Abstract
We report an immunocompetent woman with multisystem organ failure following herpes simplex virus type 2 (HSV-2) hepatitis. After she initially responded to intravenous acyclovir, she was switched to oral valacyclovir. She developed respiratory failure and opportunistic infections and died. Autopsy confirmed disseminated HSV infection, and lung tissue grew acyclovir-resistant HSV-2.
CASE REPORT
A 28-year-old woman was admitted to a local hospital with a 2-week history of abdominal pain, fever, nausea, and vomiting. Multiple bacterial and fungal cultures, including cultures of blood, urine, sputum, cervical, and bone marrow, were unrevealing. She developed fulminant hepatitis, thrombocytopenia, and acute renal failure. Serologic tests for hepatitis A, B, and C viruses and human immunodeficiency virus were negative. Serum antibodies confirmed past infection with Epstein-Barr virus, cytomegalovirus, and varicella zoster virus. Antinuclear antigen was positive at 1:2,560. In the absence of an identified pathogen, the patient was started on intravenous methylprednisolone (1 g once a day) on hospital day 6 for a presumed autoimmune hepatitis. On day 7, she developed sepsis and respiratory failure. She was intubated and transferred to the University of Washington Medical Center. Her medical history was significant for asthma and remote, steroid-responsive idiopathic thrombocytopenic purpura. She had not received corticosteroids in the year prior to admission. She was gravida 1, para 1. She had no allergies and was receiving amitriptyline and albuterol as routine medications.
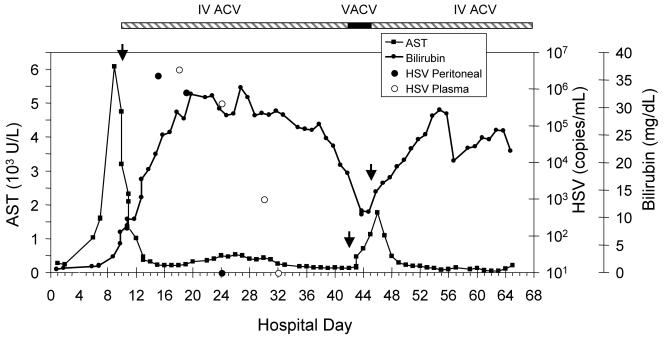
On arrival at the University of Washington Medical Center, she was noted to have 10 to 15 single small vesicles scattered on her trunk and extremities that had developed in the 24 h prior to transfer. Intravenous acyclovir was initiated for possible disseminated herpes simplex virus (HSV). An unroofed toe lesion was positive by direct fluorescent antibody testing and culture for HSV-2. Serum antibodies to HSV-1 and HSV-2 were detected by Western blotting. A sepsis workup revealed peritonitis with no evidence of bacterial or fungal infection, but 2.4 million copies of HSV DNA/ml were detected by PCR in the peritoneal fluid. Plasma HSV PCR peaked at 3.5 million copies/ml on hospital day 18 (Fig. 1). Liver biopsy showed viral inclusion bodies with confirmation of HSV by immunohistochemical staining.
FIG. 1.
Serum aspartate aminotransferase (AST) and total bilirubin during hospitalization with plasma and peritoneal HSV levels as determined by PCR. Recurrent elevation of bilirubin and AST levels coincided with a switch to valacyclovir. Arrows indicate changes in antiviral therapy. IV ACV, intravenous acyclovir; VACV, oral valacyclovir.
She improved steadily on intravenous acyclovir (10 mg/kg of body weight every 8 h) and broad-spectrum antibiotics, and her ascites and plasma showed gradual clearance of HSV (Fig. 1). On day 43, she was significantly improved with no clinical evidence of peritonitis. She was switched to oral valacyclovir (1 g three times a day) in preparation for transfer home. On the morning of day 47, she developed acute respiratory distress requiring intubation and transfer to the intensive care unit. Intravenous acyclovir was restarted at the time of transfer. Initial blood, sputum, urine, and peritoneal cultures revealed no growth, but 3 days into her intensive care unit stay she developed sepsis with Candida dubliniensis fungemia. Over the next 3 weeks, she required continued mechanical ventilation, broad-spectrum antibiotics, antifungals, hemodialysis, and blood product support for thrombocytopenia and an elevated international normalized ratio. Her course was complicated by disseminated candidal infection, vancomycin-resistant enterococcal line infection, and an upper gastrointestinal bleed. She died on hospital day 65.
