Abstract
Intrauterine infection is a recognized cause of preterm birth. The infectious organisms are believed to originate primarily from the vaginal tract and secondarily from other parts of the body. It is plausible that microbes in the oral cavity can be transmitted to the pregnant uterus. However, direct evidence supporting such a transmission is lacking. In this study, amniotic fluids of 34 pregnant women were examined by PCR using 16S and 23S rRNA universally conserved primers. Bacterial DNA was amplified from the only patient with clinical intrauterine infection and histologic necrotizing acute and chronic chorioamnionitis. One strain, Bergeyella sp. clone AF14, was detected and was 99.7% identical to a previously reported uncultivated oral Bergeyella strain, clone AK152, at the 16S rRNA level. The same strain was detected in the subgingival plaque of the patient but not in her vaginal tract. The 16S-23S rRNA sequence of clone AF14 matched exactly with the sequences amplified from the patient's subgingival plaque. These observations suggest that the Bergeyella strain identified in the patient's intrauterine infection originated from the oral cavity. This is the first direct evidence of oral-utero microbial transmission. The patient's periodontal health during pregnancy was unclear. She did not have detectable periodontal disease during postpartum examination. Bergeyella spp. had not been previously associated with preterm birth and were detected in subgingival plaque of women without clinical levels of intrauterine infection. Uncultivated species may be overlooked opportunistic pathogens in preterm birth. This study sheds new light on the implication of oral bacteria in preterm birth.
Preterm birth (PTB) is a significant public health problem and accounts for approximately 11% of all deliveries in the United States (6). It is the leading cause of prenatal death, and the surviving infants may encounter long-term health problems throughout their lives. The majority of this morbidity and mortality involves a small subset of infants born before 30 to 32 weeks of gestation (5, 6). Although advancement in medical technology has steadily improved the survival rate of the preterm infants, the PTB rate has not improved during the past 4 decades, showing instead a slight upward trend (30). Thus, identification of new risk factors for PTB could have significant social and economic impact. Periodontal disease is one of the newly identified risk factors for PTB (35). Epidemiologic and intervention studies have indicated a link between these two conditions (21, 22, 28, 29, 34-36). However, contradictory reports also exist (13, 33). The questions of whether periodontal disease is a risk factor for PTB and what is the mechanism underlying the potential link remain. Animal studies have provided evidence supporting the potential link and have suggested two possible mechanisms. (i) Periodontal disease could lead to systemic dissemination of inflammatory mediators and/or periodontal pathogens causing adverse pregnancy outcomes (9, 26, 27). (ii) Oral bacteria may translocate specifically into the pregnant uterus and induce adverse pregnancy outcomes due to localized infection (18).
One of the known causes of PTB is intrauterine infection (8, 39, 41, 44). The infection rate is inversely related to the gestational age (5, 42). Watts et al. (42) studied 105 amniotic fluid (AF) samples obtained by amniocentesis from women in preterm labor at less than 35 weeks of gestation. The frequencies of positive cultures by gestational age were as follows: 23 to 24 weeks, 67%; 25 to 26 weeks, 36%; 27 to 30 weeks, 17%; 31 to 32 weeks, 12%; and 33 to 34 weeks, 11% (42). The paradigm has been that intrauterine infections most commonly originate from the lower genital tract and that the microorganisms invade the pregnant uterus through an ascending mechanism (5, 6). A less common route of infection is hematogenous transmission, where the infectious organism may originate from other parts of the body. As one of the major microbial habitats in the human body, hosting as many as 700 different species, the oral cavity is a potential microbial reservoir for infection (1, 23, 37). Indeed, organisms with an oral origin, such as Fusobacterium nucleatum and Capnocytophaga spp., have been associated with intrauterine infections, with F. nucleatum being one of the most frequently cultivated from or detected in the infected uterus (2, 4, 7, 10, 11, 15-17, 19, 20). These observations are consistent with our recent findings with mice, where hematogenous infection with orally related F. nucleatum resulted in localized infection in the placenta, causing preterm and term stillbirths of the fetal pups (18). However, direct evidence detecting and matching the microorganisms in intrauterine infections with those in the oral cavities of the pregnant women is lacking. In this pilot study, the source of intrauterine infection was examined. We report the identification of an uncultivated oral Bergeyella sp. strain associated with PTB and the possible source of the infection.
MATERIALS AND METHODS
Study population and sample collection.
The study was approved by the Internal Review Board at the MetroHealth Medical Center (MHMC) in Cleveland, Ohio. A total of 35 pregnant women undergoing transabdominal amniocentesis at the Fetal Diagnostic Center or the Labor and Delivery Department at MHMC were recruited. Women who were pregnant with multiple fetuses, who were more than 5 cm dilated, or whose fetal membranes were ruptured and who had been on antibiotics within 2 weeks prior to amniocentesis were excluded from the study. Qualified patients were requested to sign a written consent and to fill out a questionnaire. Additional information was obtained from the patient's medical record. Three samples were collected at the time of recruitment: AF, blood, and vaginal swabs. Following amniocentesis, AF was placed directly into sterile 15-ml Corning centrifuge tubes (Corning Inc., Corning, NY). The vaginal samples were collected from the lower two-thirds of the vagina by using the S/P brand culture swab collection and transport system (Allegiance Healthcare, McGraw Park, IL), aided by a sterile speculum for visualization. The subgingival plaque samples were collected either at MHMC at the time of recruitment or later at the School of Dental Medicine at Case Western Reserve University. Full-mouth subgingival plaque was collected using sterile curettes and pooled into 2 ml sterile phosphate buffer (Sigma, St. Louis, MO). Caution was taken during sample collection to avoid traumatizing the soft tissue. All samples were placed into a PackAnaero system (Mitsubishi Gas Chemical Inc., New York, NY) within 10 to 15 min of collection and transported to the laboratory. The blood was cultured immediately for bacterial growth. AF samples were divided into 1-ml aliquots. The vaginal swabs were suspended in sterile phosphate-buffered saline. All samples were then centrifuged at 10,000 × g for 3 min. The supernatants were removed, and the pellets were stored at −80°C. The placenta was submitted to the Pathology Department at MetroHealth Medical Center for pathological examination. A total of six tissue blocks, including two sections each of umbilical cord and membranes and four full-thickness sections of placental parenchyma, were prepared. Hematoxylin- and eosin-stained slides of these blocks were later examined.
