Abstract
Toxoplasma gondii has a clonal population genetic structure with three (I, II, and III) lineages that predominate in North America and Europe. Type II strains cause most cases of symptomatic human infections in France and the United States, although few other regions have been adequately sampled. Here we determined the parasite genotype in amniotic fluid and cerebrospinal fluid samples from congenital toxoplasmosis cases in Poland. Nineteen confirmed congenital cases of toxoplasmosis were analyzed, including both severe and asymptomatic cases. The genotype of parasite strains causing congenital infection was determined by direct PCR amplification and restriction fragment length polymorphism analysis. Nested multiplex PCR analysis was used to type four independent polymorphic markers. The sensitivity of multiplex nested PCR was ≥25 parasites/ml in amniotic fluid and cerebral spinal fluid samples. Parasite DNA was successfully amplified in 9 of 19 samples (eight severely affected and one asymptomatic fetus). Only genotype II parasites were identified as the source of T. gondii infection based on restriction fragment length polymorphism analysis. Strains causing congenital infections were also typed indirectly based on detection of antibodies to strain-specific peptides. Serotyping indicated that 12 of 15 cases tested were caused by type II strains and these positives included both symptomatic and asymptomatic infections. Overall, the combined analysis indicated that 14 of the cases were caused by type II strains. Our results are consistent with the hypothesis that parasite burden is associated with severity of congenital toxoplasmosis and indicate that serological testing provides a promising method for genotypic analysis of toxoplasmosis.
Toxoplasmosis is a common parasitic disease caused by the protozoan parasite Toxoplasma gondii. Seroprevalence varies between different geographic regions: in Poland, 2,200 (41.3%) out of 4,916 pregnant women were found to have T. gondii-specific anti-immunoglobulin G (IgG) antibodies (20, 20a). Infection can be acquired by ingestion of viable tissue cysts in undercooked meat or oocysts excreted by cats (23). T. gondii is a major cause of morbidity and mortality in congenitally infected infants and immunodeficient and immunocompromised patients (15).
The population genetic structure of T. gondii is highly clonal, despite a sexual phase in the life cycle (14, 25, 26). Three predominant clonal types (I, II, and III) are recently derived from recombination between two highly similar ancestral lineages (8, 27). Recombinant genotypes are rarely found in nature, indicating infrequent sexual recombination between the three lineages (1, 14). Type II strains have been identified as the cause of more than 70% of human cases of toxoplasmosis in the United States and France (1, 5, 14). Type II strains are relatively avirulent in mice yet they readily establish chronic infections characterized by tissue cysts that are highly infectious by the oral route (25, 27). Type I strains are more virulent in mice and have a greater capacity to cross tissue barriers in vitro and in vivo (2, 26, 28).
Enhanced migration could potentially lead to greater capacity to cause congenital infection due to transplacental transmission, although such a relationship has not been directly demonstrated. A single study from Spain indicated that strains possessing the type I allele at the SAG2 locus were found in 6 of 13 cases of congenital infection (6). However, even strains that are nonvirulent in the mouse model are capable of causing severe disease in humans, as shown by the prevalence of type II strains in congenital toxoplasmosis in France (1).
While the majority of genotyping studies have been based on polymorphic DNA markers, one of the primary limitations of this method is the inability to type strains causing chronic infection. Strains of T. gondii are highly similar antigenically; however, the recent identification of serological epitopes that are strain specific raises the possibility of genotyping even chronic infections based on serological profile (17). Serological typing based on strain-specific peptides is capable of distinguishing type II strains from non-type II (typically I or III) strains and offers the promise for determining the frequency of strain types that cause both acute and chronic infections.
The presentation of congenital toxoplasmosis varies widely from subclinical to severe cases, which may cause fetal or neonatal death (23). The frequency and severity of fetal toxoplasmosis depends on the time when infection takes place during pregnancy. Early in pregnancy, infections are less likely to cross the placental barrier, yet those congenital infections that do result are more severe. While infection occurs more readily late in pregnancy, the majority of such cases are mild or asymptomatic at birth (summarized in reference 23). High parasite concentrations in the amniotic fluid (AF) have been associated with severe outcome (24).
In the present study we examined a set of well-characterized cases of congenital toxoplasmosis from Poland. We determined the genotypes of T. gondii found in AF and cerebral spinal fluid (CSF) from cases of congenital toxoplasmosis using a newly developed multiplex nested-PCR typing system (16). We also analyzed serological responses to strain-specific peptides in order to serotype infections (17).
MATERIALS AND METHODS
Clinical cases of congenital toxoplasmosis.
Nineteen cases of congenital toxoplasmosis diagnosed at the Research Institute Polish Mother's Memorial Hospital (RIPMMH, Lodz, Poland) between March 1999 and June 2003 were included in the study. The following classification of clinical forms of congenital toxoplasmosis was used. Symptomatic toxoplasmosis was described when ventricular dilatation was observed in repeated ultrasound scans and/or fetal and neonatal death was reported. Chorioretinitis was diagnosed during the neonatal period. Fetuses and/or neonates that did not present pathological symptoms were classified as asymptomatic. After birth, a team of specialists including neurologists and ophthalmologists attended symptomatic and asymptomatic neonates. Congenitally infected infants were treated with combined therapy with pyrimethamine and sulfadiazine. The studies were conducted with approval of the Ethical Committee of RIPMMH, Lodz
Confirmation of congenital infection.
