 10 to 21 months of apparent Leishmania-negative status before progression.
Zoonotic visceral leishmaniasis, caused by the protozoan parasite Leishmania infantum (=L. chagasi) (17), is a sand fly-borne disease found in the Mediterranean area, Asia, and Latin America (5). In most of this range, the domestic dog is the main reservoir host. Dogs may suffer from a severe disease characterized by chronic evolution of viscero-cutaneous signs, which occurs in fewer than 50% of infected animals (13); however, both asymptomatic and symptomatic dogs can be infectious to phlebotomine vectors (18). Canine leishmaniasis is a major veterinary and public health problem in traditional areas of endemicity, but also in areas where the disease is not endemic but outbreaks are occasionally reported, such as in the United States and Canada (7, 23) and northern Europe (24). The prevalence and incidence of canine infection are important epidemiologic parameters, the estimation of which depends on the reliable identification of infected dogs. Parasitological examination by microscopy and culture and a wide variety of serologic and PCR techniques have been extensively used in comparative assays for the diagnosis of Leishmania infection in canines (see reference 1 for a review). In general, a number of PCR-based methods exhibited higher sensitivity to detect any Leishmania infection compared to parasitological and serologic methods. Positive findings with the latter techniques, however, tend to be associated with important features, such as occurrence of clinical disease or infectiousness to phlebotomine vectors (3, 20). Most experience in the application and comparison of diagnostic tools for natural infections comes from cross-sectional studies on dogs of different ages and breeds (8, 16, 21, 22, 26). Consequently, interpretation of the laboratory data is difficult, and this may explain the different performances reported for various diagnostic techniques in cases with unknown onset and duration of the infection and with different breed-associated susceptibilities to Leishmania (25).
We have carried out a longitudinal study on 43 dogs of the same age and breed, with the aim to determine the incidence and time course of L. infantum infections acquired by natural exposure, by means of parasitological, serologic, and PCR techniques. Dogs were exposed to sand fly bites in a setting where the infection is endemic and seasonally transmitted and were periodically examined during a 32-month period which included three transmission seasons.
MATERIALS AND METHODS
Study area and dogs.
The study was performed in a rural area of the Naples province in southern Italy, where both human and canine visceral leishmaniases are highly endemic (11, 15). The local phlebotomine vector, Phlebotomus perniciosus, is usually active from the end of May through early October (14). Forty-three beagle dogs (23 males), which were born in January 2002 in an area of northern Italy where the infection is not endemic and which tested Leishmania negative by serology (immunofluorescent-antibody test [IFAT]), were moved to the study site in July 2002. There the dogs were placed in an open kennel and kept under constant veterinary care during the study period. The use of topical or environmental insecticides was avoided to allow natural exposure of dogs to sand fly bites. The collection of biological samples from the dogs was performed in accordance with the national guidelines for animal welfare.
Samples.
Every 1 to 3 months, the following samples were obtained from each dog: (i) peripheral blood for specific serology, hematological evaluation, and clinical chemistry; (ii) bone marrow (BM) aspirate for microscopy and Leishmania DNA detection by nested PCR (n-PCR); and (iii) popliteal lymph node (LN) aspirate for parasite culture.
IFAT.
The in-house antigen consisted of promastigotes of L. infantum zymodeme MON-1, and the assay procedure followed the protocol of the Office International des Epizooties (9). The cutoff dilution was set at 1:160.
Nested PCR.
Total genomic DNA was extracted from 350 μl of BM sample using the Easy-DNA kit (Invitrogen, San Diego, CA) and was stored at −20°C until use. The first PCR amplification was carried out in a 50-μl volume containing 10 μl BM DNA plus 40 μl PCR Master Mix (Promega) containing 50 pmol of the kinetoplastid-specific primers R221 and R332 of the small-subunit rRNA gene (27). For the second amplification, 3 μl of the first PCR product was added to 22 μl of PCR Master Mix (Promega) containing 3 pmol of the Leishmania-specific primers R223 and R333 of the same gene (27). Contaminations of amplicons were avoided by using physical separation (rooms and materials) as well as decontamination procedures (UV exposure and bleaching of materials and surfaces). BM samples from Leishmania-free dogs were used as negative controls in each step of the procedure. The amplification products were analyzed on 1.5% agarose gels and visualized under UV light. Positive samples yielded a predicted n-PCR product of 358 bp.