DNA extraction.
Each frozen sample pellet was suspended in 500 μl lysis buffer (50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 50 mM EDTA, 1% sodium dodecyl sulfate) containing 150 mg of 0.1-mm and 50 mg of 0.5-mm zirconia/silica beads (Biospec Products, Inc., Bartlesville, OK). Samples were vigorously mixed for 3 min by vortexing and incubated at 70°C for 15 min. After centrifugation at 10,000 × g for 5 min, the supernatant was collected and mixed with 130 μl of 10 M ammonium acetate. The mixture was incubated on ice for 5 min and centrifuged at 10,000 × g for 10 min. DNA was isolated from the supernatant by isopropanol precipitation, purified using a GeneClean Turbo kit (Qbiogene, Irvine, CA), and suspended in 30 μl of elution solution from the kit.
Amplification of the ribosomal 16S-23S rRNA gene region.
All PCRs were carried out using AccuPrime Taq DNA polymerase (Invitrogen, Carlsbad, CA) and an Applied Biosystems 2720 thermal cycler (Applied Biosystems, Foster City, CA). A total of 1 μl of DNA from each sample was used as the template in a 25-μl reaction volume. Following the first PCR, an aliquot of 5 μl of the reaction mixture was treated with 2 μl of Exo/SAP-IT (USB Co., Cleveland, OH) following the manufacturer's instruction. An aliquot of 1 μl from the Exo/SAP-IT reaction mixture was then used as the DNA template for the nested PCR. The conditions of PCR were as follows: an initial denaturing at 94°C for 3 min; 25 to 27 cycles of denaturing at 94°C for 1 min, annealing at 42°C (or at 52°C for nested PCR) for 2 min, and extension at 72°C for 3 min; and an additional extension at 72°C for 10 min. Primers used for PCR are listed in Table 1. Primer BergF is complementary to the Bergeyella-specific primer kindly provided by Bruce Paster. Primers 14BitsR1 and 14BitsR2 were designed based on the DNA sequence of the highly variable internal transcribed spacer (ITS) region between the 16S and 23S rRNA genes of the Bergeyella sp. clone AF14. The PCR products were examined by 1% agarose gel electrophoresis.
TABLE 1.
Primers used in this study
| Primer | Description | Sequence (5′-3′) | Reference or source |
|---|---|---|---|
| A17F | Forward primer in 16S region, 17 bases from 5′ end | GTTTGATCCTGGCTCAG | 25 |
| 785F | Forward primer in 16S region, 785 bases from 5′ end | GGATTAGATACCCTGGTAGTC | 25 |
| 422R | Reverse primer in 23S region, 422 bases from 5′ end of 23S | GGAGTATTTAGCCTT | 25 |
| L189R | Reverse primer in 23S region, 189 bases from 5′ end of 23S | GGTACTTAGATGTTTCAGTTC | 25 |
| 1512R | Reverse primer in 16S region, 1,512 bases from 5′ end of 16S | TACCTTGTTACGACTT | 25 |
| BergF | Forward primer for Bergeyella spp., 1,000 bases from 5′ end of 16S | GACAGCTGTAGAAATACGG | This study |
| 14BitsR1 | Reverse primer for ITS region of Bergeyella clone AF14B | TCAGCACTCGAAAGTGCTCGG | This study |
| 14BitsR2 | Reverse primer for ITS region of Bergeyella clone AF14B | CTTAGTCTCTATTAATCCCTG | This study |
| M13 Universal (−21) | For DNA sequencing | GTAAAACGACGGCCAGT | MBCa |
| M13-2 Reverse | For DNA sequencing | CAGGAAACAGCTATGAC | MBC |
MBC, Molecular Biotechnology Core, Lerner Institute, Cleveland, Ohio.
DNA cloning and sequencing.
The PCR products were cloned into the pCR2.1 vector by use of a TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions. Plasmid DNA was isolated from the transformants by using a Wizard Plus SV Minipreps DNA purification system (Promega, Madison, WI). The plasmid DNA was sequenced at the Molecular Biotechnology Core (Lerner Research Institute, Cleveland, OH) using sequencing primers M13 Universal (-21) and M13-2 Reverse (Table 1).
Nucleotide sequence accession number.
The 2,375-bp nucleotide sequence of the 16S rRNA gene, the complete ITS region, and the 5′-end portion of the 23S rRNA gene of Bergeyella sp. clone AF14 has been deposited in the GenBank database with accession number DQ241813.
RESULTS
Study population, sample collection, and medical data collection.
A total of 35 women pregnant with singletons and undergoing transabdominal amniocentesis were recruited at MHMC. One patient (patient 19) was excluded from the study due to an error in sample collection. For the remaining 34 patients, the population consisted of 50.0% African-American, 35.3% Caucasian, and 14.7% Hispanic subjects, with an average age of 26.6 years. On the basis of the reasons for amniocentesis, the patients were divided into three groups: group 1, patients in preterm labor (PTL) or threatening PTL, including those undergoing cerclage due to an incompetent cervix (19 patients); group 2, patients checking for fetal lung maturity (11 patients); and group 3, patients interested in fetal genetic diagnosis (4 patients) (Table 2). The gestational ages at the times of amniocentesis and delivery were recorded, as were the samples collected from each patient (Table 2). Among group 1 patients, approximately 58% delivered before 35 weeks of gestation, with five delivering extremely early, before 30 weeks of gestation (patients 3, 5, 14, 24, and 31). The rates of premature delivery before 35 weeks were lower in groups 2 and 3, at 0% and 25%, respectively, with only one case of extremely early delivery in group 3 (patients 9 and 30) (Table 2).