Serological testing (anti-Toxoplasma IgG, IgM, IgA, and IgG avidity) was performed in the Department of Microbiology, RIPMMH, Lodz (20a). Prior to March 2000, screening of maternal sera for Toxoplasma-specific IgG antibodies was performed with a latex agglutination kit (Toxo Screen DA; bioMérieux) (positive cutoff: 4 IU/ml), or an indirect agglutination assay (Platelia Toxo-G; Diagnostics Sanofi Pasteur) (positive cutoff: >6 IU). Testing for Toxoplasma-specific IgM was conducted using an enzyme-linked immunosorbent assay (ELISA) (Platelia Toxo M; Diagnostics Sanofi Pateur). (cutoffs: 0 to 1 IU/ml, negative; 1 to 2 IU/ml, borderline; >2 IU/ml, positive). In cases where specific IgM was detected, an immunosorbent agglutination assay was used for detecting specific IgM and IgA (bioMérieux) (cutoffs: <6, negative; 6 to 7, borderline; >7, positive). In neonates, the Platelia Toxo-M and Toxo-A tests were used with the same cutoff values as in the mothers and the immunosorbent agglutination assay was used for IgM and IgA (cutoffs: <3, negative; 3 to 6, intermediate; >6, positive). IgG avidity was tested using an ELISA (Labsystems) (cutoffs: low, <15%; borderline, 15 to 30%; high, >30%).
Beginning in March 2000, Toxoplasma-specific IgG was detected in sera from mothers and their neonates by enzyme-linked immunofluorescent assay (VIDAS Toxo-IgG; bioMerieux) (positive cutoff: >8 IU/ml). Toxoplasma-specific IgM was detected using VIDAS Toxo-IgM (bioMerieux) (positive cutoff: >0.65 IU/ml) and Toxoplasma-specific IgA was detected using the Platelia Toxo-IgA method as described above. IgG avidity was tested using the enzyme-linked immunofluorescent assay test (VIDAS Toxo-avidity; bioMerieux) (cutoffs: low, <0.2; borderline, 0.2 to 0.3; high, >0.3).
PCR detection of parasite DNA was based on the B1 gene as described previously (7). PCR assays and bioassay by inoculation of mice were conducted in the Department of Medical Parasitology National Institute of Hygiene, Warsaw. For bioassay, 1 ml of AF or CSF was injected intraperitoneally into laboratory mice (CFW/Pzh strain). Six weeks after inoculation, blood samples were examined with the Toxo-Screen DA test to detect seroconversion and establish the titer of Toxoplasma-specific IgG antibodies. The brains of these animals were homogenized and inoculated into another pair of mice that were then examined for Toxoplasma-specific antibodies as described above.
Clinical samples.
AF samples were obtained by amniopuncture under sonographic guidance in the Department of Ultrasound, RIPMMH, Lodz, Poland. Additionally, in one case (number 18) fetal CSF was obtained when hydrocephalus was decompressed in utero by insertion of a ventriculoamniotic shunt (19). In two cases (nos. 1 and 10), CSF samples were collected postnatally for genotyping. One neonate (no. 1) was confirmed infected by positive B1 PCR from CSF taken a few days after birth (Table 1). In another case (no. 10), CSF was available from the infant after long-term antiparasitic treatment. For three neonates (nos. 16, 17, and 18), AF was not available and instead, neonatal blood was used for typing. Negative controls consisted of AF or CSF samples from pregnancies with negative anti-T. gondii serology after delivery. Samples were collected and stored at −20°C at the Department of Fetal-Maternal Medicine and Gynecology, Research Institute Polish Mother's Memorial Hospital, Lodz, Poland and then sent frozen on dry ice to the Department of Microbiology, Washington University School of Medicine, Saint Louis, MO.
TABLE 1.
Clinical findings and genotype analysis in congenitally infected fetuses and infants with T. gondiia
| Case no. | Mother's serology
|
Symptomatic vs. asymptomatic congenital toxoplasmosis
|
Perinatal diagnosis
|
Genotype
|
Serotype
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GW | Anti-T. gondii antibodies | GW/NP | Clinical findings | IUD, GW/NP | PCR | MI | NS | Sample | T. gondii type | Serum | T. gondii type | |
| 1 | 34 | IgG+, IgM+, IgA+ | <35 | Hydrocephaly | Pos. | Pos. | IgG+ | CSF | II | Mother | II | |
| IgG avidity low | NP | Chorioretinitis | IgM+ | Infant | II | |||||||
| IgA+ | ||||||||||||
| 2 | 12 | IgG−, IgM− | Asymptomatic | Pos. | Pos. | IgG+ | AF | II | Mother | Uncertain | ||
| 31 | IgG−, IgM+ | IgM+ | Infant | II | ||||||||
| 38 | IgG+, IgM+, IgA+ | IgA+ | ||||||||||
| 3 | 27 | IgG+, IgM+, IgA+ | 29 | Hydrocephaly | IUD, 32 | Pos. | Pos. | ND | AF | II | Mother | II |
| IgG avidity low | Infant | II | ||||||||||
| 4 | 35 | IgG+, IgM+, IgA+ | 32 | Hydrocephaly | NP | Pos. | Pos. | IgG+ | AF | II | Mother | II |
| IgG avidity low | IgM+ | Infant | II | |||||||||
| IgA− | ||||||||||||
| 5 | 27 | IgG+, IgM+, IgA+ | 27 | Ventriculomegaly | Pos. | Pos. | IgG+ | AF | II | Mother | II | |
| IgG avidity low | IgM+ | Infant | II | |||||||||
| IgA+ | ||||||||||||
| 6 | 29 | IgG+, IgM+, IgA+ | 28 | Hydrocephaly | IUD, 35 | Pos. | Pos. | ND | AF | II | Mother | II |
| IgG avidity low | Infant | NA | ||||||||||
| 7 | 28 | IgG+, IgM+, IgA− | 27 | Hydrocephaly | Pos. | Pos. | IgG+ | AF | II | Mother | II | |
| IgG avidity low | NP | Chorioretinitis | IgM+ | Infant | NA | |||||||
| IgA+ | ||||||||||||
| 8 | 32 | IgG+, IgM+, IgA+ | 27 | Hydrocephaly | NP | Pos. | ND | IgG+ | AF | II | Mother | NA |