Microscopy and culture.
BM aspirate material was smeared onto slides and stained with Giemsa stain. LN aspirate material was cultured in Evans' modified Tobie's medium at 22.5°C. Cultures were examined for promastigote growth for 1 month.
Clinical assessment.
Clinical assessment was performed by accurate inspection of dogs for the presence of seven signs attributable to Leishmania infection (i.e., dermatitis, skin ulcers, alopecia, ocular lesions, lymphadenopathy, onychogryphosis, and weight loss) (2) and by the evaluation of nonspecific laboratory data such as full blood count, total proteins, and albumin/globulin ratio. Animals were scored for clinical and laboratory signs on a scale of 0 to 2 or 3, and the scores were added up to give a clinical score.
Statistical analysis.
Infection incidence rates and sensitivity values recorded by each diagnostic method were tested for significance by the chi-square test or one-tailed Fisher exact test as appropriate.
RESULTS
Exposure of dogs to sand fly bites.
The duration of a dog's exposure to bites of P. perniciosus sand flies was estimated through routine entomological data obtained from sand fly collection stations sited in villages of southern Italy in the years 2002, 2003, and 2004. In the transmission season in 2002, the vector was present at low density from June through late September due to unfavorable climatic conditions. In 2003 and 2004, high densities of P. perniciosus were recorded from the end of May through mid-October, with two peaks in July and September. Therefore, the dogs were exposed to sand fly bites for about 2.5 months in 2002 and for about 5 months in 2003 and 2004.
Six dogs died in the period from September 2002 to October 2003. These animals were either Leishmania negative or had subpatent leishmanial infections. Necroscopy findings suggested that the deaths were unrelated to leishmaniasis.
Comparative performance of diagnostic techniques in detecting infections.
Eighteen full samplings were performed from October 2002 through May 2005, for a total of 681 sets of samples (Table 1). Considering the cumulative results, the highest number of positives was found with the n-PCR examination of BM (371; 54.5%) and the lowest with the serologic examination (180; 26.4%). BM microscopy and LN culture (190 positive samples each; 27.9%) showed identical performance. The sensitivity of BM n-PCR proved to be 100%, in contrast to 51.2% for parasitological and 48.5% for serologic techniques.
TABLE 1.
Cumulative results of nested PCR, serology, microscopy, and culture examinations of 681 sets of canine samples collected from October 2002 to May 2005
| No. of samples | BM n-PCR | IFAT | BM microscopy | LN culture |
|---|---|---|---|---|
| 310 | − | − | − | − |
| 176 | + | − | − | − |
| 175 | + | + | + | + |
| 15 | + | − | + | + |
| 5 | + | + | − | − |
| No. positive | 371 | 180 | 190 | 190 |
| % positivea | 54.5 | 26.4 | 27.9 | 27.9 |
| Sensitivity (%)b | 100 | 48.5 | 51.2 | 51.2 |
Percentage of positive samples over total samples analyzed using the indicated method.
Percentage of positive samples with the indicated method over total positive samples with at least one method.
Considering the longitudinal results, the rate of positives in BM n-PCR recorded at each assessment (from 23.3% in March 2003 to 97.3% in May 2005) was always significantly higher than rates determined by serology and microscopy/culture examinations (from 7.0% to 75.0 to 75.7% in the same period) (P < 0.05) (Table 2). However, while BM n-PCR was found to be 100% sensitive at each assessment, the sensitivities of the parasitological and serologic techniques were not uniform over time, as both increased from 15.8% in December 2003 to 75.0 to 77.8% in May 2005, although they always were significantly lower than the BM n-PCR sensitivity (P < 0.05) (Table 2).
TABLE 2.