TABLE 2.
Summary of pertinent medical records and sample collection
| Patient no. | Reason for amniocentesisa | Hospital diagnosis of AFb
|
Gestation time (wk) at:
|
Samples obtainedc | ||||
|---|---|---|---|---|---|---|---|---|
| Culture | Gram stain | Glucose (mg/dl) | WBC (ml) | Amniocentesis | Delivery | |||
| 1 | PTL | − | − | 37 | 13 | 34 | 35 | A, B, P, V |
| 2 | PTL | − | − | 24 | 7 | 32 | 32 | A, B, P, V |
| 3 | PTL | − | − | 56 | 16 | 22 | 25 | A, B, V |
| 4 | PTL | − | − | 19 | 15 | 32 | 36 | A, B, P, V |
| 5 | PTL | − | − | 17 | 36 | 21 | 25 | A, B, P, V |
| 6 | PTL | − | − | 54 | 5 | 28 | 36 | A, B, P, V |
| 7 | PTL | − | − | 50 | NAd | 33 | 35 | A, B, V |
| 8 | PTL | − | − | 32 | 3 | 18 | 33 | A, B, V |
| 10 | PTL | − | − | NA | NA | 32 | 34 | A, B, V |
| 14 | PTL | − | − | <3 | 3,304 | 24 | 24 | A, B, P, V |
| 15 | PTL | − | − | 49 | 13 | 32 | 32 | A, B, P, V |
| 16 | PTL | − | − | 65 | 13 | 32 | 33 | A, B, P, V |
| 20 | PTL | − | − | 21 | 18 | 20 | 33 | A, B, V |
| 22 | PTL | − | − | NA | 2 | 33 | NAe | A, B, V |
| 24 | PTL | − | − | 34 | 40 | 21 | 27 | A, B, V |
| 28 | PTL | − | − | NA | 2 | 31 | 38 | A, B, V |
| 31 | PTL | − | − | 21 | 5 | 22 | 23 | A, B, P, V |
| 32 | PTL | − | − | 29 | 12 | 33 | 33 | A, B, V |
| 33 | PTL | − | − | 37 | 10 | 24 | 38 | A, B, V |
| 9 | Lung | NA | NA | NA | NA | 36 | 36 | A, B, V |
| 11 | Lung | NA | NA | NA | NA | 34 | 35 | A, B, V |
| 18 | Lung | NA | NA | NA | NA | 36 | 37 | A, B |
| 21 | Lung | NA | NA | NA | NA | 36 | 38 | A, B, P, V |
| 23 | Lung | NA | NA | NA | NA | 36 | 37 | A, B, P, V |
| 25 | Lung | NA | NA | NA | NA | 36 | 37 | A, B, P, V |
| 26 | Lung | NA | NA | NA | NA | 36 | 38 | A, B, V |
| 27 | Lung | NA | NA | NA | NA | 35 | 35 | A, B, V |
| 29 | Lung | − | − | 46 | 4 | 34 | 35 | A, B, V |
| 34 | Lung | NA | NA | NA | NA | 35 | 35 | A, B, V |
| 35 | Lung | NA | NA | NA | NA | 36 | 36 | A, B, V |
| 12 | Genet. | NA | NA | NA | NA | 21 | 40 | A, B, V |
| 13 | Genet. | NA | NA | NA | NA | 17 | NAe | A, B, V |
| 17 | Genet. | NA | NA | NA | NA | 25 | 40 | A, B, V |
| 30 | Genet. | NA | NA | NA | NA | 24 | 25 | A, B, P, V |
The reasons for amniocentesis include preterm labor or threatening preterm labor, including cervical incompetence (PTL); checking for fetal lung maturity (Lung); and fetal genetic diagnosis (Genet.).
Tests of the AF were performed at the hospital laboratory. The results were obtained from the patients' medical records.
A, AF; B, blood; P, subgingival plaque; V, vaginal swab.
NA, not available.
The patient did not deliver at MHMC.
For group 1, hospital laboratory diagnosis of infection in the AF was ordered by the attending physicians. The results of these tests were obtained retrospectively from the patients' medical records (Table 2). No such tests were usually ordered for patients in groups 2 and 3 (Table 2). No bacteria were detected in the AF of any patients by either culturing or Gram staining. Most patients showed a normal glucose concentration in AF, ranging from 17 to 65 mg/dl, and a normal white blood cell (WBC) count of 2 to 40 cells/ml. One patient (patient 14), however, showed abnormal results, with a decreased glucose concentration of <3 mg/dl, below the diagnosis criteria of 5 mg/dl (24), as well as an elevated WBC of 3,304 cells/ml with approximately 90% neutrophils. This patient was admitted to the hospital due to premature contractions. Amniocentesis was performed because she did not respond to tocolysis and her cervix continued to dilate to 3 to 4 cm. Results of AF tests indicated intrauterine infection despite the lack of detection of bacteria in the amniotic fluid. On the basis of this diagnosis, patient 14 was induced immediately, at 24 weeks of gestation, and delivered a female infant weighing 1 lb 7 oz. The infant was sent to the neonatal intensive care unit and survived. Following delivery, the patient's placenta was sent for pathological examination, which revealed necrotizing acute and chronic chorioamnionitis with a diffuse fetal inflammatory response in all umbilical and chorionic plate vessels and umbilical cord stroma, further confirming the intrauterine infection.
Analysis of blood and AF samples.