| NP | Choroidoretinitis | IgM+ | Infant | NA | ||||||||
| IgA+ | ||||||||||||
| 9 | 34 | IgG+, IgM+, IgA+ | 28 | Ventriculomegaly | Pos. | ND | IgG+ | AF | II | Mother | NA | |
| IgG avidity low | NP | Chorioretinitis | IgM+ | Infant | NA | |||||||
| IgA+ | ||||||||||||
| 10 | 28 | IgG+, IgM+, IgA+ | <28 | Hydrocephaly | ND | ND | IgG+ | CSF | NT | Mother | NA | |
| IgG avidity low | IgM+ | Infant | NA | |||||||||
| IgA+ | ||||||||||||
| 11 | 13 | IgG+, IgM+, IgA+ | Asymptomatic | Pos. | Pos. | IgG+ | AF | NT | Mother | II | ||
| IgG avidity low | IgM− | Infant | II | |||||||||
| IgA− | ||||||||||||
| 12 | 20 | IgM+ | Asymptomatic | Pos. | Pos. | IgG+ | AF | NT | Mother | II | ||
| 25 | IgG+, IgM+, IgA+ | IgM− | Infant | II | ||||||||
| IgG avidity low | IgA− | |||||||||||
| 13 | 30 | IgG+, IgM+, IgA− | Asymptomatic | Pos. | Pos. | IgG+ | AF | NT | Mother | Uncertain | ||
| IgG avidity low | IgM− | Infant | Uncertain | |||||||||
| IgA− | ||||||||||||
| 14 | 28 | IgG+, IgM+, IgA+ | Asymptomatic | Neg. | Pos. | IgG+ | AF | NT | Mother | Uncertain | ||
| IgG avidity low | IgM− | Infant | II | |||||||||
| IgA− | ||||||||||||
| 15 | 27 | IgG+, IgM+, IgA+ | Asymptomatic | Pos. | Neg. | IgG+ | AF | NT | Mother | Atypical | ||
| IgG avidity low | IgM+ | Infant | Atypical | |||||||||
| IgA+ | ||||||||||||
| 16 | 30 | IgG+, IgM+, IgA+ | Asymptomatic | ND | ND | IgG+ | NB | NT | Mother | NA | ||
| IgM+ | Infant | NA | ||||||||||
| IgA+ | ||||||||||||
| 17 | 15 | IgG+, IgM+, IgA+ | Asymptomatic | ND | ND | IgG+ | NB | NT | Mother | Atypical | ||
| IgG avidity low | IgM+ | Infant | Atypical | |||||||||
| IgA− | ||||||||||||
| 18 | 32 | IgG+, IgM+, IgA− | 29 | Hydrocephaly | Pos. | ND | IgG+ | NB | NT | Mother | II | |
| IgG avidity high | IgM+ | Infant | II | |||||||||
| IgA− | ||||||||||||
| 19 | 31 | IgG+, IgM+, IgA+ | 31 | Hydrocephaly | NP | ND | ND | IgG+ | AF | NT | Mother | II |
| IgG avidity low | IgM+ | Infant | II | |||||||||
| IgA+ | ||||||||||||
Abbreviations: GW, gestational week; NP, neonatal period; IUD, intrauterine death; MI, mouse inoculation; NS, neonatal serology; pos., positive result; neg., negative; ND, not done; NT, nontypeable; NA, not available; NB, neonatal blood. Mothers' serology results are given for the first available test. CSF was used for PCR or mouse inoculation in cases 1 and 18 and AF was used in the remaining cases. Uncertain refers to low values with all peptides, such that a strain type could not be reliably determined. Atypical refers to the reaction with peptides from different strain types, reflecting either mixed infection or possibly unusual genotypes. The tests used and their cutoff values are given in the text.
Experimental samples.
T. gondii tachyzoites were cultivated by 2-day passage in human foreskin fibroblast monolayers and purified from host cells as described previously (14). Type strains consisted of RH (type I) (American Type Culture Collection, Manassas, VA; ATCC 50174), PTG (type II) (ATCC 50841), and CTG (type III) (ATCC 50842). T. gondii cells were harvested, counted, and resuspended in phosphate-buffered saline (PBS). Two types of standards were used for testing sensitivity. First, aliquots containing 1, 0.5, 2.5, 5, or 10 parasite equivalents were made from a stock lysate of purified parasites prepared by incubation with 100 μg/ml proteinase K for 1 h at 37°C and 2 h at 50°C, followed by heat inactivation at 90°C for 15 min. Second, aliquots (10 μl) containing 5.0, 10.0, 25.0, 50.0, or 100.0 parasites were added to 1 ml of negative AF and centrifuged at 3,000 × g, and the pellet was used for analysis.
Multiplex PCR genotyping.
We utilized a recently described multiplex nested PCR for T. gondii genotyping based on four independent, unlinked markers: 5′-SAG2, 3′-SAG2, SAG3, GRA6, and BTUB (16). In the first round of PCR, all reverse and forward external primers were combined in one reaction tube. The PCR mixture consisted of 5 μl of 10× PCR buffer (Sigma, St. Louis, MO) containing 1.5 mM of MgCl2; 4 μl of deoxynucleoside triphosphates (2.5 μM each) (Roche Applied Sciences, Indianapolis, IN); 0.15 μl of 50 μM of each forward and reverse primer (Integrated DNA Technologies, Coralvile, IA); 0.5 μl of (5 U/μl) Taq DNA polymerase (Sigma); and 31.0 μl of distilled, DNase- and RNase-free water. PCR was conducted using a PTC-200 DNA engine system Peltier thermal cycler (MJ Research Inc., Watertown, MA) programmed for 94°C for 30 seconds, 55°C for 60 seconds, and 72°C for 2 min during each of 35 cycles. PCR products were digested with restriction enzymes (New England BioLabs, Inc., Beverley, MA) and restriction fragment length polymorphisms (RFLPs) were visualized using ethidium bromide staining of 2% agarose gels.
Sample processing.
Clinical samples of AF and CSF were centrifuged for 10 min at 3,000 × g and pellets were extracted with a QIAamp DNA blood minikit (QIAGEN Inc., Valencia, CA). Neonatal whole blood (200 μl) was processed directly using the QIAamp DNA blood minikit. In the final step, samples were eluted with 25 μl of buffer. Multiplex PCR was conducted using 12.5 μl of the eluted samples as described above. Representative strains were used as positive controls. Negative AF and CSF samples and distilled water served as negative controls.