Number of positive samples by and sensitivity of nested PCR, serology, and microscopy/culture at some assessmentsa
| Mo and yr | No. of dogs | BM n-PCR
|
IFAT
|
BM microscopy/LN culture
|
|||
|---|---|---|---|---|---|---|---|
| No. (%) Pos | % S | No. (%) Pos | % S | No. (%) Pos | % S | ||
| March 2003 | 43 | 10 (23.3) | 100 | 3 (7.0) | 30.0 | 3 (7.0) | 30.0 |
| September 2003 | 42 | 14 (33.3) | 100 | 3 (7.1) | 21.4 | 3 (7.1) | 21.4 |
| December 2003 | 40 | 19 (47.5) | 100 | 3 (7.5) | 15.8 | 3 (7.5) | 15.8 |
| March 2004 | 39 | 26 (66.7) | 100 | 9 (23.1) | 34.6 | 10 (25.6) | 38.5 |
| May 2004 | 38 | 33 (86.8) | 100 | 18 (47.4) | 54.5 | 17 (44.7) | 51.5 |
| December 2004 | 37 | 35 (94.6) | 100 | 27 (73.0) | 77.1 | 26 (70.3) | 74.3 |
| May 2005 | 37 | 36 (97.3) | 100 | 28 (75.7) | 77.8 | 27 (73.0) | 75.0 |
Pos, positive; S, sensitivity.
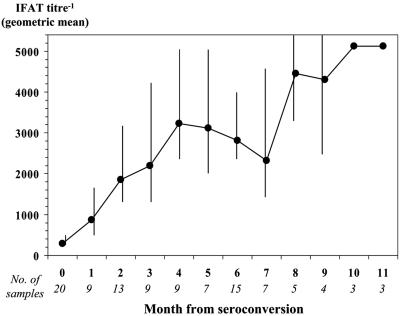
Seven of 35 dogs (20.0%) that tested positive by BM n-PCR but negative by other tests at one assessment converted to negative in the subsequent assessment. Four of them converted again to positive after a variable period of time. In contrast, all dogs detected as having an IFAT titer of ≥1:160 and/or positive BM microscopy and LN culture at one assessment were found to be positive by these tests in all subsequent assessments. In particular, IFAT titers increased progressively from the seroconversion value up to a plateau of 1:5,120 (Fig. 1). Notably, in half of these dogs (14/28) the detection of significant levels of specific antibodies was preceded by the demonstration of the organisms by microscopy/culture 1 to 2 months earlier.
FIG. 1.
Trend of IFAT titers detected in 104 serum samples from dogs with seroconversion titers of ≥1:160 and for which ≥3 serologic determinations were available. Error bars indicate lower and upper 95% confidence limits.
Occurrence of clinical manifestations.
The first symptomatic case was detected 10 months after the 2002 exposure (July 2003). Clinical disease was progressively recorded in a further 18 dogs by the end of the study. All 19 dogs had been positive in all diagnostic tests for a median period of 7 months (range, 3 to 14 months) before the onset of symptoms. Clinical scores varied from 4 (oligosymptomatic condition) in the early infection stages to 17 (polysymptomatic condition) in the late ones. None of the dogs recorded as having clinical disease showed spontaneous resolution of symptoms. Animals with polysymptomatic disease received a 1-month course of specific antileishmanial treatment, consisting of intramuscular meglumine antimoniate plus oral allopurinol.
Category of infection.
By the comparative analysis of diagnostic and clinical findings from longitudinal samples, the stage of Leishmania infection detected at each assessment was assigned to one of the following categories: (i) subpatent infection (this term is preferred to “prepatent,” as not all subjects evolved to a patent condition), meaning detection of parasite DNA in BM samples (occasionally followed by conversion to negative in a subsequent assessment[s]), an IFAT titer of <1:160, negative BM microscopy and LN culture, and a clinical score of ≤3; (ii) asymptomatic patent infection, meaning steady detection of parasite DNA in BM samples, steady or increasing IFAT titer (≥1:160), positive BM microscopy and LN culture, and a clinical score of ≤3; and (iii) symptomatic patent infection, meaning laboratory findings as for asymptomatic patent infection and a clinical score of >3.