Postmortem examination revealed multisystem organ failure secondary to disseminated HSV, bacterial, and fungal infections. The liver was normal in size but exhibited complete necrosis with only a few viable cells visualized. Liver tissue culture was negative. Multiple deep tracheal ulcerations were seen; histology revealed viral cytopathic effects consistent with HSV infection. Both lungs had multiple patches of necrosis with viral cytopathic effects consistent with extensive HSV infection. HSV-2 and Acinetobacter baumannii were isolated from lung tissue culture. The HSV isolate was resistant to acyclovir by plaque reduction testing with a 50% tissue culture infective dose of 47.67 μg/ml (>1.90 μg/ml is resistant) (ViroMed Laboratories, Minnetonka, MN). HSV-2 was isolated from cultured Vero cells, and the thymidine kinase (tk) gene was PCR amplified using PFU thermostable polymerase (Stratagene, La Jolla, CA) and primers flanking the coding sequence. Primer sequences and PCR conditions are available upon request. Amplification and internal primers were used for sequencing in both directions, as previously described (16). The sequence of the patient isolate (GenBank accession number DQ372963) was compared to the tk gene from HSV-2 strains HG52 (7) and 333 (14) and contained three single-nucleotide substitutions relative to these strains. Each substitution led to an amino acid change. The T287M change from the sequence of the HG52 reference strain (T288M, from that of reference strain 333) has been previously associated with in vivo and in vitro acyclovir resistance (10). At the polymorphic amino acid residues 39 and 78, the isolate had E and D residues, respectively. These sequence variants have been previously noted (4) and are not specifically associated with acyclovir resistance.
HSV is an uncommon cause of hepatitis, typically occurring during pregnancy or in immunocompromised hosts. In the several cases reported occurring with clinically immunocompetent hosts, diagnosis of HSV hepatitis is often delayed, as skin lesions are frequently not present or are atypical (1, 3, 8, 9, 11-13, 17, 22). This diagnosis should be considered in any patient with acute hepatitis, particularly those with fever, leukopenia, and a negative hepatitis panel. Rapid initiation of antiviral treatment is imperative and should be started empirically when disseminated HSV is suspected. Unfortunately, the diagnosis of HSV hepatitis is often made at autopsy, due to a lack of clinical suspicion. PCR has emerged as a rapid and sensitive diagnostic tool for verification of disseminated HSV disease and should be utilized on plasma, body fluids, and biopsy specimens when possible (2, 6).
The pathogenesis of HSV hepatitis is unknown. Proposed mechanisms include an unrecognized host cell-mediated immune dysfunction, a high viral inoculum, superinfection with a second strain of HSV, and infection with a hepatovirulent strain of HSV (13). This patient did not have a known immune deficiency, but her history of idiopathic thrombocytopenic purpura raises the possibility of unrecognized immune dysfunction. Serologic evidence of prior infection with Epstein-Barr virus, cytomegalovirus, and varicella zoster virus suggests that if an immune defect was present, it was specific to HSV.
Early treatment with intravenous acyclovir is associated with improved outcomes in patients with HSV hepatitis, but there is little experience to guide when, if ever, it is safe to transition to oral therapy. Despite 32 days of intravenous acyclovir and clinical resolution of signs and symptoms of active HSV infection, our patient was not able to control continued HSV replication. Oral valacyclovir may not have been sufficiently absorbed, and low levels of acyclovir in a patient with a high viral burden may have resulted in the emergence of resistance.
Acyclovir resistance is rare (<0.5%) among immunocompetent hosts (5, 15, 19-21). It is associated with mutations in the gene encoding thymidine kinase, the enzyme required for initial phosphorylation of acyclovir (18). In this case the HSV strain recovered at autopsy exhibited phenotypic acyclovir resistance and bore a predicted amino acid change (T287M) previously associated with clinical resistance to acyclovir in vivo and high-level resistance to acyclovir in vitro. While thymidine kinase mutants are often present, they become significant only in immunocompromised patients who have extensive viral replication. In this patient, low levels of acyclovir may have contributed to the emergence of resistant virus. This mechanism indicates the importance of maintaining adequate serum drug levels in the setting of disseminated HSV disease. Our patient had an impressive 3.5 million copies of plasma HSV/ml by PCR, a high viral burden that likely contributed to the development of resistance. While it is not known when the acyclovir resistance developed, her clinical course suggests that the initial virus was susceptible to acyclovir, as she had a good clinical response, and that resistance coincided with the administration of oral therapy.
In our patient, disseminated HSV was found in nearly every portion of the body, including skin, liver, lungs, trachea, peritoneal fluid, and blood, despite treatment with intravenous antiviral therapy. Serial monitoring of patients with disseminated HSV should be performed by plasma PCR, and consideration should be given to viral resistance testing if the level does not decrease during antiviral therapy. This approach will lead to early recognition of viral resistance and treatment failure and facilitate initiation of alternative therapy as indicated.
Nucleotide sequence accession number.
The sequence of the patient isolate from this study has been submitted to GenBank under accession no. DQ372963.
Acknowledgments
This work was supported by NIH grants AI-30731 (to A.W.), AI-50132 (to D.M.K.), and AI-07044 (to T.C.).
None of the authors have conflicts of interest related to the manuscript.