Serological typing.
Strain-specific polymorphic peptides derived from the T. gondii dense granule (GRA) proteins GRA6 and GRA7 were coupled to keyhole limpet hemocyanin as described previously (17). Within the abbreviated names of the peptides, 6 denotes peptides from GRA6 and 7 those from GRA7; I/III or II indicates the allele of the peptide, i.e., from which archetypal strain it was derived; and indicates a truncated version of the diagnostic peptide. Coupled peptides were diluted to 2 μg/ml in 0.1 M carbonate buffer, pH 8.5, and 50 μl of each peptide solution was loaded into a polystyrene ELISA plate well overnight at 4°C. Coated wells were blocked with 200 μl of a 2% casein solution in PBS for 2 h at room temperature.
Sera were tested by adding 50 μl of diluted human serum (typically 1:100) to each well and incubating for 2 h at room temperature. ELISA plates were washed four times with a PBS/0.1% Tween 20 solution before incubation with a horseradish peroxidase-coupled monoclonal antibody against human IgG (BD Pharmingen, San Diego, CA) for 1 h at room temperature. Plates were washed in PBS and developed with 150 μl of ABTS 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) reagents (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD). Absorbance was read at 1, 2, and 4 h using a 405-nm filter. Two control peptides served as negative controls to establish background reactivity for normalization purposes. They consisted of a randomized sequence of the GRA6 peptide and a mix of peptides derived from the human and Leishmania EF1α proteins coupled to keyhole limpet hemocyanin.
ELISA data for each infection serum were normalized by dividing the optical density (OD) value obtained at each of the eight serotyping peptides by the mean of the OD readings for the two control peptides. Negative reactivity thus yields a theoretical value of ∼1.0: values of 1.4 or greater are considered significant positive reactivity against the serotyping peptides, as defined previously (17). The four positive control sera utilized (two type II and two type I/III) were from patients from whom parasites were recovered and genotyped by PCR-RFLP.
RESULTS
Sensitivity of multiplex PCR in amniotic fluid.
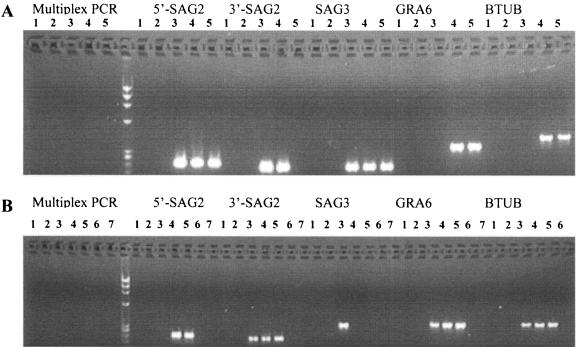
To establish the sensitivity of the multiplex PCR, 1-ml volumes of AF or CSF samples from uninfected pregnancies were spiked with lysates of T. gondii equivalent to 0.5 to 10 parasites per sample. Samples were then directly processed for PCR. The sensitivity of detection ranged from 2.5 to 10 parasites per sample in both AF (Fig. 1A) and CSF (Fig. 1B). In the primary round of multiplex PCR (left side of Fig. 1), no amplification products were seen, consistent with the low input of parasites. In the second round, specific products were detected for each marker (right side, Fig. 1). Differences in the sensitivity of detection of individual markers likely reflect efficiencies based on different PCR primers, since all of these targets are present as a single copy per genome.
FIG. 1.
Sensitivity of multiplex PCR analysis of T. gondii in AF (A) and CSF (B) samples. Multiplex PCR was performed for the four genetic markers SAG2 (5′ and 3′ amplified separately), SAG3, GRA6, and BTUB followed by electrophoresis in agarose gels containing ethidium bromide. Lanes 1, 6, and 7 are negative controls, and lanes 2 to 5 are samples spiked with 0.5, 2.5, 5.0, and 10.0 T. gondii cells, respectively.
We also tested the sensitivity of multiplex PCR under conditions that more closely approximate sample processing. In this instance, defined numbers of whole parasite cells were added to 1 ml of normal AF samples and centrifuged, and the pellet was extracted and analyzed. Under these conditions, the sensitivity was approximately 25 parasites per sample (data not shown). The decreased sensitivity under these conditions likely reflects inefficiency in recovery of parasites following centrifugation and/or extraction. However, since these conditions more closely simulate processing of clinical AF samples, they likely reflect the real sensitivity of this method versus the more efficient detection seen when small samples are spiked with parasites and assayed directly.
Genotypes of T. gondii by multiplex PCR-RFLP analysis.
Nineteen cases of confirmed congenital toxoplasmosis were examined here, as summarized in Table 1. In 14 cases, congenital toxoplasmosis was confirmed by two of the following criteria: positive results by B1 PCR with AF or CSF; positive serology in mice after inoculation of AF or CSF samples into mice; and presence of specific IgM and/or IgA in the neonate (Table 1). Four neonates were classified as congenitally infected based solely on demonstration of specific IgG, IgM, and/or IgA antibodies in repeated tests following birth (Table 1). In the remaining case, only IgG was demonstrated, however, it was included in the study based on inoculation of AF into naïve mice and their subsequent positive serology (case number 14). Ventricular dilatation (ventriculomegaly or hydrocephalus) was detected prenatally in 11 cases (Table 1). Five out of 11 symptomatic cases died, two in utero and three in early postnatal periods. Chorioretinitis was diagnosed in four neonates. Eight cases were asymptomatic.
For 14 of the cases, AF samples were used for detection of parasite DNA by multiplex PCR (Table 1). However, for several cases, only CSF (numbers 1 and 10), or only neonatal blood (numbers 16, 17, and 18) was available. PCR amplification of T. gondii genetic markers was only successful in 9 out of 19 samples; the remaining samples were negative in all tests (Tables 1 and 2). Eight of the nine cases that were positive were detected with AF samples and the remaining positive was obtained from a CSF sample.
TABLE 2.