Prevalence of different categories of infection after each exposure.
Cross-sectional findings for samples obtained in May 2003, May 2004, and May 2005 were used to estimate the prevalence and category of Leishmania infections following each transmission season and before the exposure to new infections (Table 3). From May 2003 to May 2005, the percentage of infected dogs in a subpatent condition decreased from 75.0% to 25.0%, while those that developed a patent infection associated with clinical disease increased from 0 to 52.8%.
TABLE 3.
Cross-sectional findings for dogs after each Leishmania transmission season, examined before exposure to new infections
| Mo and yr | No. of dogs | No. (%) positive | No. (%) with the following category of infection:
|
||
|---|---|---|---|---|---|
| Subpatent | Asymptomatic patent | Symptomatic patent | |||
| May 2003 | 43 | 12 (27.9) | 9 (75.0)a | 3 (25.0) | 0 |
| May 2004 | 38 | 33 (86.8) | 16 (48.5)b | 12 (36.4) | 5 (15.1) |
| May 2005 | 37 | 36 (97.3) | 9 (25.0)c | 8 (22.2) | 19 (52.8) |
Five dogs that previously tested positive were found to be negative at this assessment.
Three dogs that previously tested positive were found to be negative at this assessment.
One dog that previously tested positive was found to be negative at this assessment.
Incidence and progression of infection.
Following exposure to the 2002 transmission season, infections were first recorded in 11 dogs in mid-January 2003, i.e., 3.5 months after the end of the season. Six more dogs were found to be infected in March and May (three dogs each). As no further infections were detected through the end of the 2003 transmission season, the infection incidence attributed to the first exposure was 39.5% (17/43). Following exposure to the 2003 season, new infections were detected in two dogs at the end of September, in five in December, in seven in February 2004, and in eight in April. Hence, the incidence attributed to the second exposure was 91.7% (22 out of 24 dogs remained apparently negative after the first exposure), i.e., much higher than the 2002 incidence value (P < 0.001). Finally, 2/2 dogs still negative were found to be infected after the 2004 exposure, in November 2004 and April 2005, respectively. Therefore, if we exclude the two dogs that remained negative until they died in October 2003, 100% of our dogs showed evidence of a Leishmania infection in at least one assessment.
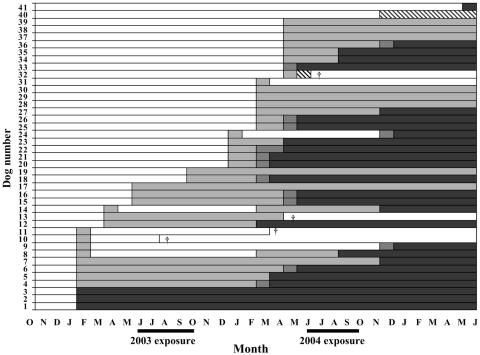
The time course of the infection was variable (Fig. 2). Excluding dogs that died during the study, three main patterns may be arbitrarily identified among the 37 surviving dogs. (i) Eleven dogs (29.7%) showed rapid establishment of a patent condition. This was characterized by the simultaneous detection of Leishmania by all tests (six dogs) or was preceded by a short period of a subpatent condition (∼2 months, corresponding to the periodicity of our assessments) (five dogs). (ii) Twenty-one dogs (56.8%) showed a prolonged subpatent condition, from 4 to 22 months, either progressing (13 dogs) or not (8 dogs) to a patent condition. In this type of pattern, however, we included animals found infected late in the study and for which a progression to patent infection could not be ascertained. In dogs with established progressive infection, the median time to seroconversion was 10.5 months (range, 4 to 22 months). (iii) Five dogs (13.5%) showed a transient subpatent condition. In these animals, the infection was detected once and then it was followed by 10 to 21 months of an apparent Leishmania-negative condition. After this period, four dogs presented infection courses described above.
FIG. 2.