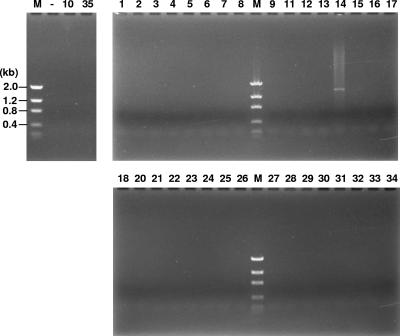
The blood samples collected from the 34 patients were cultured anaerobically at 37°C for 1 to 2 weeks. No bacterial growth was detected in any samples. The AF samples were analyzed by PCR using universal primers 785F and 422R. Using a lab working strain, Fusobacterium nucleatum 12230, as a testing organism, the limit of detection by this approach was determined to be 106 CFU (data not shown). Bacteria were detected only in patient 14 (Fig. 1). Thus, patient 14 likely had an AF infection with a significant bacterial titer, consistent with the severe intrauterine infection diagnosed clinically. The other patients were either not infected or infected at a subclinical level not detected by PCR.
FIG. 1.
PCR examination of AF samples with universal primers 785F and 442R. The numbers at the top correspond to patient numbers. Lane M, DNA molecular size markers, as indicated on the left; Lane —, negative control.
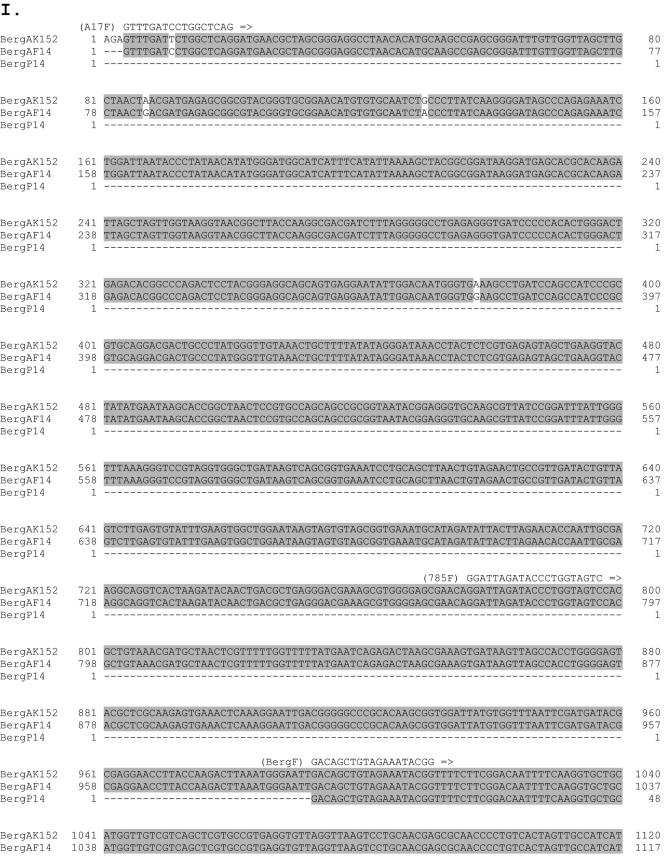
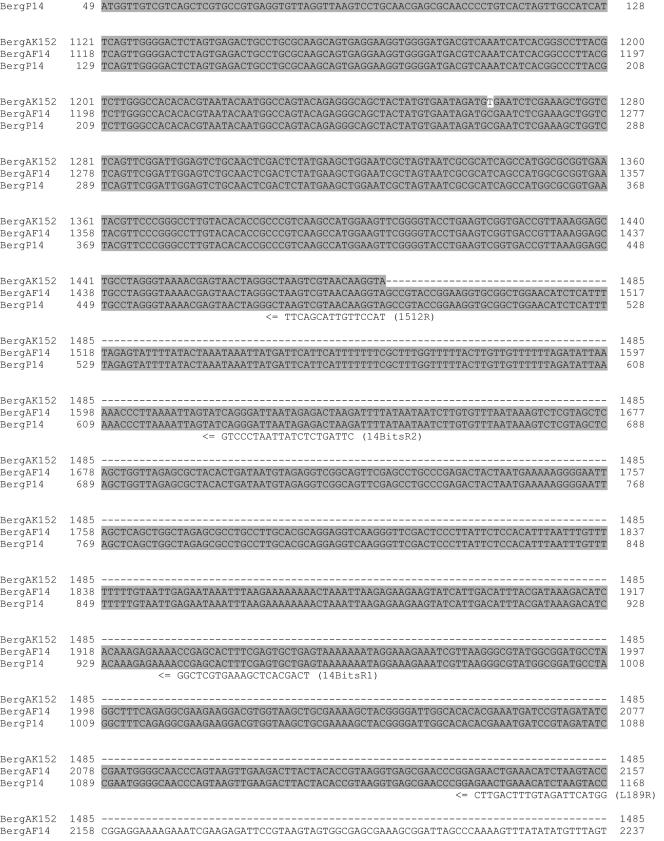
The 1,602-bp fragment amplified from patient 14 by PCR was cloned into pCR2.1, and the DNA sequences from 10 independent clones were determined. The sequences were 99.8% to 100% identical to each other, with only one or two random changes occurring at different positions in different clones, likely caused by infidelities of PCR (data not shown). When the consensus sequence derived from these 10 clones was searched against the NCBI nucleotide database, the first 709 bp, corresponding to the 3′-end region of the 16S rRNA gene, matched 99.8% with the corresponding sequences from an uncultivable oral strain, Bergeyella sp. clone AK152, deposited by B. J. Paster et al. (accession number AY008691). The remaining DNA sequence on the 1,602-bp fragment, containing ITS and 5′-end 23S rRNA sequences, could not be matched because there was no DNA sequence available from Bergeyella sp. clone AK152 in that region (Fig. 2).