Genotypes of T. gondii in clinical samples from congenitally infected fetuses and newborns based on multiplex PCRa
| Marker | Restriction enzyme | Score with sample from casec:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
|
|
|
|
6
|
|
|
|
||||||
| CSF (1 ml) | CSF (10 ml) | 2 | 3 | 4 | 5 | AF (1 ml) | AF (10 ml) | 7 | 8 | 9 | ||||
| 5′-SAG2 | Sau3AI | Negb | Neg | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 3′-SAG2 | HhaI | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| SAG3 | NciI | Neg | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| GRA6 | MseI | 2 | 2 | 2 | 2 | 2 | 2 | Neg | 2 | 2 | 2 | 2 | ||
| BTUB | TaqI | Neg | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| BspEI | Neg | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| SAG1 | Sau96I | Neg | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| HaeII | Neg | Neg | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| T. gondii genotype | II | II | II | II | II | II | II | II | II | |||||
Alleles are defined in reference 16. Genotype II is the result of allele 1 at 5′-SAG2 and allele 2 at 3′-SAG2.
Neg, negative.
Where sample type and volume are not specified for the case, the sample was 1 ml of AF.
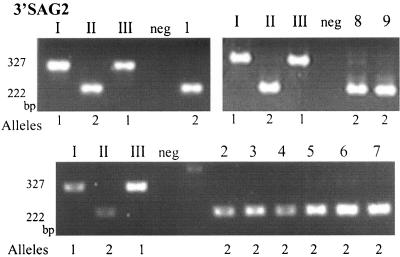
Gene-specific amplification products were subjected to restriction enzyme digestion to identify characteristic RFLPs and the genotypes were determined based on the combination of alleles at different markers (16). For eight of the nine samples, the genotype was determined to be type II for all four markers (Table 2). A representative example is shown for 3′-SAG2 in Fig. 2. In one CSF sample (number 1), genotype II was determined only for the 3′-SAG2 and GRA6 markers and the sample was negative for the remaining markers (Table 2). Genotyping was not successful with the second CSF sample, suggesting a lower parasite concentration in CSF than in AF. In all three severe cases that were not successfully genotyped, late referral complicated proper sampling and this may have caused in the negative results.
FIG. 2.
RFLP analysis of PCR products amplified from AF and CSF samples from cases of congenital toxoplasmosis. 3′-SAG2 amplification products digested with HhaI were resolved in 3% agarose gels stained with ethidium bromide. The samples loaded in lanes I, II, and III are representative of T. gondii strain types I, II, and III, respectively. Samples from pregnancies with congenitally infected fetuses were loaded in lanes 1 to 9. Genotyping revealed all nine positive samples were type II strains of T. gondii. AF samples from uninfected pregnant women (neg) served as negative controls.
Genotyping at the SAG1 locus and type X.
The genotype at SAG1 was also examined using gene-specific PCR to distinguish between parasite type II and newly described type X (18). RFLP analysis does not discriminate between type X and type II strains at the markers (SAG2, SAG3, and GRA6) used here. However, type X can be distinguished from type II by the presence of a type I RFLP pattern at SAG1 after digestion with Sau961 (18). PCR-RFLP analysis of the nine clinical samples studied here showed an RFLP pattern at SAG1 consistent with type II and distinct from type X (Table 2).
Serological typing of clinical samples.
Sera from both the mother and infant were tested for reaction to allele-specific peptides to define the genotype of parasites causing infection, as described previously (17). A total of 28 mother and infant sera from 15 different cases of congenital toxoplasmosis (two were not available as paired samples) were provided to the Toxoplasma Serology Laboratory (Palo Alto Medical Research Foundation) as a blinded set of samples without any identifying information about strain genotype, clinical disease, or dye test titer prior to analysis. Sera were tested against a set of polymorphic peptides that have previously been shown to identify strain-specific antibodies present in patient serum (17). Reaction to these peptides can thus be used to genotype the strain responsible for infection.
Positive control sera (Table 3) established that the assay was reproducible and working within the detection and cutoff limits as previously established by Kong and colleagues (17). The results show that 20 of 28 sera (representing 12 of 15 cases) produced a reactivity pattern consistent with infection by type II strains (positive reaction with one or more type II peptides designated 6II, 6 d-II, 7 II, and 7 d-II) (Table 3). Two sera (maternal sera from cases 2 and 14 in Table 1) gave no clear indication of the type responsible for infection, as reactivity against all peptides was weak. However, in both cases, sera from the infant showed reactivity to at least one type II peptide. These results for maternal and infant sera were consistently observed in repeated assays.
TABLE 3.