Time course of Leishmania infections detected in 41 beagle dogs by parasitological, serologic, and n-PCR techniques during 32 months of observations following exposure to the 2002 transmission season, starting from October 2002. Results for two dogs that remained negative until they died in October 2003 are not shown in the graph. White bars, negative by all tests; light gray bars, positive by n-PCR only; hatched bars, positive by serology and n-PCR; dark gray bars, positive by n-PCR and culture/microscopy; black bars, positive by all tests; †, dog's death.
None of the dogs that developed patent leishmaniasis showed spontaneous conversion to a subpatent condition, suggesting the progressive nature of the disease in these animals.
DISCUSSION
To our knowledge, this is the first investigation of this type carried out in a setting where canine leishmaniasis is endemic and seasonally transmitted. A similar longitudinal study was performed in an area of canine leishmaniasis endemicity in Brazil that is characterized by annual L. infantum transmission (20). Those authors, however, employed different study design and diagnosis protocols.
In our study, parasitological, serologic, and molecular techniques were used in combination to determine the course of infection in dogs after intermittent exposure to L. infantum. Hence, their relative diagnostic performances could be compared in animals with known onsets and durations of natural infection. In dogs with ascertained progressive leishmaniasis (28/37), n-PCR predicted such a condition in 100% of the cases, while both serology and microscopy/culture failed to do so in 79% (22/28) at the initial detection of infection and in 61% (17/28) 2 to 4 months after detection of infection. The high sensitivity of our PCR method may depend not only on the targeted multicopy-sequence small-subunit rRNA gene (27) but also on the type of biopsy material and protocol used. Although requiring an invasive procedure, BM biopsy is usually considered the sample of choice for a sensitive diagnosis of canine leishmaniasis, compared with less invasive materials such as peripheral blood (12). Among available PCR protocols, n-PCR is one of the most powerful techniques, theoretically being able to detect the DNA of 0.01 parasite (4).
On the other hand, n-PCR also tested positive in animals that, during the observation period, did not show any evidence of uncontrolled parasite multiplication and/or production of high-titer specific antibodies, both conditions being 100% predictive for the onset of clinical disease in a median period of 7 (range, 3 to 14) months. Furthermore, among these animals with subpatent infection there were some that converted to negative for long periods. Since repeated assays excluded the possibility that these “transient” n-PCR positives were false positives due to contamination, this finding may have two explanations: (i) after infection, the parasite load in BM dropped to undetectable for a variable period of time until the number of organisms increased again (this type of “parasite silencing,” attributable to a limited sensitivity of diagnostic tests, has been reported for experimental canine leishmaniasis [19]), or (ii) the dogs cleared the infection acquired in one transmission season, but they were reinfected in subsequent seasons. Our follow-up findings may be consistent with the second explanation, as the time courses of putative reinfections were similar to those of dogs that were infected for the first time in the same season (Fig. 2). If this is so, it may be argued that “natural vaccination” with L. infantum did not protect our dogs from further infections and from disease progression.
Whatever diagnostic method is considered, our cross-sectional findings indicated an extremely high burden of infection compared with results from cross-sectional surveys reported elsewhere for canines exposed to seasonal L. infantum transmission (8, 16, 22, 26). In those reports, which included dogs of different ages and breeds, canine leishmaniasis prevalences were found to range from 49 to 87% by BM/LN PCR, 26 to 49% by serologic tests (IFAT titer of ≥1/160, enzyme-linked immunosorbent assay, or Western blotting), and 23 to 43% by BM/LN microscopy/culture. In our survey performed on May 2005, when the dogs were 3.5 years old, prevalence values obtained by the three methods were 97%, 76%, and 73%, respectively. Furthermore, in our study area a serological survey performed among 326 owned dogs in 1998 had revealed a seroprevalence of 40.4% (15), a quite high rate but about half of the seroprevalence value detected at the end of our study. Incidence values were also high, although they showed a sharp difference between 2002 and 2003 exposures (39.5% and 91.7%, respectively). This may be attributable to both a shorter period of infection exposure (2.5 months) and a lower density of the phlebotomine vectors in the 2002 season, as well as the possible occurrence of infections undetected by our methods after this season and which became apparent only after the 2003 season.