FIG. 2.
I. DNA sequence comparison of 16S-23S rRNA genes of Bergeyella sp. clone AK152 (BergAK152), deposited by Paster et al.; clone AF14 (BergAF14), isolated from AF of patient 14; and clone P14 (BergP14), isolated from the subgingival plaque of patient 14. Identical nucleotides are highlighted in gray. Primers for PCR are indicated above or below the sequences. The dashed lines indicate that no sequence is available. II. Schematic diagram of 16S-23S rRNA genes. Primer locations and their corresponding products are indicated by arrows and lines.
In order to compare DNA sequences of the 5′ ends of 16S rRNA, AF from patient 14 was analyzed by nested PCR using primers A17F and 1512R (Fig. 2 and data not shown). The limit of detection by nested PCR was 10 CFU, as determined using F. nucleatum 12230 as a testing organism (data not shown). The fragments amplified by nested PCR were cloned, and the sequences from three independent clones were determined. Again, only Bergeyella was detected. When the DNA sequences of the entire 1,482 bp of the 16S rRNA gene were compiled and aligned against those from Bergeyella sp. clone AK152, a total of five mismatches were detected (Fig. 2). With a 99.7% match of the 16S rRNA, it is apparent that the intrauterine infection of patient 14 was caused predominantly by an oral Bergeyella strain, designated clone AF14. This strain differs from the type species Bergeyella zoohelcum because they share only 87 to 92% identity at the 16S rRNA level.
Examination of the subgingival plaque and vaginal swab of patient 14.
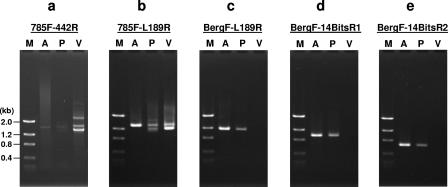
To determine the source of intrauterine Bergeyella infection in patient 14, her subgingival plaque and vaginal swab samples were analyzed by nested PCR. The first PCR using universal primers 785F and 442R detected bacteria in both samples, with more in the vaginal swab than in the subgingival plaque (Fig. 3a). This observation verified the adequacy of sample collection, which was further confirmed by nested PCR using universal primers 785F and L189R (Fig. 3b). Nested PCR using a Bergeyella-specific primer, BergF, and the universal primer L189R amplified a strong 1,168-bp fragment from both AF and subgingival plaque but not from the vaginal sample (Fig. 3c). Similar results were observed with two additional nested PCRs, using all Bergeyella-specific primers, i.e., BergF-14BitsR1 and BergF-14BitsR2. Bergeyella-specific fragments of 961 bp and 648 bp were amplified from AF and subgingival plaque but not from the vaginal swab (Fig. 3d and 3e).
FIG. 3.
Direct and nested PCR analyses of samples collected from patient 14. a. PCR using universal primers 785F and 442R. The products obtained were used as temples for nested PCR in panels b to e. b. Nested PCR using universal primers 785F and L189R. c. Nested PCR using Bergeyella-specific primer BergF and universal primer L189R. d. Nested PCR using Bergeyella-specific primers BergF and 14BitsR1. e. Nested PCR using Bergeyella-specific primers BergF and 14BitsR2. Lanes M, molecular size markers, as indicated on the left; lanes A, AF; lanes P, subgingival plaque; lanes V, vaginal swab.
All three fragments (1,168, 961, and 648 bp) amplified from the subgingival plaque of patient 14 were then cloned into pCR2.1, followed by DNA sequence analysis. A total of 28 independent clones were sequenced. For each of the three fragments, two groups of sequences were identified, with one matching exactly that from AF (Fig. 2) and the other matching by 99.0 to 99.7% (data not shown). The sequence variations in the second group could be due to the increased infidelity of nested PCR; alternatively, they could indicate different strains of Bergeyella or different 16S-23S rRNA operons of the same strain. In either case, since the Bergeyella sequences identified in AF matched those in the subgingival plaque and since no Bergeyella was detected in the patient's lower vaginal tract, it is plausible that Bergeyella sp. clone AF14 originated from her oral cavity rather than from the vagina.
Detection of Bergeyella in the subgingival plaque of women without intrauterine infection.
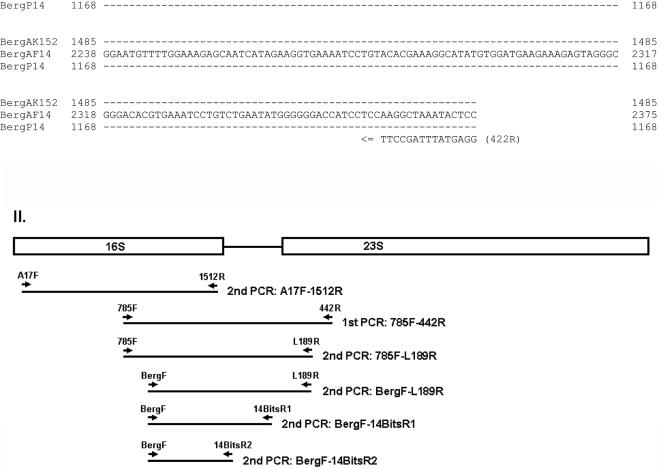
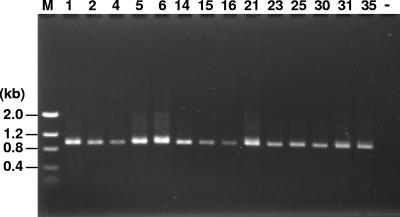
Bergeyella was detected in the subgingival plaque of all 14 patients tested by nested PCR using BergF and 14BitsR1 (Fig. 4) and BergF and 14BitsR2 (data not shown). However, patient 14 was the only subject positive for Bergeyella in AF.
FIG. 4.