Serological analysis of paired mother-infant samples with strain-specific peptidesa
| Case no. | Sample | Score with peptide:
|
Serotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (6 I/III) | 2 (6 d-I/III) | 3 (6 II) | 4 (6 d-II) | 5 (7 II) | 6 (7 d-II) | 7 (7 III) | 8 (7 d-III) | |||
| Negative control | 1.0 | 1.0 | 1.0 | 1.0 | 1.1 | 1.2 | 1.0 | 1.0 | ||
| Positive type II control | 1.2 | 1.0 | 4.1 | 1.6 | 9.1 | 1.1 | 1.0 | 1.1 | ||
| Positive type II control | 1.2 | 1.0 | 4.2 | 1.6 | 9.1 | 1.1 | 1.1 | 1.1 | ||
| Positive type I/III control | 2.4 | 1.0 | 1.1 | 1.1 | 1.4 | 1.1 | 1.1 | 1.1 | ||
| Positive type I/III control | 2.4 | 1.0 | 1.1 | 1.1 | 1.4 | 1.1 | 1.1 | 1.1 | ||
| 1 | Mother | 1.1 | 1.0 | 2.0 | 1.2 | 16.7 | 1.0 | 1.0 | 1.1 | II |
| Infant | 1.3 | 1.0 | 1.3 | 0.9 | 2.0 | 1.0 | 0.9 | 1.0 | II | |
| 2 | Mother | 1.0 | 1.0 | 1.0 | 0.9 | 1.3 | 1.0 | 1.0 | 1.0 | Uncertain |
| Infant | 1.0 | 1.0 | 1.1 | 1.0 | 1.6 | 1.0 | 1.0 | 1.0 | II | |
| 3 | Mother | 1.0 | 1.7 | 5.5 | 4.8 | 3.5 | 1.1 | 1.1 | 1.2 | II |
| Infant | 1.0 | 1.4 | 4.9 | 4.0 | 2.8 | 1.3 | 1.0 | 1.1 | II | |
| 4 | Mother | 1.1 | 0.9 | 2.4 | 1.7 | 4.5 | 1.1 | 1.1 | 1.1 | II |
| Infant | 1.1 | 1.0 | 1.7 | 1.4 | 2.4 | 1.0 | 1.1 | 1.1 | II | |
| 5 | Mother | 1.0 | 1.0 | 1.4 | 1.1 | 2.6 | 1.0 | 1.0 | 1.0 | II |
| Infant | 1.0 | 1.0 | 2.3 | 1.1 | 3.8 | 1.1 | 1.3 | 1.1 | II | |
| 6 | Mother | 1.3 | 1.0 | 2.4 | 1.0 | 7.6 | 1.0 | 1.1 | 1.1 | II |
| 7 | Mother | 1.1 | 1.0 | 2.3 | 2.0 | 4.6 | 1.3 | 1.1 | 1.0 | II |
| 11 | Mother | 1.0 | 1.1 | 1.4 | 1.4 | 8.1 | 1.1 | 1.1 | 1.1 | II |
| Infant | 1.0 | 1.0 | 1.4 | 1.3 | 6.1 | 1.0 | 1.0 | 1.1 | II | |
| 12 | Mother | 1.1 | 1.0 | 1.0 | 1.0 | 1.9 | 1.1 | 1.0 | 1.1 | II |
| Infant | 1.0 | 1.0 | 1.2 | 1.1 | 4.3 | 1.0 | 1.0 | 1.0 | II | |
| 13 | Mother | 1.1 | 1.6 | 1.1 | 1.1 | 1.3 | 0.9 | 1.0 | 1.0 | Uncertain |
| Infant | 1.0 | 1.5 | 1.1 | 1.1 | 1.2 | 1.0 | 1.0 | 1.0 | Uncertain | |
| 14 | Mother | 1.4 | 1.1 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 | 1.0 | Uncertain |
| Infant | 1.0 | 1.1 | 1.1 | 1.2 | 2.5 | 1.2 | 1.1 | 1.1 | II | |
| 15 | Mother | 12.5 | 1.7 | 1.1 | 1.4 | 3.3 | 1.2 | 2.8 | 2.2 | Atypical |
| Infant | 9.7 | 1.2 | 1.0 | 1.0 | 2.0 | 1.1 | 2.3 | 1.9 | Atypical | |
| 17 | Mother | 4.9 | 1.2 | 7.9 | 9.6 | Off scale | 1.0 | 3.2 | 2.6 | Atypical |
| Infant | 3.5 | 1.0 | 5.8 | 2.5 | 12.0 | 1.0 | 2.5 | 2.2 | Atypical | |
| 19 | Mother | 1.0 | 1.0 | 3.2 | 2.0 | 7.9 | 1.0 | 1.0 | 1.0 | II |
| Infant | 1.1 | 1.0 | 1.6 | 1.0 | 6.4 | 1.0 | 1.0 | 1.0 | II | |
Peptides utilized in testing are listed across the top of the table. The negative control value is the mean for two negative peptides (see text). Positive controls were provided by patient sera from previously typed cases of toxoplasmosis (see text). Results are shown for sera from the cases listed in Table 1. Sera were reacted against the panel of eight strain-specific peptides to identify allele-specific antibodies present in infection serum. All data were normalized by dividing the A405 reading for each serotyping peptide against the mean of the two control peptides. Values of >1.4 were considered significant (17). Uncertain refers to low values with all peptides, such that a strain type could not be reliably determined. Atypical refers to reaction with peptides from different strain types, reflecting either mixed infection or possibly unusual genotypes.
IgG antibodies detected in neonatal sera are almost certainly derived from transfer of maternal antibodies. Thus, the negative results for the mothers of these infants is likely due to degradation of the sample during storage. Paired maternal and infant sera from case number 13 reacted weakly with the 6 d-I/III peptide but none of the other I/III diagnostic peptides. All type I/III infection sera previously characterized have shown strong reactivity with peptide 6 I/III, so lack of reactivity with this peptide precludes assigning these two sera. Paired maternal and infant sera from case 15 and case 17 reacted strongly with both type II and type I/III peptides, suggesting a possible mixed infection. Mixed infections are highly unusual, and thus their reactivity is listed as atypical. Based on the serological testing, it was concluded that 12 of the 19 cases of congenital toxoplasmosis were due to type II strains.
DISCUSSION
We used multiplex PCR to genotype strains of T. gondii directly in AF and CSF samples from complicated congenital infections in Poland. We found exclusively type II strains of T. gondii and eight of nine typeable cases represented severe cases of toxoplasmosis. Serological typing was consistent with type II genotypes causing the majority of symptomatic and asymptomatic infections. Our findings indicate that type II strains can cause both benign and complicated cases and are consistent with the hypothesis that the severity of infection is primarily related to the burden of parasites.
T. gondii has a highly clonal population structure and most human infections are caused by one of three main genotypes. Type II strains cause the majority (70 to 80%) of human cases of toxoplasmosis reported previously from North America and Europe (primarily France) (1, 5, 12-14). Type I is infrequent in nature, but has been shown to be more common both in congenital toxoplasmosis (6) and in AIDS patients (14). Type III strains are largely found in animals and only rarely cause human infection for reasons that are unknown (14).