Reasons for such an elevated burden of infection could be found in the particular conditions to which our group of animals was exposed, i.e., without any sand fly control measures (15) or removal of leishmaniasis cases during three consecutive seasons (10), which may have contributed to a focal cycle of intense transmission. Furthermore, beagle dogs may be particularly susceptible to Leishmania infections compared with other breeds (25) or mongrel dogs. Dye et al. (6) estimated a 72% infection incidence among 50 beagle dogs exposed to natural sand fly bites during one transmission season (1989) in southern France. Finally, other modes of acquiring Leishmania infection in dogs living in close contact should not be ruled out, such as via mating, bites, and exposure to blood, as recently suggested by some authors (23, 24) to explain the occurrence and spread of canine leishmaniasis in vector-free areas.
Acknowledgments
This investigation was supported in part by Novartis Animal Vaccines Ltd., Braintree, United Kingdom.
REFERENCES
- 1.Alvar, J., C. Canavate, R. Molina, J. Moreno, and J. Nieto. 2004. Canine leishmaniasis. Adv. Parasitol. 57:1-88. [DOI] [PubMed] [Google Scholar]
- 2.Ciaramella, P., G. Oliva, R. De Luna, L. Gradoni, R. Ambrosio, L. Cortese, A. Scalone, and A. Persechino. 1997. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet. Rec. 141:539-543. [DOI] [PubMed] [Google Scholar]
- 3.Courtenay, O., R. J. Quinnell, L. M. Garcez, J. J. Shaw, and C. Dye. 2002. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 186:1314-1320. [DOI] [PubMed] [Google Scholar]
- 4.Cruz, I., C. Canavate, J. M. Rubio, M. A. Morales, C. Chicharro, F. Laguna, M. Jimenez-Mejias, G. Sirera, S. Videla, J. Alvar, and the Spanish HIV-Leishmania Study Group. 2002. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S185-S189. [DOI] [PubMed] [Google Scholar]
- 5.Desjeux, P. 2001. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 95:239-243. [DOI] [PubMed] [Google Scholar]
- 6.Dye, C., E. Vidor, and J. Dereure. 1993. Serological diagnosis of leishmaniasis: on detecting infection as well disease. Epidemiol. Infect. 103:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enserink, M. 2000. Infectious diseases. Has leishmaniasis become endemic in the U.S.? Science 290:1881-1883. [DOI] [PubMed] [Google Scholar]
- 8.Fisa, R., C. Riera, M. Gállego, J. Manubens, and M. Portús. 2001. Nested PCR for diagnosis of canine leishmaniosis in peripheral blood, lymph node and bone marrow aspirates. Vet. Parasitol. 99:105-111. [DOI] [PubMed] [Google Scholar]
- 9.Gradoni, L., and M. Gramiccia. 2000. Leishmaniosis, p. 803-812. In Manual of standards for diagnostic tests and vaccines. Office International des Epizooties, Paris, France.
- 10.Gradoni, L., M. Gramiccia, F. Mancianti, and S. Pieri. 1988. Studies on canine leishmaniasis control. 2. Effectiveness of control measures against canine leishmaniasis in the Isle of Elba, Italy. Trans. R. Soc. Trop. Med. Hyg. 82:568-571. [DOI] [PubMed] [Google Scholar]
- 11.Gradoni, L., R. Pizzuti, A. Scalone, M. Russo, M. Gramiccia, L. di Martino, R. Pempinello, and G. B. Gaeta. 1996. Recrudescence of visceral leishmaniasis unrelated to HIV infection in the Campania region of Italy. Trans. R. Soc. Trop. Med. Hyg. 90:234-235. [DOI] [PubMed] [Google Scholar]