Detection of Bergeyella in subgingival plaque samples by nested PCR using primers BergF and 14BitsR1. The numbers on the top correspond to the patient numbers. Lane M, DNA molecular size markers, as indicated on the left; Lane —, negative control.
DISCUSSION
In this pilot study, AF samples from a total of 34 women pregnant with singletons were analyzed. The samples were obtained via transabdominal amniocentesis before the cervix was dilated >5 cm and prior to the rupture of fetal membranes. This would minimize the possibility of microbial contamination from the lower vaginal tract. PCR analysis using universal primers specific to the 16S-23S rRNA genes detected bacteria in only one patient, patient 14 (Fig. 1). DNA sequence analysis of the PCR-amplified 16S rRNA gene revealed a Bergeyella strain, designated clone AF14, which shared 99.7% identity with the previously reported uncultivated oral clone AK152.
Information obtained retrospectively from the patients' medical records indicated that this was also the only patient in our study population with clinically diagnosed intrauterine infection, as indicated by a dramatically decreased glucose level and elevated WBC counts in AF. Approximately 90% of the WBC in the AF of patient 14 were neutrophils, further confirming the infectious status. Pathological analysis of her placenta following delivery showed histologic chorioamnionitis with fetal vasculitis involving the umbilical cord (funisitis) and chorionic plate, which was apparently the cause of her premature labor and delivery at 24 weeks of gestation. The acute and chronic nature of the maternal inflammatory response together with the finding of total necrosis of amnion epithelial cells is indicative of a longstanding infection of at least 3 days in duration. The fetal inflammatory response involving all three umbilical vessels and umbilical cord stroma demonstrates that the Bergeyella sp. is capable of activating the fetal immune system, thereby increasing the risk of central nervous system and pulmonary complications (12, 31, 43).
Little is known about the genus Bergeyella. The type species, B. zoohelcum, once named Weeksella zoohelcum, is a rod-shaped, gram-negative, aerobic bacterium that is frequently associated with dog and cat bite wounds and with respiratory diseases in cats (14, 32, 38, 40). It can be found in 38 to 90% of nasal and oral fluids and gingival scrapings of dogs (38), yet it is difficult to cultivate (40). On the basis of the sequence of the 16S rRNA gene, oral Bergeyella sp. clones AK152 and AF14 are clearly different from B. zoohelcum. No oral Bergeyella species has been cultivated to date. The difficulty in cultivation could explain why no bacteria were detected in the AF of patient 14 during route hospital testing. Although unlikely, the possibility exists that the AF of patient 14 was infected with additional species and that the AF of other patients was also infected, all at levels below the limit of detection by PCR. Since the other patients did not have clinical intrauterine infections and no bacteria were detected in their AF, PCR appears to be a reliable method for clinical diagnosis of intrauterine infections, particularly of those caused by uncultivated microorganisms. With a limit of detection of approximately 106 CFU of F. nucleatum, Bergeyella sp. clone AF14 likely reached a significant titer in AF. Thus, AF may be a useful supplement in media for enrichment or isolation of oral Bergeyella spp.
Intrauterine infection with Bergeyella has never been reported before. Where could the bacteria come from? The paradigm is that intrauterine infections originate primarily from the lower genital tract via an ascending mechanism. In the case of patient 14, however, the ascending mechanism seemed questionable due to the lack of detection of Bergeyella in her vaginal swab, even with the more sensitive method of nested PCR. The lack of detection was not due to inadequate sample collection, since ample amounts of bacteria were detected in the vaginal swab, as indicated by PCR and nested PCR using universal primers (Fig. 3a and b). There is an alternative possibility that due to uneven distribution of microbes, a single sampling in the vaginal tract could have missed a particular species. Nevertheless, the same Bergeyella strain was detected in the subgingival plaque of patient 14. DNA sequence comparison of clones obtained from AF and subgingival plaque revealed a perfect match of the 16S-23S rRNA gene region, including the highly variable ITS region (Fig. 2), indicating that Bergeyella sp. clone AF14 is of oral origin. Ideally, fecal samples from patient 14 should also be examined, because the gut is another major microbial habitat. However, since Bergeyella has never been reported to be associated with the gut flora, it is unlikely that it originated from the gut.
The PTB rate among the study population was higher than the national rate of 11%. This is due to the fact that women undergoing amniocentesis are usually experiencing certain complications or are under high risk and thus are more likely to deliver prematurely. However, the majority of PTBs in this study occurred late during the gestation, with a total of only six cases of preterm delivery before 30 weeks (Table 1). Since intrauterine infection is more prevalent in extremely early PTB (5, 42), this may explain why only one case of intrauterine infection, at a rate of 3% of the study population, was identified.
The current study differs from previous ones in that bacteria identified in AF were compared with those in the vaginal tract and the oral cavity of the same patient at the DNA level (3, 4, 10, 11, 15, 16, 20). Although other orally related microorganisms, such as F. nucleatum and Capnocytophaga spp., have been isolated from intrauterine infections and have been speculated to originate from the oral cavity, the presence (or absence) of these organisms in the oral cavities of the same patients was not known (4, 10, 11, 16, 20). Oral Bergeyella could be yet another opportunistic pathogen whose association with periodontal disease is not known but which is pathogenic when transmitted to other parts of the body such as the pregnant uterus.