In the present study, all of the cases typeable by nested PCR were type II, and eight of nine positives were obtained from severe cases. Five cases resulted in death in utero or early in the neonatal period. A total of 14 of 19 cases studied in the present report were found to be due to type II strains, based on genotyping and/or serological typing. The samples studied here come from a referral hospital that receives patients with complicated pregnancies from a wide area of Poland. Thus, our studies provide a baseline for further analysis of congenital cases of toxoplasmosis in Poland to determine if this pattern of type II infections is widespread. Ajzenberg et al. also described type II in eight out of eight cases of fetal or newborn death and in a majority (73 of 86 samples) of all congenital cases studied from France (1). In contrast to these findings, a study from Spain based on single-locus PCR-RFLP analysis reported that non-type II strains were found in 12 of 13 congenital infections (6). This difference may be primarily related to geography, since any referral bias for severe cases would appear to be the same in both studies. Collectively, these results indicate multiple strain types can be associated with severe toxoplasmosis due to congenital infection.
Clinical forms of congenital toxoplasmosis vary widely. Only 5 to 10% of all infected fetuses develop serious disease, and the majority of infections are asymptomatic (23). Chorioretinitis can result from both congenital and acquired infection (11). Human ocular toxoplasmosis is associated with different strains, including strains containing a mixture of type I and type III genotypes (9). Chorioretinitis was diagnosed in four neonates presented in our study, all due to type II infections. Recently, Miller et al. described T. gondii genotype X that was associated with brain lesions and mortality in sea otters in the western United States (18). Our samples from cases with severe clinical signs of the disease (hydrocephalus and fetal/neonatal death) were found to match the type II, not the type X, genotype.
Semiquantitative PCR with the B1 gene has been used previously to estimate the parasite burden in AF during congenital infection (10, 24). The parasite load estimated using real-time PCR was less then 10 parasites/ml in 40 to 46% of AF samples and 11 to 100 parasites/ml in 30 to 40% of samples (4, 24) Previous studies using real-time PCR in AF established the relationship between high parasite burden and severity of disease (24). PCR detection with B1 is more sensitive than the methods used here; however, it is not sufficiently polymorphic to allow simple RFLP typing of alleles. For these reasons, we developed nested PCR-RFLP typing of several single-copy genes that are polymorphic (16). While these markers are well suited for typing, they are less sensitive overall than detection methods based on B1 PCR.
In our study, the sensitivity of parasite detection by multiplex nested PCR was ≥25 parasites/ml in AF and CSF. Thus, the negative status of many of our samples may have resulted from small amounts of parasite DNA in these samples. Consistent with this, most of those strains that were typeable by PCR (>25 parasites/ml) were from severe cases. These findings are consistent with an association between parasite burden and disease association established previously by more quantitative methods (10, 24). The failure to type some cases by nested PCR-RFLP analyses also raises the possibility that the present samples have an inherent bias. However, our conclusion that type II strains cause the majority of infections in this sample group is supported by similar results obtained by serological testing, which also identified five additional cases of type II infections that were only typeable by serology. The failure to detect type I/III samples among these samples is not due to assay bias, since both PCR-based and serological typing perform well with standards that included samples from patients with infections of known genotypes in the case of serology (Table 3).
Severe symptoms of congenital toxoplasmosis occur mostly as a result of infections that occur early in pregnancy. Results of real-time PCR showed that severe outcome was associated with high parasite load and the highest parasite concentrations were found when seroconversion occurred early in pregnancy (24). In our study, the timing of the mother's primary infection was uncertain due to the lack of serological monitoring. However, all of the mothers had serological results suggesting primary infection during the first or second trimester of pregnancy (IgM and/or IgA and/or low IgG avidity). In Poland, serological screening in pregnancy is not routinely performed, although anti-T. gondii testing is recommended. Routine ultrasound screening during pregnancy is well established in Poland.
In our study, detection of symptoms in a fetus was typically followed by prenatal diagnosis of congenital toxoplasmosis. The prevalence of T. gondii (41.3% seropositive) in pregnant women in Poland is high (20, 20a), and screening of cord blood from neonates, which per se does not detect all congenitally infected cases, indicates a frequency of infection of ∼1 in 1,000 to 2,000 live births (21, 22). Most of these cases are likely not severe, and the high rate of fetal defects among the cases studied here is mainly due to the referral of complicated pregnancies to RIPMMH, suggesting that the majority of asymptomatic cases are not detected. Elective interruption of pregnancies in Poland is very rare, so the emphasis is placed on possible treatment in utero and postnatally (19).
Strain type has been suggested to play a role in determining the outcome of T. gondii infection (3). In our study, T. gondii type II was identified in eight AF and one CSF sample from Polish fetuses and neonates with severe forms of disease. Importantly, for all samples for which PCR genotype data were available, the serotyping assay accurately predicted infection by a type II strain (Tables 2 and 3). While most asymptomatic cases were generally not typeable by PCR, serological analysis indicated a majority of these were also type II. The reaction between mothers and infants was highly correlated, likely reflecting the fact that this assay detects IgG that is acquired by the infant by transfer across the placental barrier in utero. Serum from several cases (13 and 15) reacted to peptides of both type II and I/III, raising the possibility that these patients were multiply infected either with a mix of different strains or by a novel strain(s) that possesses alleles at GRA6 and GRA7 that induce antibodies that react to peptides of both alleles. Interpretation of these sera would require parasite genotype information, but unfortunately, insufficient parasite DNA was recovered from these patients to allow PCR-RFLP genotyping.
Our findings demonstrate that type II strains can be associated with either mild and severe disease and support the hypothesis that high parasite concentration is primarily responsible for the severity of congenital disease. They also demonstrate a close concordance of parasite genotyping by PCR-based genetic markers and serological testing using strain-specific epitopes. Expanded use of serological typing should enable more comprehensive analysis of the contribution of parasite genotype to clinical severity of toxoplasmosis.
Acknowledgments
D.N. was supported by a Fulbright Senior Fellowship Grant. This work was also partially supported by an NIH grant to L.D.S. (AI059176).
We thank Tadeusz H. Dzbenski for PCR testing and mouse inoculation studies conducted in the Department of Medical Parasitology, National Institute of Hygiene, in Warsaw, Poland, Asis Khan for technical advice, and Julie Suetterlin and Dorota Stodolnik for technical assistance.