- 12.Lachaud, L., S. Marchergui-Hammami, E. Chabbert, J. Dereure, J. P. Dedet, and P. Bastien. 2002. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J. Clin. Microbiol. 40:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanotte, G., J. A. Rioux, J. Perières, and Y. Vollhardt. 1979. Ecologie des leishmanioses dans le sud de la France. 10. Les formes evolutives de la leishmaniose viscèrale canine. Ann. Parasitol. Hum. Comp. 54:277-295. [PubMed] [Google Scholar]
- 14.Maroli, M., M. Gramiccia, L. Gradoni, M. Troiani, and R. Ascione. 1994. Natural infection of Phlebotomus perniciosus with MON 72 zymodeme of Leishmania infantum in the Campania region of Italy. Acta Trop. 57:333-335. [DOI] [PubMed] [Google Scholar]
- 15.Maroli, M., V. Mizzoni, C. Siragusa, A. D'Orazi, and L. Gradoni. 2001. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med. Vet. Entomol. 15:358-863. [DOI] [PubMed] [Google Scholar]
- 16.Martín-Sanchez, J., M. C. Lopez-Lopez, C. Acedo-Sanchez, J. J. Castro-Fajardo, J. A. Pineda, and F. Morillas-Marquez. 2001. Diagnosis of infections with Leishmania infantum using PCR-ELISA. Parasitology 122:607-615. [DOI] [PubMed] [Google Scholar]
- 17.Mauricio, I. L., M. K. Howard, J. R. Stothard, and M. A. Miles. 1999. Genomic diversity in the Leishmania donovani complex. Parasitology 119:237-246. [DOI] [PubMed] [Google Scholar]
- 18.Molina, R., C. Amela, J. Nieto, M. San-Andrés, F. Gonzàles, J. A. Castillo, J. Lucientes, and J. Alvar. 1994. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans. R. Soc. Trop. Med. Hyg. 88:491-493. [DOI] [PubMed] [Google Scholar]
- 19.Paranhos-Silva, M., G. G. Oliveira, E. A. Reis, R. M. de Menezes, O. Fernandes, I. Sherlock, R. B. Gomes, L. C. Pontes-de-Carvalho, and W. L. dos-Santos. 2003. A follow-up of beagle dogs intradermally infected with Leishmania chagasi in the presence or absence of sand fly saliva. Vet. Parasitol. 114:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinnell, R. J., O. Courtenay, S. Davidson, L. Garcez, B. Lambson, P. Ramos, J. J. Shaw, M. A. Shaw, and C. Dye. 2001. Detection of Leishmania infantum by PCR, serology and cellular immune response in a cohort study of Brazilian dogs. Parasitology 122:253-261. [DOI] [PubMed] [Google Scholar]
- 21.Reale, S., L. Maxia, F. Vitale, N. S. Glorioso, S. Caracappa, and G. Vesco. 1999. Detection of Leishmania infantum in dogs by PCR with lymph node aspirates and blood. J. Clin. Microbiol. 37:2931-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roura, X., A. Sánchez, and L. Ferrer. 1999. Diagnosis of canine leishmaniasis by a polymerase chain reaction technique. Vet. Rec. 144:262-264. [DOI] [PubMed] [Google Scholar]
- 23.Schantz, P. M., F. J. Steurer, Z. H. Duprey, K. P. Kurpel, S. C. Barr, J. E. Jackson, E. B. Breitschwerdt, M. G. Levy, and J. C. Fox. 2005. Autochthonous visceral leishmaniasis in dogs in North America. J. Am. Vet. Med. Assoc. 226:1316-1322. [DOI] [PubMed] [Google Scholar]
- 24.Slappendel, R. J., and E. Teske. 1999. A review of canine leishmaniasis presenting outside endemic areas, p. 54-59. In R. Killick-Kendrick (ed.), Canine leishmaniosis: an update. Hoechst Roussel Vet, Wiesbaden, Germany.
- 25.Solano-Gallego, L., J. Llull, G. Ramos, C. Riera, M. Arboix, J. Alberola, and L. Ferrer. 2000. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet. Parasitol. 90:37-45. [DOI] [PubMed] [Google Scholar]
- 26.Solano-Gallego, L., P. Morell, M. Arboix, J. Alberola, and L. Ferrer. 2001. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J. Clin. Microbiol. 39:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eys, G. J., G. J. Schoone, N. C. Kroon, and S. B. Ebeling. 1992. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol. Biochem. Parasitol. 51:133-142. [DOI] [PubMed] [Google Scholar]