How are oral bacteria transmitted from the oral cavity to the uterus? Like F. nucleatum and Capnocytophaga spp., Bergeyella appears to be commonly present in the subgingival plaque (Fig. 4), albeit at low levels (unpublished results; Bruce Paster, personal communication). It is unclear why it can be transmitted to the uterus in certain patients but not in others. Orogenital contact had been proposed as a possible route of transmission of oral bacteria (3, 15). The lack of detection of Bergeyella in patient 14's vaginal tract did not support such a transmission (Fig. 3). Thus, we speculate that the bacteria were transmitted hematogenously, as previously demonstrated in mice (18). Is periodontal disease a prerequisite for the transmission? It is plausible that periodontal disease may facilitate the oral-utero transfer because of the increased bacterial load in the oral cavity and the altered host immune responses during disease. Although patient 14 reported gum bleeding during pregnancy, she appeared to be in good periodontal health at postpartum examination, with no significant signs of periodontal disease. Thus, the role of periodontal disease in the oral-utero transmission is unclear. It is worth pointing out that the organisms most capable of oral-utero transmission may not be those most critically implicated in periodontal disease. Host factors and the oral microflora as a whole may play important roles in the transmission process. The current study identifies a potential link between oral bacteria and preterm birth. Follow-up studies with a much larger patient population are required to address the above-mentioned questions in depth.
Acknowledgments
We thank Cathy Sulzman, Saad Salem Ai-Anizi, Khaldoun Alkatma, and Mei Li for assistance in sample collection; Sheela Pramaraj for discussions of PCR and nested PCR conditions; Jianhong Zhou for preparing the placental samples for pathological analysis; and Bruce Paster for providing Bergeyella-specific 16S rRNA nucleotide sequences and for critically reading the manuscript.
This work was supported in part by NIH/NIDCR grants DE 14447 and DE 14924 and a grant from the Philip Morris External Research Program to Y.W.H.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akcali, K. C., G. Gibori, and S. A. Khan. 2003. The involvement of apoptotic regulators during in vitro decidualization. Eur. J. Endocrinol. 149:69-75. [DOI] [PubMed] [Google Scholar]
- 3.Alanen, A., and E. Laurikainen. 1999. Second-trimester abortion caused by Capnocytophaga sputigena: case report. Am. J. Perinatol. 16:181-183. [DOI] [PubMed] [Google Scholar]
- 4.Altshuler, G., and S. Hyde. 1985. Fusobacteria. An important cause of chorioamnionitis. Arch. Pathol. Lab. Med. 109:739-743. [PubMed] [Google Scholar]
- 5.Andrews, W. W., R. L. Goldenberg, and J. C. Hauth. 1995. Preterm labor: emerging role of genital tract infections. Infect. Agents Dis. 4:196-211. [PubMed] [Google Scholar]
- 6.Andrews, W. W., J. C. Hauth, and R. L. Goldenberg. 2000. Infection and preterm birth. Am. J. Perinatol. 17:357-365. [DOI] [PubMed] [Google Scholar]
- 7.Bearfield, C., E. S. Davenport, V. Sivapathasundaram, and R. P. Allaker. 2002. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br. J. Obstet. Gynaecol. 109:527-533. [DOI] [PubMed] [Google Scholar]
- 8.Bobitt, J. R., C. C. Hayslip, and J. D. Damato. 1981. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am. J. Obstet. Gynecol. 140:947-952. [DOI] [PubMed] [Google Scholar]
- 9.Boggess, K. A., P. N. Madianos, J. S. Preisser, K. J. Moise, Jr., and S. Offenbacher. 2005. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am. J. Obstet. Gynecol. 192:554-557. [DOI] [PubMed] [Google Scholar]
- 10.Cahill, R. J., S. Tan, G. Dougan, P. O'Gaora, D. Pickard, N. Kennea, M. H. Sullivan, R. G. Feldman, and A. D. Edwards. 2005. Universal DNA primers amplify bacterial DNA from human fetal membranes and link Fusobacterium nucleatum with prolonged preterm membrane rupture. Mol. Hum. Reprod. 11:761-766. [DOI] [PubMed] [Google Scholar]
- 11.Chaim, W., and M. Mazor. 1992. Intraamniotic infection with fusobacteria. Arch. Gynecol. Obstet. 251:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Dammann, O., E. N. Allred, A. Leviton, S. Shen-Schwarz, D. Heller, D. R. Genest, and M. H. Collins. 2004. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta 25:788-796. [DOI] [PubMed] [Google Scholar]
- 13.Davenport, E. S., C. E. Williams, J. A. Sterne, S. Murad, V. Sivapathasundram, and M. A. Curtis. 2002. Maternal periodontal disease and preterm low birthweight: case-control study. J. Dent. Res. 81:313-318. [DOI] [PubMed] [Google Scholar]
- 14.Decostere, A., L. A. Devriese, R. Ducatelle, and F. Haesebrouck. 2002. Bergeyella (Weeksella) zoohelcum associated with respiratory disease in a cat. Vet. Rec. 151:392. [DOI] [PubMed] [Google Scholar]
- 15.Dixon, N. G., D. Ebright, M. A. Defrancesco, and R. E. Hawkins. 1994. Orogenital contact: a cause of chorioamnionitis? Obstet. Gynecol. 84:654-655. [PubMed] [Google Scholar]
- 16.Douvier, S., C. Neuwirth, L. Filipuzzi, and J. P. Kisterman. 1999. Chorioamnionitis with intact membranes caused by Capnocytophaga sputigena. Eur. J. Obstet. Gynecol. Reprod. Biol. 83:109-112. [DOI] [PubMed] [Google Scholar]
- 17.Ernest, J. M., and B. Wasilauskas. 1985. Capnocytophaga in the amniotic fluid of a woman in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 153:648-649. [DOI] [PubMed] [Google Scholar]