REFERENCES
- 1.Baxter, R. P., L. E. Phillips, S. Faro, and L. Hoffman. 1986. Hepatitis due to herpes simplex virus in a nonpregnant patient: treatment with acyclovir. Sex. Transm. Dis. 13:174-176. [DOI] [PubMed] [Google Scholar]
- 2.Brice, S. L., S. S. Stockert, J. D. Jester, J. C. Huff, J. D. Bunker, and W. L. Weston. 1992. Detection of herpes simplex virus DNA in the peripheral blood during acute recurrent herpes labialis. J. Am. Acad. Dermatol. 26:594-598. [DOI] [PubMed] [Google Scholar]
- 3.Chase, R. A., J. C. Pottage, Jr., M. H. Haber, G. Kistler, D. Jensen, and S. Levin. 1987. Herpes simplex viral hepatitis in adults: two case reports and review of the literature. Rev. Infect. Dis. 9:329-333. [DOI] [PubMed] [Google Scholar]
- 4.Chibo, D., J. Druce, J. Sasadeusz, and C. Birch. 2004. Molecular analysis of clinical isolates of acyclovir resistant herpes simplex virus. Antiviral Res. 61:83-91. [DOI] [PubMed] [Google Scholar]
- 5.Danve-Szatanek, C., M. Aymard, D. Thouvenot, F. Morfin, G. Agius, I. Bertin, S. Billaudel, B. Chanzy, M. Coste-Burel, L. Finkielsztejn, H. Fleury, T. Hadou, C. Henquell, H. Lafeuille, M. E. Lafon, A. Le Faou, M. C. Legrand, L. Maille, C. Mengelle, P. Morand, F. Morinet, E. Nicand, S. Omar, B. Picard, B. Pozzetto, J. Puel, D. Raoult, C. Scieux, M. Segondy, J. M. Seigneurin, R. Teyssou, and C. Zandotti. 2004. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J. Clin. Microbiol. 42:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond, C., K. Mohan, A. Hobson, L. Frenkel, and L. Corey. 1999. Viremia in neonatal herpes simplex virus infections. Pediatr. Infect. Dis. J. 18:487-489. [DOI] [PubMed] [Google Scholar]
- 7.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahy, R. J., E. Crouser, and E. R. Pacht. 2000. Herpes simplex type 2 causing fulminant hepatic failure. South. Med. J. 93:1212-1216. [PubMed] [Google Scholar]
- 9.Farr, R. W., S. Short, and D. Weissman. 1997. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin. Infect. Dis. 24:1191-1194. [DOI] [PubMed] [Google Scholar]
- 10.Gaudreau, A., E. Hill, H. H. Balfour, Jr., A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, Z. D., K. G. Ishak, and I. A. Sesterhenn. 1986. Herpes simplex hepatitis in apparently immunocompetent adults. Am. J. Clin. Pathol. 85:694-699. [DOI] [PubMed] [Google Scholar]
- 12.Gutmann, D. H., B. A. Beard, and M. L. Collinge. 1988. Nonfatal disseminated mucocutaneous herpes simplex type 2 infection in a healthy woman. Obstet. Gynecol. 72:506-508. [PubMed] [Google Scholar]
- 13.Kaufman, B., S. A. Gandhi, E. Louie, R. Rizzi, and P. Illei. 1997. Herpes simplex virus hepatitis: case report and review. Clin. Infect. Dis. 24:334-338. [DOI] [PubMed] [Google Scholar]
- 14.Kit, S., M. Kit, H. Qavi, D. Trkula, and H. Otsuka. 1983. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim. Biophys. Acta 741:158-170. [DOI] [PubMed] [Google Scholar]
- 15.Kriesel, J. D., S. L. Spruance, M. Prichard, J. N. Parker, and E. R. Kern. 2005. Recurrent antiviral-resistant genital herpes in an immunocompetent patient. J. Infect. Dis. 192:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 17.McMinn, P. C., I. S. Lim, P. E. McKenzie, A. G. van Deth, and A. Simmons. 1989. Disseminated herpes simplex virus infection in an apparently immunocompetent woman. Med. J. Aust. 151:588-590, 592, 594. [DOI] [PubMed] [Google Scholar]
- 18.Morfin, F., G. Souillet, K. Bilger, T. Ooka, M. Aymard, and D. Thouvenot. 2000. Genetic characterization of thymidine kinase from acyclovir-resistant and -susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J. Infect. Dis. 182:290-293. [DOI] [PubMed] [Google Scholar]
- 19.Morfin, F., and D. Thouvenot. 2003. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 26:29-37. [DOI] [PubMed] [Google Scholar]
- 20.Stranska, R., R. Schuurman, E. Nienhuis, I. W. Goedegebuure, M. Polman, J. F. Weel, P. M. Wertheim-Van Dillen, R. J. Berkhout, and A. M. van Loon. 2005. Survey of acyclovir-resistant herpes simplex virus in The Netherlands: prevalence and characterization. J. Clin. Virol. 32:7-18. [DOI] [PubMed] [Google Scholar]
- 21.Tyring, S. K., D. Baker, and W. Snowden. 2002. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J. Infect. Dis. 186(Suppl. 1):S40-S46. [DOI] [PubMed] [Google Scholar]
- 22.Velasco, M., E. Llamas, M. Guijarro-Rojas, and M. Ruiz-Yague. 1999. Fulminant herpes hepatitis in a healthy adult: a treatable disorder? J. Clin. Gastroenterol. 28:386-389. [DOI] [PubMed] [Google Scholar]