REFERENCES
- 1.Ajzenberg, D., N. Cogné, L. Paris, M. H. Bessieres, P. Thulliez, D. Fillisetti, H. Pelloux, P. Marty, and M. L. Dardé. 2002. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis and correlation with clinical findings. J. Infect. Dis. 186:684-689. [DOI] [PubMed] [Google Scholar]
- 2.Barragan, A., and L. D. Sibley. 2002. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 195:1625-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boothroyd, J. C., and M. E. Grigg. 2002. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr. Opin. Microbiol. 5:438-442. [DOI] [PubMed] [Google Scholar]
- 4.Costa, J. M., P. Ernault, E. Gautier, and S. Bretagne. 2001. Prenatal diagnosis of congenital toxoplasmosis by duplex real-time PCR using fluorescence resonance energy transfer hybridization probes. Prenat. Diagn. 21:85-88. [DOI] [PubMed] [Google Scholar]
- 5.Dardé, M. L., B. Bouteille, and M. Pestre-Alexandre. 1992. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J. Parasitol. 78:786-794. [PubMed] [Google Scholar]
- 6.Fuentes, I., J. M. Rubio, C. Ramírez, and J. Alvar. 2001. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J. Clin. Microbiol. 39:1566-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golab, E. 1995. Detection of Toxoplasma gondii DNA in body fluids by polymerase chain reaction. Wiad. Parazytol. 41:13-18. [PubMed] [Google Scholar]
- 8.Grigg, M. E., S. Bonnefoy, A. B. Hehl, Y. Suzuki, and J. C. Boothroyd. 2001. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 294:161-165. [DOI] [PubMed] [Google Scholar]
- 9.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184:633-639. [DOI] [PubMed] [Google Scholar]
- 10.Hohlfeld, P., F. Daffos, J. Costa, P. Thulliez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 11.Holland, G. N. 1999. Reconsidering the pathogenesis of ocular toxoplasmosis. Am. J. Ophthalmol. 128:502-505. [DOI] [PubMed] [Google Scholar]
- 12.Honoré, S., A. Couvelard, Y. J. Garin, C. Bedel, D. Hénin, M. L. Dardé, and F. Derouin. 2000. Genotyping of Toxoplasma gondii strains from immunocompromised patients. Pathol. Biol. (Paris) 48:541-547. [PubMed] [Google Scholar]
- 13.Howe, D. K., S. Honoré, F. Derouin, and L. D. Sibley. 1997. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 35:1411-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 15.Joynson, D. H., and T. J. Wreghitt. 2001. Toxoplasmosis: a comprehensive clinical guide. Cambridge University Press, Cambridge, England.
- 16.Khan, A., C. Su, M. German, G. A. Storch, D. Clifford, and L. D. Sibley. 2005. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J. Clin. Microbiol. 43:5881-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong, J. T., M. E. Grigg, L. Uyetake, S. F. Parmley, and J. C. Boothroyd. 2003. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J. Infect. Dis. 187:1484-1495. [DOI] [PubMed] [Google Scholar]
- 18.Miller, M. A., M. E. Grigg, C. Kreuder, E. R. James, A. C. Melli, P. R. Crosbie, D. A. Jessup, J. C. Boothroyd, D. Brownstein, and P. A. Conrad. 2004. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 34:275-284. [DOI] [PubMed] [Google Scholar]
- 19.Nowakowska, D., M. Respondek-Liberska, E. Golab, B. Stray-Pedersen, K. Szaflik, T. H. Dzbenski, and J. Wilczynski. 2005. Too late prenatal diagnosis of fetal toxoplasmosis: a case report. Fetal Diagn. Ther. 29:190-193. [DOI] [PubMed] [Google Scholar]
- 20.Nowakowska, D., M. Slaska, E. Kostrzewska, and J. Wilczynski. 2001. Anti-Toxoplasma gondii antibody concentration in sera of pregnant women in the sample of Lódz population. Wiad. Parazytol. 47(Suppl. 1):83-89. [PubMed] [Google Scholar]
- 20a.Nowakowska, D., B. Stray-Pedersen, E. Spiewak, W. Sobala, E. Malafiej, and J. Wilczyski. Prevalence and estimated incidence of Toxoplasma infection among pregnant women in Poland: a decreasing trend in the younger population. Clin. Microbiol. Infect., in press. [DOI] [PubMed]
- 21.Paul, M., E. Petersen, Z. S. Pawlowski, and J. Szczapa. 2000. Neonatal screening for congenital toxoplasmosis in the Poznan region of Poland by analysis of Toxoplasma gondii-specific IgM antibodies eluted from filter paper blood spots. Pediatr. Infect. Dis. 19:30-36. [DOI] [PubMed] [Google Scholar]
- 22.Paul, M., E. Petersen, and J. Szczapa. 2001. Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznan region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. J. Clin. Microbiol. 39:1912-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remington, J. S., R. McLeod, P. Thulliez, and G. Desmonts. 2001. Toxoplasmosis, p. 205-346. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders Company, Philadelphia, Pa.
- 24.Romand, S., M. Chosson, J. Franck, M. Wallon, F. Kieffer, K. Kaiser, H. Dumon, F. Peyron, P. Thulliez, and S. Picot. 2004. Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am. J. Obstet. Gynecol. 190:797-802. [DOI] [PubMed] [Google Scholar]
- 25.Sibley, L. D. 2003. Recent origins among ancient parasites. Vet. Parasitol. 115:185-198. [DOI] [PubMed] [Google Scholar]
- 26.Sibley, L. D., and J. C. Boothroyd. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature (London) 359:82-85. [DOI] [PubMed] [Google Scholar]
- 27.Su, C., D. Evans, R. H. Cole, J. C. Kissinger, J. W. Ajioka, and L. D. Sibley. 2003. Recent expansion of Toxoplasma through enhanced oral transmission. Science 299:414-416. [DOI] [PubMed] [Google Scholar]
- 28.Su, C., D. K. Howe, J. P. Dubey, J. W. Ajioka, and L. D. Sibley. 2002. Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 99:10753-10758. [DOI] [PMC free article] [PubMed] [Google Scholar]