- 18.Han, Y. W., R. W. Redline, M. Li, L. Yin, G. B. Hill, and T. S. McCormick. 2004. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect. Immun. 72:2272-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, G. B. 1993. Investigating the source of amniotic fluid isolates of fusobacteria. Clin. Infect. Dis. 16(Suppl. 4):S423-S424. [DOI] [PubMed] [Google Scholar]
- 20.Hill, G. B. 1998. Preterm birth: associations with genital and possibly oral microflora. Ann. Periodontol. 3:222-232. [DOI] [PubMed] [Google Scholar]
- 21.Jeffcoat, M. K., N. C. Geurs, M. S. Reddy, S. P. Cliver, R. L. Goldenberg, and J. C. Hauth. 2001. Periodontal infection and preterm birth: results of a prospective study. J. Am. Dent. Assoc. 132:875-880. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoat, M. K., J. C. Hauth, N. C. Geurs, M. S. Reddy, S. P. Cliver, P. M. Hodgkins, and R. L. Goldenberg. 2003. Periodontal disease and preterm birth: results of a pilot intervention study. J. Periodontol. 74:1214-1218. [DOI] [PubMed] [Google Scholar]
- 23.Kazor, C. E., P. M. Mitchell, A. M. Lee, L. N. Stokes, W. J. Loesche, F. E. Dewhirst, and B. J. Paster. 2003. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 41:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiltz, R. J., M. S. Burke, and R. P. Porreco. 1991. Amniotic fluid glucose concentration as a marker for intra-amniotic infection. Obstet. Gynecol. 78:619-622. [PubMed] [Google Scholar]
- 25.Kumar, P. S., A. L. Griffen, J. A. Barton, B. J. Paster, M. L. Moeschberger, and E. J. Leys. 2003. New bacterial species associated with chronic periodontitis. J. Dent. Res. 82:338-344. [DOI] [PubMed] [Google Scholar]
- 26.Lin, D., M. A. Smith, C. Champagne, J. Elter, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 71:5156-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, D., M. A. Smith, J. Elter, C. Champagne, C. L. Downey, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect. Immun. 71:5163-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez, N. J., P. C. Smith, and J. Gutierrez. 2002. Higher risk of preterm birth and low birth weight in women with periodontal disease. J. Dent. Res. 81:58-63. [DOI] [PubMed] [Google Scholar]
- 29.Lopez, N. J., P. C. Smith, and J. Gutierrez. 2002. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J. Periodontol. 73:911-924. [DOI] [PubMed] [Google Scholar]
- 30.Martin, J. A., B. E. Hamilton, P. D. Sutton, S. J. Ventura, F. Menacker, and M. L. Munson. 2005. Births: final data for 2003. Natl. Vital Stat. Rep. 54:1-116. [PubMed] [Google Scholar]
- 31.Mittendorf, R., A. G. Montag, W. MacMillan, S. Janeczek, P. G. Pryde, R. E. Besinger, J. G. Gianopoulos, and N. Roizen. 2003. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am. J. Obstet. Gynecol. 188:1438-1446. [DOI] [PubMed] [Google Scholar]
- 32.Montejo, M., K. Aguirrebengoa, J. Ugalde, L. Lopez, J. A. Saez Nieto, and J. L. Hernandez. 2001. Bergeyella zoohelcum bacteremia after a dog bite. Clin. Infect. Dis. 33:1608-1609. [DOI] [PubMed] [Google Scholar]
- 33.Moore, S., M. Ide, P. Y. Coward, M. Randhawa, E. Borkowska, R. Baylis, and R. F. Wilson. 2004. A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. Br. Dent. J 197:251-258, 247. [DOI] [PubMed] [Google Scholar]
- 34.Offenbacher, S., H. L. Jared, P. G. O'Reilly, S. R. Wells, G. E. Salvi, H. P. Lawrence, S. S. Socransky, and J. D. Beck. 1998. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann. Periodontol. 3:233-250. [DOI] [PubMed] [Google Scholar]
- 35.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher, S., S. Lieff, K. A. Boggess, A. P. Murtha, P. N. Madianos, C. M. Champagne, R. G. McKaig, H. L. Jared, S. M. Mauriello, R. L. Auten, Jr., W. N. Herbert, and J. D. Beck. 2001. Maternal periodontitis and prematurity. I. Obstetric outcome of prematurity and growth restriction. Ann. Periodontol. 6:164-174. [DOI] [PubMed] [Google Scholar]
- 37.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reina, J., and N. Borrell. 1992. Leg abscess caused by Weeksella zoohelcum following a dog bite. Clin. Infect. Dis. 14:1162-1163. [DOI] [PubMed] [Google Scholar]
- 39.Romero, R., M. Sirtori, E. Oyarzun, C. Avila, M. Mazor, R. Callahan, V. Sabo, A. P. Athanassiadis, and J. C. Hobbins. 1989. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 161:817-824. [DOI] [PubMed] [Google Scholar]
- 40.Shukla, S. K., D. L. Paustian, P. J. Stockwell, R. E. Morey, J. G. Jordan, P. N. Levett, D. N. Frank, and K. D. Reed. 2004. Isolation of a fastidious Bergeyella species associated with cellulitis after a cat bite and a phylogenetic comparison with Bergeyella zoohelcum strains. J. Clin. Microbiol. 42:290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahbeh, C. J., G. B. Hill, R. D. Eden, and S. A. Gall. 1984. Intra-amniotic bacterial colonization in premature labor. Am. J. Obstet. Gynecol. 148:739-743. [DOI] [PubMed] [Google Scholar]
- 42.Watts, D. H., M. A. Krohn, S. L. Hillier, and D. A. Eschenbach. 1992. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet. Gynecol. 79:351-357. [DOI] [PubMed] [Google Scholar]
- 43.Yoon, B. H., R. Romero, K. S. Kim, J. S. Park, S. H. Ki, B. I. Kim, and J. K. Jun. 1999. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am. J. Obstet. Gynecol. 181:773-779. [DOI] [PubMed] [Google Scholar]
- 44.Yoon, B. H., R. Romero, J. H. Lim, S. S. Shim, J. S. Hong, J. Y. Shim, and J. K. Jun. 2003. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am. J. Obstet. Gynecol. 189:919-924. [DOI] [PubMed] [Google Scholar]